Abstract
The normal function of lymphatic vessels is to facilitate the trafficking of antigen presenting cells to draining lymph nodes where they evoke an immune response. Donor lymphatic vessels are not connected to that of recipients’ during organ transplantation. The pathophysiology of this disruption has received little attention. Murine heterotopic cardiac transplantation has been used extensively in transplantation research. Following vascularised organ transplantation, the main site of allosensitisation is thought to be in the spleen of the recipient as a result of migration of donor passenger leukocytes via blood. Here, using Single Photon Emission Computed Tomography/Computerised Tomography (SPECT/CT) lymphoscintigraphy, we studied the pattern of lymphatic flow from mouse heterotopic abdominal cardiac grafts and identified mediastinal lymph nodes as the draining nodes for the donor graft. Staining with HY tetramer after transplantation of HY mismatched heart grafts and ELISPOT following allogeneic grafts to detect donor specific T cells revealed them as important sites for allosensitisation. Our data indicates that mediastinal lymph nodes play a crucial role in the alloimmune response in this model, and should be used for ex-vivo and adoptive transfer studies after transplantation in addition to the spleen.
Keywords: SPECT/CT, heart transplant, lymphatics, alloresponse
Introduction
During organ transplantation, the donor artery and vein are anastomosed to suitable counterparts of the recipient. However, due to the technical difficulties posed by their small size and fragility, the lymphatic vessels of the donor organ are not surgically re-connected, despite their vital function in the immune response. Under normal physiological conditions, lymphatics facilitate the trafficking of antigen presenting cells to draining lymph nodes to elicit acquired immune responses. Donor lymphatic vessels re-connect to those of the recipient 2 to 4 weeks after transplantation (1). This period when donor lymphatic vessels are disconnected coincides with the priming of the alloimmune response.
For vascularised organs, with the disruption of lymphatics after transplantation, an alternative route of donor passenger leukocytes migration from donor grafts has been demonstrated. Donor passenger leukocytes enter the bloodstream, presumably through reverse transmigration across capillaries, and can be found in the spleens of recipients within 24 hours of transplantation (2–5).
In addition to exit via blood, it is also possible that donor passenger leukocytes can leave donor organs via the open severed ends of the lymphatic vessels, into the local vicinity, although this has not been well studied. From here, they could then be taken up by local lymphatic capillaries and traffic to draining lymph nodes.
Heterotopic cardiac transplantation in mice has been an invaluable model in transplantation immunobiology. Traditionally, spleens of recipients are harvested to study the immune response of different cell populations ex vivo (6–8), or for isolation of specific cell populations such as regulatory T cells for adoptive transfer (9, 10). If lymph and donor passenger leukocytes do traffic from the transplanted organ to local draining lymph nodes, these may act as sites of allorecognition in addition to the spleen. However, the existence and identity of the draining lymph nodes for heterotopic cardiac transplants (and other vascularised organ grafts) are unknown. The aim of this work was to study the pattern of lymphatic flow from heterotopic cardiac transplants to determine whether secondary lymphoid compartments other than the spleen take part in the priming of the adaptive immune response.
Materials and Methods
Animals
C57BL/6 (H-2b), DBA/2 (H-2d) and CBA (H-2k) mice were purchased from Harlan UK Ltd. (Oxon, UK), kept in specific pathogen free animal facilities and used in accordance with the Animals (Scientific Procedures) Act 1986. Institutional review board approval was obtained. Cardiac transplantation into the abdomen and neck was performed as previously described (11, 12). All grafts were syngeneic unless otherwise stated.
Single Photon Emission Computed Tomography/CT (SPECT/CT) lymphoscintigraphy
One or 28 days after transplantation, under general anaesthesia, donor hearts were exposed. Nanocoll (GE Healthcare, Buckinghamshire, UK), consisting of 30 megabecquerels (MBq) of Technetium-99m (Tc-99m) labelled human albumin nanoparticle in 0.1ml saline, was injected directly into the left ventricular wall at the apex. Injection of nanocoll is a validated approach to detect lymphatic flow in humans. The incision was then closed and the animals positioned in a nanoSPECT/CT pre-clinical imager (Bioscan, Washington, DC, USA) equipped with a multiplexed multi-pinhole (nine pinholes, aperture 1.0 mm) collimator. Mice were imaged at various time points and acquisition times were defined to obtain 100 000 counts for each projection with 24 projections. Images and maximum intensity projections were reconstructed using the dedicated software Invivoscope (Bioscan, Inc., Washington, USA).
After the final scan, the mice were immediately sacrificed. Samples of blood and organs were removed, weighed, and counted for radioactivity using a Compugamma 1281 gamma counter (LKB Wallac, Turku, Finland). Standard uptake values were calculated using the formula: (cpm (organ)/weight (organ))/(cpm (whole mouse)/weight (whole mouse)).
Immunohistochemistry
Lymph nodes and spleens were harvested in optimal cutting temperature embedding compound (RA Lamb, Eastbourne, UK) and snap frozen in liquid nitrogen. Five µm tissue sections were stained with an antibody against the donor class II molecule I-Ad (Clone AMS-32.1, BD Biosciences, San Jose, USA) as previously described (5).
