Abstract
Breast cancer susceptibility gene 1 (BRCA1) and binding partner BRCA1-associated RING domain protein 1 (BARD1) form an essential E3 ubiquitin ligase important for DNA damage repair and homologous recombination. The Caenorhabditis elegans orthologs, BRC-1 and BRD-1, also function in DNA damage repair, homologous recombination, as well as in meiosis. Using functional GFP fusions we show that in mitotically-dividing germ cells BRC-1 and BRD-1 are nucleoplasmic with enrichment at foci that partially overlap with the recombinase RAD-51. Co-localization with RAD-51 is enhanced under replication stress. As cells enter meiosis, BRC-1-BRD-1 remains nucleoplasmic and in foci, and beginning in mid-pachytene the complex co-localizes with the synaptonemal complex. Following establishment of the single asymmetrically positioned crossover on each chromosome pair, BRC-1-BRD-1 concentrates to the short arm of the bivalent. Localization dependencies reveal that BRC-1 and BRD-1 are interdependent and the complex fails to properly localize in both meiotic recombination and chromosome synapsis mutants. Consistent with a role for BRC-1-BRD-1 in meiotic recombination in the context of the synaptonemal complex, inactivation of BRC-1 or BRD-1 enhances the embryonic lethality of mutants defective in chromosome synapsis. Our data suggest that under meiotic dysfunction, BRC-1-BRD-1 stabilizes the RAD-51 filament and alters the recombination landscape; these two functions can be genetically separated from BRC-1-BRD-1’s role in the DNA damage response. Together, we propose that BRC-1-BRD-1 serves a checkpoint function at the synaptonemal complex where it monitors and modulates meiotic recombination.
Author summary
Our genomes are passed down from one generation to the next through the specialized cell division program of meiosis. Meiosis is highly regulated to coordinate both the large scale chromosomal and fine scale DNA events to ensure fidelity. While the tumor suppressor BRCA1-BARD1 is essential for genome integrity, its specific role in meiosis has been difficult to uncover. Taking advantage of attributes of the Caenorhabditis elegans system, we have analyzed the function of the BRCA1-BARD1 complex in meiosis in this simple metazoan. We find that BRCA1 and BARD1 localize dynamically to the proteinaceous structure that aligns maternal and paternal chromosomes, where it regulates homologous recombination. Although BRCA1 and BARD1 mutants have only subtle meiotic defects, we show that this complex plays critical roles in meiotic recombination when meiosis is perturbed, and this is separable from BRCA1-BARD1’s function in response to DNA damage in somatic cells. These results highlight the complexity of ensuring accurate transmission of the genome and uncover the requirement for this conserved complex in meiosis.
Introduction
BRCA1 was identified twenty-eight years ago as the causative agent of early-onset familial breast cancer [1]. Subsequently, BRCA1 was shown to interact with BARD1 through their RING domains [2], to form an E3 ubiquitin ligase, which adds the small polypeptide ubiquitin to protein substrates [3] (hereafter referred to as BRCA1-BARD1). While BRCA1-BARD1 has been extensively studied with respect to its crucial tumor suppressor activities, we still do not fully understand how this protein complex mediates the diverse functions that have been ascribed to it (e.g., DNA metabolism, checkpoint signaling, chromatin dynamics, centrosome amplification, and transcriptional and translational regulation [4, 5]). This is due in part to the diversity of protein-protein interactions involved in generating numerous distinct BRCA1-BARD1 multi-protein complexes [6]. An additional impediment to understanding BRCA1-BARD1 function is that the corresponding mouse knockouts are embryonic lethal [7, 8].
The simple metazoan Caenorhabditis elegans offers several advantages to the study of this key complex. First, unlike in mammals, C. elegans BRCA1 and BARD1 orthologs, BRC-1 and BRD-1, are not essential yet play critical roles in DNA replication and the DNA damage response, as well as in homologous recombination, which is critical for repairing programmed double strand breaks (DSBs) during meiosis [9–14]. Additionally, attributes of the C. elegans system, including sophisticated genetics, ease of genome editing, and the spatio-temporal organization of the germ line allow us to overcome some challenges inherent in studying this complex in mammalian meiosis.
Meiosis is essential for sexual reproduction and results in the precise halving of the genome for packaging into gametes. During meiosis, homologous chromosomes are connected by crossover recombination to facilitate their alignment and segregation on the meiotic spindle. Recombination is integrated and reinforced with chromosome pairing and synapsis, although the extent of dependencies of these critical meiotic processes are distinct in different organisms (reviewed in [15, 16]). While it is well established that BRCA1-BARD1 plays an important role in DNA repair and recombination [5], the specific function of BRCA1-BARD1 in meiotic recombination is not known. In mice, partial deletions of BRCA1 result in early apoptosis of male germ cells due to failures in meiotic sex chromosome inactivation [17, 18]. BRCA1 has been shown to co-localize with RAD51 on asynapsed chromosomes in mouse spermatocytes, suggesting it functions in meiotic recombination [19]. In C. elegans, brc-1 and brd-1 mutants have mild meiotic phenotypes consistent with a role in some aspect of meiotic recombination [9, 10]. However, the relationship between BRC-1-BRD-1 function in synapsis and recombination has not been explored.
Here, we assessed BRC-1 and BRD-1 dynamics in the C. elegans germ line. Surprisingly, BRC-1-BRD-1 localizes to the synaptonemal complex (SC), becomes concentrated onto chromosome regions upon crossover designation, and at late meiotic prophase is restricted to the short arm of each bivalent as defined by the single crossover site on C. elegans chromosomes. BRC-1 and BRD-1 are interdependent for localization to the SC and proper localization is dependent on meiotic recombination and chromosome synapsis. Further, our data suggest that the BRC-1-BRD-1 complex promotes homologous recombination under meiotic dysfunction by stabilizing the RAD-51 filament and altering the patterning of crossovers. Similar findings are reported by Janisiw et al. in the accompanying paper.
Results
GFP::BRC-1 and BRD-1::GFP are expressed in embryos and the germ line
To examine BRC-1 and BRD-1 expression and localization in C. elegans, we engineered GFP::BRC-1 and BRD-1::GFP fusions at the endogenous loci using CRISPR-Cas9 [20]. brc-1 and brd-1 mutants have low levels of embryonic lethality, produce slightly elevated levels of male progeny (X0), a readout of X chromosome nondisjunction, and display sensitivity to γ-irradiation (IR) [10]. Worms expressing these fusions as the only source of BRC-1 or BRD-1 produced wild-type levels of viable progeny and males, and were not sensitive to IR (S1A–S1C Fig), suggesting that the fusions are fully functional.
We monitored the localization of GFP::BRC-1 and BRD-1::GFP by live cell imaging. In whole worms, GFP fluorescence was observed in embryos and in the germ line, with very little signal in the soma (note auto-fluorescence of gut granules also observed in wild type; Fig 1A). Immunoblots of whole worm extracts of gfp::brc-1; fog-2, which are true females [21] and therefore do not contain embryos, compared to self-fertilizing gfp::brc-1 hermaphrodites containing embryos, revealed that <10% of the GFP::BRC-1 signal is due to expression in embryos (S1E Fig). Thus, BRC-1 and BRD-1 are expressed predominantly in the germ line.
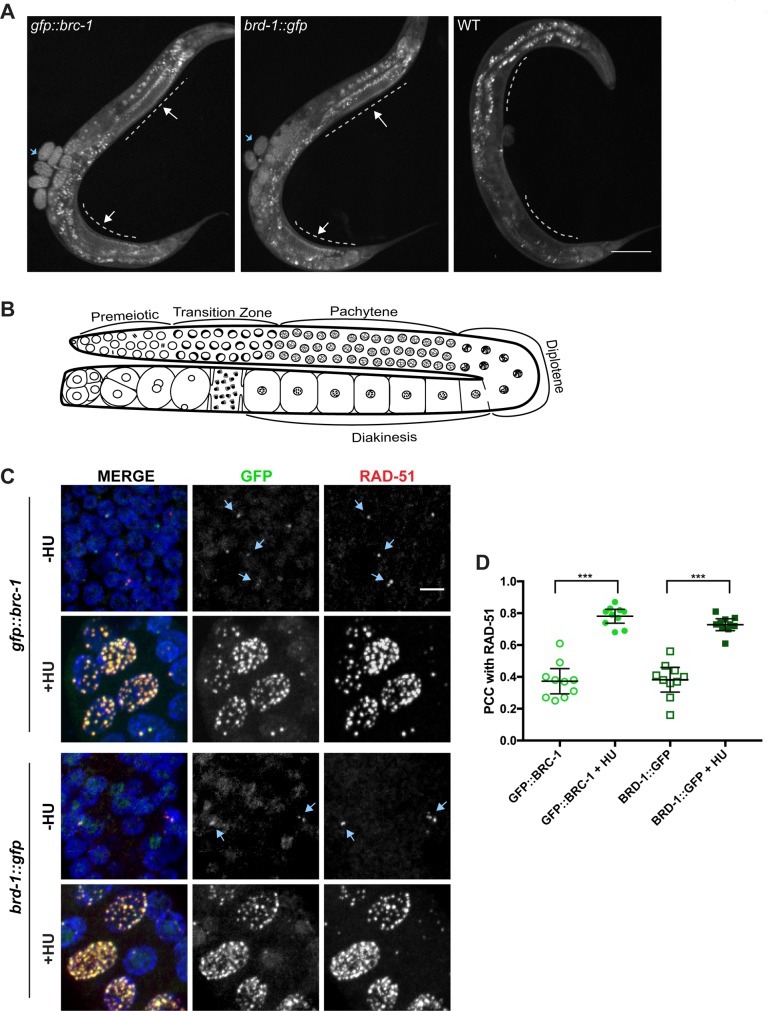
Fig 1. GFP::BRC-1 and BRD-1::GFP are expressed in the germ line and respond to stalled/collapsed replication forks.
A) GFP fluorescence of whole worms expressing GFP::BRC-1, BRD-1::GFP, or no GFP (WT). Dashed line denotes germ line with arrows to indicate GFP fluorescence; blue arrows denote GFP signal in embryos; gut granules auto-fluoresce. Scale bar = 100μm. B) Schematic of the spatiotemporal organization of the hermaphrodite germ line with meiotic stages indicated. C) Proliferating germ cells (premeiotic) expressing GFP::BRC-1 or BRD-1::GFP (green), stained with antibodies against RAD-51 (red), and counterstained with DAPI (blue) in the absence (-HU) and presence of 5mM hydroxyurea (+HU). Blue arrows denote co-localization between GFP and RAD-51. Images are projections through half of the gonad. Scale bar = 5μm. D) Pearson’s Correlation Coefficient (PCC) measurements between RAD-51 and GFP::BRC-1 or BRD-1::GFP in the absence and presence of HU. 95% Confidence Intervals are shown. *** p< 0.0001, Mann-Whitney test; n = 10 nuclei.
BRC-1-BRD-1 and RAD-51 become concentrated in foci upon replication stress
The C. elegans germ line is arranged in a spatio-temporal gradient, with proliferating germ cells (premeiotic) and all stages of meiosis arrayed from the distal to proximal end [22] (Fig 1B). We first focused on the premeiotic zone, where germ cells are mitotically proliferating. GFP::BRC-1 and BRD-1::GFP were observed diffusely throughout the nucleus, with occasional foci that partially co-localized with the recombinase RAD-51 (Fig 1C and 1D). In mammalian cells RAD51 marks stalled/collapsed replication forks [23], and BRCA1/BRC-1 has been implicated in repair of damaged forks in both mammals and C. elegans [14, 24]. To determine whether BRC-1-BRD-1 responds to stalled/collapsed replication forks, we treated worms with the ribonucleotide reductase inhibitor, hydroxyurea (HU). HU slows replication causing fork stalling and collapse, and cell cycle arrest leading to enlarged nuclei [23, 25]. GFP::BRC-1 and BRD-1::GFP fluorescence became enriched in many foci following exposure to HU, and these exhibited substantial co-localization with RAD-51 (Fig 1C and 1D). Consistent with a role in resolving collapsed replication forks, both brc-1 and brd-1 mutants were sensitive to HU as measured by embryonic lethality (S1D Fig). These results suggest that BRC-1-BRD-1 responds to replication stress and concentrates in foci where it co-localizes with RAD-51, presumably to resolve stalled/collapsed replication forks.
BRC-1 and BRD-1 localize to the SC and concentrate to the short arm of the bivalent during meiotic prophase
In early meiotic prophase (transition zone/early pachytene), GFP::BRC-1 and BRD-1::GFP direct fluorescence were observed diffusely on chromatin and in foci (Fig 2A). These foci partially overlapped with RAD-51, which marks meiotic DSBs [26]. We noticed that the relative intensity of the foci was weaker in fixed versus live imaging (see Figs 3 and 4), suggesting that these foci were sensitive to fixation conditions. Beginning at mid-pachytene, GFP::BRC-1 and BRD-1::GFP were observed in tracks along the entire chromosome length, and then concentrated to a portion of each chromosome at late pachytene (Fig 2A). In diplotene and diakinesis, GFP::BRC-1 and BRD-1::GFP were further restricted to six short stretches on the six pairs of homologous chromosomes (Fig 2A). As oocytes continued to mature, GFP::BRC-1 and BRD-1::GFP were disassembled from chromosomes in an asynchronous manner, with some chromosomes losing signal before others. Thus, in diakinesis nuclei we did not always observe six stretches of fluorescence, and the fluorescence intensity varied between chromosomes.
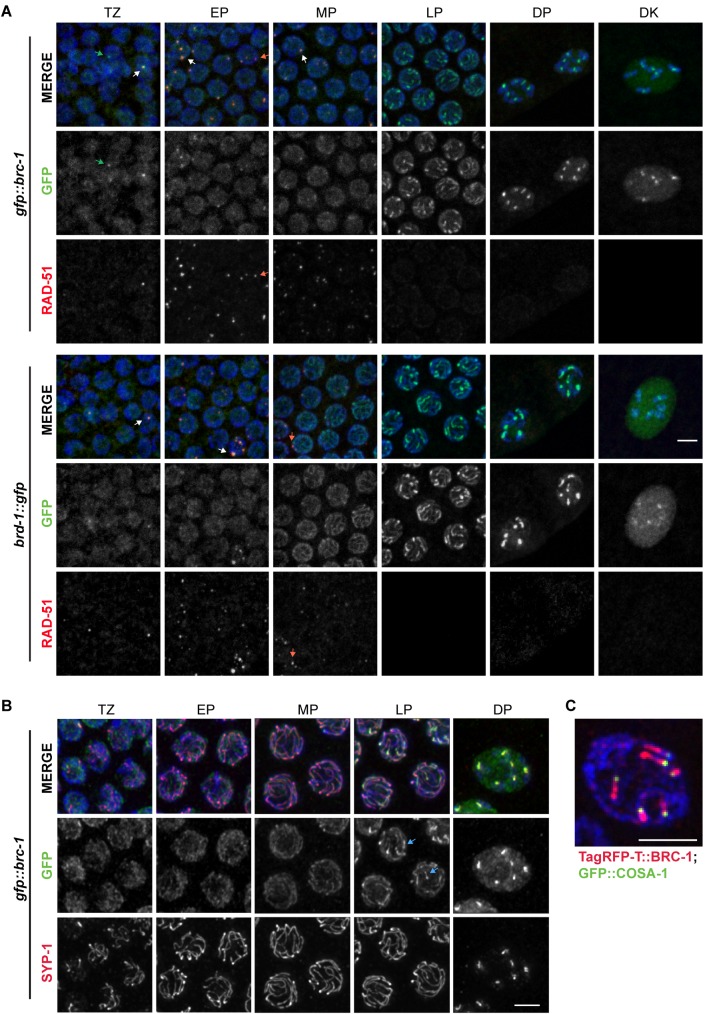
Fig 2. GFP::BRC-1 and BRD-1::GFP localize to the SC in meiotic prophase.
