Abstract
Background
Self-reported maternal active smoking has been associated with reduced offspring birth length and shorter stature in early and late childhood.
Objective
To use circulating cotinine as an objective biomarker to investigate the association between smoking and environmental tobacco smoke (ETS) exposure in pregnancy, and longitudinal measures of offspring length/height from birth to 60 months.
Methods
In 969 maternal-offspring dyads from the GUSTO cohort, maternal plasma cotinine at 26-28 weeks’ gestation was measured by LC/MS/MS and categorized into 4 groups: Group 1: cotinine <0.17 ng/mL (the assay’s detection limit) and no ETS exposure; Group 2: cotinine <0.17 ng/mL but self-reported ETS; Group 3: cotinine 0.17-13.99 ng/mL (ETS or light smoking); Group 4: cotinine ≥14 ng/mL (active smoking).
Results
Adjusting for infant sex, gestational age at birth, ethnicity, maternal age, education, parity, BMI and height, Group 4 offspring were shorter at birth [z-score β= -0.42 SD units (SDs) (95% CI -0.77, -0.06)] than Group 1 offspring. Group 4 offspring continued to be shorter at older ages, with similar effect sizes at 3 months [-0.57 SDs (0.95, -0.20)], 36 months [-0.53 SDs (-0.92, -0.15)], 48 months [-0.43 SDs (-0.81, -0.04)], and 60 months [-0.57 SDs (-0.96, -0.17)]. Associations were particularly marked in boys. No significant differences in stature were observed in Groups 2 or 3 compared with Group 1.
Conclusions
This Asian longitudinal study associated high prenatal cotinine with persistently shorter stature in offspring from birth and into early childhood, whilst low prenatal cotinine levels and ETS exposure showed no such association.
Implications
Little is known about the long-term effects of prenatal tobacco exposure on offspring stature in Asia where passive smoking is common. This study has used an objective biomarker to reveal that the association of prenatal tobacco exposure with offspring length/height mainly occurs at a high maternal cotinine level of greater than 14 ng/mL in pregnancy, consistent with active smoking, but no significant associations were found with lower cotinine levels, consistent with passive smoking. Encouraging women to quit smoking prior to or during pregnancy may avert the long-term negative impact on their child’s height despite appreciable prenatal environmental tobacco smoke exposure.
Introduction
Maternal smoking during pregnancy is known to increase the risk of intrauterine growth restriction.1,2 Newborn size has classically been considered in terms of birth weight, but recent data have suggested that length at birth may differ from birth weight in risk and prognostic factors.3 Persistent effects of maternal active smoking on the length/height of infants and children have been reported in several prospective birth cohort studies conducted in predominantly Caucasian populations.4–8 Most report shorter birth length, and a few documented reduced child and later height attainment,9–11 but reports are inconsistent with respect to the degree of “catch-up”.6,12,13 To our knowledge, the only published Asian study (from a Japanese cohort) found that maternal smoking was weakly associated with shorter stature in late childhood, but the effect size was very small.14
Less consistent evidence suggests that mothers exposed to passive smoking, otherwise known as environmental tobacco smoke (ETS) exposure, in pregnancy may also give birth to smaller infants.2 In Asian culture, young women rarely smoke,15 but smoking is more common among men. Published Asian data on prenatal ETS effects, mainly of maternal passive smoking exposure to a husband who smokes, have reported an associated reduction in offspring birth weight in Chinese,16 Malay17 and Indian18 populations, but we are aware of no reports of effects on child stature, except for a single Chinese study that found no change in offspring height at age 7 and 11 years old.19
Smoking and ETS exposure during pregnancy is of course a continuum. Most studies are based on the mother’s self-reported history of active smoking or ETS exposure, rather than more objective and quantitative measures such as circulating cotinine concentration or other biomarkers of nicotine exposure. Errors in recall may lead to misclassification of prenatal maternal smoking or ETS exposure status in those studies,20,21 and it is difficult to quantify passive smoking, and thus to assess dose-dependent effects.
Maternal smoking during pregnancy has been reported to have a larger impact on the growth of male fetuses than on females,22–24 which may be explained by a greater intrauterine growth velocity and a different hormonal milieu in males.25 Sex-specific differences in the impact of prenatal tobacco exposure on post-natal physical growth have been infrequently studied, however.
Our group has previously reported that maternal self-reported smoking was associated with significantly reduced offspring birthweight in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort.26 Within this same cohort, we have now quantified maternal circulating cotinine concentrations during pregnancy. In this paper, we have hypothesized that there is also a persistent negative association between maternal circulating cotinine concentration and offspring length/height from birth and into early childhood. We also hypothesized that the magnitude of association would decline with age, as the impact of other genetic and environmental determinants of growth predominate. We also postulated that susceptibility to the effects of maternal active and passive smoking would be greater in boys than in girls at birth and in early childhood.
We used a combination of questionnaires recording maternal self-reported history and maternal plasma cotinine at 26-28 weeks of pregnancy to classify both active and passive smoking exposure. We examined the dose-response association between prenatal tobacco exposure and length/height of offspring at birth and at 11 post-natal ages during the first 60 months of life. In particular, we assessed whether a negative association with length/height persisted post-natally and whether length/height growth rate differed according to prenatal tobacco exposure.
Methods
Study design and participants
Our study is based in the ongoing prospective birth cohort study, Growing Up in Singapore Towards healthy Outcomes (GUSTO), which has been previously described.27 The study was ethically approved by the Institutional Review Boards of the KK Women’s and Children’s Hospital, and the National University Hospital, Singapore. Informed written consent was obtained from all participants.
Briefly, study participants who met eligibility criteria and agreed to participate were recruited (Supplementary Figure 1, n=1247) between June 2009 and September 2010 at 10-14 weeks of gestation when they attended their dating ultrasound scan. Participants and their offspring were followed up at multiple time points through the pregnancy, at birth and post-natally. For our study, participants were restricted to those who conceived spontaneously with singleton pregnancies, with available maternal plasma cotinine measurements at 26-28 weeks of pregnancy, and delivered at a gestational age of 32 weeks or more, thus yielding a final study cohort of 969 maternal-offspring dyads (Supplementary Figure 1). All participants were interviewed during pregnancy (at recruitment and at 26-28 weeks’ gestation) by trained research coordinators using structured questionnaires to collect demographic data, document lifestyle (dietary pattern, cigarette smoking, physical activity, alcohol consumption), and individual and family medical history.
