Abstract
A large body of literature links risk of cognitive decline, mild cognitive impairment (MCI) and dementia with Type 2 Diabetes (T2D) or pre-diabetes. Accumulating evidence implicates a close relationship between the brain insulin receptor signaling pathway (IRSP) and the accumulation of amyloid beta and hyperphosphorylated and conformationally abnormal tau. We showed previously that the neuropathological features of Alzheimer’s disease (AD were reduced in patients with diabetes who were treated with insulin and oral antidiabetic medications. To understand better the neurobiological substrates of T2D and T2D medications in AD, we examined IRSP and endothelial cell markers in the parahippocampal gyrus of controls (N = 30), of persons with AD (N = 19), and of persons with AD and T2D, who, in turn, had been treated with anti-diabetic drugs (insulin and or oral agents; N = 34). We studied the gene expression of selected members of the IRSP and selective endothelial cell markers in bulk postmortem tissue from the parahippocampal gyrus and in endothelial cell enriched isolates from the same brain region. The results indicated that there are considerable abnormalities and reductions in gene expression (bulk tissue homogenates and endothelial cell isolates) in the parahippocampal gyri of persons with AD that map directly to genes associated with the microvasculature and the IRSP. Our results also showed that the numbers of abnormally expressed microvasculature and IRSP associated genes in diabetic AD donors who had been treated with anti-diabetic agents were reduced significantly. These findings suggest that anti-diabetic treatments may reduce or normalize compromised microvascular and IRSP functions in AD.
Introduction
A significant body of literature links risk of cognitive decline, mild cognitive impairment (MCI), and dementia with Type 2 Diabetes (T2D) or pre-diabetes[1, 2]. T2D or impaired fasting glucose may be present in up to 80% of persons with Alzheimer’s disease (AD)[3]. Modifications in brain insulin metabolism are thought to be among the pathophysiological factors underlying dementia, whether due to AD[4] or to vascular cognitive impairment and dementia (VCID). Several studies suggest that pre-diabetes[5] and T2D may anticipate conversion to MCI[2, 6]. Imaging studies suggest significant changes in the brain microvasculature and in metabolic dysfunction[7] in persons with T2D, or even simple hyperglycemia in the absence of full blown diabetes, and dementia[8–10]. Accumulating evidence implicates a close relationship between the brain insulin receptor signaling pathway (IRSP) and the major neurobiological abnormalities of AD, amyloid beta (Aβ) and hyperphosphorylated and conformationally abnormal tau. Both pathologies have been shown to lower neuronal IR responses to insulin and to cause rapid and substantial loss of neuronal surface insulin receptors (IRs)[11]. Disruption of brain insulin signaling is one of the explanations for the consistently higher risk of AD and dementia in type 2 diabetic elderly[12]. Fat-feed laboratory transgenic mice models of AD that overexpress brain amyloidogenic genes develop glucose intolerance and insulin resistance, illustrating the potential bidirectional complexity of this relationship[13]. In AD patients, monotherapy with insulin[14] or with single representatives of other classes of hypoglycemic medications[15, 16] have been shown to not alter the risk of AD[17], but to potentially improve memory performance and slow cognitive decline. That cognitive impairment and AD neuropathology have been linked with T2D and even pre-diabetes suggests that the mechanisms underlying the relationship of T2D with dementia may be generalizable to non-T2D individuals. Although not performed on brain tissue, a recent study strongly supports an association between the molecular mechanisms of AD, insulin regulation, and T2D[18]. Integrative systems analysis of multiple tissues and organs in ob/ob mice identified APP as a top regulator of islet cell functions with the potential to regulate plasma insulin levels. This and other evidence for the complexity of the T2D-dementia interaction was comprehensively reviewed by Arnold and colleagues[19].
Recent evidence[20] suggests that metformin, and by extension other hypoglycemic medications[21], can significantly improve health- and lifespan. For example, metformin has the unique ability to correct the aging-related missorting of nuclear and cytoplasmic proteins[22]. In non-diabetic AD patients, monotherapy with insulin[23, 24] or with other hypoglycemic medications[14, 25] has shown some, albeit inconsistent, improvement in memory performance and slowing of AD symptom progression. In a series of studies[26, 27], our group showed that, when taken as a whole, T2D did not significantly affect the neuropathological sequelae of AD, but that the absence of a “T2D X AD neuropathology” interaction was apparently driven in large measure by the presence of antidiabetic drugs. Our studies showed that elderly persons with T2D treated with insulin plus other hypoglycemic agents (i.e., combination therapy) have dramatically less AD neuropathology (reduced densities of neuritic plaques and neurofibrillary tangles in the cortex) than otherwise similar non-T2D persons2. In an effort to understand better the neurobiological substrates of T2D and T2D medications in AD, we examined in the current study IRSP and endothelial cell markers in the parahippocampal gyri of persons with and without AD, T2D and T2D medications.
