Abstract
Recent work by several groups has undoubtedly shown that we can produce cancer vaccines targeting neoantigens. However, each vaccine is essentially a single-use, patient-specific product, making this approach resource-intensive. For this reason, it is important to ask whether this approach will be any more successful than what has been attempted during the last 30 years using vaccines targeting self-epitopes. Here, we discuss what might be expected from neoantigen vaccines based on our experience in chronic viral infections, and how this new approach may be applied to cancer immunotherapy.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
IMMUNOTHERAPY AND VACCINATION
Using the immune system has long been proposed as a method to treat cancer, although the field only really gained momentum in the late 1980s and early 1990s when specific mechanisms underpinning this approach were established. The identification of tumor-associated antigens began with MAGE-A1 in melanoma and was then followed by the discovery of additional antigens in several other cancers, which highlighted that T cells could recognize and respond to self-antigens in tumors (van der Bruggen et al. 1991; Vigneron et al. 2013). Concurrent work from Rosenberg and team at the National Institutes of Health (NIH) showed that the adoptive transfer of large numbers of tumor antigen–specific T cells could cause regression of widely metastatic disease (Rosenberg et al. 1988; Hinrichs and Rosenberg 2014). Finally, a number of key experiments showed T-cell recognition and killing of tumor cells (Dighe et al. 1994; Engel et al. 1997; Shankaran et al. 2001; Dunn et al. 2004). Together, these studies established the theoretical basis required for a viable therapeutic vaccine; tumor-specific antigens existed, and the immune system could kill cancer when tumor antigen–specific T cells were present in sufficient numbers.
Following these discoveries, several approaches have been pursued in the development of vaccines against various cancers with many recently completing phase III trials, although recent results from these trials have been less successful than expected. Patients treated with Provenge, an autologous dendritic cell vaccine loaded with the prostate cancer antigen prostatic acid phosphatase (PAP), showed an improved overall survival of 4 months in three separate phase III trials (Small et al. 2006; Higano et al. 2009; Kantoff et al. 2010a). The only other cancer vaccine to show positive results in a phase III trial is a peptide derived from the melanoma antigen, gp100 (premelanosone protein [PMEL]). However, this vaccine only generated a small improvement in a progression-free survival of 0.6 months (p = 0.008), and did not show any significant improvement in overall survival (Schwartzentruber et al. 2011). Most recently, vaccines targeting the MAGE-A3 antigen for melanoma and lung cancer also showed no significant improvement in survival (Vansteenkiste et al. 2016). Although there are many factors that could be contributing to this, ultimately these vaccines were only able to induce a relatively poor T-cell response against the cancer antigens. Provenge, which showed the best performance in clinical trials, only generated a fivefold increase in antigen-specific T cells to 20 cells per million PBMCs (peripheral blood mononuclear cells) (Fong et al. 2014). Prostvac, a modified vaccine virus targeting prostate-specific antigen (PSA), also increased antigen-specific T cells by fivefold to produce 30 vaccine-specific cells per million PBMCs (Kaufman et al. 2004; Gulley et al. 2014). Similarly, the maximum response observed for the gp100 peptide vaccine for melanoma was a twofold increase in antigen-specific T cells, and this was only achieved by a small percentage of patients (Sosman et al. 2008). The message from these trials is clear. Although we can induce an immune response against cancer epitopes with various vaccine strategies, the response to these self-epitopes in cancer patients is simply not at the magnitude needed for a consistent antitumor effect.
CAN TARGETING NEOANTIGENS OVERCOME THE PROBLEMS FACED BY SELF-ANTIGEN CANCER VACCINES?
Although the previously tested cancer vaccines target a normally occurring peptide (self-antigen) that is present in cancer, targeting of neoantigens has been less well studied. Neoantigens are the epitopes generated by mutations in tumors. Mutations are a universal feature of cancer, and tumors can have anywhere from a hundred to several thousand protein-coding mutations (Vogelstein et al. 2013). Each of these mutations is a potential nonself epitope that could be recognized by T cells. The appeal of targeting neoantigens for vaccination is clear; because these epitopes are nonself, central tolerance mechanisms will not limit the affinity and number of T cells responding, as is the case with vaccines targeting self-epitopes. Additionally, as the mutations are limited to the tumor, off-target autoimmune side effects will be reduced.