Prenatal smoking and environmental tobacco smoke (ETS) exposure
Structured questionnaires were interviewer administered at 26-28 weeks’ gestation to obtain a smoking and ETS exposure history. To establish if participants were current smokers or had recently stopped smoking, and to assess the possibility of fetal exposure at conception and at the start of pregnancy, participants were asked whether they had ever smoked regularly and from what age, whether they smoked during the one year prior to conception, whether they were still smoking at 26-28 weeks of pregnancy and the number of cigarettes smoked per day. To establish if there was significant ETS exposure, participants were asked to consider exposure just prior to pregnancy and, in separate questions, exposure during pregnancy, and specifically about ETS exposure at home and at work. At 24 months post-delivery, the child’s ETS exposure history was obtained through parental reporting in interviewer administered questionnaires. Parents were asked if the child lived with at least one smoker in the house and, if so, whether smoking occurred in front of the child, within the house, just outside the house or far away from the house.
The concentration of plasma cotinine, obtained at 26-28 weeks’ of pregnancy, was analyzed by liquid chromatography-tandem mass spectrometry at BEVITAL AS (www.bevital.no), as previously described.28 Plasma samples were collected in an EDTA tube, prepared by prompt centrifugation and stored at -80°C until analysis in a single batch. The limit of detection (LOD) for cotinine was 0.17 ng/mL.
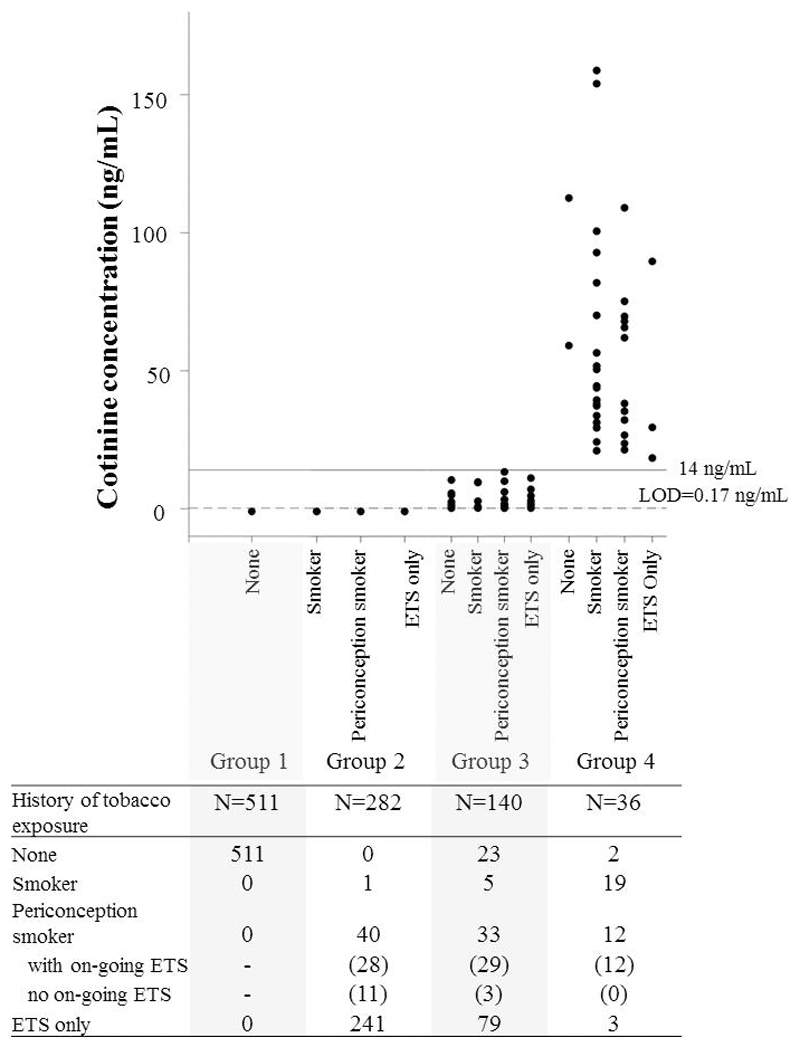
Participants were categorized into four tobacco exposure groups based primarily on cotinine concentration (Figure 1). When maternal plasma cotinine was below the LOD, maternal self-reported smoking and ETS exposure history were used to differentiate participants who had never smoked and who had not been exposed to ETS (Group 1) from those who had been exposed to tobacco either through smoking peri-conceptually and in early pregnancy or through ETS exposure (Group 2). Since the half-life of circulating cotinine is only 8-9 hours in pregnancy as opposed to 16 hours in the non-pregnant state,29 we used maternal self-reported history to account for participants who were exposed to prenatal tobacco outside 12-24 hours prior to blood collection.
Figure 1.
Categorization of the study population into four tobacco exposure groups, depicting the correlation between plasma cotinine concentrations and self-reported tobacco exposure. The grouping was primarily based on plasma cotinine concentrations (Groups 1 and 2 had cotinine levels below the limit of detection (LOD) of 0.17 ng/ml, Group 3: 0.17-13.99 ng/ml, Group 4: >14 ng/ml). Those with cotinine below the LOD were further divided into two groups based on self-reported history: Group 1 comprised those who had never smoked and had not been exposed to environmental tobacco smoke (ETS) [None], and Group 2 comprised those who were self-reported active smokers at 26-28 weeks’ gestation [smoker], those who had smoked regularly during the one year prior to conception [periconception smoker], and those who were exposed to ETS at home or at work. Self-reported history in Groups 3 and 4 are indicated here, although it was not used as criteria for categorization. The number of subjects in each subgroup within each category is shown in the accompanying table.
When the cotinine level was detectable, participants were stratified according to concentration: 0.17 to 13.99 ng/mL, which indicates appreciable exposure due to ETS or light smoking (Group 3), and 14 ng/mL or more (Group 4), a level that had been used to identify active smokers with greater than 95% sensitivity and specificity in a non-pregnant population.30 Given that the half-life of cotinine in pregnancy is shorter than in the non-pregnant, the cotinine threshold that could define an active smoker is likely to be lower than 14 ng/mL during pregnancy. So those in Group 4 were more than likely to be active smokers.
Length/height measures
Recumbent infant length at birth and at 3, 6, 9, 12, 15, 18, 24 months of age was measured from the top of the head to the soles of the feet to the nearest 0.1 cm, using an infant mat (SECA 210 Mobile Measuring Mat; SECA Corp.). Standing height at 36, 48, 54 and 60 months of age was measured with the use of the SECA 213 stadiometer from the top of the participant’s head to his or her heels to the nearest 0.1 cm. All measurements were taken in duplicate and averaged.
Maternal standing height and weight were measured at recruitment in the first trimester and again at 26-28 weeks of pregnancy, and paternal height was taken at either the 24- or 36-month postnatal home visits using a SECA 213 stadiometer (SECA corp.) and a SECA 803 weighing scale, respectively.