A) Nuclei from indicated meiotic stages stained with RAD-51 antibodies (red), DAPI (blue) and imaged for GFP fluorescence (green). White arrows demark foci positive for both GFP fluorescence and RAD-51 signal; green arrows demark foci containing GFP but not RAD-51; red arrows demark foci containing only RAD-51. Scale bar = 5 μm. B) Co-localization between GFP::BRC-1 (green) and SC central component SYP-1 (red) by antibody staining; germ lines at indicated stages were counterstained with DAPI. Blue arrows at late pachytene show chromosomal regions where GFP::BRC-1 concentrates before SYP-1. Scale bar = 2 μm. C) TagRFP-T::BRC-1 (red) and GFP::COSA-1 (green) at late pachytene showing TagRFP-T::BRC-1 on one side of the GFP::COSA-1 focus, which marks the persumptive crossover. Scale bar = 2 μm. Images are projections through half of the gonad. TZ = transition zone; EP = early pachytene; MP = mid pachytene; LP = late pachytene; DP = diplotene; DK = diakinesis.
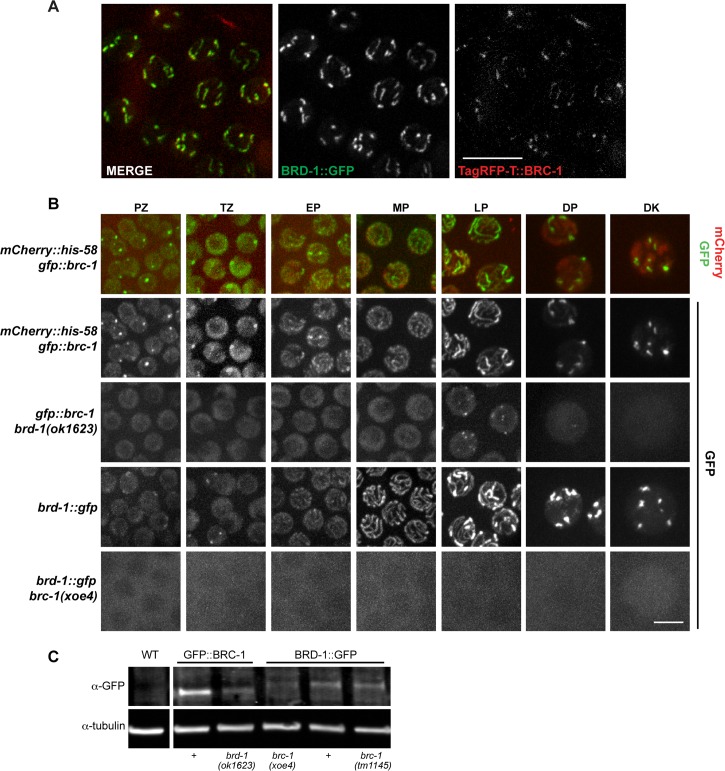
Fig 3. BRC-1 and BRD-1 are inter-dependent for localization.
A) Co-localization between BRD-1::GFP (green) and TagRFP-T::BRC-1 (red) at late pachytene in live worms. Scale bar = 10 μm. B) Stills of germline nuclei from live worms expressing GFP::BRC-1 and mCherry::Histone H2B (mCherry::his-58; gfp::brc-1); merge and GFP channel; top two panels, respectively. GFP::BRC-1 expression in brd-1(ok1623) mutant at indicated meiotic stages. Bottom two panels show BRD-1::GFP localization in wild type and the brc-1(xoe4) mutant. Images are projections through half of the gonad. TZ = transition zone; EP = early pachytene; MP = mid pachytene; LP = late pachytene; DP = diplotene; DK = diakinesis. Scale bar = 5 μm. C) Immunoblot of whole worm extracts from indicated worms probed with anti-GFP and α-tubulin antibodies. Lane 1 = N2: wild type; Lane 2 = JEL515: gfp::brc-1; Lane 3 = JEL520: gfp::brc-1 brd-1(ok1623); Lane 4 = JEL744: brc-1(xoe4) brd-1::gfp; Lane 5 = JEL657: brd-1::gfp; Lane 6 = JEL678: brc-1(tm1145) brd-1::gfp.
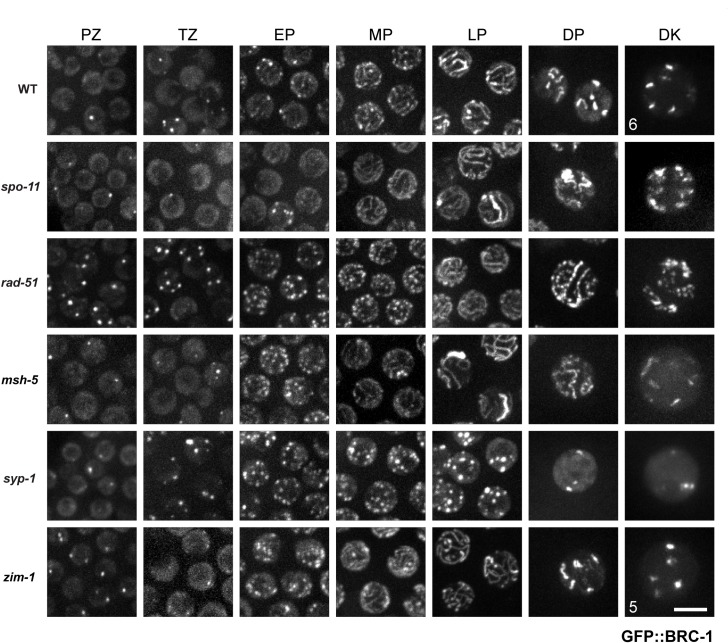
Fig 4. GFP::BRC-1 localization is perturbed when either meiotic recombination or chromosome synapsis is impaired.
High-magnification images of live C. elegans expressing GFP::BRC-1 from the indicated genetic backgrounds and gonad region (PZ = Proliferative Zone, TZ = Transition Zone, EP = Early Pachytene, MP = Mid Pachytene, LP = Late Pachytene, DP = Diplotene, DK = Diakinesis). In wild-type worms GFP::BRC-1 localizes to chromatin and in a small number of foci in the proliferative and transition zones. GFP::BRC-1 localizes to long tracks corresponding to the SC in mid-pachytene. In late pachytene, GFP::BRC-1 becomes condensed to one side of each chromosome, demarcating what will become the short arms of the six bivalents in diakinesis (WT = 6). This localization pattern is perturbed when synapsis and crossover formation are disrupted. A zim-1 diakinesis nucleus with 5 GFP::BRC-1 short stretches is shown. Images are projections through half of the gonad. Scale bar = 5 μm.
The concentration of BRC-1-BRD-1 into tracks at mid-pachytene suggested that the complex localized to the SC. To investigate this, we co-stained with antibodies against GFP and the SC central region component, SYP-1 [27]. Homologous chromosomes begin synapsing early in meiotic prophase (29); however, GFP::BRC-1 was not observed on tracks until after the SC appeared to be fully formed (Fig 2B). Interestingly, the concentration of GFP::BRC-1 to a portion of each chromosome preceded the relocalization of SYP-1 to the short arm of the bivalent (arrows in late pachytene images of GFP::BRC-1; Fig 2B). As the SC reorganizes as a consequence of crossover maturation [28], we examined worms co-expressing TagRFP-T::BRC-1 (TagRFP-T is a RFP variant with improved photostability [20, 29]) and GFP::COSA-1, a cyclin related protein that marks presumptive crossover sites [30]. TagRFP-T::BRC-1 also appeared to be fully functional (S1A–S1C Fig), although the fluorescent signal was weaker than GFP, and could only be detected in mid-late pachytene through diakinesis. GFP::COSA-1 was observed at one end of each TagRFP-T::BRC-1 stretch (Fig 2C). Thus, BRC-1 and BRD-1 localize to the SC and are redistributed concomitant with crossover designation, suggesting that BRC-1-BRD-1 functions in one or more aspects of meiotic recombination within the context of the SC.
BRC-1 and BRD-1 are interdependent for localization
In both mammalian cells and C. elegans, BRCA1/BRC-1 and BARD1/BRD-1 form a stable complex [2, 31]. To probe the relationship between C. elegans BRC-1 and BRD-1 in vivo, we imaged live worms heterozygous for both TagRFP-T::BRC-1 and BRD-1::GFP (brc-1 and brd-1 are linked). In the heterozygous state the TagRFP-T signal could only be detected at late pachytene through early diakinesis when BRC-1 and BRD-1 are concentrated on short tracks. The TagRFP-T and GFP signals overlapped, suggesting that BRC-1 and BRD-1 are localized together on the SC (Fig 3A).
To examine localization dependencies between BRC-1 and BRD-1 in C. elegans germ cells, we monitored GFP::BRC-1 and BRD-1::GFP in the corresponding brd-1(ok1623) and brc-1(xoe4) null mutant backgrounds by live cell imaging. In the absence of BRD-1 we observed diffuse GFP::BRC-1 fluorescence within the nucleoplasm from proliferative zone to mid-pachytene, with no evidence of tracks (Fig 3B). In late pachytene, weak GFP::BRC-1 foci were observed; however, in diplotene and diakinesis only a diffuse nucleoplasmic signal was detected, with no concentrated regions of GFP::BRC-1. This result suggests that BRD-1 is required for the correct localization of BRC-1 in meiotic cells. In worms harboring a null allele of brc-1, BRD-1::GFP was largely cytosolic, except at diakinesis where it was observed in the nucleoplasm. Analysis of steady state protein levels by immunoblot revealed that BRC-1 and BRD-1 were present, albeit at reduced levels, in the absence of the other partner (in brc-1(xoe4), BRD-1::GFP = 60% of wild-type levels; in brd-1(ok1623), GFP::BRC-1 = 50% of wild-type levels; Fig 3C). Thus, BRC-1 and BRD-1 are mutually dependent for localization to meiotic chromosomes.
Impairment of either meiotic recombination or synaptonemal complex formation alters GFP::BRC-1 localization
To provide insight into the relationship between BRC-1-BRD-1 and the progression of meiotic recombination, we monitored the localization of GFP::BRC-1 in mutants that impair different steps of meiotic recombination: spo-11 mutants are unable to form meiotic DSBs [32, 33], rad-51 mutants are blocked prior to strand invasion [34–36], and msh-5 mutants fail to form crossovers [37, 38]. In live spo-11 mutants, we observed many fewer GFP::BRC-1 foci in transition zone and early pachytene compared to WT (TZ: 1.29±0.12 vs. 0.18±0.05; EP: 4.61±0.36 vs. 0.91±0.22 foci/nucleus in WT and spo-11, respectively; p <0.0001; S2A Fig). At mid-pachytene GFP::BRC-1 was observed in tracks in the spo-11 mutant similar to wild type, as synapsis occurs in the absence of meiotic DSB formation in C. elegans [32] (Fig 4). In late pachytene, GFP::BRC-1 fluorescence did not concentrate on a portion of each chromosome pair nor retract to the short arm of the bivalent as in wild type, consistent with these events being dependent on meiotic recombination. However, in 20.23±1.78% of nuclei (n = 4 germ lines) there was enrichment of GFP::BRC-1 on one or sometimes two tracks, in addition to weak staining on other tracks. This is similar to what has been previously reported for synapsis markers, including the phosphorylated form of SYP-4 [39–41], and likely represents spo-11-independent lesions capable of recruiting meiotic DNA repair components and altering SC properties. Consistent with this, we observed GFP::BRC-1 enrichment on the phospho-SYP-4-marked chromosome in spo-11 mutants (S2B Fig). However, GFP::BRC-1 did not retract to chromosome subdomains as in wild type in diplotene and diakinesis, suggesting that the relocalization of BRC-1-BRD-1 is dependent on formation of meiotic DSBs. As expected, BRD-1::GFP was observed in a similar pattern to GFP::BRC-1 in spo-11 mutants throughout meiotic prophase (S2C Fig).
Following DSB formation and processing, RAD-51 is loaded onto resected single-stranded DNA and facilitates strand exchange [36]. GFP::BRC-1 localization was altered in the rad-51 mutant (Fig 4). Significantly increased levels of GFP::BRC-1 foci were observed throughout the germ line. In the proliferative zone, wild type had 0.55±0.04, while rad-51 had 0.96±0.10 foci per nucleus (S2A Fig). These most likely represent concentration of GFP::BRC-1 at stalled/collapsed replication forks. In transition zone, wild type had 1.29±0.12, while rad-51 had 3.98±0.31 foci/nucleus, and this was further increased in early pachytene (WT: 4.6±0.4 vs. rad-51: 13.3±0.7; S2A Fig). These foci presumably represent resected meiotic DSBs that fail to undergo strand invasion in the absence of RAD-51, as they cannot be solely accounted for by the elevated foci observed in proliferating cells. Track-like structures were not observed until late pachytene in the absence of RAD-51. The punctate nature of GFP::BRC-1 was particularly pronounced in diplotene and diakinesis, with no clear concentration to six regions. This is consistent with the disorganized chromatin masses observed in rad-51 diakinesis nuclei [35], and suggests that RAD-51 is required for the proper organization and retraction of GFP::BRC-1.
In msh-5 mutants, GFP::BRC-1 appeared similar to wild type from the proliferative zone to mid pachytene, localizing in the nucleoplasm and concentrating in foci before converging on tracks (Fig 4; S2A Fig). Similar to spo-11, 26.27±2.25% of msh-5 late pachytene nuclei (n = 4 germ lines) contained enrichment of GFP::BRC-1 on one or occasionally two chromosomes. In diplotene, GFP::BRC-1 was observed in long tracks, with no evidence of retraction. The presence of more than six stretches of GFP::BRC-1 in diakinesis suggests that BRC-1 remains associated with the univalents in msh-5 mutants. Taken together, our data suggest that GFP::BRC-1 localizes to the SC and its retraction to the short arm of the bivalent is dependent on processing of meiotic DSBs into crossovers.
We also examined localization of GFP::BRC-1 when synapsis is blocked by mutation of a component of the central region of the SC, syp-1 [27]. GFP::BRC-1 in syp-1 looked similar to wild type in proliferating germ cells (Fig 4). However, as cells entered meiosis GFP::BRC-1 was observed in many foci (in TZ, WT: 1.29±0.12 vs. syp-1: 7.29±0.36 foci/nucleus; S2A Fig). The number of foci increased through early and mid pachytene but GFP::BRC-1 never attained nuclear track staining, supporting a dependency on the SC for track localization. Similarly, the GFP::BRC-1 signal did not localize to sub-regions of condensed (DP and DK) chromosomes, but rather was found in a small number of nuclear foci. Thus, GFP::BRC-1 localization to tracks is dependent on SC formation.
To examine localization under conditions where a subset of chromosomes fail to synapse and recombine, we monitored GFP::BRC-1 localization in the zim-1 mutant, in which chromosomes II and III cannot synapse [42]. In transition zone and early pachytene, GFP::BRC-1 was observed in many foci in the zim-1 mutant, similar to the syp-1 mutant (TZ: WT: 1.29±0.12 vs. zim-1: 4.5±0.36 foci/nucleus; Fig 4; S2A Fig). However, as meiosis progressed GFP::BRC-1 was observed on tracks that condensed to the short arm of the bivalent on multiple chromosomes. Many times we observed more than four stretches of GFP::BRC-1 fluorescence at diplotene/diakinesis (Fig 4), suggesting that there are more than four chiasmata in the zim-1 mutant. We address the role of BRC-1 in chiasmata formation in the zim-1 mutant below.
The BRC-1-BRD-1 complex is important when chromosome synapsis and crossover formation are perturbed
Given the association of GFP::BRC-1 and BRD-1::GFP with the SC (Fig 4), we next examined the functional consequence of removing BRC-1-BRD-1 when synapsis is perturbed. For these studies we focused on the zim-1 mutant, as the appearance of more than four short tracks of GFP::BRC-1 at diplotene/diakinesis (Fig 4) suggested that these BRC-1-BRD-1-associated regions were altered in the absence of zim-1. Additionally, unlike mutants such as syp-1 that result in a complete failure in synapsis and therefore 95% embryonic lethality [27], loss of ZIM-1 results in 73.9% inviable progeny [42], allowing us to determine whether removal of BRC-1-BRD-1 enhances embryonic lethality.