Flow cytometry
HY mismatched male heart grafts were transplanted into the abdomen of female C57BL/6 mice. Fourteen days post transplant, lymph nodes and spleens were harvested, stained with anti-CD8 PerCP-Cy5.5 (clone 53-6.7, BD Biosciences) and an HY tetramer (PE conjugated HY derived peptide UTY in the context of the class I MHC molecule Db, Beckman Coulter, Brea, Ca, USA) as previously described (13). Cells were acquired using a FACScan flow cytometer (Becton Dickinson, Oxford, UK) and analyzed using Cellquest software version V3.3 (Becton Dickinson).
ELISPOT
Lymph nodes and spleens of recipients were harvested from C57BL/6 recipients of DBA/2 allografts for enzyme-linked immunosorbent spot (ELISPOT) assays as previously described (14), using splenocytes from CBA mice as 3rd party stimulators.
Results
Lymphatic flow post cardiac transplantation in the abdomen
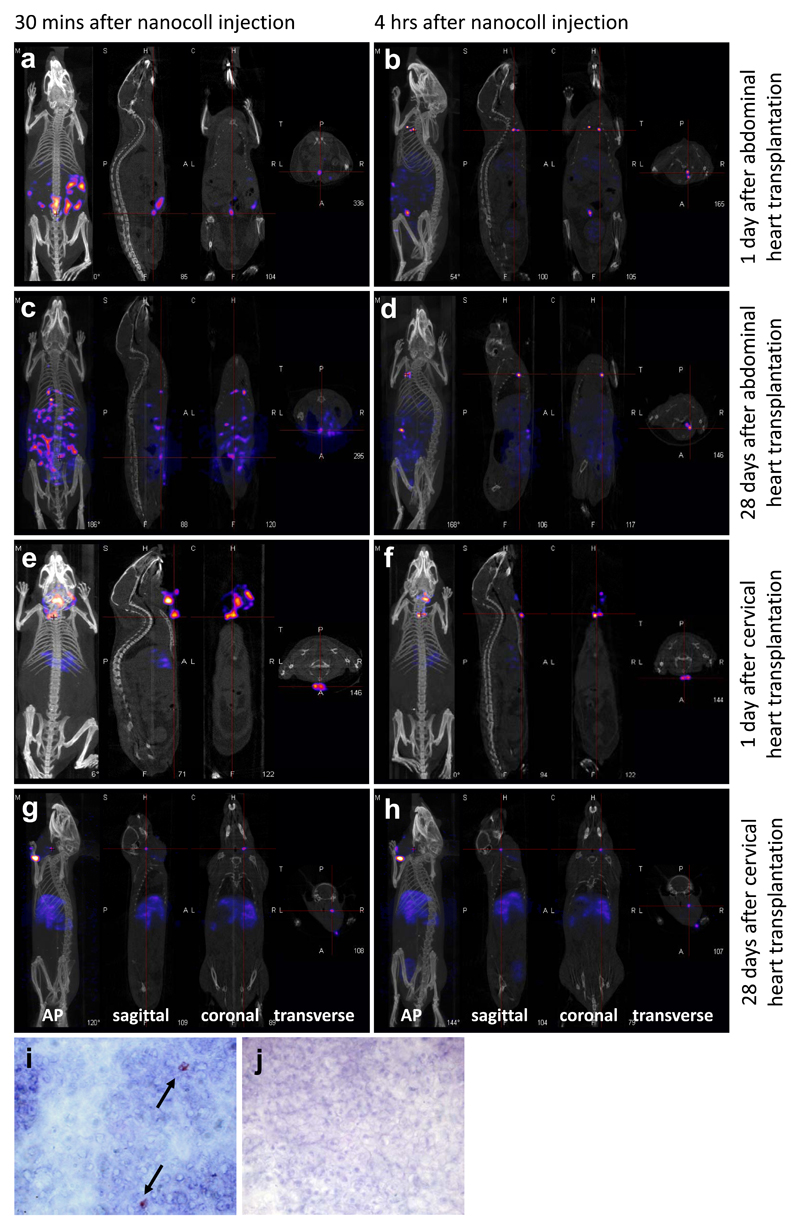
One day after transplantation of syngeneic heart grafts into the abdomen, recipients underwent SPECT/CT lymphoscintigraphy to determine the pattern of lymphatic flow. All recipient mice showed a similar pattern of lymphatic drainage, with representative examples shown in figure 1. Please also refer to videos uploaded as supplementary information for 3D reconstructions which show more detailed images demonstrating the important anatomical structures highlighted by the scans. Thirty minutes after injection, there was an intense region of radioactivity at the injection site within the transplanted heart, and also diffuse radioactivity within the peritoneal cavity, which we have interpreted as lymphatic leak through the open severed ends of donor lymphatic vessels (fig 1a, video S1, supplementary information). Four hours after injection, radioactivity was seen at 3 distinct “hot spots” in the thoracic cavity (fig 1b, video S2, supplementary information) corresponding to the mediastinal lymph nodes and confirmed upon dissection for biodistribution studies (fig 2a).