Statistical analysis
Age- and sex-specific z-scores for length/height were calculated using the WHO Child Growth Standards published by the World Health Organization in 2006.31 Outliers were excluded from the baseline analysis following the instructions of WHO.
Intergroup differences in characteristics were examined by χ2-tests or ANOVA. Multiple linear regression models were used to assess associations between the 4 groups of prenatal tobacco exposure and offspring length/height at each time point from birth to 60 months.
Covariates which could potentially confound the association of prenatal tobacco exposure and child growth 4,8,32,33 were prospectively collected and adjusted for in the analysis: ethnicity (Chinese, Malay, Indian), maternal age at delivery (≤30 or >30 years), maternal education level (secondary or below, diploma, university), parity (nulliparous [0], primiparous [1] or multiparous [2 or more]), maternal BMI in the 1st trimester (<18.5, 18.5-22.9, 23-27.4, ≥27.5 kg/m2; representing underweight, normal weight, overweight and obese categories respectively in an Asian population as recommended by WHO 34), maternal height (as a continuous variable), and gestational age at birth.
Father’s height was also adjusted for in sensitivity analyses, following multiple imputation for missing data (in 28.3% of cases), with 100 imputations using the PROC MI procedure with the Markov chain Monte Carlo method. The interaction between sex and cotinine exposure level was tested. In a secondary analysis, the cohort was then stratified by sex to assess sex-dependent effects of prenatal tobacco exposure on child length/height. Pregnancy complications such as gestational diabetes mellitus and pre-eclampsia were not adjusted for, because these factors that occur after (or during) tobacco exposure are not confounders (i.e. those that can lead to both tobacco exposure and to altered offspring stature) and may lie on the causal pathway between tobacco exposure and child growth.
Longitudinal changes in the magnitude of association between prenatal tobacco exposure and z-scores for length/height were analyzed using linear mixed-effects models with an unstructured working covariance matrix for random intercept and random slope modeling. Mixed model analysis provides a flexible approach in longitudinal studies with a repeated measurement design, because it can accommodate randomly missing measurements due to incomplete follow-up of the cohort at each time point. This approach allowed us to estimate the impact of prenatal tobacco exposure on the average z-score for length/height over 60 months. With introduction of the interaction term of tobacco exposure*age at time of visit with random intercept and random slope modelling, the rate of length/height growth over time (represented by the slope) was calculated. All analyses were carried out with SAS software (SAS Institute Inc., Cary, NC) version 9.4.
Results
The distribution of maternal cotinine concentrations and self-reported tobacco exposure history are shown in Figure 1. Overall, 459 (47.4%) women self-reported active and/or passive smoking in pregnancy. Group 4 (cotinine ≥14ng/mL) comprised 36 (3.7% of the cohort) women and included light to heavy smokers with a median (range) cotinine of 47.4 (18.3, 158.7) ng/mL. Of these 36 women in Group 4, only 19 (53%) admitted to active smoking at 26-28 weeks’ gestation, 12 (33%) reported smoking in the one year prior to pregnancy but not at 26-28 weeks’ and experienced on-going ETS exposure, 3 (8%) reported ETS exposure only and 2 (5%) reported no history of active smoking nor any ETS exposure. Group 3 comprised 140 (14.4% of the cohort) women with appreciable exposure based on their cotinine levels. Of these women, 5 (4%) self-reported active smoking at 26-28 weeks’ gestation, and a further 33 (24%) admitted to being smokers in the one year preceding pregnancy but had stopped by 26-28 weeks’ gestation although 29 of them experienced on-going ETS exposure. Importantly, 23 (16%) women in Group 3 reported no tobacco exposure (active or ETS) history despite their detectable levels of cotinine. Among the 282 women in Group 2 where cotinine was below the LOD, only 1 self-reported current active smoking, while 40 (14%) declared themselves as peri-conception smokers who had stopped by mid-gestation but of whom the majority continued to be exposed to ETS, and the remainder declared exposure to ETS only.
Maternal and offspring characteristics across the four prenatal tobacco exposure groups are shown in Table 1. High maternal cotinine (≥14 ng/mL) was associated with younger maternal age (p<0.001), Malay ethnicity (p<0.001), lower maternal education level (p<0.001) and higher parity (p<0.001) but was not associated with maternal body mass index (BMI) in the first trimester nor with maternal height. High maternal cotinine was significantly associated with a lower crude birthweight (p<0.001) and a shorter crude birth length (p<0.001). Of note, the infant sex distribution was similar between groups.
Table 1.
Characteristics of participants
| All | Maternal Fasting Plasma Cotinine concentration (ng/mL) | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |||
| <0.17 | <0.17 | 0.17-13.99 | ≥14 | |||
| Self-reported ETS Exposure | No | Yes | - | - | p value | |
| No. of Subjects, N (%) | 969 | 511 (52.7) | 282 (29.1) | 140 (14.4) | 36 (3.7) | |