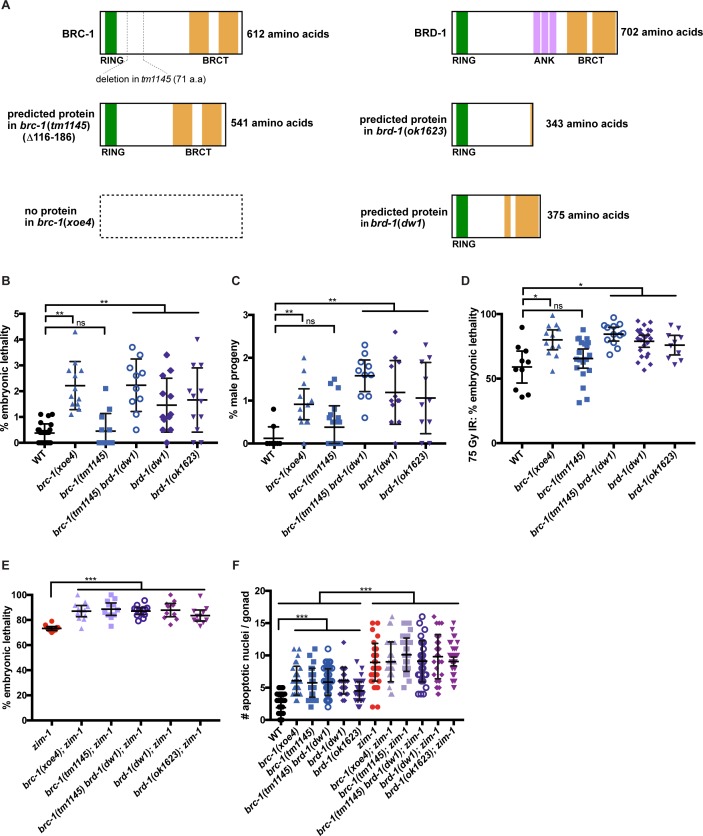
In the course of our experiments we discovered that strain DW102 [31] harbors mutations in both brc-1 and brd-1; sequence analysis revealed that brc-1(tm1145) is an in-frame deletion, removing 71 amino acids (116–186) C-terminal to the predicted RING domain, which in the mammalian ortholog is responsible for E3 ubiquitin ligase activity and dimerization with BARD1 [3, 43, 44] (Fig 5A). The brd-1 mutation in DW102 is identical to brd-1(dw1) [31]; cDNA analysis revealed that the mutation results in the use of an alternative splice site to generate a protein missing 327 amino acids, leaving the RING domain intact (Fig 5A and S3A Fig). To discern the contributions of BRC-1 and BRD-1 we used CRISPR-Cas9 to generate a complete deletion of BRC-1, brc-1(xoe4) (Fig 5A and S3A Fig). We also examined the brc-1(tm1145) and brd-1(dw1) single mutants, the brc-1(tm1145) brd-1(dw1) double mutant and brd-1(ok1623), which results in the removal of 359 amino acids C terminal of the RING domain (Fig 5A and S3A Fig). As expected, brc-1(xoe4), brd-1(dw1), brc-1(tm1145) brd-1(dw1), and brd-1(ok1623) displayed slightly elevated embryonic lethality (Fig 5B), male progeny (Fig 5C), and IR sensitivity (Fig 5D). On the other hand, brc-1(tm1145) was not statistically different from wild type for embryonic lethality, production of male progeny or IR sensitivity, suggesting that this allele is not a null mutation (Fig 5B–5D). Consistent with this, BRD-1::GFP was stable (Fig 3C) and localized similarly to wild type in the brc-1(tm1145) mutant background (S3B Fig).
Fig 5. Differential effect of brc-1 and brd-1 alleles on the DNA damage response and meiosis.
A) Cartoon of predicted proteins produced from the different brc-1 and brd-1 mutant alleles based on cDNA analysis (see also S3A Fig). RING (green), BRCT (gold) and Ankyrin (ANK; purple) domains are indicated. B) % embryonic lethality of brc-1 and brd-1 mutants; numbers of animals scored: WT = 26; brc-1(xoe4) = 12; brc-1(tm1145) = 12; brc-1(tm1145) brd-1(dw1) = 10; brd-1(dw1) = 12; brd-1(ok1623) = 12. C) % male progeny produced from brc-1 and brd-1 mutants; numbers of animals scored: WT = 10; brc-1(xoe4) = 12; brc-1(tm1145) = 22; brc-1(tm1145) brd-1(dw1) = 10; brd-1(dw1) = 12; brd-1(ok1623) = 10. D) % embryonic lethality following 75 Gy IR; numbers of animals scored: WT = 10; brc-1(xoe4) = 12; brc-1(tm1145) = 20; brc-1(tm1145) brd-1(dw1) = 12; brd-1(dw1) = 23; brd-1(ok1623) = 10. E) % embryonic lethality of zim-1 in the presence and absence of BRC-1-BRD-1. Numbers of animals scored: zim-1 = 12; brc-1(xoe4); zim-1 = 12; brc-1(tm1145); zim-1 = 22; brc-1(tm1145) brd-1(dw1); zim-1 = 10; brd-1(dw1); zim-1 = 12; brd-1(ok1623); zim-1 = 10. The genetic interaction between brc-1/brd-1 and zim-1 is significant by a one-way ANOVA (p<0.0001). F) Number of apoptotic nuclei/gonad as scored by acridine orange. Numbers of gonads scored: WT = 43; brc-1(xoe4) = 29; brc-1(tm1145) = 25; brc-1(tm1145) brd-1(dw1) = 41; brd-1(dw1) = 24; brd-1(ok1623) = 50; zim-1 = 42; brc-1(xoe4); zim-1 = 30; brc-1(tm1145); zim-1 = 36; brc-1(tm1145) brd-1(dw1); zim-1 = 30; brd-1(dw1); zim-1 = 31; brd-1(ok1623); zim-1 = 46. 95% Confidence Intervals are shown. Statistical comparisons by Mann-Whitney: * p<0.05; ** p<0.001; *** p<0.0001; ns = not significant.
In contrast to the differential impact of the alleles on embryonic lethality, male progeny, and IR sensitivity, loss of zim-1 in any of the brc-1 or brd-1 mutants resulted in enhanced embryonic lethality compared to the single zim-1 mutant (p<0.0001; Fig 5E). These results suggest that the region C-terminal to the BRC-1 RING domain, which is deleted in brc-1(tm1145), is important for promoting embryonic viability when chromosome pairing and synapsis are perturbed.
To determine the nature of the enhanced embryonic lethality of zim-1 mutants when BRC-1-BRD-1 is impaired, we first monitored germline apoptosis. Apoptosis is an output of checkpoint signaling and is important for culling defective germ cells [45–47]. Previous studies had established that both brc-1 [9] and zim-1 [48] have elevated checkpoint-dependent germline apoptosis. We found that all brc-1 and brd-1 alleles, including brc-1(tm1145), had elevated apoptosis (Fig 5F). Loss of zim-1 resulted in higher levels of apoptosis than brc-1 and brd-1 mutants; however, the levels of apoptosis in the double brc-1; zim-1 and brd-1; zim-1 mutants were not significantly different than zim-1 alone. We also analyzed SUN-1 phosphorylated on Serine12 (Sun-1 S12P), which is dephosphorylated following establishment of the obligate crossover, and serves as a readout of meiotic progression [49]. Loss of ZIM-1 resulted in persistent SUN-1 S12P, which was unaltered in the absence of BRC-1 (S3C Fig). These results suggest that BRC-1-BRD-1 does not function in known signaling pathways responsible for monitoring unrepaired DSBs or crossovers leading to apoptosis or cell cycle delay.
We next monitored RAD-51 assembly/disassembly in the spatiotemporal organization of the germ line. Previous analyses revealed that brc-1 and brd-1 mutant hermaphrodites have elevated RAD-51 foci in late pachytene, suggesting that repair of a subset of meiotic DSBs is delayed in the absence of BRC-1-BRD-1 [9]; this was also observed in the brc-1(tm1145) brd-1(dw1) and brd-1(ok1623) mutants (S4A Fig). Further, blocking synapsis on some or all chromosomes results in elevated RAD-51 levels genome wide [26, 50], as observed in the zim-1 mutant (Fig 6A and 6B). Surprisingly, brc-1; zim-1 and brd-1; zim-1 double mutants resulted in fewer RAD-51 at mid-late pachytene: RAD-51 foci appeared at similar levels compared to the zim-1 single mutant early in meiotic prophase, but in the latter half of pachytene many fewer RAD-51 were detected on chromosomes (Fig 6A and 6B and S4B Fig). High levels of RAD-51 were observed again at the gonad bend, as nuclei exited pachytene and entered diplotene (Fig 6A and 6B and S4B Fig). Similar patterns were observed when BRC-1-BRD-1 was removed in other mutants that perturb synapsis (i.e., syp-1; S4B Fig). These results suggest that when synapsis and therefore crossover formation is impaired, BRC-1-BRD-1 plays a role in DSB formation, DNA end resection, RAD-51 loading, and/or stabilization of the RAD-51 filament in mid-late pachytene.
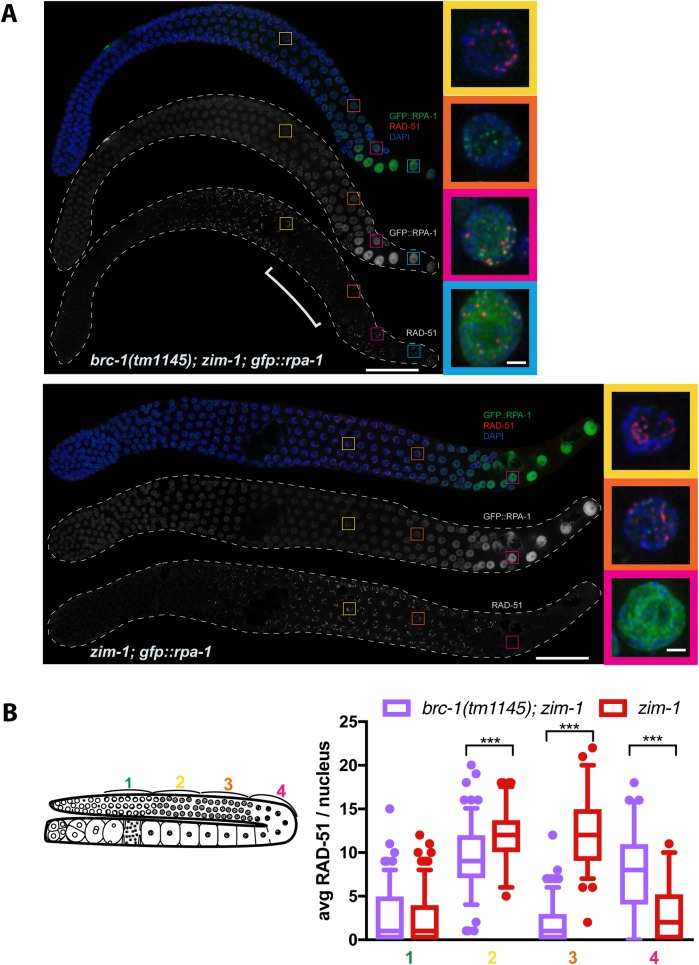
Fig 6. Inactivation of BRC-1-BRD-1 alters the pattern of RAD-51 foci in the zim-1 mutant.
A) Dissected germ lines from brc-1(tm1145); zim-1; gfp::rpa-1 and zim-1; gfp::rpa-1 worms stained with anti-RAD-51 (red), counterstained with DAPI (blue) imagined for GFP::RPA-1 fluorescence (green). Scale bar = 20 μm. Insets show selected nuclei from different regions of the germ line; bracket indicates RAD-51 “dark zone”. Images are projections through half of the gonad. A minimum of 3 germ lines were examined for each genotype. Scale bar = 1 μm. B) Schematic of germ line indicating zones for analysis of RAD-51 foci. Box whisker plots show average number of RAD-51 foci per nucleus in the different zones. Horizontal line of each box indicates the median, the top and bottom of the box indicates medians of upper and lower quartiles, lines extending above and below boxes indicate standard deviation and individual data points are outliers from 5–95%. Statistical comparisons by Mann-Whitney of brc-1(tm1145); zim-1 versus zim-1 in the different regions of the germ line; *** p<0.0001. A minimum of 3 germ lines were analyzed. Numbers of nuclei scored in each zone for brc-1; zim-1: 1 = 177; 2 = 138; 3 = 161; 4 = 61; zim-1: 1 = 159; 2 = 88; 3 = 103; 4 = 78.
To differentiate between these possible meiotic functions of BRC-1-BRD-1, we analyzed the pattern of the single-stranded binding protein RPA-1 (GFP::RPA-1; [51]). RPA-1 binds resected ends prior to RAD-51 loading [52, 53] and is also associated with recombination events at a post-strand-exchange step, which can be observed in chromosome spreads [54]. In the brc-1(tm1145); zim-1 germ line we observed an inverse pattern between RAD-51 and RPA-1 at mid-late pachytene: GFP::RPA-1 foci were prevalent in the region where RAD-51 foci were reduced (Fig 6A). In the zim-1 single mutant, fewer GFP::RPA-1 foci were observed at this stage, while RAD-51 remained prevalent. We also observed very few RPA-1 foci at mid-late pachytene in wild type or brc-1(tm1145) brd-1(dw1) double mutant whole mount gonads (S4C Fig). These results suggest that BRC-1-BRD-1 is not required for DSB formation per se in this region of the germ line, as we observed an increase in GFP::RPA-1 foci, not a decrease as would be expected if BRC-1-BRD-1 mediates DSB formation. Additionally, this result argues against a role for BRC-1-BRD-1 in promoting resection as RPA-1 loads on exposed single stranded DNA [52]. Thus, at mid to late pachytene BRC-1-BRD-1 either facilitates the assembly of RAD-51 on new breaks, and/or stabilizes the RAD-51 filament.