Insulin receptors (IR) are particularly abundant in brain regions supporting cognition, with recent evidence implicating a close relationship between the brain IR signaling pathway (IRSP) and the major neurobiological abnormalities of accumulations of Aβ and of conformationally abnormal and hyperphosphorylated tau (pTau)e.g.,[28]. In vitro studies3-5 provide support for our findings identifying the IRSP as an underlying mechanism by which combination of insulin with non-insulin T2D medications may modulate AD neuropathology even in the absence of comorbid T2D. Insulin binds to IR subunits (member of the receptor tyrosine kinase family engaging Akt and mTOR pathways). Phosphorylated insulin receptor substrate (IRS) scaffolding proteins link the IR to downstream signaling effectors including Akt. Akt activation inhibits pro-apoptotic signaling molecules such as BCL2-antagonist of cell death (BAD), B cell lymphoma 2 (BCL2), Forkhead Box O (FoxO), nuclear factor kappa B (NF-κB) and glycogen synthase kinase-3 beta (GSK3β). GSK3β by itself inhibits Akt by controlling mTORC, a key activating kinase for AKT[29]. GSK3β also phosphorylates β-catenin, targeting it for ubiquitination and proteasome dependent degradation[30]. Independent evidence implicates elements of this pathway in neuronal dysfunction, neurodegeneration and dementia[31–34]. In addition to this canonical “metabolic and anti-apoptotic” pathway of the IRSP, insulin binding to IR can also activate a second, SHC-ERK1/2, pro-survival pathway culminating in the activation of PPAR, and its coactivator PGC-1α, and interactions with the master cholesterol regulators RxR and LxR[35, 36]. Notably, these are the molecules that our studies indicate to be dysregulated in postmortem human AD brain[37, 38]. The IRSP in the brain contributes to the control of processes such as synaptic plasticity, e.g.,[27] [33, 34], neuroprotection, neurodegeneration, survival, growth, and energy metabolism, e.g.,[39], all of which are particularly relevant to cognition.
The mechanism by which insulin is delivered to the brain is uncertain, but insulin receptors (IR) on endothelial cells are the leading candidates[40]. Several non-mutually exclusive mechanisms may underlie the association between T2D and dementia, but increased cerebrovascular compromise and blood-brain barrier disruption[41–43]; defective signal transduction mechanism of central nervous system insulin receptors[44, 45]; and their potential interactions with amyloidogenic processes[44, 46–48] are among the most prominent see also, [19]. Multiple studies have suggested that microvascular dysfunction, including permeability of the blood-brain barrier[49–52], may be a significant contributor to cognitive impairment in the elderly and in VCID or AD[53]. This evidence has come not only from numerous neuroimaging studies of microvascular dysfunction[54–56], but also from direct neuropathological investigations[57–65] and animal model systems[66, 67]. Similarly, microvascular damage and involvement, including dysfunction of endothelial cells in T2D, is undeniable[23, 55, 56, 68–71]. On the other hand, IRs in the brain do not appear to downregulate dramatically in response to high concentrations of insulin, and—in contrast to its role in glia and peripheral cells—insulin has a relatively limited role in regulating glucose metabolism in neurons[20, 72] and densities of IRs are not adversely affected in AD[47] when studied in bulk tissue assays. However, the vast majority of the studies of the brain IRSP in AD and T2D have been conducted in homogenized bulk tissue where even large changes and abnormalities in one or more cell types can be diluted or completely obscured when different cell types are intermixed in bulk-homogenate studies. In order to overcome this limitation and to address more directly the roles of T2D and antidiabetic medication in AD, we developed a method of endothelial cell enrichment from bulk human postmortem brain tissue and studied components of the IRSP and markers of endothelial cell function.
Methods
Tissue processing
This study involved analysis of postmortem human brain samples only. Consent for research use of the tissue was obtained from all donors. The collection and consent procedures were reviewed by the Mount Sinai (HS#: 13–00709 PS) and JJ Peters VA (ID01527) IRBs and were exempted from further review. The parahippocampal gyrus (Brodmann 36), was dissected from snap-frozen ~8 mm thick coronal sections. The dissected block was then pulverized using liquid nitrogen cooled mortar and pestle. The crushed homogenate was aliquoted into 50 and 100 mg aliquots. The general procedures for tissue acquisition, dissection and aliquot preparation have been described previously[73]. Some aliquots were used for bulk tissue analyses, whereas other sister aliquots from the same brain region of each donor were used for endothelial cell enrichment.