Figure 1.
a-h: SPECT/CT images taken after injection of nanocoll into the wall of the transplanted hearts. Representative images from one mouse are shown (n=3/4 per group). Each show images taken in 4 planes – (from left to right) the anterior posterior view, and images of the sagittal, coronal and transverse planes taken at the point of the red cross. Green arrows indicate the transplanted heart, and yellow arrows indicate the draining lymph nodes. a,b: one day after abdominal heart transplantation, the animal was scanned 30 minutes (a) and 4 hours (b) after nanocoll injection. c,d: 28 days after abdominal heart transplantation, the animal was scanned 30 minutes (c) and 4 hours (d) after nanocoll injection. e,f: one day after cervical heart transplantation, the animal was scanned 30 minutes (e) and 4 hours (f) after nanocoll injection. g,h: 28 days after cervical heart transplantation, the animal was scanned 30 minutes (g) and 4 hours (h) after nanocoll injection. All views shown are as labelled, except where oblique (b), and lateral (g, h) views are shown instead of AP to demonstrate the draining lymph nodes. Immunohistochemical staining for the donor MHC class II molecules I-Ad on the right anterior inferior mediastinal lymph node (i) and an axillary lymph node (j), harvested 8 days after abdominal transplantation of a DBA/2 (H-2d) heart into a C57BL/6 (H-2b) recipient. Representative examples of 4 independent experiments are shown. Black arrows indicate positive staining. Original magnifications x1000.
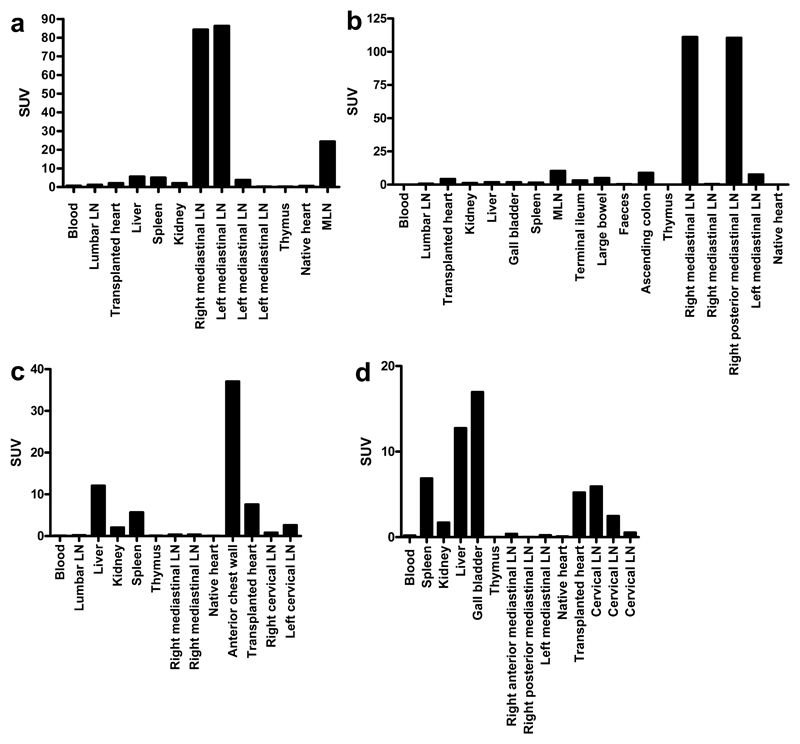
Figure 2.
Biodistribution studies carried out after the final SPECT/CT scan. Radioactivity is expressed as SUV (standard uptake value), calculated using the formula: (cpm (organ)/weight (organ))/(cpm (whole mouse)/weight (whole mouse)). Representative data from one mouse per group is shown (n=3/4 per group). Standard uptake values of each tissue 1 day (a) and 28 days (b) after abdominal heart transplantation, 5 hours after nanocoll injection. Standard uptake values of each tissue 1 day (c) and 28 days (d) after cervical heart transplantation, 5 hours after nanocoll injection.
To determine differences in lymphatic flow after the re-connection of donor lymphatics with those of the recipient, SPECT/CT lymphoscintigraphy was performed 28 days after transplantation on 4 recipients. Again, radioactivity was seen in the mediastinal lymph nodes in the thoracic cavity after 4 hours (fig 1c, d, videos S3 and S4, supplementary information). Biodistribution studies confirmed this (fig 2b).