| Maternal Characteristics | ||||||
| Ethnicity | <0.001b | |||||
| Chinese | 527 (54.4)a | 325 (61.7) | 141 (26.8) | 47 (8.9) | 14 (2.6) | |
| Malay | 260 (26.8)a | 62 (23.9) | 102 (39.2) | 78 (30.0) | 18 (6.9) | |
| Indian | 182 (18.8)a | 124 (68.1) | 39 (21.4) | 15 (8.2) | 4 (2.2) | |
| Maternal Age (years) | <0.001b | |||||
| 30 and below | 423 (43.7)a | 169 (40.0) | 139 (32.9) | 93 (22.0) | 22 (5.2) | |
| Above 30 | 546 (56.4)a | 342 (62.6) | 143 (26.2) | 47 (8.6) | 14 (2.6) | |
| Education level | <0.001b | |||||
| Secondary and below | 294 (30.3)a | 87 (29.6) | 114 (38.8) | 67 (22.8) | 26 (8.8) | |
| Diploma | 335 (34.6)a | 156 (46.6) | 116 (34.6) | 54 (16.1) | 9 (2.7) | |
| University and above | 327 (33.8)a | 263 (80.4) | 46 (14.1) | 18 (5.5) | 0 (0.0) | |
| Unknown | 13 (1.3)a | 5 (38.5) | 6 (46.2) | 1 (7.7) | 1 (7.7) | |
| Parity | <0.001b | |||||
| 0 | 412 (42.5)a | 212 (51.5) | 123 (29.9) | 70 (17.0) | 7 (1.7) | |
| 1 | 344 (35.5)a | 210 (61.1) | 86 (25.0) | 35 (10.2) | 13 (3.8) | |
| ≥ 2 | 213 (22.0)a | 89 (41.8) | 73 (34.3) | 35 (16.4) | 16 (7.5) | |
| Early pregnancy BMI (kg/m2) | 0.246b | |||||
| Less than 18.5 | 84 (8.7)a | 47 (56.0) | 21 (25.0) | 11 (13.1) | 5 (6.0) | |
| 18.5-22.9 | 439 (45.3)a | 242 (55.1) | 123 (28.0) | 57 (13.0) | 17 (3.9) | |
| 23-27.4 | 269 (27.8)a | 138 (51.3) | 84 (31.2) | 43 (16.0) | 4 (1.5) | |
| 27.5 and above | 168 (17.3)a | 78 (46.4) | 52 (31.0) | 29 (17.3) | 9 (5.4) | |
| Unknown | 9 (0.9)a | |||||
| Maternal height (cm)f | 158.3 (5.6) | 158.5 (5.7) | 158.1 (5.5) | 157.8 (5.7) | 158.6 (5.4) | 0.46 c |
| Offspring characteristics | ||||||
| Sex | 0.884b | |||||
| Female | 465 (48.0)a | 249 (53.6) | 131 (28.2) | 69 (14.8) | 16 (3.4) | |
| Male | 504 (52.0)a | 262 (52.0) | 151 (30.0) | 71 (14.1) | 20 (4.0) | |
| Birth Weight (gram)f | 3110±433 | 3138 ±429 | 3092 ±449 | 3112 ±382 | 2843 ± 467 | <0.001c |
| Birth Length (cm)f | 48.7 ±2.2 | 49.0 ±2.1 | 48.4 ±2.3 | 48.5 ±2.0 | 47.4 ±2.3 | <0.001c |
| Gestational age at birth (weeks)d, f | 38.8 ±1.3 | 39.0 ±1.2 | 38.7 ±1.2 | 38.7 ±1.2 | 38.2 ±1.6 | <0.001c |
| Postnatal ETS exposuree | ||||||
| No | 534 (55.1)a | 407 (76.2) | 92 (17.2) | 30 (5.6) | 5 (0.9) | <0.001b |
| Yes | 289 (29.8)a | 36 (12.5) | 146 (50.5) | 84 (29.1) | 23 (8.0) | |
| Unknown (N=146) | 146 (15.1)a | |||||
Percentage of all study subjects (N=969) across categories of each characteristic, otherwise the percentage (%) indicates distribution of each category across the four different prenatal tobacco exposure groups.
p value determined by chi-square (χ2) test
p value determined by ANOVA test
Pregnancy dating was done by first trimester crown rump length on ultrasound scan.
Without postnatal ETS exposure was defined as either having no smokers at home or if there is a smoker at home they only smoked far away from the house; With postnatal ETS exposure was defined as having at least a smoker at home who either smoked in front of the subject or who smoked within or just outside the house.
mean ± SD
Missing data: Unknown data for maternal BMI in the 1st trimester (n=49) were imputed by self-reported pre-pregnancy BMI (n=40) or estimated using the maternal pregnancy BMI at 26-28 week (n=5); for maternal education level (n=13) were imputed by mode (diploma) in further analysis.
Amongst the 822 out of the 969 (85%) mothers who responded to the postnatal ETS survey at 24 months post-delivery, 288 (35%) of the children lived with at least one smoker who smoked in front of the child, or within or just outside the house (Table 1). The pattern of smoking among household smokers and the likelihood of child ETS exposure remained consistent across the first 24 months of life. Among all children with at least one household smoker (N=310), 40 (13%) had household smokers who smoked in the presence of the children, 121 (39%) smoked indoors in the house, 127 (41%) smoked outside the house and only 22 (7%) smoked far away from the house. Self-reported prenatal tobacco smoke exposure and parental reports of child ETS exposures at 24 month were highly correlated (data not shown).
Associations of maternal cotinine with offspring length/height
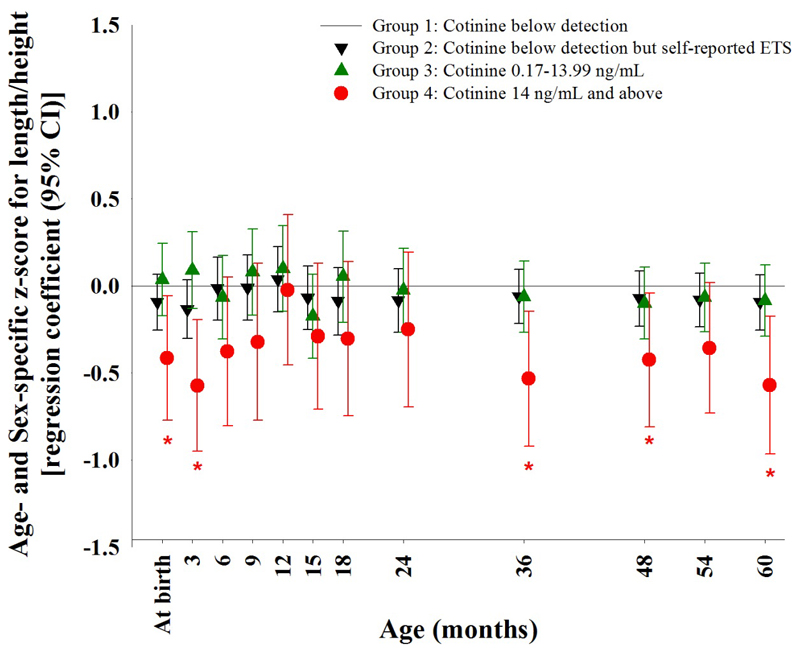
Compared with Group 1, maternal cotinine ≥14 ng/mL (Group 4) was associated with a lower z-score for length/height of the offspring, with significant associations found at birth [β (95% CI) -0.42 SD units (SDs) (-0.77, -0.06)], 3 months [-0.57 SDs (-0.95, -0.20)], 36 months [-0.53 SDs (-0.92, -0.15)], 48 months [-0.43 SDs (-0.81, -0.04)], and 60 months [-0.57 SDs (-0.96, -0.17)] after adjustment for ethnicity, maternal age at delivery, education level, parity, 1st trimester maternal BMI, maternal height and gestational age at birth (Figure 2). In absolute terms the reduction of mean birth length and mean height at 60 months between Group 1 and Group 4 was 0.77 cm and 2.7 cm, respectively. Sensitivity analysis with further adjustment for paternal height, with multiple imputation for missing data, showed similar results for Group 4 compared to Group 1, with additional significant associations at 54 months [-0.36 SDs (-0.74, -0.01)] (Supplementary Table 1). Even though results from 6 months to 24 months failed to reach statistical significance, a consistent (except at 12 months) trend of shorter offspring length was observed in Group 4 compared to Group 1.