BRC-1-BRD-1 stabilizes the RAD-51 filament when crossover formation is impaired
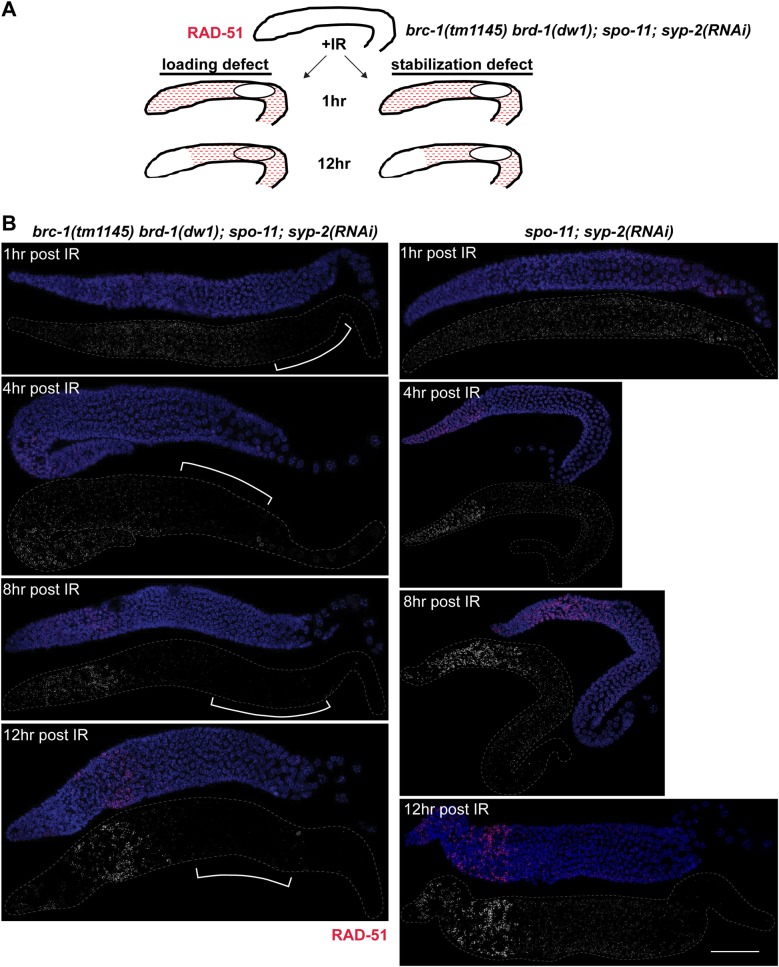
The lack of RAD-51 in mid to late pachytene in brc-1; zim-1 and brd-1; zim-1 mutants is reminiscent of the RAD-51 “dark zone” observed in the rad-50 mutant following exposure to IR, which likely reflects a requirement for RAD-50 in loading RAD-51 at resected DSBs on meiotic chromosomes [55]. However, the distal boundary of the dark zone in the brc-1; zim-1 double mutant is distinct from the rad-50 mutant: the dark zone in rad-50 extends from meiotic entry to late pachytene [55], while in the brc-1; zim-1 and brd-1; zim-1 mutants reduction in RAD-51 was limited to mid-late pachytene (Fig 6A and 6B and S4B Fig), suggesting that the nature of the dark zone is different in these mutant situations. If BRC-1-BRD-1 is required for loading RAD-51 on breaks in mid-late pachytene, then a time course analysis would reveal a diminution of the dark zone by twelve hours following IR exposure, as was observed for rad-50 mutants (Fig 7A, loading defect on left) [55]. On the other hand, if BRC-1-BRD-1 was important for protecting RAD-51 from disassembly, then the dark zone should be maintained throughout the time course as RAD-51 would be disassembled as nuclei with pre-installed RAD-51 move through the mid-late pachytene region of the germ line (Fig 7A, stabilization defect on right). SPO-11 remains active under conditions where crossovers have not formed on all chromosomes [56, 57], making it difficult to distinguish a RAD-51 loading defect onto new breaks in this region of the germ line versus a defect in RAD-51 stability. Therefore, we performed these experiments in the spo-11 mutant background [32], as IR will induce breaks uniformly in the germ line at a single point in time and as nuclei move through the germ line, no new breaks will be formed. spo-11 is tightly linked to zim-1; consequently, we used RNAi against SYP-2, which in our hands is more efficient than zim-1(RNAi), to block synapsis and crossover formation. To that end, we exposed spo-11 and brc-1(tm1145) brd-1(dw1); spo-11 mutants depleted for SYP-2 to 10 Gy of IR and examined RAD-51 over time. At one, four, eight, and twelve hours following IR, the dark zone was maintained in the absence of BRC-1-BRD-1 (Fig 7B). This result is consistent with the hypothesis that BRC-1-BRD-1 stabilizes the RAD-51 filament rather than facilitates loading of RAD-51 on new DSBs at mid-late pachytene.
Fig 7. BRC-1 promotes the stability of RAD-51 filaments when crossover formation is impaired.
A) Schematic of potential outcomes of IR-induced RAD-51 (red) over time in brc-1(tm1145) brd-1(dw1); spo-11; syp-2(RNAi) worms: a defect in RAD-51 loading would result in loss of the “dark zone” (circle) by 12 hrs (left), while a defect in RAD-51 stabilization would manifest in the maintenance of the dark zone over 12 hrs (right). B) Projections of whole germ lines at indicated times after IR treatment in brc-1(tm1145) brd-1(dw1); spo-11; syp-2(RNAi) (left) and spo-11; syp-2(RNAi) (right). DAPI (blue), RAD-51 (red). A minimum of 3 germ lines were imaged for each condition. Scale bar = 30 μm.
BRC-1-BRD-1 alters recombination patterning under meiotic dysfunction
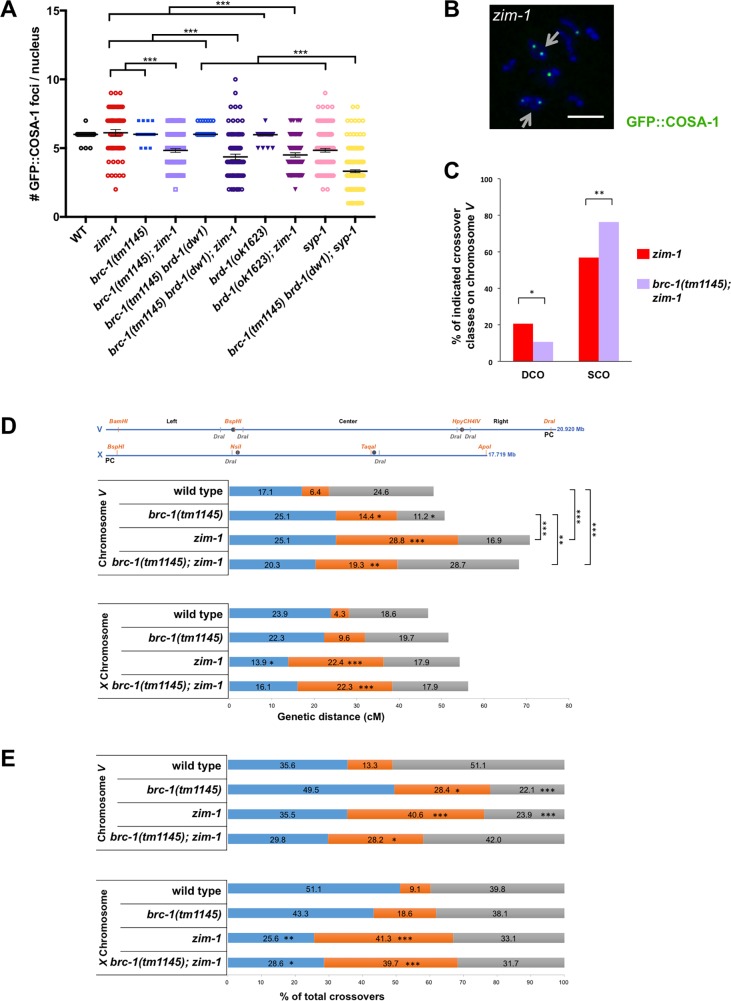
A subset of RAD-51 strand invasions are processed into crossovers, which are marked by CNTD1/COSA-1 [30, 58]. Given the reduction in RAD-51 in mid-late pachytene in brc-1; zim-1 and brd-1; zim-1 mutant hermaphrodites, we next analyzed crossover precursor formation in the various mutants. In C. elegans, each of the six chromosome pairs forms a single crossover; consequently, there are six COSA-1 foci in hermaphrodite germ cells at late pachytene [30] (Fig 8A). We also observed six COSA-1 foci in late pachytene nuclei in the brc-1 and brd-1 mutants (Fig 8A), indicating that breaks are efficiently processed into crossovers in the absence of BRC-1-BRD-1 in an otherwise wild-type worm. This is consistent with the presence of six bivalents at diakinesis and the low embryonic lethality of brc-1 and brd-1 [9, 10] (Fig 5B). In zim-1 mutants we expected to observe four COSA-1 foci per nucleus, one on each of the four paired chromosomes, but not on the unpaired chromosomes II and III. Contrary to our expectations, zim-1 had an average of 6.12±0.12 COSA-1 foci (χ2: p<0.005), with a very broad distribution ranging from 2 to 9 foci; such a wide distribution is never observed in wild type [30] (Fig 8A; S5 Fig). Inactivation of BRC-1 and/or BRD-1 in zim-1 reduced the number of GFP::COSA-1 foci to a range of 4.3–4.8 in the various mutants, closer to expectations although still significantly different than expected (χ2: p<0.005), and the distribution remained broad (p <0.0001; Fig 8A). These results suggest that when crossovers are unable to form between some homologs, additional COSA-1-marked crossover precursors are generated, and some of these are dependent on BRC-1-BRD-1.
Fig 8. BRC-1-BRD-1 alters the crossover landscape when meiosis is impaired.
A) Number of GFP::COSA-1 foci in mid-late pachytene in indicated mutants. Number of nuclei from a minimum of 4 germ lines scored: gfp::cosa-1 = 458, brc-1(tm1145) brd-1(dw1); gfp::cosa-1 = 815, brc-1(tm1145) brd-1(dw1); zim-1; gfp::cosa-1 = 169; brc-1(tm1145); gfp::cosa-1 = 235; brc-1(tm1145); zim-1; gfp::cosa-1 = 255, zim-1; gfp::cosa-1 = 120, brd-1(ok1623); zim-1; gfp::cosa-1 = 164, brd-1(ok1623); gfp::cosa-1 = 145, syp-1; gfp::cosa-1 = 292, brc-1(tm1145) brd-1(dw1); syp-1; gfp::cosa-1 = 487. The genetic interaction between brc-1/brd-1 and zim-1 is significant by a one-way ANOVA (*** p<0.0001). B) Diplotene zim-1; gfp::cosa-1 nucleus showing ring-shaped chromosomes (arrows) and GFP::COSA-1 (green). Scale bar = 2 μm. C) Percent recombinants for double crossover (DCO) [100 x DCO/(SCO+DCO+TCO)], p = 0.024, and single crossover (SCO) classes [100 x SCO/(SCO+DCO+TCO)], p = 0.0007, in zim-1 and brc-1(tm1145); zim-1 mutants. Statistical analyses were conducted using Fisher exact test on 2-by-2 contingency tables of DCO or SCO and total recombinants. D) Top: SNP markers: orange sites were analyzed from all embryos, grey sites were used to confirm potential double and triple COs; Bottom: CO frequency on chromosome V in wild type (n = 187), brc-1(tm1145) (n = 187), zim-1 (n = 219) and brc-1(tm1145); zim-1 (n = 192) mutants. CO frequency on the X chromosome in wild type (n = 188), brc-1(tm1145) (n = 188), zim-1 (n = 223) and brc-1(tm1145); zim-1 (n = 112) mutants. n = number of embryos analyzed per genotype. E) CO distribution among recombinants on chromosome V in wild type (n = 90), brc-1(tm1145) (n = 95), zim-1 (n = 155) and brc-1(tm1145); zim-1 (n = 131) and on the X chromosome in wild type (n = 88), brc-1(tm1145) (n = 97), zim-1 (n = 121) and brc-1(tm1145); zim-1 (n = 63). n = total number of COs per genotype; statistical analyses were conducted using χ2 on 2-by-2 contingency tables, * p<0.05; ** p<0.001; *** p<0.0001.
The higher than expected numbers of COSA-1 foci observed in zim-1 mutants could reflect recombination intermediates that do not go on to form chiasmata (i.e., non-crossovers or inter-sister crossovers). Alternatively, COSA-1 could mark bona fide inter-homolog crossovers, such that some chromosomes have more than one chiasma, as has been observed in mutants where the X chromosomes fail to pair and synapse [50]. As these two possibilities are not mutually exclusive, the extra COSA-1 foci could be due to a combination of both recombination outcomes. To provide insight into the nature of the extra COSA-1 foci, we analyzed COSA-1 in syp-1 mutants, where no chiasmata can form as all chromosomes fail to synapse, and found that there were on average 4.85±0.07 COSA-1 foci at late pachytene (Fig 8A; S5 Fig). These results suggest that under conditions of meiotic dysfunction when chromosomes are unable to pair/synapse, COSA-1 is recruited to recombination intermediates that are processed into non-crossovers and/or inter-sister crossovers. Similar numbers of COSA-1 foci, associated with MSH-5, were observed in syp-3 mutants; high resolution cytological analyses indicated that these recombination sites are non-randomly distributed but with some abnormalities, consistent with the formation of nonproductive intermediates or inter-sister crossovers [59]. As with zim-1 mutants, inactivation of BRC-1-BRD-1 in the syp-1 mutant background led to fewer COSA-1 foci (Fig 8A; S5 Fig), suggesting that BRC-1-BRD-1 promotes COSA-1-associated recombination processing when chiasma formation is impaired.
To determine whether the extra COSA-1 foci on synapsed chromosomes could form chiasmata, we examined zim-1 and brc-1(tm1145); zim-1 diplotene/diakinesis nuclei, where chromosomes are individualized and cross-shaped structures indicative of crossovers between homologs can be observed. Consistent with the formation of extra chiasmata in the zim-1 mutant background, we observed 52% of diplotene/diakinesis nuclei (n = 52) containing at least one ring-shaped structure, and six had two ring-shaped structures. The simplest interpretation is that there was a chiasma on each end of the chromosome pair (arrow; Fig 8B). This was reduced to 21% of diplotene/diakinesis nuclei (n = 43) containing ring-shaped chromosomes in the brc-1(tm1145); zim-1 double mutant (zim-1 vs. brc-1(tm1145); zim-1, p = 0.0028 Mann-Whitney). These results suggest that BRC-1-BRD-1 promotes chiasma formation when some chromosomes are unable to interact with their partner.
To examine genetic crossovers, we monitored linkage between SNP markers on chromosomes V and X in Bristol/Hawaiian hybrid strains to assess both crossover numbers and distribution. While inactivation of brc-1 had no effect on crossover numbers on chromosome V (WT = 48.1cM; brc-1 = 50.8cM), we observed an altered distribution compared to wild type (Fig 8D and 8E; S1 Table). In C. elegans, crossovers are enriched on the arms [28, 60–62]; in the brc-1(tm1145) mutant we observed a more even distribution, with more crossovers in the center and fewer on the right arm (Fig 8E; S1 Table). On the other hand, in brc-1(tm1145), neither crossover frequency nor distribution were significantly different on the X chromosome (Fig 8D and 8E), which has an altered crossover landscape compared to the autosomes [63, 64].
We next monitored linkage between SNP markers in the zim-1 and brc-1(tm1145); zim-1 mutants. We observed a significant increase in the recombination map on chromosome V in zim-1 (70.8cM), and multiple double crossovers were observed (Fig 8D; S1 Table). Extra crossovers were also observed on autosomes in worms unable to pair and synapse X chromosomes [50]. Inactivation of BRC-1 in the zim-1 background resulted in significantly fewer double crossovers (DCOs) on chromosome V (p = 0.0242; Fig 8C, S1 Table), although the overall genetic map length was not significantly different compared to the zim-1 single mutant (68.2cM; Fig 8D). This is most likely a consequence of an increase in the single crossover class (SCO; zim-1 vs. brc-1(tm1145); zim-1, p = 0.0007; Fig 8C, S1 Table). On the X chromosome crossover frequency and distribution were altered in the center region in both zim-1 and brc-1(tm1145); zim-1 and in the left interval in zim-1; however, the overall map lengths were not statistically different between any of the strains.
C. elegans exhibits strong interference, which is the phenomenon that a crossover at one position on a chromosome decreases the probability of formation of a crossover nearby, resulting in a single crossover per chromosome [62]. Given the detection of DCOs on chromosome V in the zim-1 and brc-1(tm1145); zim-1 mutants, we calculated the interference ratio. While wild type and brc-1 had absolute intereference of 1, as no double crossovers were observed, the zim-1 mutant displayed reduced interference in the left-center and left-right intervals and negative interference in the center-right interval (Table 1). Inactivation of BRC-1 in the zim-1 mutant restored positive interference in the center-right interval; however, this fell short of statistical significant (p = 0.064). In addition to the non-randomness in the number and position of crossovers, interference also operates on the level of chromatids such that a crossover between any two non-sister chromatids can affect the probability of those chromatids being involved in other crossovers [65]. Chromatid interference has been shown to occur in fungi, Drosophila, maize and humans [65–70]. Since we assayed single products of meiosis, the SCO class includes single crossovers as well as recombinants that are the result of three- or four-strand double crossovers, while only two strand-events can be detected as DCOs. The elevated numbers of SCOs and reduction in two-strand DCOs on chromosome V in the brc-1(tm1145); zim-1 mutant compared to the zim-1 single mutant (Fig 8C), suggest that there may be more three- and/or four-strand double crossovers when BRC-1 is inactivated. Thus, BRC-1 may counteract chromatid interference under meiotic dysfunction, such that more two-strand double crossovers occur. Taken together, the reduced number of COSA-1 foci and alteration in the genetic map in the brc-1(tm1145); zim-1 mutant suggest that BRC-1-BRD-1 modifies recombination patterning under meiotic dysfunction.