Anatomical identification of mediastinal lymph nodes
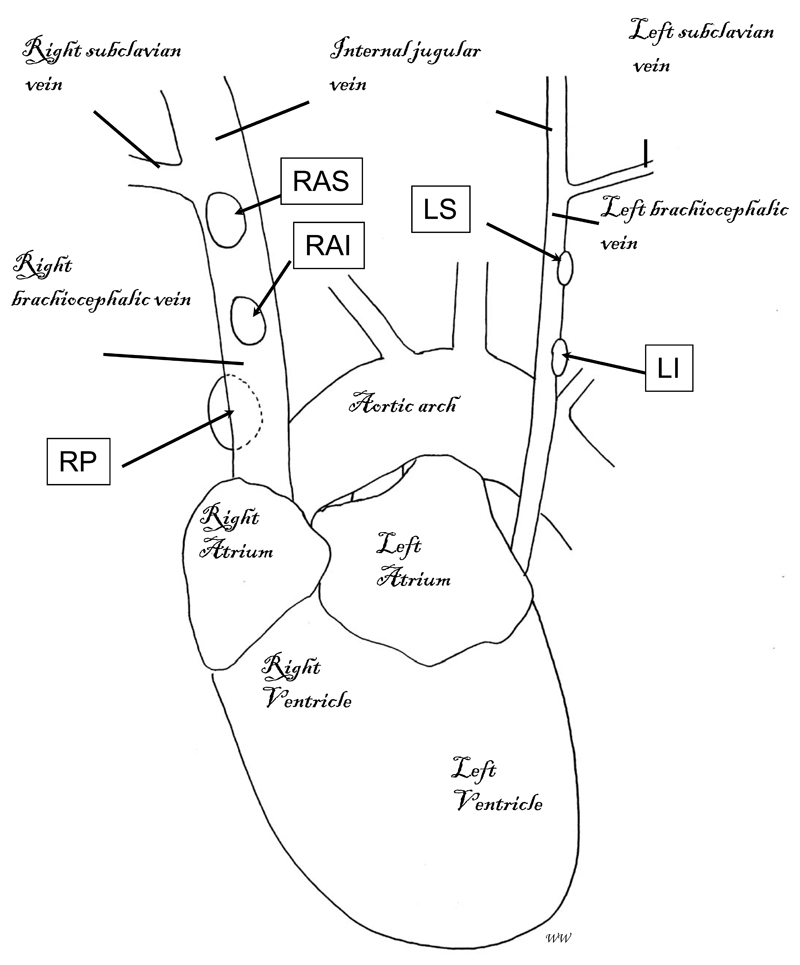
Careful dissection of recipients for biodistribution studies revealed the mediastinal lymph nodes highlighted by SPECT/CT lymphoscintigraphy to be closely related to the left and right brachiocephalic (innominate) veins. We have named them according to their relation to these vessels: right anterior superior (RAS); right anterior inferior (RAI); right posterior (RP); left superior (LS); and left inferior (LI) (fig 3).
Figure 3.
Schematic representation of the position of the mediastinal lymph nodes in the thoracic cavity of a mouse. Numbers and exact positions of these lymph nodes vary slightly between individual mice, but normally consist of: the right anterior superior (RAS) and right anterior inferior (RAI) mediastinal lymph nodes, located on top of the right brachiocephalic vein; the right posterior (RP) mediastinal lymph node, located underneath the right brachiocephalic vein; and the left superior (LS) and left inferior (LI) mediastinal lymph nodes, located on top of the left brachiocephalic vein.
Lymphatic flow post cardiac transplantation in the neck
Second heterotopic heart grafts can be transplanted in the neck of recipients that have received a primary heart allograft in the abdomen as challenge grafts to determine whether donor specific tolerance is achieved. In addition, in humans, some organ grafts are transplanted into the peritoneal cavity, e.g. liver; while others are transplanted outside, e.g. kidney. Antigens administered through different routes are known to elicit different immune responses (15), which may be related to lymphatic trafficking. For these reasons, we performed SPECT/CT lymphoscintigraphy on hearts transplanted subcutaneously into the necks of recipients to investigate the pattern of lymphatic flow one and 28 days after transplantation (n=3 and n=4 respectively).
At day one after transplantation, 30 minutes after injection of nanocoll, radioactivity was seen within the injection site, and in the immediate surrounding area, suggesting again leakage of lymph from the donor organ through severed lymphatics (fig 1e, video S5, supplementary information). Four hours after injection, radioactivity was also observed in discrete hot spots of the anterior chest wall with the appearance of lymph nodes. Unlike after abdominal cardiac transplantation there was no activity seen in mediastinal lymph nodes (fig 1f, 2c, video S6, supplementary information).
Twenty-eight days post transplantation, no lymphatic leakage was seen 30 minutes after nanocoll injection, presumably due to re-connection of lymphatic vessels (fig 1g, video S7, supplementary information). At both 30 minutes and 4 hours after injection, radioactivity could be seen within a deep cervical lymph node (fig 1h, video S8, supplementary information), which was confirmed by biodistribution studies (fig 2d).
Presence of donor antigen within mediastinal lymph nodes
An important function of the lymphatic system is the trafficking of antigen bearing dendritic cells from sites of inflammation to draining lymph nodes to elicit an immune response. To determine whether lymphatic flow from the transplanted organ to the mediastinal lymph nodes observed here resulted in the trafficking of donor antigens to these lymph nodes, DBA/2 heart allografts were transplanted into the abdomen of C57BL/6 recipients. Mediastinal lymph nodes were harvested at day 8 for immunohistochemistry and stained for the donor MHC class II molecule I-Ad.
Positive staining was seen in at least one mediastinal lymph node identified in previous recipients by SPECT/CT lymphoscintigraphy (fig 1i) in all recipients examined (n=4), but not in an irrelevant axillary lymph node (fig 1j), suggesting that lymphatic flow has resulted in the trafficking of donor passenger leukocytes to the mediastinal lymph nodes.
Even in recipients with evidence of donor passenger leukocytes, not all the mediastinal lymph nodes showed positive staining, despite the fact that all the SPECT/CT lymphoscintigraphy consistently demonstrated lymphatic drainage into these lymph nodes. It is possible that small number of donor passenger leukocytes can be missed in immunohistochemistry. Therefore, we sought further, more definitive evidence to support the importance of the mediastinal lymph nodes following heterotopic cardiac transplantation in the abdomen. The generation of donor specific T cells in these lymph nodes, but not in other non-draining nodes early after transplantation would serve this purpose.