Because of these potential benefits, significant work has been conducted to determine the value of targeting neoantigens to enhance the efficacy of anticancer vaccines. Several groups have recently found T cells specific to neoantigens in cancer patients (Robbins et al. 2013; Gros et al. 2016; McGranahan et al. 2016; Parkhurst et al. 2016; Stronen et al. 2016). Additionally, vaccines targeting neoantigens can induce an immune response in melanoma patients (Ott et al. 2017; Sahin et al. 2017). Although encouraging, significant challenges remain. In particular, the relative rarity of common mutations between individual patients coupled with differences in human leukocyte antigen (HLA) types and T-cell repertoire means that each vaccine has to be truly personalized. Several excellent reviews have discussed how these problems are being addressed and the potential benefits of doing so, but it is reasonable to say that a substantial effort would be required to produce neoantigen-specific vaccines for every patient (Schumacher and Hacohen 2016; Tran et al. 2017; Yarchoan et al. 2017). As the feasibility of this approach has become clearer, the question now is not whether we can develop vaccines against neoantigens, but whether the substantial effort required to make a personalized vaccine is reflected in a significantly better outcome than that observed with self-antigen vaccines.
WHAT TO EXPECT FROM A NEOANTIGEN VACCINE
Before discussing the benefits of neoantigens as cancer vaccine targets, it is worth considering the features of an ideal cancer vaccine. First, the vaccine should induce a significant increase in the number of antigen-specific T cells, as it is reasonable to assume that the antitumor effect will be greater when more antigen-specific T cells are present. Second, it is also important for high-quality T cells to be produced, as we know that higher-affinity T cells generally show a higher efficacy (Varela-Rohena et al. 2008; Zhong et al. 2013). Finally, it would theoretically be ideal for the antigen to be highly tumor-specific to minimize the off-target effects of the vaccine. Although we do not know yet how neoantigens may affect these parameters of a vaccine, we can make some inferences from observations made in chronic viral infections. Epitopes of chronic viral infections are somewhat analogous to neoantigens in cancer in the sense that they are all nonself, but have been present for a long period leading to T-cell exhaustion, and the disease is often spread throughout the body (summarized in Fig. 1).
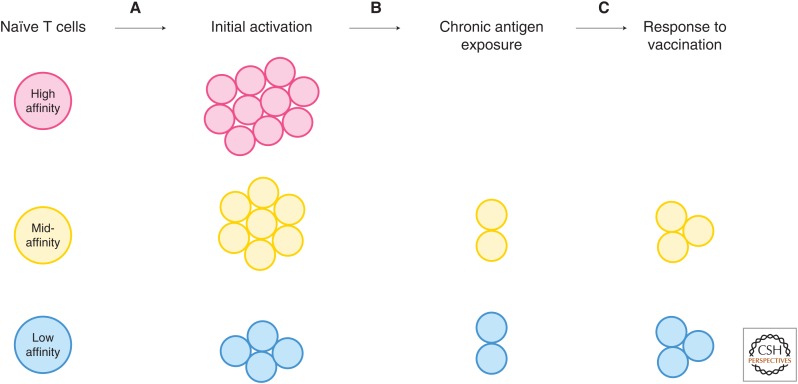
Figure 1.
Loss of T-cell proliferative capacity after long-term antigen exposure. (A) Activation of naïve T cells results in different levels of expansion based on many factors, including affinity. Generally, this is more than a 1 × 104-fold increase in the number of CD8 T cells specific for any epitope. (B) If the antigen is not cleared, over time, the numbers of T cells specific for an epitope declines. In some cases, T cells responding to high-affinity epitopes are completely deleted after long-term exposure. (C) Vaccination after chronic exposure to antigen, even in the case of viral nonself epitopes, is ineffective. High-affinity cells may not be present to respond to the vaccine, and expansion ranges from a two- to fivefold increase in epitope-specific cells. The number of T cells after vaccination is never restored to the number of T cells induced by the initial activation.
WILL NEOANTIGENS VACCINES PRODUCE A LARGER ANTITUMOR T-CELL RESPONSE?