Table 1. Crossover interference on chromosome V.
| WT (V) | expected DCO | observed DCO | c.o.c. | interference |
| LC | 0.011 | 0.00 | 0.00 | 1.00 |
| CR | 0.016 | 0.00 | 0.00 | 1.00 |
| LR | 0.042 | 0.00 | 0.00 | 1.00 |
| brc-1 (V) | expected DCO | observed DCO | c.o.c. | interference |
| LC | 0.036 | 0.00 | 0.00 | 1.00 |
| CR | 0.016 | 0.00 | 0.00 | 1.00 |
| LR | 0.028 | 0.00 | 0.00 | 1.00 |
| zim-1(V) | expected DCO | observed DCO | c.o.c. | interference |
| LC | 0.072 | 0.055 | 0.75 | 0.25 |
| CR | 0.049 | 0.087 | 1.79 | -0.79 |
| LR | 0.042 | 0.018 | 0.43 | 0.57 |
| brc-1;zim-1(V) | expected DCO | observed DCO | c.o.c. | interference |
| LC | 0.039 | 0.026 | 0.67 | 0.33 |
| CR | 0.055 | 0.031 | 0.57 | 0.43 |
| LR | 0.058 | 0.031 | 0.54 | 0.46 |
LC = left-center interval; CR = center-right interval; LR = left-right interval. DCO: double crossover; expected DCO: (crossover frequency at interval “A”) x (crossover frequency at interval “B”). c.o.c. (coefficient of coincidence) = actual DCO frequency/ expected DCO frequency; Interference = 1- c.o.c. See S1 Table for data used for calculations. Statistical analyses of interference using χ2 on 2-by-2 contingency tables of observed and expected DCOs [119], indicated that interference in the CR interval fell short of statistical significance between zim-1 and brc-1(tm1145); zim-1, p = 0.064.
Discussion
Here we show that C. elegans BRC-1 and BRD-1 orthologs localize to the SC and regulate recombination when meiosis is perturbed. Our results suggest that BRC-1-BRD-1 plays an important role in monitoring and modulating processing of meiotic DSBs into crossovers in the context of the specialized meiotic chromosome structure.
BRC-1-BRD-1 undergoes dynamic localization that is coupled to crossover recombination
In mouse spermatocytes BRCA1 is associated with RAD51 and enriched on asynapsed regions of meiotic chromosomes, including the X-Y sex body [18, 19]. Here we show that C. elegans BRC-1 and BRD-1 partially co-localize with RAD-51 in early meiotic prophase, but become enriched on synapsed chromosomes as meiosis progresses, co-localizing with SYP-1, a SC central region component (Fig 2B). The enrichment of mammalian BRCA1 on asynapsed chromosomes versus BRC-1 on synapsed chromosomes in C. elegans most likely reflects alteration in the relationship between meiotic recombination and SC formation in these organisms. Meiotic chromosomes can pair and synapse in the absence of meiotic recombination in C. elegans [32], while these events are interdependent in mammals [15, 16]. The HORMAD axial components also show differences in chromosome association in mice and worms: in mice, HORMAD1 and HORMAD2 are enriched on asynapsed chromosomes [71, 72], while C. elegans HORMADS, HIM-3, HTP-1/2, and HTP-3, remain associated with synapsed chromosomes [73–76]. However, the function of HORMADs in preventing inter-sister recombination and in checkpoint signaling appears to be similar in these different organisms [77–82]. Thus, the association of BRC-1-BRD-1 to the SC in C. elegans is likely a consequence of the inter-relationship between SC formation and meiotic recombination in this organism and not due to different functions for this complex in worm versus mammalian meiosis.
Another difference between C. elegans and mammals is the nature of the kinetochore. C. elegans chromosomes are holocentric while in many organisms, including yeast and mice, chromosomes are monocentric. Holocentricity dictates that a single off-centered crossover is formed on each homolog pair to define the long and short arms necessary to ensure regulated sister chromatid cohesion release at meiosis I and II [60–62, 83]. Interestingly, BRC-1-BRD-1 becomes restricted to the short arm of the bivalent, as defined by the crossover site, and this precedes SC reorganization. While the absence of BRC-1-BRD-1 alone does not affect crossover formation on chromosome V and the X chromosome, it does have a subtle effect on the distribution of crossovers along chromosome V such that more occur in the middle of the chromosome (Fig 8D and 8E). The change in crossover distribution in brc-1 mutants may contribute to the slightly increased nondisjunction observed in the absence of the BRC-1-BRD-1 complex.
We show that the concentration of BRC-1-BRD-1 to a portion of each chromosome track in late pachytene is dependent on meiotic DSB formation and processing into crossovers (spo-11, rad-51 and msh-5; Fig 4). Interestingly, in both spo-11 and msh-5 mutants there are occasional chromosomal tracks in late pachytene, which are highly enriched for BRC-1. While synapsis markers also show occasional enrichment to single tracks in the absence of spo-11, and these partially overlap with BRC-1 (S2B Fig), no enrichment of synapsis markers is observed when crossover factors (i.e., msh-5, cosa-1 or zhp-3) are removed [39–41]. While it has been proposed that spo-11-independent lesions can recruit meiotic DNA repair components [39–41], the enrichment of BRC-1 in the absence of such crossover factors suggests that BRC-1-BRD-1 can respond to other repair intermediates in addition to those leading to inter-homolog crossovers. One possibility is that when inter-homolog crossover formation is blocked, DSBs are repaired through site-specific nucleases [84–86], a subset of which leads to the concentration of BRC-1-BRD-1 on chromosomes in late pachytene. This is also consistent with the observation that BRC-1 is maintained on chromosomes in spo-11, rad-51 and msh-5 mutants in diakinesis nuclei. Perhaps the failure to form interhomolog crossovers in these mutants leads to continued association of BRC-1-BRD-1 on chromosomes.
BRC-1 and BRD-1 associate and are mutually dependent for localization to meiotic chromosomes
BRCA1 forms a potent E3 ubiquitin ligase only in complex with its partner BARD1 [2, 3]. Biochemical and structural studies have defined the RING domains and associated helices of these proteins as critical for catalytic activity and BRCA1-BARD1 interaction [43]. However, while the BRCA1-BARD1 heterodimer exhibits substantially greater E3 ligase activity in vitro than BRCA1 alone, only the BRCA1 RING domain interacts with the E2 for ubiquitin transfer, suggesting that BRCA1 is the critical subunit for E3 ubiquitin ligase activity [3, 87]. Structure-function analysis of the BARD1 RING domain suggests that BARD1 may serve to attenuate BRCA1 E3 ligase activity [88]. Interestingly, while the localization of BRC-1 and BRD-1 were interdependent (Fig 3B), BRD-1 appeared to be excluded from the nucleus in the absence of BRC-1, while BRC-1 was nucleoplasmic and formed foci in late pachytene in the absence of BRD-1 (Fig 3B). These differences may reflect the nature of the alleles examined: brc-1(xoe4) produces no protein, while the two brd-1 alleles are predicted to produce truncated proteins, where the RING domain and associated helices remain intact (Fig 5A). In humans, BRCA1 nuclear localization signals in the middle of the protein can directly mediate nuclear import, or import can occur indirectly through interaction with BARD1 [89]. Thus, the truncated BRD-1 protein produced from the brd-1(ok1623) allele could associate with BRC-1 and facilitate nuclear localization of the albeit nonfunctional complex. Alternatively, C. elegans BRC-1 may be uniquely required for nuclear localization or retention, and in its absence BRD-1 cannot enter or be retained in the nucleus. The weak nucleoplasmic BRD-1 signal observed at the end of meiotic prophase in the absence of BRC-1 most likely reflects differences in the nuclear membrane as oogenesis proceeds [90, 91].
In addition to the N-terminal RING domains, both BRC-1 and BRD-1 contain long linker and phosphoprotein binding BRCA1 C-terminal (BRCT) domains. BRCT domains are phosphorylation-dependent interacting modules that have been implicated in tumor suppressor activity [92]. Interestingly, only BRD-1 contains Ankyrin (ANK) repeat interaction domains. Recent structural and functional analyses of the ANK domain in TONSL-MMS22L, a complex involved in homologous recombination, revealed that the ANK domain interacts with histone H4 tails [93]. The BARD1 ANK domains have a very similar fold [93], suggesting that BARD1 ANK domains may be important for association with chromatin. The predicted truncated proteins produced in the brd-1 mutants, which behave as nulls (Fig 5 and S4 Fig), lack at least part of the BRCT domains and all of the ANK domains, suggesting that some combination of these domains are critical for BRD-1 function with respect to both DNA damage signaling and meiosis.
BRC-1-BRD-1 function in meiotic recombination can be genetically separated from its established role in the DDR
It has long been appreciated that BRCA1-BARD1 mediates its tumor suppressor activity at least in part through regulating homologous recombination [6]. Given the importance of homologous recombination in repairing DSBs during meiosis, it is not surprising that removing BRC-1-BRD-1 impinges on meiotic recombination. Unexpectedly, we identified a small region C-terminal to the BRC-1 RING and associated helices as being important specifically for meiosis, suggesting that the function of BRC-1-BRD-1 in DNA damage response and meiosis are distinct. While containing no specific fold or homology, this region has several potential phosphorylation sites based on prediction algorithms that may mediate its interaction with key meiotic proteins.
BRCA1-BARD1 associates with the recombinase RAD51 in both mammals and C. elegans [19, 31, 94]. BRCA1 has also been shown to be required for the assembly of DNA damage induced RAD51 foci on chromatin [95], and this has been interpreted as a requirement for BRCA1 in RAD51 filament assembly. However, recent biochemical analyses using purified proteins found that BRCA1 is not required for RAD51 assembly on RPA coated single stranded DNA and instead promotes DNA strand invasion [94]. Further, a BARD1 mutant that cannot interact with RAD51 does not promote DNA strand invasion, and also does not form foci in vivo. Thus, it is likely that BRCA1-BARD1 is not required for RAD51 filament assembly per se. Our IR time course analysis of C. elegans brc-1 brd-1 mutants is consistent with a function for this complex in stabilizing the RAD-51 filament. It is possible that similar to the mammalian complex, BRC-1-BRD-1 promotes RAD-51 strand invasion; however, in vivo the RAD-51 filament may be subject to disassembly by other proteins in the absence of BRC-1-BRD-1, which would not be recapitulated in vitro. One such protein is the FANCJ/DOG-1 helicase, which interacts with BRCA1 [96], and can disassemble RAD51 on ssDNA in vitro [97]. It is also likely that BRCA1-BARD1 plays multiple roles during homologous recombination and interacts with, and coordinates the activity, of many proteins, including RAD51, and these interactions are modulated under different conditions, including DNA damage, meiosis, meiotic dysfunction, as well as at different stages of the cell cycle. Consistent with this, Janisiw et al. found that BRC-1 associates with the pro-crossover factor MSH-5.
BRCA1-BARD1 and meiotic checkpoint signaling
brc-1 and brd-1 mutants have very subtle defects in meiosis. These include low levels of X chromosome nondisjunction [10] (Fig 5C), a delay in repair of a subset of DSBs through the inter-sister pathway [9], and elevated heterologous recombination [12]. However, removing BRC-1-BRD-1 when meiosis is perturbed in mutants that impair chromosome pairing, synapsis and crossover recombination leads to enhanced meiotic dysfunction, including elevated embryonic lethality (Fig 5E), impaired RAD-51 stability (Fig 7), and alteration of COSA-1 numbers and the crossover landscape (Fig 8). These results suggest that BRC-1-BRD-1 plays a critical role in meiotic recombination when meiosis is impaired.
In both C. elegans and Drosophila melanogaster, preventing crossover formation on a subset of chromosomes leads to additional events on other chromosomes, and is referred to as the interchromosomal effect [50, 98–101]. There is also evidence in humans that Robertsonian translocations elicit the interchromosomal effect [102]. Our analyses of the zim-1 mutant, where chromosomes II and III fail to recombine, revealed elevated COSA-1 foci genome wide and an increase in genetic crossovers on chromosome V (but not the X chromosome, Fig 8), consistent with the interchromosomal effect. Further, our data suggest that when meiosis is impaired as in syp-1, and perhaps zim-1 mutants, COSA-1 can mark events that do not ultimately become interhomolog crossovers (see also [59]). Interestingly, removal of BRC-1-BRD-1 in zim-1 and syp-1 mutants decreased the number of COSA-1 foci. On the other hand, in the brc-1(tm1145); zim-1 mutant we detected elevated levels of single crossovers but reduced levels of two-strand double crossovers on chromosome V compared to the zim-1 single mutant, with no change in the overall map length (Fig 8D). One possibility to explain the observed COSA-1 and crossover pattern is that COSA-1 does not become enriched on a subset of crossovers in brc-1; zim-1 mutants even though these events are dependent on the canonical meiotic crossover pathway, as observed in the rtel-1 and dyp-28 mutants [30, 103–105]. Alternatively, the extra crossovers that are not marked by COSA-1 in the absence of BRC-1-BRD-1 may be the result of activation of alternative crossover formation pathways. In either scenario, BRC-1, and presumably BRD-1, appear to dictate the patterning of crossovers among non-sister chromatids. As interference is mediated by meiotic chromosome structure [106], perhaps SC-associated BRC-1-BRD-1 counteracts chromatid interference in the context of meiotic dysfunction.
In conclusion, our results suggest that BRC-BRD-1 serves a critical role in monitoring the progression of meiotic recombination in the context of the SC when meiosis cannot proceed normally, suggesting that BRC-1-BRD-1 serves a checkpoint function. When crossover formation is blocked, BRC-1-BRD-1 stabilizes the RAD-51 filament and promotes processing of recombination intermediates marked by COSA-1. In this context, BRC-1-BRD-1 joins a growing list of proteins that monitor meiotic recombination to promote accurate chromosome segregation, including protein kinases and ubiquitin/SUMO E3 ligases [39, 56, 57, 107–110]. Future work will examine the relationship between BRC-1-BRD-1 and other meiotic checkpoint pathways and identify substrates of BRC-1-BRD-1-ubiquitination to understand how this complex modulates recombination under conditions when meiosis is perturbed.
Materials and methods
Generation of gfp::brc-1, tag-rfp-T::brc-1, brd-1::gfp and brc-1(xoe4)
Fluorescent protein knock-ins were generated using CRISPR-mediated homology dependent repair with self-excising cassette containing hygromycin resistant as selection [20]. The brc-1(xoe4) deletion allele was generated using Cas9-snRNPs and an single strand oligonucleotide repair template [111]. Cas9 protein was purchased from Innovative Genomics Institute, UC Berkeley. For a list of sgRNAs and repair templates refer to S2 Table. All CRISPR-generated lines were back crossed a minimum of three times, with the exception of JEL744 brc-1(xoe4) brd-1::gfp, which was only back crossed once.
Genetics
C. elegans var. Bristol (N2), was used as the wild-type strain. Other strains used in this study are listed in S3 Table. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. Strains were maintained at 20°C.
Embryonic lethality and production of male progeny
Embryonic lethality in the absence or presence of 5mM hydroxyurea (HU) (16 hrs), or 75 Grays (Gy) of γ-irradiation (IR) from a 137Cs source, was determined over 3 days by counting eggs and hatched larvae 24 hr after removing the hermaphrodite and calculating percent as eggs/ (eggs + larvae); male progeny was assessed 48 hr after removing the hermaphrodite. A minimum of 10 worms were scored for each condition.