Donor specific response within mediastinal lymph nodes
To determine whether lymphatic flow and donor passenger leukocyte trafficking to mediastinal lymph nodes after abdominal cardiac transplantation resulted in the initiation of an anti-donor immune response within them, male C57BL/6 hearts were transplanted into female recipients (n=5). This combination results in donor vasculopathy rather than acute rejection (16). A T cell mediated immune response is generated against the male HY antigen which can be identified by flow cytometry using an HY tetramer, which is recognised by CD8+ T cells specific for the HY derived peptide UTY. Fourteen days after transplantation, tetramer positive, donor-specific CD8+ T cells were found convincingly in at least one “dominant” mediastinal lymph node in all recipients tested. For example, in representative recipient mouse 1 and 2, the right posterior mediastinal and right anterior superior lymph nodes had the highest percentages (1.4 and 2.17% respectively) of donor specific CD8+ T cells (fig 4c, h) while other mediastinal lymph nodes had lower percentages (fig 4a, b, I, j, k), sometimes difficult to distinguish from background. By contrast, no donor specific CD8+ T cells could be found in other lymph nodes (examples of which are shown in fig 4e,l, s, z, θ) except some mesenteric lymph nodes where a small population of donor specific T cells may be present (fig 4f, α, ι). Donor specific CD8+ T cells could also be found in spleens of recipients (fig 4g, n, u, β, κ) Tetramer positive cells are not detected in recipients of gender matched grafts (data not shown).
Figure 4.
Flow cytometry of secondary lymphoid organs after abdominal transplantation of a male heart into a female recipient. Cells were stained with anti-CD8 (vertical axis) and an HY tetramer (horizontal axis) to identify CD8+ donor specific T cells. Mediastinal lymph nodes (top 5 rows) were named according to the scheme shown in figure 3. Due to minor anatomical variations between individuals, not all mediastinal lymph nodes were found in all recipients, shown as blank spaces. Where a donor specific population of CD8+ T cells was observed, as a well defined cell population in the upper right quadrant, its percentage the total CD8+ T cell population are shown in the top right hand corner.
To further demonstrate the presence of donor specific T cells in the mediastinal lymph nodes, and to see if the pattern of lymphatic drainage is the same following fully MHC mismatched, rather than syngeneic or single minor angiten mismatched grafts, DBA/2 heart allografts were transplanted into C57BL/6 recipients (n=6). Draining (mediastinal) lymph nodes and non draining lymph nodes were harvested at days 3 and 4 for IL-2 and IFN-γ ELISPOT analysis. Three days after transplantation, the frequencies of IFN-γ producing cells in mediastinal lymph nodes and non draining lymph nodes were similar (15.3±9.4 and 14.3±6.3 spots/2x105 cells respectively), at a level similar to third party CBA cells stimulated control. Con A stimulated positive control, however, produced 347.3+129.2 spots/2x105 cells. Four days after transplantation, mediastinal lymph nodes had more IFN-γ producing cells compared with non draining nodes (75.1±59.7 vs 42.6±29.3 spots/2x105 cells respectively). Splenocytes contained 33.4±32.9 and 177.6±35.2 spots/2x105 cells at these 2 time points, respectively. ELISPOT assays for IL-2 in all recipients showed low levels of cells similar to that of 3rd party control at these 2 early time points.
Discussion
Our data reveals that lymphatic flow from heterotopic cardiac grafts in the abdominal cavity results initially in leakage into the peritoneal cavity. The route that it follows from here is somewhat tortuous. Detailed studies in non transplant sheep models to study lymphatic drainage from the peritoneal cavity have shown that lymph flows either: (i) into diaphragmatic lymphatics that pass into caudal sternal lymph nodes or mediastinal lymph nodes; or (ii) through the mesothelial lining of the abdominal viscera and then is removed from the interstitium by afferent visceral lymphatics (17). The pattern of lymphatic flow we observed from donor heart grafts in the abdomen appears to be similar to route (i). The flow of lymphatics from the peritoneal cavity to mediastinal lymph nodes has also been demonstrated in rats (18, 19) although another study had suggested flow towards parathymic nodes, where accumulation of plasma cells was observed after heterotopic cardiac allografts transplantation rats (20, 21). The demonstration that lymph drains into mediastinal lymph nodes both before and after the re-connection of donor lymphatic vessels suggests that intact lymphatic vessels are not essential for effective lymph flow towards draining lymph nodes.
In contrast to the large distance travelled by lymph from abdominal cardiac grafts to the mediastinal lymph nodes, lymph from cervical cardiac grafts remains within the local vicinity at all time points analysed. Importantly, no radioactivity was seen in the mediastinal lymph nodes as in the case of abdominal heart transplantation, indicating that drainage to these lymph nodes is specific to the abdominal site. A small amount of nanocoll did enter the bloodstream in some cases, almost certainly due to direct entrance to the bloodstream via blood vessels at the site of injection. As expected, this was entirely taken up by the liver (fig 1g,h), as nanocoll contains nanoparticles. No activity was seen elsewhere, suggesting that nanocoll can only traffic to lymph nodes through lymphatic vessels, and not indirectly via blood. From our data, it is not possible to determine whether the nanocoll trafficked to the draining lymph nodes unchanged or after they have been phagocytosed, or both. No matter which mechanism is involved, it allows the accurate tracking of lymphatic flow.