Gene expression studies
Custom 51-plex QuantiGene assays (Life Technologies/Thermo, CA) were used for gene expression studies. Tissue and microvessel isolate homogenization, proteinase K treatment, probe hybridization and signal amplification were performed according to the manufacturer manual. Measurement of 51 genes (Table 1, and S1 File) was performed on Luminex 200 (Millipore, MA) instrument. Relative expression values were calculated using standard curve method and normalized to geometric means of four housekeeping genes included in the panel: HMBS, NONO, PPIB and RPLP0.
Table 1. Characteristics of the primary study cohorts.
| Mean PMI (Hours) | Mean CDR | Mean Age | Sex | Mean Cortical Plaques per mm^2 | Mean Braak & Braak Score | Race (white; Black; Hispanic | Mean Blood glucose (mg/dl) | |
| Control (N = 30) | 12.85 (1.4) | 0.43 (0.13) | 83.17 (1.48) | 14F; 16M | 0.83 (0.32) | 1.5 (0.32) | 26; 3; 1 | 95.5 (22) |
| AD (N = 19)* | 6.88 (1.17) | 3.0 (0.28) | 88.26 (2.01) | 15F; 4M | 9.58 (1.38) | 4.89 (0.29) | 17; 2; 1 | 119.4 (8) |
| AD+DM+Meds (N = 34)* | 7.67 (0.98) | 2.89 (0.26) | 84.61 (1.49) | 23F; 11M | 8.39 (0.86) | 4.64 (0.26) | 26; 5; 3 | 148.8 (14) |
| Medication Characteristics of the AD+DM+Meds (N = 34 cohort) | ||||||||
| Medication Subset | Percent of Subset | Medication class | ||||||
| Insulin Only | N = 15 (45%) | Insulin | ||||||
| Oral Only | N = 12 (35%) | N = 9 (75%) Sulfonylurea N = 2 (17%) Metformin N = 1 (8%) Thiazolidinedione |
||||||
| Insulin + Oral | N = 7 (21%) | N = 5 (79%) Sulfonylurea N = 2 (21%) Metformin |
||||||
* 17% (N = 9) of cases with AD (with or without DM medications) were treated with medications for AD (one was treated with memantine and 8 received donepezil) which were terminated at least 14 months prior to death.
Selection of mRNA transcripts for expression studies
Twenty-four to twenty-six mRNA transcripts were selected for study. The selection of transcripts was based on several factors that included: known association with the IRSP; known and relatively enriched expression in endothelial cells[74], and relatively high or altered expression in bulk tissue microarray studies of AD[75, 76]. Twenty-three other transcripts associated with immune-inflammation, neurons, oligodendrocytes, astrocytes and microglia were also studied to survey other systems that may have been affected by T2D medications. The results of these later assays are shown in S1 File.
Microvascular/endothelial cell enrichment
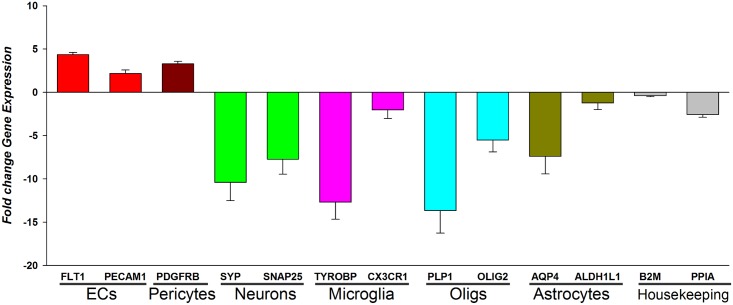
One hundred milligrams of brain tissue was gently homogenized in cold 18% dextran/PBS containing protectRNA RNase inhibitor (Sigma, MO) (10 ml/g of tissue) using a Potter-Elvejehm with a loose-fit Teflon pestle (6–8 strokes at low speed)[77]. The homogenate was overlaid onto an equal volume of a discontinuous gradient of Ficoll-Paque PLUS™ and centrifuged for 30 min at 1,500 x g and 4°C. The resulting microvascular enriched pellet was resuspended and washed twice with PBS. The isolated microvascular/endothelial cell fragment was frozen and stored at -80C until further assays. In preliminary multiple gene expression studies, we have found this protocol to results in a significant (3–7 fold depending on the endothelial cell marker used) enrichment of microvascular/endothelial cell markers and a many-fold reduction in the levels of non-endothelial cell markers. Fig 1 shows one such example.