Apoptosis assay
Acridine orange (AO) staining of apoptotic germ cells in WT (N2), brc-1 and brd-1 alleles as well as zim-1 and corresponding double and triple mutants were performed as in [48]. Briefly, 0.5 ml of 50 mg/ml AO (Molecular Probes, Invitrogen; Carlsbad, CA) in M9 was added to 60-mm plates containing 48 hr post L4 worms and incubated at room temperature for 1 hr. Worms were transferred to new 60-mm plates, allowed to recover 15 min, and then mounted under coverslips in M9 on 3% agarose pads containing 1 mM tetramisole (Sigma-Aldrich; St. Louis). Apoptotic bodies were scored by fluorescence microscopy and DIC.
Cytological analysis
Gonads were dissected and fixed with 1% paraformaldehyde in egg buffer plus 0.01% Tween20 for 5 min, freeze-cracked and post-fixed in either ice-cold 100% methanol for indirect immunofluorescence, or ice-cold 100% ethanol for direct fluorescence (GFP::BRC-1, TagRFP-T::BRC-1, BRD-1::GFP, GFP::RPA-1, GFP::COSA-1) for 1 min [112]. For staining with antibodies against phospho-SYP-4, gonads were dissected, freeze-cracked, incubated in 100% methanol for 1 min and post-fixed in 4% paraformaldehyde in PBS, 80mM HEPES(pH7.4), 0.8mM EDTA, 1.6mM MgS04 for 30 min [40]. The following primary antibodies were used at the indicated dilutions: rabbit anti-RAD-51 (1:10,000; Catalog #29480002; Novus Biologicals, Littleton, CO), rabbit anti-GFP (1:500; NB600-308; Novus Biologicals, Littleton, CO), goat anti-SYP-1 (1:200; generously provided by Anne Villeneuve); rabbit anti-phospho-SYP-4 (1:100; [40]), and guinea pig anti-SUN-1 S12P (1:1,000; generously provided by Verena Jantsch). Secondary antibodies Alexa Fluor 594 donkey anti-rabbit IgG, Alexa Fluor 555 donkey anti-goat IgG, Alexa Fluor 488 donkey anti-rabbit IgG, Alexa Fluor 488 goat anti-guinea pig IgG from Life Technologies were used at 1:500 dilutions. DAPI (2μg/ml; Sigma-Aldrich) was used to counterstain DNA.
Collection of fixed images was performed using an API Delta Vision deconvolution microscope, a Nikon TiE inverted microscope stand equipped with an 60x, NA 1.49 objective lens and Andor Clara interline camera, or were captured on a spinning-disk module of an inverted objective fluorescence microscope [Marianas spinning-disk confocal (SDC) real-time 3D Confocal-TIRF (total internal reflection) microscope; Intelligent Imaging Innovations] equipped with an 63x, NA 1.46 objective lens using a Photometrics QuantiEM electron multiplying charge-coupled device (EMCCD) camera. Z stacks (0.2 μm) were collected from the entire gonad. A minimum of three germ lines was examined for each condition. Images were deconvolved using Applied Precision SoftWoRx or Nikon NIS Elements Offline batch deconvolution software employing either “Automatic3D” or “Richardson-Lucy” deconvolution modes and subsequently processed and analyzed using Fiji (ImageJ) (Wayne Rasband, NIH).
RAD-51 foci were quantified in a minimum of three germ lines of age-matched hermaphrodites (18–24 hr post-L4). As zim-1 mutants have an extended transition zone [42], we divided germ lines into four equal zones from the beginning of the transition zone (leptotene/zygotene), as counted from the first row with three or more crescent-shaped nuclei, through diplotene (Fig 6B). The number of foci per nucleus was scored for each region.
To assess formation of RAD-51 foci following IR treatment, 18–24 hrs post-L4 worms were exposed to 10 Gy of IR; gonads were dissected 1, 4, 8, and 12 hr following IR treatment and fixed for immunofluorescence as above.
GFP::COSA-1 foci were quantified from deconvolved 3D data stacks; nuclei were scored individually through z-stacks to ensure that all foci within each individual nucleus were counted. Nuclei with features indicative of apoptosis (compact and DAPI-bright) were excluded. Foci were counted in the last five rows of pachytene nuclei as in [30].
For live cell imaging, 18–24 hr post L4 hermaphrodites were anesthetized in 1mM tetramisole and immobilized between a coverslip and an 2% agarose pad on a glass slide. Z-stacks (0.33 μm) were captured on a spinning-disk module of an inverted objective fluorescence microscope (NIH 1S10RR024543) with a 100×, NA 1.46 objective, and EMCCD camera. Z-projections of stacks were generated, cropped, and adjusted for brightness in Fiji.
Pearson’s Correlation Coefficient (PCC) was determined by drawing a Region of Interest (ROI) around a nucleus and using the co-localization function in Fiji.
Immunoblot analysis
Whole worm lysates were generated from indicated worms; unmated fog-2(q71) worms were used to eliminate embryos. ~100 worms were collected, washed in M9 buffer and resuspended in equal volume of 2X Laemmli sample buffer (Bio-RAD). Lysates were resolved on 4–15% SDS-PAGE gradient gels (Bio-RAD) and transferred to Millipore Immobilon-P PVDF membranes. Membranes were blocked with 5% nonfat milk and probed with rabbit anti-GFP (1:1000; NB600-308; Novus Biologicals, Littleton, CO) and mouse anti-α-tubulin (1:1000; Sigma-Aldrich; T9026) as loading control, followed by IRDye680LT- and IRDye800-conjugated anti-rabbit and anti-mouse IgG secondary antibodies (1:20000; LI-COR Bioscience Lincoln, NE). Immunoblots were imaged on a LI-COR Odyssey Infrared Imager, signal was quantified using Fiji and normalized with the α-tubulin signal.
RNA-mediated interference analysis
RNA-mediated interference (RNAi) was performed at 20°C, using the feeding method [113]. Cultures were plated onto NGM plates containing 25 μg/ml carbenicillin and 1 mM IPTG and were used within 2 weeks. L4 worms were transferred to RNAi plates, and resulting progeny were exposed to IR as described above. The efficiency of RNAi was tested in parallel by examining embryonic lethality.
Meiotic mapping
Meiotic crossover frequencies and distribution were assayed utilizing single-nucleotide polymorphism (SNP) markers as in [114]. The SNP markers located at the boundaries of the chromosome domains were chosen based on data from WormBase (WS231) and [64], and are indicated in Fig 8D. The SNP markers and primers used are listed in [86]. PCR and restriction digests of single embryo lysates were performed and confirmed with additional SNPs as described in [115, 116] (Fig 8D). Statistical analyses were performed using the two-tailed Fisher's Exact test, 95% C.I., as in [117, 118]. For statistical analyses of interference we conducted χ2 tests on 2-by-2 contingency tables of observed and expected DCOs [119].
Supporting information
(XLSX)
(DOCX)
(DOCX)
A) % embryonic lethality; B) % male progeny; C) % embryonic lethality following 75 Gy IR; D) % embryonic lethality following treatment with 5mM HU for 16 hrs of indicated strains. 95% Confidence Intervals shown; * p<0.05; ** p<0.001; *** p<0.0001. gfp::brc-1, tag-rfp-t::brc-1 and brd-1::gfp are not statistically different compared to WT. A minimum of 10 worms were analyzed for each condition. E) Immunoblot of whole worm extracts from WT, gfp::brc-1 and gfp::brc-1; fog-2 probed with rabbit anti-GFP and mouse anti-α-tubulin. Ratio determined by fluorescent intensities from three independent experiments.
(TIF)
A) Number of GFP::BRC-1 foci in indicated mutants in Proliferative Zone, Transition Zone and Early Pachytene. Number of foci examined in a minimum of 3 germ lines: PZ: WT (n = 412); spo-11 (n = 177); rad-51(n = 114); msh-5 (n = 175); syp-1 (n = 140); zim-1 (n = 142); TZ: WT (n = 287); spo-11 (n = 103); rad-51(n = 52); msh-5 (n = 94); syp-1 (n = 83); zim-1 (n = 112); EP: WT (n = 202); spo-11 (n = 106); rad-51(n = 57); msh-5 (n = 57); syp-1 and zim-1 had too many foci to accurately count. *** p<0.0001. B) Late pachytene region of the germ line stained with anti-GFP (green) and anti-phosphoSYP-4 (SYP-4P) (red) and counterstained with DAPI. Scale bar = 10 μm. C) High-magnification images of live C. elegans expressing BRD-1::GFP in the spo-11 background. Images are projections through half of the gonad. PZ = Proliferative Zone, TZ = Transition Zone, EP = Early Pachytene, MP = Mid Pachytene, LP = Late Pachytene, DP = Diplotene, DK = Diakinesis. Scale bar = 5 μm.
(TIF)
A) Genomic regions of brc-1 and brd-1 from WormBase Version: WS265 (https://wormbase.org/#012-34-5), with the region deleted in the different alleles indicated. Color dotted lines indicate the resulting splicing of brd-1(ok1623) (pink; splicing of exon 7–12, which introduces a stop codon and results in a 343 a. a. protein) and brd-1(dw1) (orange; cryptic splice site within intron 11 spliced to exon 12, resulting in a 375 a. a. protein) as determined by cDNA analysis. B) High-magnification images of live brc-1(tm1145) worms expressing BRD-1::GFP (PZ = Proliferative Zone, TZ = Transition Zone, EP = Early Pachytene, MP = Mid Pachytene, LP = Late Pachytene, DP = Diplotene, DK = Diakinesis). Scale bar = 5 μm. C) Indicated germ lines stained with antibodies against SUN-1 S12P (green) and counterstained with DAPI (blue). Numbers beneath genotype indicate the percentage of cell rows with SUN-1 S12P staining normalized to gonad length as in [49]; 3 germ lines were examined. Images are projections through half of the gonad. Scale bar = 20 μm.
(TIF)
A) Box whisker plots show average number of RAD-51 foci per nucleus in the different zones of meiotic prophase (see Fig 6B). Horizontal line of each box indicates the median, the top and bottom of the box indicates medians of upper and lower quartiles, lines extending above and below boxes indicate standard deviation and individual data points are outliers from 5–95%. Numbers of nuclei scored in each zone for WT: 1 = 186; 2 = 343; 3 = 292; 4 = 166; brc-1(tm1145) brd-1(dw1): 1 = 233; 2 = 303; 3 = 261; 4 = 68; brd-1(ok1623): 1 = 186; 2 = 135; 3 = 162; 4 = 117. * p<0.05; ** p<0.001; *** p<0.0001. B) Dissected germ lines from brd-1(ok1623); zim-1, brc-1(tm1145) brd-1(dw1); syp-1, syp-1, brd-1(dw1); zim-1, brc-1(tm1145) brd-1(dw1); zim-1, and brc-1(xoe4); zim-1 worms stained with anti-RAD-51 (red) and counterstained with DAPI (blue); white bracket indicates region of reduced RAD-51 foci. A minimum of 4 germ lines were imaged for each genotype. Full projections of the gonads are shown. Scale bar = 20 μm. C) Mid-late pachytene region of gonad from gfp::rpa-1 and brc-1(tm1145) brd-1(dw1); gfp::rpa-1 worms stained with anti-RAD-51 (red) and imaged for GFP::RPA-1 fluorescence (green), counterstained with DAPI (blue). Images are projections through half of the gonad. Scale bar = 8 μm.
(TIF)
Late pachytene region of the germ line in indicated mutants expressing GFP::COSA-1 (green) and counterstained with DAPI (blue). Images are projections through half of the gonad. Scale bar = 5 μm.