The particular lymph nodes that had taken up nanocoll did vary slightly from recipient to recipient, but were always a group of mediastinal lymph nodes (after abdominal transplantation) or a cervical lymph node (after cervical transplantation). This variation may have been due to (i) minor anatomical differences between individual animals, (ii) varying degree of surgical disruption to the local lymphatics, (iii) variation in the surgical procedure and lymphatic re-connection after transplantation.
Lymphoscintigraphy at day one showed a diffuse area of radioactivity surrounding the transplanted heart, in both abdominal and cervical transplantation. This was interpreted as being leakage from the open severed ends of the lymphatic vessels. Surprisingly, similar appearances of activity within the peritoneal cavity were also seen 28 days after abdominal heart transplantation (fig 1c, video S3, supplementary information), when donor lymphatics vessels were expected to have re-connected to those of the recipient, as demonstrated by others (1) and as was the case after cervical heart transplantation here (fig 1g, video S7, supplementary information). Preliminary investigation reveals this was likely due to drainage of lymphatics to lymphoid patches within the bowel (fig 2b).
To determine whether the lymph nodes identified by SPECT/CT lymphoscintigraphy were sites where immune priming takes place after transplantation, and therefore have physiological relevance, mediastinal lymph nodes were harvested from female recipients of male heart grafts. Donor specific CD8+ T cells were present within these lymph nodes, in far greater proportions than in mesenteric or axillary lymph nodes from the same recipients. The percentages of CD8+ T cells specific for donor antigen in the mediastinal lymph nodes were comparable to the spleen as demonstrated by our tetramer study; in the ELISPOT study, the frequency of alloreactive T cells was higher in the spleen compared with mediastinal lymph nodes. Put together, these data suggest that these draining lymph nodes are also sites of anti-donor response in addition to the spleen.
Mice without lymph nodes but with intact spleens can reject allografts, but cannot do so if a splenectomy has been performed (22). Conversely, splenectomised recipients with intact lymph nodes can also reject allografts. These observations, together with our data, suggest that lymph is an important additional route to blood for antigen trafficking after vascularised organ transplantation. In the absence of one, the other can compensate and still elicit an effective immune response, demonstrating the principle of redundancy in immunity.
All the SPECT/CT lymphoscintigraphy shown here (figure 1 and supplementary information) were on recipients of syngeneic grafts. However, the finding of donor MHC class II positive cells in recipients of allogeneic DBA/2 heart grafts, our data from the ELISPOT experiments, and demonstration of HY tetramer positive CD8+ T cells in mediastinal lymph nodes of recipients suggest the pattern of lymphatic flow is the same in allogeneic and minor antigen mismatched transplantation. Indeed, SPECT/CT lymphoscintigraphy on MHC mismatched grafts showed the same drainage pattern 1 and 4 days after transplantation. During the early post transplant period, physical factors, e.g. severing of the donor lymphatics, surgical trauma etc. are more important factors influencing the lymphatic flow than immunological factors, which may take time to be activated, especially the adaptive response. Therefore, the pattern of lymphatic drainage from allografts is not expected to be different to that of the syngeneic graft.
It is interesting to note that within the mediastinal lymph nodes identified by SPECT/CT lymphoscintigraphy, usually one “dominant” node (commonly the right posterior mediastinal) is identified as having a much larger donor specific CD8+ T cell population (fig 4c,q,x). This may be because lymph flows towards major lymphatic vessels such as the thoracic duct via a series of lymph nodes connected by lymphatic vessels. It is possible that most donor passenger leukocytes, expressing the appropriate chemokine receptors, are attracted to remain in the first of these lymph nodes they encounter, and therefore, do not enter the next lymph node in the chain, while lymph fluid continues to flow to other lymph nodes downstream.
Mouse heterotopic cardiac transplantation is a widely used experimental model in transplantation. The site of T cell priming after vascularised organ transplantation is generally thought to be the spleen. Our data suggests that mediastinal lymph nodes may be additional sites for this process, which may be more prominent than the spleen. These lymph nodes are easily identified (fig 3) and should be relatively simple to harvest for ex vivo studies or purification into subsets, e.g. regulatory T cells, memory T cells etc. for adoptive transfer into secondary recipients.
Supplementary Material
Additional Supporting Information may be found in the online version of this article.
SPECT/CT of mouse transplanted with donor heart in the abdomen 1 day earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart in the mid abdominal cavity and at distinct hot spots within the peritoneal cavity, suggesting that lymph from the donor organ was draining freely through severed ends of lymphatic vessels.
SPECT/CT of mouse transplanted with donor heart in the abdomen 1 day earlier (4 hours after nanocoll injection). Four hours after injection, Tc-99m activity migrated from the peritoneal cavity to 3 distinct hot spots in the thoracic cavity, suggesting that lymph from the donor organ has flowed towards the mediastinal lymph nodes. The identity of these lymph nodes were confirmed by biodistribution studies at post mortem examination (fig 2a). Tc-99m activity was also seen in the liver after 4 hours. This was most likely to be due to lymph from the donor organ entering the blood stream via the lymphatic route described above.