Fig 1. Enrichment of endothelial cell transcripts in microvascular isolates.
The expression levels of selected endothelial cell markers and non-endothelial markers are expressed as fold change ratios relative to the levels of each transcript in bulk tissue homogenates.
Characteristics of brain tissue donors and molecular pathways of interest
The demographic characteristics and group stratification of study cases is shown in Table 1. All postmortem brain tissue donors were derived from the Mount Sinai NIH Neurobiobank (http://icahn.mssm.edu/research/nih-brain-tissue-repository/about). All brain tissue donations were derived from cases with written consent from the next of kin of each donor for research use. Each brain underwent a detailed neuropathological assessment as described previously[78, 79]. Donors were selected from over 1,900 cases for short postmortem interval (under 24 hours), high brain tissue pH (>6.0), the presence of either only AD pathology (as defined by CERAD[80]) or no evidence of neuropathology. Brodmann area 36 (the parahippocampal gyrus) was selected for study because transcriptome-wide analysis of 17 different brain regions showed it to be among the most transcriptionally vulnerable brain region in AD[76]. To enable direct comparison of assayed IRSP and endothelial cell mRNA expression, cases with AD and no known history of T2D were selected to match those with AD and a history of T2D as closely as possible with respect to both pre-agonal cognitive function and severity of neuropathology as defined by Braak score and density of cortical neuritic plaques. Cases with neuropathological evidence of non-AD pathology (e.g., Lewy body pathology, significant vascular pathology such as stroke or amyloid angiopathy, etc.) were excluded from all studies as described previously. Donors were included as presenting with T2D based on the American Diabetes Association criteria (symptoms of diabetes plus casual plasma glucose concentration > 200mg/dl; fasting plasma glucose > 126mg/dl; 2h plasma glucose>200mg/dl during OGTT); and/or record of receiving anti-diabetes medication; history of diabetes in the medical record. Diagnoses of T2D were ascertained from detailed structured review (>275 items) of all medical records and medical history. Similarly, medication use history and laboratory test results were derived from detailed reviews of medical records and semi-structured guided interviews of the next of kin or caregivers intimately acquainted with the donor.
Statistical methods
Differential expression between disease status (control, AD) and treatment (insulin and/or oral anti-diabetes agents) was assessed using the limma package in R, with normalized gene expression matrix and the final covariate model, which included gender and PMI. Note that the analysis was run separately for gene expression derived from vessels and homogenate tissue. For each gene, least-squares linear regression was performed using limma to yield coefficients for the effect on gene expression of each variable on the right-hand side:
where Group is defined based on combination of diagnosis and treatment [i.e., control, AD (no T2D, no T2D medications), and AD+T2D treated with insulin and/or oral anti-diabetes agents]. P values were adjusted for multiple hypothesis testing using false discovery rate (FDR) estimation, and the differentially expressed genes were determined as those with an estimated FDR ≤ 5%, or where specified FDR ≤ 7%.
Results
The main analyses were performed on the expression of the genes shown in Table 2 with the primary goal of determining the extent to which IRSP and endothelial cell related transcripts were altered in AD vs. controls and whether these disease-associated changes were normalized in the brain of persons with AD receiving one or more antidiabetic medications of any kind and in any combination (i.e., insulin only, oral agents only, insulin plus oral agents).
Table 2. Abbreviations, brief function and cell-type expression and expression levels of IRSP and endothelial cell transcripts in AD-tissue and vessels.