A key determinant of the size of an immune response generated is the number of T cells specific for the vaccine. Although central tolerance mechanisms are not 100% effective at deleting self-reactive T cells, they do reduce the frequency of these cells (Zhen et al. 2006; Enouz et al. 2012; Yu et al. 2015). Therefore, one advantage of neoantigens may be the potential for a higher frequency of T cells specific to the nonself-epitopes. Significant investigation of therapeutic vaccine strategies to expand T-cell epitopes against chronic viral infections like human immunodeficiency virus (HIV) and hepatitis C virus (HCV), along with extensive investigation of therapeutic vaccines in animal models of chronic viral infections, have been conducted.
Vaccines are generally ineffective at expanding T cells during a chronic viral infection. For example, immunization of mice chronically infected with the clone 13 strain of lymphocytic choriomeningitis virus (LCMV) using a modified vaccinia virus containing the LCMV glycoprotein (GP) epitope fails to induce significant expansion of the T-cell population (Wherry et al. 2005; Ha et al. 2008). Likewise, simian immunodeficiency virus (SIV)-infected primates do not respond to any epitopes in a modified poxvirus vaccine containing gag, pol, and env proteins. In these animals, only 1% of the T cells in the blood are specific for the gag protein, no different from unimmunized animals. In contrast, prior reduction of the viral load by administration of antiretroviral therapy led to 7% of the total CD8 T cells in the animals being gag-specific after vaccination (Hel et al. 2000). Similar effects are seen in human subjects with chronic viral infections receiving vaccinations. A peptide vaccine against the hepatitis B virus (HBV) core protein was only able to induce a threefold increase in the HBV-specific response in HBV-infected patients, but a more than 30-fold increase in uninfected patients (Heathcote et al. 1999). In human HCV infections, a modified vaccinia virus containing HCV proteins was also unable to induce a detectable response in most patients, with those that did respond only showing a minimal increase in T-cell numbers (Habersetzer et al. 2011). Another approach in patients with HCV using a multivalent peptide vaccine found that less than a quarter of the targeted peptides induced a detectable immune response against HCV with the number of induced T cells being very low (Klade et al. 2008). These results, in addition to the poor responses observed in the studies of cancer vaccines, show that the frequency of the T cells responding to the vaccine is not a key factor in determining the subsequent expansion of the population. Instead, it is simply very difficult to expand T cells using vaccines against chronically stimulated epitopes. Because viral antigens and self-epitope vaccines for cancer suffer from similarly low rates of T-cell expansion, and tumor-associated neoantigens are often present in the body for a long time leading to exhaustion of the immune response, it seems unlikely that neoantigen vaccines would be able to generate a much stronger response.
WILL NEOANTIGEN VACCINES PRODUCE A HIGHER QUALITY ANTITUMOR T-CELL RESPONSE?
Many studies in both cancer and viral infections have indicated that higher-affinity T cells generally have a better capacity to kill target cells (Varela-Rohena et al. 2008; Zhong et al. 2013). As central tolerance mechanisms delete the highest-affinity T cells against self-antigens, it is reasonable to assume that the population of T cells expanded by self-antigen-targeting vaccines are generally lower affinity. In contrast, the targeting of neoantigens may allow the expansion of a higher-affinity T-cell population. Although this is an entirely logical hypothesis in a healthy individual, this may not be the case in patients who have experienced long-term exposure to the tumor. Again, we can learn a lot about what to expect from previous analyses of chronic viral infections.
It is a well-described feature of chronic viral infections that high-affinity strongly activated CD8 T cells persist for the shortest time. For example, in the chronic infection clone 13 LCMV model, the highest-affinity epitope, NP396, is deleted after long-term exposure to the virus, while the lower-affinity GP33 epitope persists in an exhausted state (Gallimore et al. 1998; Zajac et al. 1998). Similar observations are reported in chronic γ-herpesvirus, MHV-68, in which the higher-affinity p56 epitope undergoes a large expansion in the initial phase of the virus but then declines to very low levels after long- term stimulation. Meanwhile, the lower-affinity epitope p79, expands much less in the early phase of the infection, but is maintained at a comparable level even in the late stages of the infection (Stevenson et al. 1998). Similarly, loss of the highest-affinity epitopes in the chronic stages of HIV has also been reported (Lichterfeld et al. 2007). These findings indicate that in a chronic antigen setting, although T cells targeting the highest-affinity epitopes will activate very strongly in the initial phases of the response, they often drop far below the levels of T cells targeting lower-affinity epitopes and, in many cases, are deleted entirely. The implications for this in developing a vaccine to treat cancer is that by the time a vaccine is administered, it is likely that the highest-affinity epitopes will be significantly reduced in number or deleted. The result being that neoantigen vaccines may be just as unlikely to induce a high-affinity T-cell response as self-epitope vaccines.