SPECT/CT of mouse transplanted with donor heart in the abdomen 28 days earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart in the abdominal cavity left of midline and at distinct hot spots within the peritoneal cavity.
SPECT/CT of mouse transplanted with donor heart in the abdomen 28 days earlier (4 hours after nanocoll injection). Four hours after injection, Tc-99m activity migrated from the peritoneal cavity to 2 distinct hot spots in the thoracic cavity, suggesting that lymph from the donor organ has flowed towards the mediastinal lymph nodes in the same way as 1 day after transplantation (supplementary video 2).
SPECT/CT of mouse transplanted with donor heart in the neck 1 day earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart, at distinct hot spots in the surrounding tissue and anterior to the upper chest wall.
SPECT/CT of mouse transplanted with donor heart in the neck 1 day earlier (4 hours after nanocoll injection). Four hours after injection, the high intensity areas in the neck have subsided while the 2 hot spots on the chest wall intensified, suggesting that lymph from the transplanted organ has flowed out of severed lymphatic vessels and travelled to the local draining lymph nodes. The liver also took up Tc-99m activities most likely due to small amount of nanocoll entering the bloodstream.
SPECT/CT of mouse transplanted with donor heart in the neck 28 days earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart. In sharp contrast to the 1 day post transplant scan (supplementary video 5), there was no leak of Tc-99m into the surrounding tissue, demonstrating that the donor lymphatic vessels have completely re-connected with that of the recipient. There was one small superficial hot spot above the transplanted heart. There was also one hot spot in the deep cervical region under the right mandible.
SPECT/CT of mouse transplanted with donor heart in the neck 28 days earlier (4 hours after nanocoll injection). Again, there was one small superficial hot spot above the transplanted heart and one hot spot in the deep cervical region under the right mandible. These were confirmed to be a surgical knot formed by suture used to close the wound which was contaminated by Tc-99m and a deep cervical lymph node respectively in biodistribution studies. Tc-99m activity was also seen in the liver, most likely due to entry of nanocoll into the bloodstream.
Acknowledgements
The nanoSPECT/CT pre-clinical imager was funded by a grant from the Wellcome Trust. MAF was supported by an IMB capacity building award from the BBSRC. The authors thank Mr Jim Ballinger (Dept. of Nuclear Medicine, Guy’s Hospital, London) for providing the Nanocoll.
List of Abbreviations
- cpm
counts per minute
- ELISPOT
enzyme-linked immunosorbent spot
- LI
left inferior mediastinal lymph node
- LS
left superior mediastinal lymph node
- MBq
mega Becquerels
- RAI
right anterior inferior mediastinal lymph node
- RAS
right anterior superior mediastinal lymph node
- RP
right posterior mediastinal lymph node
- SPECT/CT
Single Photon Emission Computed Tomography/Computerised Tomography
- SUV
standard uptake value
- Tc-99m
Technetium-99m
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ruggiero R, Muz J, Fietsam R, Jr, Thomas GA, Welsh RJ, Miller JE, et al. Reestablishment of lymphatic drainage after canine lung transplantation. Journal of Thoracic & Cardiovascular Surgery. 1993;106(1):167–71. [PubMed] [Google Scholar]
- 2.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. Journal of Experimental Medicine. 1990;171(1):307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saiki T, Ezaki T, Ogawa M, Matsuno K. Trafficking of host- and donor-derived dendritic cells in rat cardiac transplantation: allosensitization in the spleen and hepatic nodes. Transplantation. 2001;71(12):1806–15. doi: 10.1097/00007890-200106270-00017. [DOI] [PubMed] [Google Scholar]
- 4.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 5.Brown K, Sacks SH, Wong W. Extensive and bidirectional transfer of major histocompatibility complex class II molecules between donor and recipient cells in vivo following solid organ transplantation. FASEB Journal. 2008;22(11):3776–84. doi: 10.1096/fj.08-107441. [DOI] [PubMed] [Google Scholar]
- 6.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 7.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. Journal of Immunology. 2001;166(6):3789–96. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. Journal of Immunology. 2007;179(8):5238–45. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 9.Hall BM, Jelbart ME, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. Mediation of specific suppression by T helper/inducer cells. Journal of Experimental Medicine. 1985;162(5):1683–94. doi: 10.1084/jem.162.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZK, Cobbold SP, Waldmann H, Metcalfe S. Amplification of natural regulatory immune mechanisms for transplantation tolerance. Transplantation. 1996;62(9):1200–6. doi: 10.1097/00007890-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Wong W, Morris PJ, Wood KJ. Pretransplant administration of a single donor class I major histocompatibility complex molecule is sufficient for the indefinite survival of fully allogeneic cardiac allografts: evidence for linked epitope suppression. Transplantation. 1997;63(10):1490–4. doi: 10.1097/00007890-199705270-00020. [DOI] [PubMed] [Google Scholar]