| Symbol | Description | System |
|---|---|---|
| ANGPT1 |
Angiopoietin 1 is involved in vascular development and angiogenesis. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=ANGPT1 |
Endothelial related |
| CD59 |
CD59 glycoprotein, aka MAC-inhibitory protein is mostly expressed in endothelial cells and oligodendrocytes. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=CD59 |
Endothelial related |
| CTNNB1 |
Catenin beta-1, AKA β-catenin, is involved in regulation and coordination of cell–cell adhesion and gene transcription. It is a member of the Wnt signaling pathway. Highest expression is in endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=CTNNB1 |
Endothelial related |
| FLT1 |
Vascular endothelial growth factor receptor 1 shows tyrosine protein kinase activity that is important for the control of cell proliferation and differentiation. It is exclusively expressed in endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=flt1 |
Endothelial related |
| FOXF2 |
Forkhead box protein F2. FOXF2 functions are not understood well, but it is expressed almost exclusively in endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=FOXF2 |
Endothelial related |
| ICAM1 |
Intercellular Adhesion Molecule 1 aka D54 (Cluster of Differentiation 54) encodes a cell surface glycoprotein which is expressed on endothelial cells and cells of the immune system. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=ICAM1 |
Endothelial related |
| IGF1R |
Insulin-like growth factor 1 (IGF-1) receptor belongs to the large class of tyrosine kinase receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 is highly expressed in endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=IGF1R |
Endothelial related |
| PDGFRB |
Beta-type platelet-derived growth factor receptor Activation of PDGFRβ requires de-repression of the receptor’s kinase activity which is accomplished during PDGFRβ dimerization. Two of the five PDGF isoforms activate PDGFRβ (PDGF-B and PDGF-D) which phosphorylates itself and other proteins, and engages intracellular signaling pathways associated with migration and proliferation. PDGFRβ is mostly expressed in astrocytes and endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=PDGFRB |
Endothelial related |
| PECAM1 |
Platelet endothelial cell adhesion molecule aka CD31, plays a key role in removing aged neutrophils from the body. PECAM-1 is found on the surface of platelets, monocytes, neutrophils, and some types of T-cells, and makes up a large portion of endothelial cell intercellular junctions. The encoded protein is a member of the immunoglobulin superfamily, it is expressed almost exclusively on endothelial cells and is likely involved in leukocyte transmigration, angiogenesis, and integrin activation. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=PECAM1 |
Endothelial related |
| SLC2A1 |
Glucose transporter 1 (or GLUT1), aka solute carrier family 2, facilitated glucose transporter member 1 is expressed mainly in endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=SLC2A1 |
Endothelial related |
| VCAM1 |
Vascular cell adhesion protein 1 is expressed on both large and small blood vessels only after the endothelial cells are stimulated by cytokines. The VCAM-1 protein mediates the adhesion of lymphocytes, monocytes, eosinophils, and basophils to the vascular endothelium. Upregulation of VCAM-1 in endothelial cells by cytokines occurs as a result of increased gene transcription (e.g., in response to Tumor necrosis factor-alpha (TNF-α) and Interleukin-1 (IL-1)). VCAM-1 protein is an endothelial ligand for VLA-4 (Very Late Antigen-4 or integrin α4β1) of the β1 subfamily of integrins. It is almost exclusively expressed in astroglial. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=VCAM1 |
Endothelial related |
| VEGFA |
Vascular endothelial growth factor A is a member of the platelet-derived growth factor (PDGF) family. It acts on endothelial cells to increase permeability. It is expressed in mostly in astrocytes but also in neurons and OPCs. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=VEGFA |