Figure 2.
Maternal cotinine concentration with prenatal ETS exposure status, and offspring z-score for length/height.
Examination of the association between maternal plasma cotinine concentration with prenatal ETS exposure, and offspring z-score for length/height at each single time point from birth to age 60 months with adjustment for the following covariates: ethnicity, maternal age at delivery, educational level, parity, 1st trimester maternal BMI, maternal height and gestational age at birth. *:p<0.05; ETS: Environmental tobacco smoke exposure
Offspring in Groups 2 and 3, whose mothers were light smokers, periconception smokers or who experienced ETS exposure with or without detectable plasma cotinine at 26-28 weeks’ gestation, did not demonstrate any significant differences in length/height compared with Group 1 (Figure 2).
Influence of offspring sex and ethnicity
Overall, there was no significant interaction between sex and prenatal tobacco exposure on offspring length/height. However, given the previously documented sex-specific effects of maternal smoking on fetal growth, we interrogated our data further. When the data was stratified by sex a similar direction of change was seen for both sexes with exposure to a high plasma cotinine ≥14ng/mL (Group 4) compared to Group 1 controls. The intergroup z-score reduction was pronounced in boys at birth [β (95% CI) -0.47 SDs (-0.94, -0.003)], 3 months [-0.69 SDs (-1.21, -0.18)], 36 months [-0.76 SDs (-1.29, -0.23)], 48 months [-0.59 SDs (-1.14, -0.03)] and 60 months [-0.87 SDs (-1.44, -0.30)]. However, z-score reductions among girls failed to reach statistical significance (Supplementary Table 2). Between the three ethnic groups, there was no significant difference in the association between a high maternal cotinine concentration (≥14ng/mL) and offspring length/height (data not shown).
Associations of maternal cotinine with height growth rate
Overall, there was also no significant interaction between time point of measurement and prenatal tobacco exposure on offspring length/height. Consistent with results of multiple linear regression models, using linear mixed-effects modeling we found that over the first 60 months, offspring exposed to maternal plasma cotinine ≥14ng/mL (Group 4) were shorter, on average, than Group 1 controls [-0.35 SDs (-0.64, -0.06), p<0.05]. In contrast, offspring in Group 2 and Group 3 showed no significant difference in average length/height over the first 60 months compared with Group 1 (Table 2). When considering the overall length/height growth rate from birth to 60 months, there was no statistically significant difference between Group 4 and Group 1 [β (95% CI) 0.06 SDs/year (-0.01, 0.14), p=0.097]. Only offspring in Group 2 showed a marginally faster length/height growth rate [0.03 SDs/yr (<0.01, 0.06); p=0.046] compared with Group 1, which is likely a chance finding (Table 2).
Table 2.
Linear mixed regression analysis: Association between prenatal tobacco exposure and (A) average length/height z-score over 60 months (B) relative length/height growth rate of offspring
| (A) Average length/height z-score | (B) Relative growth rate (SDs/year) b |
||||
|---|---|---|---|---|---|
| birth to 60 monthsa | birth to 60 months | 0-12 months | 12-36 months | 36-60 months | |
| Maternal plasma cotinine (ng/mL) and ETS status |
|||||
| <0.17, ETS (-) | Reference | Reference | Reference | Reference | Reference |
| <0.17, ETS (+) | -0.08 (-0.21 ,0.05) | 0.03 (<0.01 ,0.06)* | 0.08 (-0.11 ,0.27) | 0.03 (-0.03 ,0.09) | 0.01 (-0.02 ,0.04) |
| 0.17-13.99 | -0.01 (-0.17 ,0.16) | 0.02 (-0.02 ,0.05) | -0.05 (-0.29 ,0.19) | 0.05 (-0.02 ,0.12) | 0.02 (-0.02 ,0.05) |
| 14 and Above | -0.35 (-0.64 ,-0.06)* | 0.06 (-0.01 ,0.14) | 0.43 (-0.02 ,0.87) | -0.05 (-0.20 ,0.09) | 0.03 (-0.04 ,0.11) |
p value <0.05
Models adjusted for prenatal tobacco exposure grouping (Groups 1 to 4 based on maternal plasma cotinine and ETS status), ethnicity, maternal age at delivery, maternal education level, parity, 1st trimester maternal BMI, maternal height, gestational age at birth and age of visit, with random intercept and random slope model.
Models adjusted for prenatal tobacco exposure grouping (Groups 1 to 4 based on maternal plasma cotinine and ETS status), ethnicity, maternal age at delivery, maternal education level, parity, 1st trimester maternal BMI, maternal height and gestational age at birth, age of visit and interaction of ETS*age of visit with random intercept and random slope model.
ETS: Environmental tobacco smoke exposure
When we examined the growth rate over the first 12 month period only we found that a high plasma cotinine (≥14ng/mL; Group 4) was associated with a non-statistically significant trend of accelerated growth compared with Group 1 [growth rate 0.43 SDs/year (-0.02, 0.87)] (Table 2). This was followed by no significant difference in growth rate during the periods of 12 to 36 months and 36 to 60 months.
No significant interaction was observed between sex or ethnicity and prenatal tobacco exposure and growth rate (data not shown).
Discussion
Our results demonstrate a persistent, long-term association of prenatal exposure to high maternal cotinine (≥14 ng/mL), consistent with active maternal smoking during pregnancy, with offspring length and height, particularly in boys. The association was of similar magnitude on the z-score (SD) scale at birth and at 60 months of life. With exposure to ETS (passive smoking) and lower levels of maternal cotinine, no statistically significant differences in offspring length/height were observed, compared with the unexposed group. To the best of our knowledge, ours is the first longitudinal study reporting the magnitude of association between high maternal cotinine in late pregnancy and offspring length/height growth deficits in a multiethnic Asian population.
A reduction in offspring stature of approximately 0.5 SD in a population of smoking mothers would significantly increase the number of children classed as stunted (below 2 SD of the WHO child growth standards 31). Stunting is now internationally recognized as a major public health problem associated with negative impact on development and long-term health. Although a common global cause of stunting is thought to be relative malnutrition, reducing maternal smoking in pregnancy may also contribute to attainment of the WHO target of reduced global stunting by 2025 35.