WILL NEOANTIGENS BE ABLE TO PRODUCE A MORE TUMOR-SPECIFIC T-CELL RESPONSE?
The final potential advantage of using neoepitopes for cancer immunotherapy is to reduce the chance of off-target effects on other tissues expressing the target antigen. Because mutations are entirely specific to the tumor, it is fair to assume that vaccines targeting neoantigens should generate a highly tumor-specific response with minimal off-target effects. Although chronic viral infections offer minimal insight in this case, we do have extensive experience with this problem in cancer patients.
Autoimmunity has certainly had some devastating effects on patients receiving transgenic T-cell therapies. In particular, chimeric antigen receptor (CAR) T cells, and affinity-matured T-cell receptors (TCRs) have shown off-target effects in several trials, and in some cases have caused deaths (Johnson et al. 2009; Morgan et al. 2010; Parkhurst et al. 2011; Lamers et al. 2013). In an extreme case, a patient suffered respiratory distress caused by pulmonary infiltrate only 15 min after receiving 1 × 1010 CAR T cells recognizing the ERB-B2 antigen. This patient died after 5 days, presumably the result of the off-target effect of the cells against the low levels of ERB-B2 expressed by the lung tissue (Morgan et al. 2010). Patients with melanoma who were treated with T cells selected for having the highest-affinity endogenous TCR against the MART1 epitope also suffered severe, although manageable, off-target autoimmune side effects (Morgan et al. 2006). In comparison, noncellular vaccine strategies targeting self-epitopes have shown minimal evidence of autoimmune side effects. For example, patients in the final phase III trial of sipuleucel-T only had a minor increase in grade 3 adverse events more than placebo controls (Kantoff et al. 2010a). A phase II trial of the modified vaccinia virus Prostvac also showed a mostly safe profile with only two out of 88 patients having to stop treatment because of adverse events (recurrent lip edema; myocardial infarction) (Kantoff et al. 2010b).
The different prevalence and severity of autoimmune side effects between the endogenous response targeted by vaccination as compared with ex vivo expanded affinity-matured receptor therapies can be explained by cell affinity and cell number, as we have discussed above. The affinity of CAR T cells is 3 to 4 orders of magnitude higher than normal endogenous T cells. Herceptin, which is used as the receptor for the ERB-B2 trial (causing liver toxicity) has a Kd value of 100 pm, whereas natural TCR affinities are ∼100,000 times higher, ranging from 1 to 100 μm (Matsui et al. 1991; Carter et al. 1992). Meanwhile, the T cells used in the high-affinity MART-1 trial discussed above had an affinity within the normal range, but toward the higher end of TCR affinities at 6 μm (Malecek et al. 2014). However, patients that suffered autoimmunity had up to 5 × 1010 of these cells circulating in their blood, far beyond what would be expected even in a powerful viral infection. Simply put, the adoptive T-cell therapies generate an immune response that is not remotely physiological. This effect can be beneficial in terms of the anticancer effect generated, but it is also responsible for the severe side effects observed. It remains unclear whether this nonphysiological level of immune response is required for an effective antitumor response. However, positive results from checkpoint blockade trials, which rely on the endogenous response, suggest that normal-affinity T cells at a normal physiological number are sufficient to cause an antitumor effect. Nonetheless, if a large number of very high-affinity T cells provide the best response in patients, it is almost certain that selecting patient-specific neoantigens would significantly improve the safety of this approach.
WILL NEOANTIGEN VACCINES COMBINE MORE EFFECTIVELY WITH CHECKPOINT BLOCKADE STRATEGIES?
As our therapeutic options to use the immune system to treat cancer expand, it has become clear that combining therapies will yield more successful results. Although checkpoint blockade has been extremely successful to treat cancer, it is not without side effects. As we start to combine more treatments, the side effects will likely be a limiting factor to the types of combinations that can be administered. For this reason, it is important to consider how these treatments may interact with cancer vaccines.