- 13.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, et al. Homeostatic Proliferation of Lymphocytes Results in Augmented Memory-Like Function and Accelerated Allograft Rejection. J Immunol. 2008;180(6):3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 14.Shariff H, Tanriver Y, Brown KL, Meader L, Greenlaw R, Mamode N, et al. Intermittent antibody-based combination therapy removes alloantibodies and achieves indefinite heart transplant survival in presensitized recipients. Transplantation. 2010;90(3):270–8. doi: 10.1097/TP.0b013e3181e228bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCluskie MJ, Brazolot Millan CL, Gramzinski RA, Robinson HL, Santoro JC, Fuller JT, et al. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Molecular Medicine. 1999;5(5):287–300. [PMC free article] [PubMed] [Google Scholar]
- 16.He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, et al. Effects of T cell frequency and graft size on transplant outcome in mice. Journal of Immunology. 2004;172(1):240–7. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- 17.Abernethy NJ, Chin W, Hay JB, Rodela H, Oreopoulos D, Johnston MG, et al. Lymphatic drainage of the peritoneal cavity in sheep. American Journal of Physiology. 1991;260(3 Pt 2):F353–8. doi: 10.1152/ajprenal.1991.260.3.F353. [DOI] [PubMed] [Google Scholar]
- 18.Bennett HS, Shivas AA. The visualization of lymph nodes and vessels by ethyl iodostearate (Angiopac) and its effect on lymphoid tissue. A preliminary radiological and histological study. J Fac Radiol. 1953–1954;5:261–266. doi: 10.1016/s0368-2242(54)80014-5. [DOI] [PubMed] [Google Scholar]
- 19.Olin T, Saldeen T. The lymphatic pathways from the peritoneal cavity: a lymphangiographic study in the rat. Cancer Research. 1700;24:1700–11. [PubMed] [Google Scholar]
- 20.Baldwin WM, 3rd, Hendry W, Birinyi LK, Jr, Tilney NL. Immune responses to organ allografts. I. Intense B-cell response to heart allografts in lymphoid tissues of unmodified rats. Laboratory Investigation. 1979;40(6):695–702. [PubMed] [Google Scholar]
- 21.Kupiec-Weglinski JW, Tilney NL. Lymphocyte migration patterns in organ allograft recipients. Immunological Reviews. 1989;108:63–82. doi: 10.1111/j.1600-065x.1989.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 22.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y, et al. Immunologic 'ignorance' of vascularized organ transplants in the absence of secondary lymphoid tissue. Nature Medicine. 2000;6(6):686–8. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPECT/CT of mouse transplanted with donor heart in the abdomen 1 day earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart in the mid abdominal cavity and at distinct hot spots within the peritoneal cavity, suggesting that lymph from the donor organ was draining freely through severed ends of lymphatic vessels.
SPECT/CT of mouse transplanted with donor heart in the abdomen 1 day earlier (4 hours after nanocoll injection). Four hours after injection, Tc-99m activity migrated from the peritoneal cavity to 3 distinct hot spots in the thoracic cavity, suggesting that lymph from the donor organ has flowed towards the mediastinal lymph nodes. The identity of these lymph nodes were confirmed by biodistribution studies at post mortem examination (fig 2a). Tc-99m activity was also seen in the liver after 4 hours. This was most likely to be due to lymph from the donor organ entering the blood stream via the lymphatic route described above.
SPECT/CT of mouse transplanted with donor heart in the abdomen 28 days earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart in the abdominal cavity left of midline and at distinct hot spots within the peritoneal cavity.
SPECT/CT of mouse transplanted with donor heart in the abdomen 28 days earlier (4 hours after nanocoll injection). Four hours after injection, Tc-99m activity migrated from the peritoneal cavity to 2 distinct hot spots in the thoracic cavity, suggesting that lymph from the donor organ has flowed towards the mediastinal lymph nodes in the same way as 1 day after transplantation (supplementary video 2).
SPECT/CT of mouse transplanted with donor heart in the neck 1 day earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart, at distinct hot spots in the surrounding tissue and anterior to the upper chest wall.
SPECT/CT of mouse transplanted with donor heart in the neck 1 day earlier (4 hours after nanocoll injection). Four hours after injection, the high intensity areas in the neck have subsided while the 2 hot spots on the chest wall intensified, suggesting that lymph from the transplanted organ has flowed out of severed lymphatic vessels and travelled to the local draining lymph nodes. The liver also took up Tc-99m activities most likely due to small amount of nanocoll entering the bloodstream.
SPECT/CT of mouse transplanted with donor heart in the neck 28 days earlier (30 minutes after nanocoll injection). Thirty minutes after injection, Tc-99m could be seen at the injection site within the transplanted heart. In sharp contrast to the 1 day post transplant scan (supplementary video 5), there was no leak of Tc-99m into the surrounding tissue, demonstrating that the donor lymphatic vessels have completely re-connected with that of the recipient. There was one small superficial hot spot above the transplanted heart. There was also one hot spot in the deep cervical region under the right mandible.
SPECT/CT of mouse transplanted with donor heart in the neck 28 days earlier (4 hours after nanocoll injection). Again, there was one small superficial hot spot above the transplanted heart and one hot spot in the deep cervical region under the right mandible. These were confirmed to be a surgical knot formed by suture used to close the wound which was contaminated by Tc-99m and a deep cervical lymph node respectively in biodistribution studies. Tc-99m activity was also seen in the liver, most likely due to entry of nanocoll into the bloodstream.