The association we observed between maternal active smoking and birth length in this South East Asian cohort comprising participants of Chinese, Malay and Indian descent is consistent with previous findings from Caucasian and East Asian populations.5–8,13,14,36,37 In fact, the magnitude of association we observed on birth length is remarkably similar to that reported in previous studies from Europe. Between Group 4 and the Group 1 controls, we found a reduction of 0.77 cm in mean birth length and a reduction of 0.43 SDs in z-score, similar to the 0.67 cm difference documented in the UK ALSPAC cohort6 and the reduction of 0.36 SDs in z-score in light smokers (1-5 cigarettes/day) in the Netherlands’ Generation R cohort.4 This constellation of findings suggests that geographical and ethnic variation has a limited influence on the impact of maternal smoking on fetal statural growth and is also consistent with the lack of difference in association we observed across our three Asian ethnic groups.
However, our finding of boys’ stature being particularly susceptible to maternal smoking both at birth and up to 60 months of age are in contrast to those from the ALSPAC cohort, in which boys’ and girls’ lengths at birth were equally affected by maternal smoking, but by 10 years of age girls’ showed a greater attenuation in height than boys following prenatal exposure.6 Such a discrepancy may be partly attributed to population differences. Our results are, however, consistent with several other human fetal studies.22–24 It has been postulated that higher androgen levels and accompanying changes in the hormonal milieu in the male fetus could lead to differential expression of fetal liver enzymes important in regulating the metabolism of drugs and xenotoxicants25 with consequences on fetal growth and development.38 Others have suggested that given the faster rate of growth of male fetuses, they have a greater demand for oxygen and nutrients, hence, a relative reduction in transplacental delivery associated with maternal smoking could have a greater impact on growth,24 hence males may be more susceptible. From our data, this particular impact during fetal life appeared to then have persistent effects in early childhood.
Several reports have confirmed that the impact of heavy smoking on offspring stature is greater than that of light smoking.4,6 However, the impact of exposure to passive smoking has been difficult to quantify by self-reported history. In Singapore, the vast majority (95%) of the population live in multiunit housing or apartments and can be exposed to significant tobacco smoke even without active smoking.39 One Singaporean study reported that 13% of fetuses had detectable cotinine in meconium at birth, yet in 65% of the cases the mother reported no active or passive smoking during pregnancy.40 We have used the more objective measure of prenatal tobacco smoke exposure by quantifying maternal cotinine and combining this with self-reported history for more accurate categorization and found that low to intermediate levels of exposure had no significant relationship with offspring stature. A sizeable proportion of exposed women (Groups 2 and 3) in our study reported stopping smoking before or in early pregnancy, which probably averted a detrimental impact on offspring stature, as reported previously.4 The lack of significant effect of passive smoking on birth length is in contrast to its impact on birth weight in Asian populations of similar ethnicity to our study population,16–18 further supporting the notion that birth weight and birth length may be differentially regulated in utero.3
Our results were not consistent with our hypothesis that the magnitude of effect of maternal smoking on stature would decline with post-natal age. We observed that the association with birth length was sustained to a similar degree through 60 months of age, with no significant difference in the average growth rate between the high cotinine group and unexposed group over this period. This result could support the argument that persistent short stature is simply a persistent effect of shorter birth length,5 i.e., that no separate ongoing biological mechanisms affected by prenatal tobacco exposure continue to affect post-natal growth in stature.6 In contrast, others suggest that the persistent association is in part due to specific intrauterine programming effects of maternal smoking in pregnancy that can play out as an ongoing independent regulator of child height growth postnatally,4 well into late childhood and beyond.7,8 This notion could then potentially explain inter-study variations in offspring statural growth associated with maternal smoking, with some reporting “catch-up” growth.
Discrepancies in the extent of possible ‘catch-up’ among reported studies may be due to the timing of postnatal measurements. A study that reported complete “catch-up” ended at the age of 8 months12 and may well have missed a later detrimental effect on stature. The timing of when a possible early “catch-up” growth begins has also varied across studies. In our study we found that offspring length remained significantly shorter at 3 months and that height growth only started to approach the unexposed group after this. In contrast, studies of predominantly Caucasians showed that catch-up had already started by 2-3 months4,6 but a Turkish study showed a persistence of shorter stature even at 6 months.13 Our cohort, which includes three different ethnicities known to have differential infant growth velocity41 but display similar susceptibility to prenatal tobacco exposure in terms of offspring stature, suggests that differences in findings between previous studies are due predominantly to genetically determined factors of growth and timing of measurements, and may not relate to differential responses to prenatal tobacco exposure per se. Although there was suggestion of a somewhat faster rate of height growth in the first 12 months with high prenatal tobacco exposure in our cohort, this trend was not sustained and the average height over 60 months remained lower in exposed offspring.
The causal relationship of maternal smoking during pregnancy and smaller birth size is well established. The suggested physiological changes triggered by prenatal smoking that may directly and immediately affect fetal growth include maternal hypoxemia secondary to vasoconstriction or reduced oxygen carriage by hemoglobin; lower concentrations of insulin, insulin-like growth factor (IGF)-I, and IGF binding protein-3;42 increased toxins and heavy metals;43 reduced maternal nutrition; and poorer placental health,44,45 all of which may impair fetal skeletal growth and therefore birth length.46 Differential susceptibility to prenatal tobacco exposure may also be determined by newborn gene polymorphism.47 However, when seeking potential mechanisms for the long-term in utero programming effects on post-natal growth, proposed mechanisms include programming of skeletal growth;46 alterations in metabolic and satiety homeostasis involving the hypothalamo-pituitary-adrenal axis, growth hormone, ghrelin and leptin response pathways;48 and epigenetic changes such as telomere lengths49 and DNA methylation.49–53 Additionally, significant co-morbidities and neurological deficits which may be associated with prenatal tobacco exposure could also affect child growth, and would be a subject for further investigation.
The main strengths of our study are its prospective design, the use of plasma cotinine as an objective measure of tobacco exposure and the frequent, standardized, accurate measurements of length/height across the first 60 months of life. We observed substantial discrepancies between self-reported smoking history and cotinine levels. Using self-reported history on its own would have led to misclassification of a significant number of women. This finding is consistent with other studies reporting that the combination of history of self-reported smoking/ETS exposure and biomarker quantification in pregnancy improves the accurate documentation of active and/or passive tobacco smoke exposure and minimizes misclassification.20,21,54
On the other hand, the primary limitation of our study was the single cotinine measurement at 26-28 week in pregnancy. Cotinine has a relatively short half-life and reflects only recent tobacco exposure,55 but misclassification was mitigated by prospective collection of self-reported active and/or passive tobacco smoke exposure history. Our inferences are limited to the impact of cotinine exposure at 26-28 weeks of pregnancy, which may underestimate the total effect of smoking on length/height status, as some study women may have transiently quit smoking either during early or late pregnancy. Furthermore, with the lack of a post-natal cotinine measure, we cannot separate the effect of prenatal from post-natal tobacco exposure, which are highly correlated. Our GUSTO data confirm that parents who smoked during pregnancy generally continue to smoke after child birth. Nonetheless, previous published studies all suggest that prenatal tobacco exposure has a far greater impact on offspring growth than post-natal ETS exposure.4–6,52 Although, there was incomplete follow up of the cohort over the study duration there was no selection bias towards smaller infants from Group 4 remaining in the study as the birth lengths and birth weights of those who attended the visit at 60 months were similar to the respective measurements of those who did not attend. Finally, unmeasured lifestyle differences, genetic, parenting and other social factors may have confounded the associations we observed with child statural growth.