(TIF)
Acknowledgments
We thank Anne Villenueve and Verena Jantsch for generously providing antibodies and the Caenorhabditis Genetic Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for providing strains. We are grateful to Foxy Robinson, Merri-Grace Allard, Jaren Spotten, and Lorena Cruz-Gutierrez for help with embryonic lethality assays and construction of strains, and the Engebrecht and Colaiacovo labs for thoughtful discussions. We also thank MCB Light Imaging Facility Director, Michael Paddy, and Michael Kirmiz for their help and patience with collection and processing of images, and N. Silva, V. Jantsch and R. van Schendel for sharing unpublished data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health GM103860, UC Cancer Research Coordinating Committee CRR-17-426816 and Agricultural Experimental Station California-Davis grant *MCB-7237-H to JE, National Institutes of Health T32GM0070377 to AJD and KSL, National Institutes of Health T32CA10849 to PMC, and National Institutes of Health R01GM105853 and R01GM072551 to MPC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–9. . [DOI] [PubMed] [Google Scholar]
- 2.Wu LC, Wang ZW, Tsan JT, Spillman MA, Phung A, Xu XL, et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14(4):430–40. 10.1038/ng1296-430 . [DOI] [PubMed] [Google Scholar]
- 3.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276(18):14537–40. 10.1074/jbc.C000881200 . [DOI] [PubMed] [Google Scholar]
- 4.Kais Z, Parvin JD. Regulation of centrosomes by the BRCA1-dependent ubiquitin ligase. Cancer Biol Ther. 2008;7(10):1540–3. ; PubMed Central PMCID: PMCPMC2628548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage KI, Harkin DP. BRCA1, a 'complex' protein involved in the maintenance of genomic stability. FEBS J. 2015;282(4):630–46. 10.1111/febs.13150 . [DOI] [PubMed] [Google Scholar]
- 6.Li ML, Greenberg RA. Links between genome integrity and BRCA1 tumor suppression. Trends Biochem Sci. 2012;37(10):418–24. 10.1016/j.tibs.2012.06.007 ; PubMed Central PMCID: PMCPMC3459146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11(10):1226–41. . [DOI] [PubMed] [Google Scholar]
- 8.McCarthy EE, Celebi JT, Baer R, Ludwig T. Loss of Bard1, the heterodimeric partner of the Brca1 tumor suppressor, results in early embryonic lethality and chromosomal instability. Mol Cell Biol. 2003;23(14):5056–63. 10.1128/MCB.23.14.5056-5063.2003 ; PubMed Central PMCID: PMCPMC162231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamo A, Montemauri P, Silva N, Ward JD, Boulton SJ, La Volpe A. BRC-1 acts in the inter-sister pathway of meiotic double-strand break repair. EMBO Rep. 2008;9(3):287–92. 10.1038/sj.embor.7401167 ; PubMed Central PMCID: PMCPMC2267377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton SJ, Martin JS, Polanowska J, Hill DE, Gartner A, Vidal M. BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans. Curr Biol. 2004;14(1):33–9. . [DOI] [PubMed] [Google Scholar]
- 11.Hong Y, Sonneville R, Agostinho A, Meier B, Wang B, Blow JJ, et al. The SMC-5/6 complex and the HIM-6 (BLM) helicase synergistically promote meiotic recombination intermediate processing and chromosome maturation during Caenorhabditis elegans meiosis. PLoS Genet. 2016;12(3):e1005872 10.1371/journal.pgen.1005872 ; PubMed Central PMCID: PMCPMC4807058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leon-Ortiz AM, Panier S, Sarek G, Vannier JB, Patel H, Campbell PJ, et al. A Distinct class of genome rearrangements driven by heterologous recombination. Mol Cell. 2018;69(2):292–305 e6. Epub 2018/01/21. 10.1016/j.molcel.2017.12.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontier DB, Tijsterman M. A robust network of double-strand break repair pathways governs genome integrity during C. elegans development. Curr Biol. 2009;19(16):1384–8. Epub 2009/08/04. 10.1016/j.cub.2009.06.045 . [DOI] [PubMed] [Google Scholar]
- 14.Wolters S, Ermolaeva MA, Bickel JS, Fingerhut JM, Khanikar J, Chan RC, et al. Loss of Caenorhabditis elegans BRCA1 promotes genome stability during replication in smc-5 mutants. Genetics. 2014;196(4):985–99. 10.1534/genetics.113.158295 ; PubMed Central PMCID: PMCPMC3982690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahoon CK, Hawley RS. Regulating the construction and demolition of the synaptonemal complex. Nat Struct Mol Biol. 2016;23(5):369–77. Epub 2016/05/05. 10.1038/nsmb.3208 . [DOI] [PubMed] [Google Scholar]
- 16.Zickler D, Kleckner N. A few of our favorite things: Pairing, the bouquet, crossover interference and evolution of meiosis. Semin Cell Dev Biol. 2016;54:135–48. Epub 2016/03/02. 10.1016/j.semcdb.2016.02.024 ; PubMed Central PMCID: PMCPMC4867269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broering TJ, Alavattam KG, Sadreyev RI, Ichijima Y, Kato Y, Hasegawa K, et al. BRCA1 establishes DNA damage signaling and pericentric heterochromatin of the X chromosome in male meiosis. J Cell Biol. 2014;205(5):663–75. 10.1083/jcb.201311050 ; PubMed Central PMCID: PMCPMC4050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, et al. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14(23):2135–42. 10.1016/j.cub.2004.11.032 . [DOI] [PubMed] [Google Scholar]
- 19.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88(2):265–75. . [DOI] [PubMed] [Google Scholar]
- 20.Dickinson DJ, Pani AM, Heppert JK, Higgins CD, Goldstein B. Streamlined genome engineering with a self-excising drug selection cassette. Genetics. 2015;200(4):1035–49. 10.1534/genetics.115.178335 ; PubMed Central PMCID: PMCPMC4574250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119(1):43–61. ; PubMed Central PMCID: PMCPMC1203344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillers KJ, Jantsch V, Martinez-Perez E, Yanowitz JL. Meiosis. WormBook. 2015:1–54. 10.1895/wormbook.1.178.1 ; PubMed Central PMCID: PMCPMC5215044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell. 2010;37(4):492–502. 10.1016/j.molcel.2010.01.021 ; PubMed Central PMCID: PMCPMC2958316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathania S, Nguyen J, Hill SJ, Scully R, Adelmant GO, Marto JA, et al. BRCA1 is required for postreplication repair after UV-induced DNA damage. Mol Cell. 2011;44(2):235–51. 10.1016/j.molcel.2011.09.002 ; PubMed Central PMCID: PMCPMC3200447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacQueen AJ, Villeneuve AM. Nuclear reorganization and homologous chromosome pairing during meiotic prophase require C. elegans chk-2. Genes Dev. 2001;15(13):1674–87. 10.1101/gad.902601 ; PubMed Central PMCID: PMCPMC312723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colaiacovo MP, MacQueen AJ, Martinez-Perez E, McDonald K, Adamo A, La Volpe A, et al. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell. 2003;5(3):463–74. . [DOI] [PubMed] [Google Scholar]
- 27.MacQueen AJ, Colaiacovo MP, McDonald K, Villeneuve AM. Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev. 2002;16(18):2428–42. 10.1101/gad.1011602 ; PubMed Central PMCID: PMCPMC187442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabeshima K, Villeneuve AM, Colaiacovo MP. Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC. J Cell Biol. 2005;168(5):683–9. Epub 2005/03/02. 10.1083/jcb.200410144 ; PubMed Central PMCID: PMCPMC2171829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R, Liang QN, Du SQ, Hu XJ, Ding Y. The crystal structure of red fluorescent protein TagRFP-T reveals the mechanism of its superior photostability. Biochem Biophys Res Commun. 2016;477(2):229–34. 10.1016/j.bbrc.2016.06.047 . [DOI] [PubMed] [Google Scholar]
- 30.Yokoo R, Zawadzki KA, Nabeshima K, Drake M, Arur S, Villeneuve AM. COSA-1 reveals robust homeostasis and separable licensing and reinforcement steps governing meiotic crossovers. Cell. 2012;149(1):75–87. 10.1016/j.cell.2012.01.052 ; PubMed Central PMCID: PMCPMC3339199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polanowska J, Martin JS, Garcia-Muse T, Petalcorin MI, Boulton SJ. A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J. 2006;25(10):2178–88. 10.1038/sj.emboj.7601102 ; PubMed Central PMCID: PMCPMC1462971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dernburg AF, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve AM. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell. 1998;94(3):387–98. . [DOI] [PubMed] [Google Scholar]
- 33.Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88(3):375–84. . [DOI] [PubMed] [Google Scholar]
- 34.Alpi A, Pasierbek P, Gartner A, Loidl J. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma. 2003;112(1):6–16. 10.1007/s00412-003-0237-5 . [DOI] [PubMed] [Google Scholar]
- 35.Rinaldo C, Bazzicalupo P, Ederle S, Hilliard M, La Volpe A. Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics. 2002;160(2):471–9. ; PubMed Central PMCID: PMCPMC1461995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69(3):457–70. . [DOI] [PubMed] [Google Scholar]
- 37.Hollingsworth NM, Ponte L, Halsey C. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 1995;9(14):1728–39. . [DOI] [PubMed] [Google Scholar]
- 38.Kelly KO, Dernburg AF, Stanfield GM, Villeneuve AM. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics. 2000;156(2):617–30. ; PubMed Central PMCID: PMCPMC1461284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machovina TS, Mainpal R, Daryabeigi A, McGovern O, Paouneskou D, Labella S, et al. A aurveillance aystem wnsures crossover formation in C. elegans. Curr Biol. 2016;26(21):2873–84. Epub 2016/10/11. 10.1016/j.cub.2016.09.007 ; PubMed Central PMCID: PMCPMC5104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadarajan S, Lambert TJ, Altendorfer E, Gao J, Blower MD, Waters JC, et al. Polo-like kinase-dependent phosphorylation of the synaptonemal complex protein SYP-4 regulates double-strand break formation through a negative feedback loop. Elife. 2017;6 10.7554/eLife.23437 ; PubMed Central PMCID: PMCPMC5423773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pattabiraman D, Roelens B, Woglar A, Villeneuve AM. Meiotic recombination modulates the structure and dynamics of the synaptonemal complex during C. elegans meiosis. PLoS Genet. 2017;13(3):e1006670 10.1371/journal.pgen.1006670 ; PubMed Central PMCID: PMCPMC5384771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips CM, Dernburg AF. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev Cell. 2006;11(6):817–29. 10.1016/j.devcel.2006.09.020 . [DOI] [PubMed] [Google Scholar]
- 43.Brzovic PS, Rajagopal P, Hoyt DW, King MC, Klevit RE. Structure of a BRCA1-BARD1 heterodimeric RING-RING complex. Nat Struct Biol. 2001;8(10):833–7. 10.1038/nsb1001-833 . [DOI] [PubMed] [Google Scholar]
- 44.Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci U S A. 1999;96(20):11364–9. ; PubMed Central PMCID: PMCPMC18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhalla N, Dernburg AF. A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science. 2005;310(5754):1683–6. 10.1126/science.1117468 . [DOI] [PubMed] [Google Scholar]
- 46.Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage—induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5(3):435–43. Epub 2000/07/06. . [DOI] [PubMed] [Google Scholar]
- 47.Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126(5):1011–22. Epub 1999/02/03. . [DOI] [PubMed] [Google Scholar]
- 48.Jaramillo-Lambert A, Engebrecht J. A single unpaired and transcriptionally silenced X chromosome locally precludes checkpoint signaling in the Caenorhabditis elegans germ line. Genetics. 2010;184(3):613–28. 10.1534/genetics.109.110338 ; PubMed Central PMCID: PMCPMC2845332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woglar A, Daryabeigi A, Adamo A, Habacher C, Machacek T, La Volpe A, et al. Matefin/SUN-1 phosphorylation is part of a surveillance mechanism to coordinate chromosome synapsis and recombination with meiotic progression and chromosome movement. PLoS Genet. 2013;9(3):e1003335 Epub 2013/03/19. 10.1371/journal.pgen.1003335 ; PubMed Central PMCID: PMCPMC3591285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlton PM, Farruggio AP, Dernburg AF. A link between meiotic prophase progression and crossover control. PLoS Genet. 2006;2(2):e12 10.1371/journal.pgen.0020012 ; PubMed Central PMCID: PMCPMC1359072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonneville R, Querenet M, Craig A, Gartner A, Blow JJ. The dynamics of replication licensing in live Caenorhabditis elegans embryos. J Cell Biol. 2012;196(2):233–46. 10.1083/jcb.201110080 ; PubMed Central PMCID: PMCPMC3265957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Muse T, Boulton SJ. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 2005;24(24):4345–55. Epub 2005/12/02. 10.1038/sj.emboj.7600896 ; PubMed Central PMCID: PMCPMC1356337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koury E, Harrell K, Smolikove S. Differential RPA-1 and RAD-51 recruitment in vivo throughout the C. elegans germline, as revealed by laser microirradiation. Nucleic Acids Res. 2018;46(2):748–64. Epub 2017/12/16. 10.1093/nar/gkx1243 ; PubMed Central PMCID: PMCPMC5778493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woglar A, Villeneuve AM. Dynamic architecture of DNA repair complexes and the synaptonemal complex at sites of meiotic recombination. Cell 2018;1678–1691.e16. Epub 2018/5/10. 10.1016/j.cell.2018.03.066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi M, Chin GM, Villeneuve AM. C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression. PLoS Genet. 2007;3(11):e191 10.1371/journal.pgen.0030191 ; PubMed Central PMCID: PMCPMC2048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosu S, Zawadzki KA, Stamper EL, Libuda DE, Reese AL, Dernburg AF, et al. The C. elegans DSB-2 protein reveals a regulatory network that controls competence for meiotic DSB formation and promotes crossover assurance. PLoS Genet. 2013;9(8):e1003674 10.1371/journal.pgen.1003674 ; PubMed Central PMCID: PMCPMC3738457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamper EL, Rodenbusch SE, Rosu S, Ahringer J, Villeneuve AM, Dernburg AF. Identification of DSB-1, a protein required for initiation of meiotic recombination in Caenorhabditis elegans, illuminates a crossover assurance checkpoint. PLoS Genet. 2013;9(8):e1003679 10.1371/journal.pgen.1003679 ; PubMed Central PMCID: PMCPMC3749324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holloway JK, Sun X, Yokoo R, Villeneuve AM, Cohen PE. Mammalian CNTD1 is critical for meiotic crossover maturation and deselection of excess precrossover sites. J Cell Biol. 2014;205(5):633–41. 10.1083/jcb.201401122 ; PubMed Central PMCID: PMCPMC4050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woglar A, Villeneuve AM. Dynamic Architecture of DNA repair complexes and the synaptonemal complex at sites of meiotic recombination. Cell. 2018;173(7):1678–91 e16. Epub 2018/05/15. 10.1016/j.cell.2018.03.066 ; PubMed Central PMCID: PMCPMC6003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albertson DG, Rose AM, Villeneuve AM. Chromosome Organization, Mitosis, and Meiosis In: nd, Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor (NY)1997. [PubMed] [Google Scholar]
- 61.de Carvalho CE, Zaaijer S, Smolikov S, Gu Y, Schumacher JM, Colaiacovo MP. LAB-1 antagonizes the Aurora B kinase in C. elegans. Genes Dev. 2008;22(20):2869–85. Epub 2008/10/17. 10.1101/gad.1691208 ; PubMed Central PMCID: PMCPMC2569883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hillers KJ, Villeneuve AM. Chromosome-wide control of meiotic crossing over in C. elegans. Curr Biol. 2003;13(18):1641–7. Epub 2003/09/19. . [DOI] [PubMed] [Google Scholar]
- 63.Lim JG, Stine RR, Yanowitz JL. Domain-specific regulation of recombination in Caenorhabditis elegans in response to temperature, age and sex. Genetics. 2008;180(2):715–26. Epub 2008/09/11. 10.1534/genetics.108.090142 ; PubMed Central PMCID: PMCPMC2567375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rockman MV, Kruglyak L. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 2009;5(3):e1000419 Epub 2009/03/14. 10.1371/journal.pgen.1000419 ; PubMed Central PMCID: PMCPMC2652117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teuscher F, Brockmann GA, Rudolph PE, Swalve HH, Guiard V. Models for chromatid interference with applications to recombination data. Genetics. 2000;156(3):1449–60. ; PubMed Central PMCID: PMCPMC1461339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawthorne DC, Mortimer RK. Chromosome mapping in Saccharomyces: centromere-linked genes. Genetics. 1960;45(8):1085–110. ; PubMed Central PMCID: PMCPMC1210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou Y, Fan W, Yan L, Li R, Lian Y, Huang J, et al. Genome analyses of single human oocytes. Cell. 2013;155(7):1492–506. 10.1016/j.cell.2013.11.040 . [DOI] [PubMed] [Google Scholar]
- 68.Li X, Li L, Yan J. Dissecting meiotic recombination based on tetrad analysis by single-microspore sequencing in maize. Nat Commun. 2015;6:6648 10.1038/ncomms7648 ; PubMed Central PMCID: PMCPMC4383000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strickland WN. An analysis of interference in Aspergillus nidulans. Proc R Soc Lond B Biol Sci. 1958;149(934):82–101. . [DOI] [PubMed] [Google Scholar]
- 70.Strickland WN. Tetrad analysis of short chromosome regions of Neurospora crassa. Genetics. 1961;46:1125–41. ; PubMed Central PMCID: PMCPMC1210263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukuda T, Daniel K, Wojtasz L, Toth A, Hoog C. A novel mammalian HORMA domain-containing protein, HORMAD1, preferentially associates with unsynapsed meiotic chromosomes. Exp Cell Res. 2010;316(2):158–71. Epub 2009/08/19. 10.1016/j.yexcr.2009.08.007 . [DOI] [PubMed] [Google Scholar]