The side effects of these checkpoint inhibitors are ultimately the result of a reduced activation threshold against antigens that we are mostly tolerant to. This complication is well illustrated in transplant recipients who have received checkpoint blockade therapies to treat various tumor types. Cases have been reported of heart, corneal, renal, and liver transplant recipients who have acutely rejected the transplanted organs after receiving checkpoint blockade therapies (Le Fournis et al. 2016; Spain et al. 2016; Friend et al. 2017; Owonikoko et al. 2017). Because almost every self-antigen vaccine targets a molecule expressed elsewhere in the body, these vaccines rely on a therapeutic window in which the antigen is expressed more in the tumor than other locations of the body. In early clinical trials, checkpoint blockade seems to enhance vaccine-induced responses (Madan et al. 2012; van den Eertwegh et al. 2012; Romano et al. 2014). However, based on the rejection of transplanted organs, it is certainly plausible that checkpoint blockade may close that therapeutic window so that T cells induced by vaccines may be just as likely to hit peripheral tissue as the tumor. For this reason, it may be that because of the highly tumor-specific nature of neoantigens, these targets become much more able to be combined with checkpoint blockade as a result of the reduced side effects of the combination.
CONCLUSION
The ideal cancer vaccine would induce large numbers of T cells with high affinity and specificity for tumor antigens, but standard therapeutic vaccines aiming to boost the endogenous response by targeting self-antigens have been unable to achieve this goal. Data from chronic viral infections also indicate that vaccines targeting nonself viral epitopes will face similar challenges trying to expand T-cell populations. For this reason, we predict that targeting neoantigens will not generate a response far exceeding current self-epitope vaccines. However, targeting neoantigens may be an effective strategy to circumvent the autoimmunity caused by high-affinity T cells introduced via CAR methods or adoptive transfer of genetically modified TCRs that have currently only been tested against self-antigens. Furthermore, combination therapy of checkpoint blockade and vaccines targeting neoantigens may have reduced side effects allowing more aggressive combination therapy. Ultimately, neoantigen-targeting cancer vaccines are unlikely to be widely adopted unless they can induce more T cells that can more effectively kill tumor cells, without causing off-target effects. If the vaccines achieve a comparable response to what can already be achieved using self-epitopes, it will certainly not be worth the significant extra effort required to generate these individualized vaccines.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. 1992. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci 89: 4285–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD. 1994. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFNγ receptors. Immunity 1: 447–456. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. 2004. The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360. [DOI] [PubMed] [Google Scholar]
- Engel AM, Svane IM, Rygaard J, Werdelin O. 1997. MCA sarcomas induced in scid mice are more immunogenic than MCA sarcomas induced in congenic, immunocompetent mice. Scand J Immunol 45: 463–470. [DOI] [PubMed] [Google Scholar]
- Enouz S, Carrie L, Merkler D, Bevan MJ, Zehn D. 2012. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J Exp Med 209: 1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA, et al. 2014. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Institute 106: dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend BD, Venick RS, McDiarmid SV, Zhou X, Naini B, Wang H, Farmer DG, Busuttil RW, Federman N. 2017. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Ped Blood Cancer 10.1002/pbc.26682. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 187: 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al. 2016. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 22: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM, et al. 2014. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res 2: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. 2008. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med 205: 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habersetzer F, Honnet G, Bain C, Maynard-Muet M, Leroy V, Zarski JP, Feray C, Baumert TF, Bronowicki JP, Doffoel M, et al. 2011. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology 141: 890–899.e1–4. [DOI] [PubMed] [Google Scholar]
- Heathcote J, McHutchison J, Lee S, Tong M, Benner K, Minuk G, Wright T, Fikes J, Livingston B, Sette A, et al. 1999. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology 30: 531–536. [DOI] [PubMed] [Google Scholar]
- Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, Chougnet C, Shearer G, Altman JD, Watkins D, et al. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med 6: 1140–1146. [DOI] [PubMed] [Google Scholar]
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. 2009. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115: 3670–3679. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Rosenberg SA. 2014. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 257: 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. 2009. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. 2010a. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. 2010b. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28: 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM. 2004. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): A trial of the Eastern Cooperative Oncology Group. J Clin Oncol 22: 2122–2132. [DOI] [PubMed] [Google Scholar]
- Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, Blum H, Buschle M, Jelovcan S, Buerger V, et al. 2008. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology 134: 1385–1395. [DOI] [PubMed] [Google Scholar]
- Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R, et al. 2013. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol Ther 21: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fournis S, Gohier P, Urban T, Jeanfaivre T, Hureaux J. 2016. Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer 102: 28–29. [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Yu XG, Mui SK, Williams KL, Trocha A, Brockman MA, Allgaier RL, Waring MT, Koibuchi T, Johnston MN, et al. 2007. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J Virol 81: 4199–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, et al. 2012. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol 13: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecek K, Grigoryan A, Zhong S, Gu WJ, Johnson LA, Rosenberg SA, Cardozo T, Krogsgaard M. 2014. Specific increase in potency via structure-based design of a TCR. J Immunol 193: 2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Boniface JJ, Reay PA, Schild H, Fazekas de St Groth B, Davis MM. 1991. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science 254: 1788–1791. [DOI] [PubMed] [Google Scholar]
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. 2016. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. 2006. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owonikoko TK, Kumar M, Yang S, Kamphorst AO, Pillai RN, Akondy R, Nautiyal V, Chatwal MS, Book WM, Sahu A, et al. 2017. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: A case report. Cancer Immunol Immunother 66: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, et al. 2011. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19: 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst M, Gros A, Pasetto A, Prickett T, Crystal JS, Robbins P, Rosenberg SA. 2016. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin Cancer Res 23: 2491–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. 2013. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 19: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Michielin O, Voelter V, Laurent J, Bichat H, Stravodimou A, Romero P, Speiser DE, Triebel F, Leyvraz S, et al. 2014. MART-1 peptide vaccination plus IMP321 (LAG-3Ig fusion protein) in patients receiving autologous PBMCs after lymphodepletion: Results of a phase I trial. J Transl Med 12: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. 1988. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 319: 1676–1680. [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, et al. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547: 222–226. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Hacohen N. 2016. Neoantigens encoded in the cancer genome. Curr Opin Immunol 41: 98–103. [DOI] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. 2011. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 364: 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. 2001. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111. [DOI] [PubMed] [Google Scholar]
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. 2006. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24: 3089–3094. [DOI] [PubMed] [Google Scholar]
- Sosman JA, Carrillo C, Urba WJ, Flaherty L, Atkins MB, Clark JI, Dutcher J, Margolin KA, Mier J, Gollob J, et al. 2008. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol 26: 2292–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain L, Higgins R, Gopalakrishnan K, Turajlic S, Gore M, Larkin J. 2016. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol 27: 1135–1137. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Belz GT, Altman JD, Doherty PC. 1998. Virus-specific CD8+ T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc Natl Acad Sci 95: 15565–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, Donia M, Boschen ML, Lund-Johansen F, Olweus J, et al. 2016. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 352: 1337–1341. [DOI] [PubMed] [Google Scholar]
- Tran E, Robbins PF, Rosenberg SA. 2017. “Final common pathway” of human cancer immunotherapy: Targeting random somatic mutations. Nat Immunol 18: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, et al. 2012. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol 13: 509–517. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. 1991. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254: 1643–1647. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, Jassem J, Yoshimura M, Dahabreh J, Nakayama H, et al. 2016. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17: 822–835. [DOI] [PubMed] [Google Scholar]
- Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, et al. 2008. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med 14: 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. 2013. Database of T cell-defined human tumor antigens: The 2013 update. Cancer Immun 13: 15. [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. 2013. Cancer genome landscapes. Science 339: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Ahmed R. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol 79: 8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Johnson BAIII, Lutz ER, Laheru DA, Jaffee EM. 2017. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, et al. 2015. Clonal deletion prunes but does not eliminate self-specific αβ CD8+ T lymphocytes. Immunity 42: 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188: 2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen FS, Du HL, Xu HP, Luo QB, Zhang XQ. 2006. Tissue and allelic-specific expression of hsp70 gene in chickens: Basal and heat-stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. Br Poultry Sci 47: 449–455. [DOI] [PubMed] [Google Scholar]
- Zhong S, Malecek K, Johnson LA, Yu Z, Vega-Saenz de Miera E, Darvishian F, McGary K, Huang K, Boyer J, Corse E, et al. 2013. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci 110: 6973–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]