In conclusion, the association of maternal cotinine in pregnancy with offspring length/height mainly occurs at a maternal cotinine level of 14ng/mL or more, consistent with active smoking. Encouraging women to stop smoking during pregnancy may avert the long-term negative impact on their child’s height despite persistent ETS exposure. Longer follow-up of these children will be required to assess the impact on final height attainment.
Supplementary Material
Acknowledgements
We thank the GUSTO study group for their contribution. The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Marielle V. Fortier, Mark Hanson, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Neerja Karnani, Ngee Lek, P. C. Wong, Paulin Tay Straughan, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, S. Sendhil Velan, Seng Bin Ang, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, and Yin Bun Cheung.
Funding
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR).
Footnotes
Declaration of Interests:
KMG, Y-SC, and YSL have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG, S-YC and Y-SC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors have no financial or personal conflict of interest to declare.
References
- 1.Varvarigou AA, Fouzas S, Beratis NG. Effect of prenatal tobacco smoke exposure on fetal growth potential. J Perinat Med. 2010;38(6):683–687. doi: 10.1515/jpm.2010.101. [DOI] [PubMed] [Google Scholar]
- 2.Reeves S, Bernstein I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev Obstet Gynecol. 2008;3(6):719–730. doi: 10.1586/17474108.3.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG, Villar J, Barros FC, et al. Anthropometric Characterization of Impaired Fetal Growth: Risk Factors for and Prognosis of Newborns With Stunting or Wasting. JAMA Pediatr. 2015;169(7):e151431. doi: 10.1001/jamapediatrics.2015.1431. [DOI] [PubMed] [Google Scholar]
- 4.Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. Am J Clin Nutr. 2011;94(1):164–171. doi: 10.3945/ajcn.110.009225. [DOI] [PubMed] [Google Scholar]
- 5.Fox NL, Sexton M, Hebel JR. Prenatal exposure to tobacco: I. Effects on physical growth at age three. Int J Epidemiol. 1990;19(1):66–71. doi: 10.1093/ije/19.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Howe LD, Matijasevich A, Tilling K, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol. 2012;41(3):722–732. doi: 10.1093/ije/dys025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Mesa J, Menezes AM, Gonzalez DA, et al. Life course association of maternal smoking during pregnancy and offspring's height: data from the 1993 Pelotas (Brazil) birth cohort. J Adolesc Health. 2012;51(6 Suppl):S53–57. doi: 10.1016/j.jadohealth.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matijasevich A, Brion MJ, Menezes AM, Barros AJ, Santos IS, Barros FC. Maternal smoking during pregnancy and offspring growth in childhood: 1993 and 2004 Pelotas cohort studies. Arch Dis Child. 2011;96(6):519–525. doi: 10.1136/adc.2010.191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2006;35(1):121–130. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- 10.Fogelman KR, Manor O. Smoking in pregnancy and development into early adulthood. BMJ. 1988;297(6658):1233–1236. doi: 10.1136/bmj.297.6658.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshy G, Delpisheh A, Brabin BJ. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health. 2011;21(3):286–291. doi: 10.1093/eurpub/ckq173. [DOI] [PubMed] [Google Scholar]
- 12.Barr HM, Streissguth AP, Martin DC, Herman CS. Infant size at 8 months of age: relationship to maternal use of alcohol, nicotine, and caffeine during pregnancy. Pediatrics. 1984;74(3):336–341. [PubMed] [Google Scholar]
- 13.Fenercioglu AK, Tamer I, Karatekin G, Nuhoglu A. Impaired postnatal growth of infants prenatally exposed to cigarette smoking. Tohoku J Exp Med. 2009;218(3):221–228. doi: 10.1620/tjem.218.221. [DOI] [PubMed] [Google Scholar]
- 14.Ino T, Shibuya T, Saito K, Ohtani T. Effects of maternal smoking during pregnancy on body composition in offspring. Pediatr Int. 2011;53(6):851–857. doi: 10.1111/j.1442-200X.2011.03383.x. [DOI] [PubMed] [Google Scholar]
- 15.Tan PY, Utravathy V, Ho LY, Foo SG, Tan K. Prevalence of Tobacco Smoking and Accuracy of Self-Reporting in Pregnant Women at a Public Hospital for Women and Children. Ann Acad Med Singapore. 2016;45(5):184–190. [PubMed] [Google Scholar]
- 16.Tsui HC, Wu HD, Lin CJ, et al. Prenatal smoking exposure and neonatal DNA damage in relation to birth outcomes. Pediatr Res. 2008;64(2):131–134. doi: 10.1203/PDR.0b013e3181799535. [DOI] [PubMed] [Google Scholar]
- 17.Norsa'adah B, Salinah O. The Effect of Second-Hand Smoke Exposure during Pregnancy on the Newborn Weight in Malaysia. Malays J Med Sci. 2014;21(2):44–53. [PMC free article] [PubMed] [Google Scholar]
- 18.Mathai M, Vijayasri R, Babu S, Jeyaseelan L. Passive maternal smoking and birthweight in a south Indian population. Br J Obstet Gynaecol. 1992;99(4):342–343. doi: 10.1111/j.1471-0528.1992.tb13736.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwok MK, Schooling CM, Lam TH, Leung GM. Paternal smoking and childhood overweight: evidence from the Hong Kong "Children of 1997". Pediatrics. 2010;126(1):e46–56. doi: 10.1542/peds.2009-2642. [DOI] [PubMed] [Google Scholar]
- 20.Jhun HJ, Seo HG, Lee DH, et al. Self-reported smoking and urinary cotinine levels among pregnant women in Korea and factors associated with smoking during pregnancy. J Korean Med Sci. 2010;25(5):752–757. doi: 10.3346/jkms.2010.25.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki S, Braimoh TS, Yila TA, Yoshioka E, Kishi R. Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy--a validation study in Northern Japan. Sci Total Environ. 2011;412–413:114–118. doi: 10.1016/j.scitotenv.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Spinillo A, Capuzzo E, Nicola SE, Colonna L, Egbe TO, Zara C. Factors potentiating the smoking-related risk of fetal growth retardation. Br J Obstet Gynaecol. 1994;101(11):954–958. doi: 10.1111/j.1471-0528.1994.tb13038.x. [DOI] [PubMed] [Google Scholar]
- 23.Wertelecki W, Hoff C, Zansky S. Maternal smoking: greater effect on males, fetal tobacco syndrome? Teratology. 1987;35(3):317–320. doi: 10.1002/tera.1420350305. [DOI] [PubMed] [Google Scholar]