- 72.Wojtasz L, Daniel K, Roig I, Bolcun-Filas E, Xu H, Boonsanay V, et al. Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet. 2009;5(10):e1000702 Epub 2009/10/24. 10.1371/journal.pgen.1000702 ; PubMed Central PMCID: PMCPMC2758600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Couteau F, Zetka M. HTP-1 coordinates synaptonemal complex assembly with homolog alignment during meiosis in C. elegans. Genes Dev. 2005;19(22):2744–56. Epub 2005/11/18. 10.1101/gad.1348205 ; PubMed Central PMCID: PMCPMC1283966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodyer W, Kaitna S, Couteau F, Ward JD, Boulton SJ, Zetka M. HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev Cell. 2008;14(2):263–74. Epub 2008/02/13. 10.1016/j.devcel.2007.11.016 . [DOI] [PubMed] [Google Scholar]
- 75.Martinez-Perez E, Villeneuve AM. HTP-1-dependent constraints coordinate homolog pairing and synapsis and promote chiasma formation during C. elegans meiosis. Genes Dev. 2005;19(22):2727–43. Epub 2005/11/18. 10.1101/gad.1338505 ; PubMed Central PMCID: PMCPMC1283965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zetka MC, Kawasaki I, Strome S, Muller F. Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev. 1999;13(17):2258–70. Epub 1999/09/15. ; PubMed Central PMCID: PMCPMC317003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Couteau F, Nabeshima K, Villeneuve A, Zetka M. A component of C. elegans meiotic chromosome axes at the interface of homolog alignment, synapsis, nuclear reorganization, and recombination. Curr Biol. 2004;14(7):585–92. Epub 2004/04/06. 10.1016/j.cub.2004.03.033 . [DOI] [PubMed] [Google Scholar]
- 78.Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, et al. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol. 2011;13(5):599–610. Epub 2011/04/12. 10.1038/ncb2213 ; PubMed Central PMCID: PMCPMC3087846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim Y, Rosenberg SC, Kugel CL, Kostow N, Rog O, Davydov V, et al. The chromosome axis controls meiotic events through a hierarchical assembly of HORMA domain proteins. Dev Cell. 2014;31(4):487–502. Epub 2014/12/03. 10.1016/j.devcel.2014.09.013 ; PubMed Central PMCID: PMCPMC4254552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kogo H, Tsutsumi M, Ohye T, Inagaki H, Abe T, Kurahashi H. HORMAD1-dependent checkpoint/surveillance mechanism eliminates asynaptic oocytes. Genes Cells. 2012;17(6):439–54. Epub 2012/04/26. 10.1111/j.1365-2443.2012.01600.x . [DOI] [PubMed] [Google Scholar]
- 81.Shin YH, McGuire MM, Rajkovic A. Mouse HORMAD1 is a meiosis I checkpoint protein that modulates DNA double- strand break repair during female meiosis. Biol Reprod. 2013;89(2):29 Epub 2013/06/14. 10.1095/biolreprod.112.106773 ; PubMed Central PMCID: PMCPMC4076362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva N, Ferrandiz N, Barroso C, Tognetti S, Lightfoot J, Telecan O, et al. The fidelity of synaptonemal complex assembly is regulated by a signaling mechanism that controls early meiotic progression. Dev Cell. 2014;31(4):503–11. Epub 2014/12/03. 10.1016/j.devcel.2014.10.001 . [DOI] [PubMed] [Google Scholar]
- 83.Ferrandiz N, Barroso C, Telecan O, Shao N, Kim HM, Testori S, et al. Spatiotemporal regulation of Aurora B recruitment ensures release of cohesion during C. elegans oocyte meiosis. Nat Commun. 2018;9(1):834 Epub 2018/02/28. 10.1038/s41467-018-03229-5 ; PubMed Central PMCID: PMCPMC5827026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agostinho A, Meier B, Sonneville R, Jagut M, Woglar A, Blow J, et al. Combinatorial regulation of meiotic holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet. 2013;9(7):e1003591 Epub 2013/08/01. 10.1371/journal.pgen.1003591 ; PubMed Central PMCID: PMCPMC3715425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Neil NJ, Martin JS, Youds JL, Ward JD, Petalcorin MI, Rose AM, et al. Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9(7):e1003582 Epub 2013/07/23. 10.1371/journal.pgen.1003582 ; PubMed Central PMCID: PMCPMC3715453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saito TT, Lui DY, Kim HM, Meyer K, Colaiacovo MP. Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9(7):e1003586 Epub 2013/07/23. 10.1371/journal.pgen.1003586 ; PubMed Central PMCID: PMCPMC3715419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brzovic PS, Keeffe JR, Nishikawa H, Miyamoto K, Fox D 3rd, Fukuda M, et al. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc Natl Acad Sci U S A. 2003;100(10):5646–51. 10.1073/pnas.0836054100 ; PubMed Central PMCID: PMCPMC156255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stewart MD, Duncan ED, Coronado E, DaRosa PA, Pruneda JN, Brzovic PS, et al. Tuning BRCA1 and BARD1 activity to investigate RING ubiquitin ligase mechanisms. Protein Sci. 2017;26(3):475–83. 10.1002/pro.3091 ; PubMed Central PMCID: PMCPMC5326557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson ME. BRCA1 16 years later: nuclear import and export processes. FEBS J. 2010;277(15):3072–8. 10.1111/j.1742-4658.2010.07733.x [DOI] [PubMed] [Google Scholar]
- 90.Link J, Paouneskou D, Velkova M, Daryabeigi A, Laos T, Labella S, et al. Transient and partial nuclear lamina disruption promotes chromosome movement in early meiotic prophase. Dev Cell. 2018;45(2):212–25 e7. 10.1016/j.devcel.2018.03.018 ; PubMed Central PMCID: PMCPMC5920155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.VanGompel MJ, Nguyen KC, Hall DH, Dauer WT, Rose LS. A novel function for the Caenorhabditis elegans torsin OOC-5 in nucleoporin localization and nuclear import. Mol Biol Cell. 2015;26(9):1752–63. 10.1091/mbc.E14-07-1239 ; PubMed Central PMCID: PMCPMC4436785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu Q, Jubb H, Blundell TL. Phosphopeptide interactions with BRCA1 BRCT domains: More than just a motif. Prog Biophys Mol Biol. 2015;117(2–3):143–8. 10.1016/j.pbiomolbio.2015.02.003 ; PubMed Central PMCID: PMCPMC4728184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saredi G, Huang H, Hammond CM, Alabert C, Bekker-Jensen S, Forne I, et al. H4K20me0 marks post-replicative chromatin and recruits the TONSL-MMS22L DNA repair complex. Nature. 2016;534(7609):714–8. 10.1038/nature18312 ; PubMed Central PMCID: PMCPMC4939875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao W, Steinfeld JB, Liang F, Chen X, Maranon DG, Jian Ma C, et al. BRCA1-BARD1 promotes RAD51-mediated homologous DNA pairing. Nature. 2017;550(7676):360–5. Epub 2017/10/05. 10.1038/nature24060 ; PubMed Central PMCID: PMCPMC5800781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275(31):23899–903. Epub 2000/06/14. 10.1074/jbc.C000276200 . [DOI] [PubMed] [Google Scholar]
- 96.Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105(1):149–60. . [DOI] [PubMed] [Google Scholar]
- 97.Sommers JA, Rawtani N, Gupta R, Bugreev DV, Mazin AV, Cantor SB, et al. FANCJ uses its motor ATPase to destabilize protein-DNA complexes, unwind triplexes, and inhibit RAD51 strand exchange. J Biol Chem. 2009;284(12):7505–17. 10.1074/jbc.M809019200 ; PubMed Central PMCID: PMCPMC2658046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crown KN, Miller DE, Sekelsky J, Hawley RS. Local inversion heterozygosity alters recombination throughout the Genome. Curr Biol. 2018. 10.1016/j.cub.2018.07.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Joyce EF, McKim KS. Chromosome axis defects induce a checkpoint-mediated delay and interchromosomal effect on crossing over during Drosophila meiosis. PLoS Genet. 2010;6(8). Epub 2010/08/17. 10.1371/journal.pgen.1001059 ; PubMed Central PMCID: PMCPMC2920846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roberts P. Interchromosomal effects and the relation between crossing-over and nondisjunction. Genetics. 1962;47:1691–709. Epub 1962/12/01. ; PubMed Central PMCID: PMCPMC1210314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki DT. Interchromosomal effects on crossing over in Drosophila melanogaster. Ii.A reexamination of X chromosome inversion effects. Genetics. 1963;48:1605–17. Epub 1963/12/01. ; PubMed Central PMCID: PMCPMC1210445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anton E, Vidal F, Blanco J. Interchromosomal effect analyses by sperm FISH: incidence and distribution among reorganization carriers. Syst Biol Reprod Med. 2011;57(6):268–78. Epub 2011/11/19. 10.3109/19396368.2011.633682 . [DOI] [PubMed] [Google Scholar]
- 103.Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O'Neil NJ, Petalcorin MI, et al. RTEL1 maintains genomic stability by suppressing homologous recombination. Cell. 2008;135(2):261–71. Epub 2008/10/30. 10.1016/j.cell.2008.08.016 ; PubMed Central PMCID: PMCPMC3726190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsai CJ, Mets DG, Albrecht MR, Nix P, Chan A, Meyer BJ. Meiotic crossover number and distribution are regulated by a dosage compensation protein that resembles a condensin subunit. Genes Dev. 2008;22(2):194–211. 10.1101/gad.1618508 ; PubMed Central PMCID: PMCPMC2192754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Youds JL, Mets DG, McIlwraith MJ, Martin JS, Ward JD, NJ ON, et al. RTEL-1 enforces meiotic crossover interference and homeostasis. Science. 2010;327(5970):1254–8. Epub 2010/03/06. 10.1126/science.1183112 ; PubMed Central PMCID: PMCPMC4770885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Libuda DE, Uzawa S, Meyer BJ, Villeneuve AM. Meiotic chromosome structures constrain and respond to designation of crossover sites. Nature. 2013;502(7473):703–6. Epub 2013/10/11. 10.1038/nature12577 ; PubMed Central PMCID: PMCPMC3920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deshong AJ, Ye AL, Lamelza P, Bhalla N. A quality control mechanism coordinates meiotic prophase events to promote crossover assurance. PLoS Genet. 2014;10(4):e1004291 Epub 2014/04/26. 10.1371/journal.pgen.1004291 ; PubMed Central PMCID: PMCPMC3998905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim Y, Kostow N, Dernburg AF. The chromosome axis mediates feedback control of CHK-2 to ensure crossover formation in C. elegans. Dev Cell. 2015;35(2):247–61. Epub 2015/10/28. 10.1016/j.devcel.2015.09.021 ; PubMed Central PMCID: PMCPMC4624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mateo AR, Kessler Z, Jolliffe AK, McGovern O, Yu B, Nicolucci A, et al. The p53-like protein CEP-1 Is required for meiotic fidelity in C. elegans. Curr Biol. 2016;26(9):1148–58. Epub 2016/05/07. 10.1016/j.cub.2016.03.036 ; PubMed Central PMCID: PMCPMC5215890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang L, Kohler S, Rillo-Bohn R, Dernburg AF. A compartmentalized signaling network mediates crossover control in meiosis. Elife. 2018;7 10.7554/eLife.30789 ; PubMed Central PMCID: PMCPMC5906097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paix A, Folkmann A, Rasoloson D, Seydoux G. High Efficiency, Homology-directed genome editing in Caenorhabditis elegans ssing CRISPR-Cas9 ribonucleoprotein complexes. Genetics. 2015;201(1):47–54. 10.1534/genetics.115.179382 ; PubMed Central PMCID: PMCPMC4566275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaramillo-Lambert A, Ellefson M, Villeneuve AM, Engebrecht J. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev Biol. 2007;308(1):206–21. 10.1016/j.ydbio.2007.05.019 . [DOI] [PubMed] [Google Scholar]
- 113.Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263(1–2):103–12. . [DOI] [PubMed] [Google Scholar]
- 114.Nabeshima K, Villeneuve AM, Hillers KJ. Chromosome-wide regulation of meiotic crossover formation in Caenorhabditis elegans requires properly assembled chromosome axes. Genetics. 2004;168(3):1275–92. Epub 2004/12/08. 10.1534/genetics.104.030700 ; PubMed Central PMCID: PMCPMC1448768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davis MW, Hammarlund M, Harrach T, Hullett P, Olsen S, Jorgensen EM. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics. 2005;6:118 Epub 2005/09/15. 10.1186/1471-2164-6-118 ; PubMed Central PMCID: PMCPMC1242227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smolikov S, Schild-Prufert K, Colaiacovo MP. CRA-1 uncovers a double-strand break-dependent pathway promoting the assembly of central region proteins on chromosome axes during C. elegans meiosis. PLoS Genet. 2008;4(6):e1000088 Epub 2008/06/07. 10.1371/journal.pgen.1000088 ; PubMed Central PMCID: PMCPMC2408554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meneely PM, McGovern OL, Heinis FI, Yanowitz JL. Crossover distribution and frequency are regulated by him-5 in Caenorhabditis elegans. Genetics. 2012;190(4):1251–66. Epub 2012/01/24. 10.1534/genetics.111.137463 ; PubMed Central PMCID: PMCPMC3316641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mets DG, Meyer BJ. Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell. 2009;139(1):73–86. 10.1016/j.cell.2009.07.035 ; PubMed Central PMCID: PMCPMC2785808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brady MM, McMahan S, Sekelsky J. Loss of Drosophila Mei-41/ATR Alters Meiotic Crossover Patterning. Genetics. 2018;208(2):579–88. 10.1534/genetics.117.300634 ; PubMed Central PMCID: PMCPMC5788523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
A) % embryonic lethality; B) % male progeny; C) % embryonic lethality following 75 Gy IR; D) % embryonic lethality following treatment with 5mM HU for 16 hrs of indicated strains. 95% Confidence Intervals shown; * p<0.05; ** p<0.001; *** p<0.0001. gfp::brc-1, tag-rfp-t::brc-1 and brd-1::gfp are not statistically different compared to WT. A minimum of 10 worms were analyzed for each condition. E) Immunoblot of whole worm extracts from WT, gfp::brc-1 and gfp::brc-1; fog-2 probed with rabbit anti-GFP and mouse anti-α-tubulin. Ratio determined by fluorescent intensities from three independent experiments.
(TIF)
A) Number of GFP::BRC-1 foci in indicated mutants in Proliferative Zone, Transition Zone and Early Pachytene. Number of foci examined in a minimum of 3 germ lines: PZ: WT (n = 412); spo-11 (n = 177); rad-51(n = 114); msh-5 (n = 175); syp-1 (n = 140); zim-1 (n = 142); TZ: WT (n = 287); spo-11 (n = 103); rad-51(n = 52); msh-5 (n = 94); syp-1 (n = 83); zim-1 (n = 112); EP: WT (n = 202); spo-11 (n = 106); rad-51(n = 57); msh-5 (n = 57); syp-1 and zim-1 had too many foci to accurately count. *** p<0.0001. B) Late pachytene region of the germ line stained with anti-GFP (green) and anti-phosphoSYP-4 (SYP-4P) (red) and counterstained with DAPI. Scale bar = 10 μm. C) High-magnification images of live C. elegans expressing BRD-1::GFP in the spo-11 background. Images are projections through half of the gonad. PZ = Proliferative Zone, TZ = Transition Zone, EP = Early Pachytene, MP = Mid Pachytene, LP = Late Pachytene, DP = Diplotene, DK = Diakinesis. Scale bar = 5 μm.
(TIF)
A) Genomic regions of brc-1 and brd-1 from WormBase Version: WS265 (https://wormbase.org/#012-34-5), with the region deleted in the different alleles indicated. Color dotted lines indicate the resulting splicing of brd-1(ok1623) (pink; splicing of exon 7–12, which introduces a stop codon and results in a 343 a. a. protein) and brd-1(dw1) (orange; cryptic splice site within intron 11 spliced to exon 12, resulting in a 375 a. a. protein) as determined by cDNA analysis. B) High-magnification images of live brc-1(tm1145) worms expressing BRD-1::GFP (PZ = Proliferative Zone, TZ = Transition Zone, EP = Early Pachytene, MP = Mid Pachytene, LP = Late Pachytene, DP = Diplotene, DK = Diakinesis). Scale bar = 5 μm. C) Indicated germ lines stained with antibodies against SUN-1 S12P (green) and counterstained with DAPI (blue). Numbers beneath genotype indicate the percentage of cell rows with SUN-1 S12P staining normalized to gonad length as in [49]; 3 germ lines were examined. Images are projections through half of the gonad. Scale bar = 20 μm.
(TIF)
A) Box whisker plots show average number of RAD-51 foci per nucleus in the different zones of meiotic prophase (see Fig 6B). Horizontal line of each box indicates the median, the top and bottom of the box indicates medians of upper and lower quartiles, lines extending above and below boxes indicate standard deviation and individual data points are outliers from 5–95%. Numbers of nuclei scored in each zone for WT: 1 = 186; 2 = 343; 3 = 292; 4 = 166; brc-1(tm1145) brd-1(dw1): 1 = 233; 2 = 303; 3 = 261; 4 = 68; brd-1(ok1623): 1 = 186; 2 = 135; 3 = 162; 4 = 117. * p<0.05; ** p<0.001; *** p<0.0001. B) Dissected germ lines from brd-1(ok1623); zim-1, brc-1(tm1145) brd-1(dw1); syp-1, syp-1, brd-1(dw1); zim-1, brc-1(tm1145) brd-1(dw1); zim-1, and brc-1(xoe4); zim-1 worms stained with anti-RAD-51 (red) and counterstained with DAPI (blue); white bracket indicates region of reduced RAD-51 foci. A minimum of 4 germ lines were imaged for each genotype. Full projections of the gonads are shown. Scale bar = 20 μm. C) Mid-late pachytene region of gonad from gfp::rpa-1 and brc-1(tm1145) brd-1(dw1); gfp::rpa-1 worms stained with anti-RAD-51 (red) and imaged for GFP::RPA-1 fluorescence (green), counterstained with DAPI (blue). Images are projections through half of the gonad. Scale bar = 8 μm.
(TIF)
Late pachytene region of the germ line in indicated mutants expressing GFP::COSA-1 (green) and counterstained with DAPI (blue). Images are projections through half of the gonad. Scale bar = 5 μm.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.