- 24.Zaren B, Lindmark G, Bakketeig L. Maternal smoking affects fetal growth more in the male fetus. Paediatr Perinat Epidemiol. 2000;14(2):118–126. doi: 10.1046/j.1365-3016.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- 25.Stubbs AP, Engelman JL, Walker JI, Faik P, Murphy GM, Wilkinson ML. Measurement of androgen receptor expression in adult liver, fetal liver, and Hep-G2 cells by the polymerase chain reaction. Gut. 1994;35(5):683–686. doi: 10.1136/gut.35.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin X, Lim IY, Wu Y, et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC medicine. 2017;15(1):50. doi: 10.1186/s12916-017-0800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soh SE, Tint MT, Gluckman PD, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 28.Midttun O, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid communications in mass spectrometry : RCM. 2009;23(9):1371–1379. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. The Journal of pharmacology and experimental therapeutics. 2002;301(2):594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77(11):1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Multicentre Growth Reference Study Group. WHO child growth standards: length/heightfor-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization; 2006. [Google Scholar]
- 32.Leary S, Davey Smith G, Ness A. Smoking during pregnancy and components of stature in offspring. Am J Hum Biol. 2006;18(4):502–512. doi: 10.1002/ajhb.20518. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Kondo N, Sato M, Tanaka T, Ando D, Yamagata Z. Maternal smoking during pregnancy and childhood growth trajectory: a random effects regression analysis. J Epidemiol. 2012;22(2):175–178. doi: 10.2188/jea.JE20110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 35.Organization WH. Global Nutrition Targets 2025: Stunting policy brief. WHO/NMH/NHD/14.3. 2014 [Google Scholar]
- 36.Abraham M, Alramadhan S, Iniguez C, et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS One. 2017;12(2):e0170946. doi: 10.1371/journal.pone.0170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durmus B, Ay L, Hokken-Koelega AC, et al. Maternal smoking during pregnancy and subcutaneous fat mass in early childhood. The Generation R Study. Eur J Epidemiol. 2011;26(4):295–304. doi: 10.1007/s10654-010-9544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krauer B, Dayer P. Fetal drug metabolism and its possible clinical implications. Clin Pharmacokinet. 1991;21(1):70–80. doi: 10.2165/00003088-199121010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Orazine CI, Arias WA, Magee SR, King E. Non-smoking pregnant women and their fetuses are exposed to environmental tobacco smoke as a result of living in multiunit housing. Journal of exposure science & environmental epidemiology. 2016 doi: 10.1038/jes.2016.34. [DOI] [PubMed] [Google Scholar]
- 40.Ostrea EM, Jr, Villanueva-Uy E, Ngerncham S, et al. An epidemiologic study comparing fetal exposure to tobacco smoke in three Southeast Asian countries. International journal of occupational and environmental health. 2008;14(4):257–262. doi: 10.1179/oeh.2008.14.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aris IM, Bernard JY, Chen LW, et al. Infant body mass index peak and early childhood cardio-metabolic risk markers in a multi-ethnic Asian birth cohort. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingvarsson RF, Bjarnason AO, Dagbjartsson A, Hardardottir H, Haraldsson A, Thorkelsson T. The effects of smoking in pregnancy on factors influencing fetal growth. Acta Paediatr. 2007;96(3):383–386. doi: 10.1111/j.1651-2227.2007.00103.x. [DOI] [PubMed] [Google Scholar]
- 43.Wrzesniak M, Kepinska M, Krolik M, Milnerowicz H. The Influence of Tobacco Smoke on Protein and Metal Levels in the Serum of Women during Pregnancy. PLoS One. 2016;11(8):e0161342. doi: 10.1371/journal.pone.0161342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83(11):699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Wrzesniak M, Kepinska M, Krolik M, Milnerowicz H. Influence of tobacco smoking on transferrin sialylation during pregnancy in smoking and non-smoking women with iron deficiency. Environmental toxicology and pharmacology. 2016;46:95–102. doi: 10.1016/j.etap.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Godfrey K, Walker-Bone K, Robinson S, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16(9):1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi S, Sata F, Sasaki S, et al. Combined effects of AHR, CYP1A1, and XRCC1 genotypes and prenatal maternal smoking on infant birth size: Biomarker assessment in the Hokkaido Study. Reprod Toxicol. 2016;65:295–306. doi: 10.1016/j.reprotox.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 48.McDonald SD, Walker M, Perkins SL, et al. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. BJOG. 2006;113(11):1289–1295. doi: 10.1111/j.1471-0528.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- 49.Ip P, Chung BH, Ho FK, et al. Prenatal Tobacco Exposure Shortens Telomere Length in Children. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw139. [DOI] [PubMed] [Google Scholar]
- 50.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morales E, Vilahur N, Salas LA, et al. Genome-wide DNA methylation study in human placenta identifies novel loci associated with maternal smoking during pregnancy. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw196. [DOI] [PubMed] [Google Scholar]
- 52.Robinson O, Martinez D, Aurrekoetxea JJ, et al. The association between passive and active tobacco smoke exposure and child weight status among Spanish children. Obesity (Silver Spring) 2016;24(8):1767–1777. doi: 10.1002/oby.21558. [DOI] [PubMed] [Google Scholar]
- 53.Rotroff DM, Joubert BR, Marvel SW, et al. Maternal smoking impacts key biological pathways in newborns through epigenetic modification in Utero. BMC Genomics. 2016;17(1):976. doi: 10.1186/s12864-016-3310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie C, Wen X, Niu Z, et al. Comparison of secondhand smoke exposure measures during pregnancy in the development of a clinical prediction model for small-for-gestational-age among non-smoking Chinese pregnant women. Tob Control. 2015;24(e3):e179–187. doi: 10.1136/tobaccocontrol-2014-051569. [DOI] [PubMed] [Google Scholar]
- 55.Pichini S, Basagana XB, Pacifici R, et al. Cord serum cotinine as a biomarker of fetal exposure to cigarette smoke at the end of pregnancy. Environ Health Perspect. 2000;108(11):1079–1083. doi: 10.1289/ehp.001081079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.