Endothelial related |
| VWF |
Von Willebrand factor is expressed mostly in endothelial cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=VWF |
Endothelial related |
| AKT1 |
V-akt murine thymoma viral oncogene homolog 1; AKT1 gene encodes an enzyme in the serine/threonine kinase family and is a key signaling molecule in the IRSP. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=AKT1 |
IRSP related |
| AKT3 |
v-akt murine thymoma viral oncogene homolog 3; AKT3, regulates cell signaling in response to insulin and growth factors. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=AKT3 |
IRSP related |
| DDIT4 |
DNA-damage-inducible transcript 4 protein (DDIT4) acts as a negative regulator of mTOR. Metformin increases DDIT4 expression. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=DDIT4 |
IRSP related |
| FTO |
Fat mass and obesity-associated protein. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=FTO |
IRSP related |
| GSK3B |
Glycogen synthase kinase 3 beta, is a kinase and part of the IRSP. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=GSK3B |
IRSP related |
| INSR |
Insulin receptor (IR) is an insulin, IGF-I and IGF-II activated transmembrane receptor that is expressed in most cells. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=INSR |
IRSP related |
| IRS1 |
Insulin receptor substrate participates in transmitting signals from the insulin and insulin-like growth factor-1 (IGF-1) receptors to intracellular pathways PI3K / Akt and Erk MAP kinase pathways. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=IRS1 |
IRSP related |
| IRS2 |
Insulin receptor substrate 2 is a cytoplasmic signaling molecule that mediates effects of insulin, insulin-like growth factor 1, and some cytokines by acting as a molecular adaptor between diverse receptor tyrosine kinases. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=irs2 |
IRSP related |
| MTOR |
mechanistic target of
rapamycin (mTOR), (formerly mammalian target of rapamycin) is a serine/threonine kinase and is part of the IRSP. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=MTOR |
IRSP related |
| PPARGC1A |
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha regulates genes associated with energy metabolism. PPARGC1A interacts with the nuclear receptor PPAR-γ and is a downstream member of the IRSP. It is expressed in astrocytes, neurons and oligodendrocytes. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=PPARGC1A |
IRSP related |
| RICTOR |
Rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) is a subunit of mTOR and part of the IRSP. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=rictor |
IRSP related |
| RPS6KB1 |
Ribosomal protein S6 kinase beta-1 (S6K1), aka p70S6 kinase (p70S6K, p70-S6K), is a protein kinase that phosphorylates threonine 389 and activates mTOR and correlated with autophagy inhibition. The kinase activity of RPS6KB1 protein leads to an increase in protein synthesis and cell proliferation. It is expressed in most cell types. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=RPS6KB1 |
IRSP related |
| RPTOR |
Regulatory-associated protein of mTOR AKA KIAA1303 encodes part of a signaling pathway regulating cell growth responding to nutrient and insulin levels. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=RPTOR |
IRSP related |
| SLC2A4 |
Glucose transporter type 4, aka GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle (skeletal and cardiac). It is expressed in most cell types, but especially in astrocytes. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=SLC2A4 |
IRSP related |
| TBC1D4 |
TBC1 domain family member 4 now known as AS160 encodes Rba GTPase-activating protein and is a substrate for Akt2. http://web.stanford.edu/group/barres_lab/cgi-bin/geneSearch.py?geneNameIn=TBC1D4 |
IRSP related |
Altered mRNA expression in whole tissue homogenates of the parahippocampal gyrus in AD and anti-T2D medicated AD
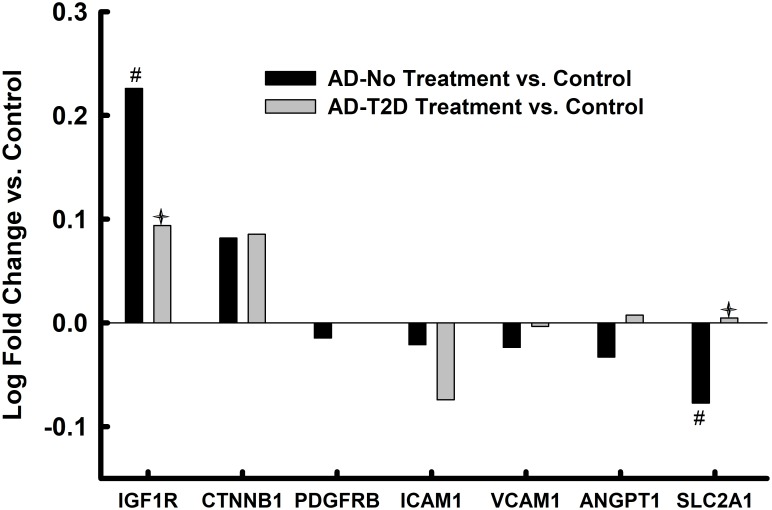
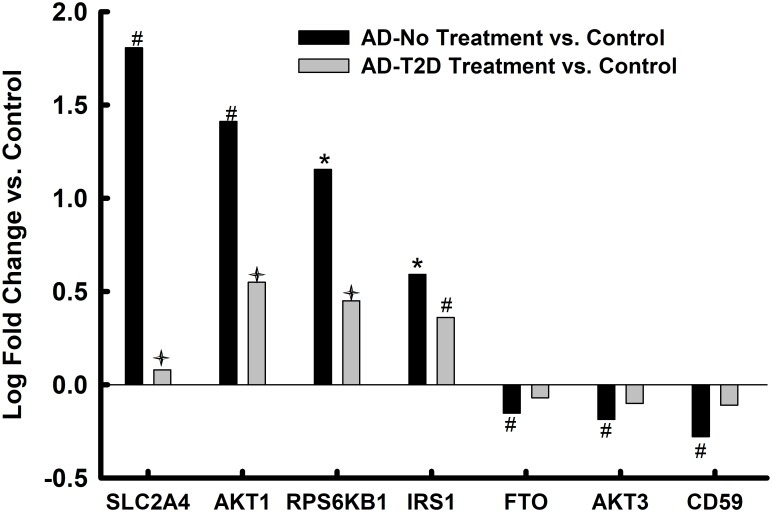
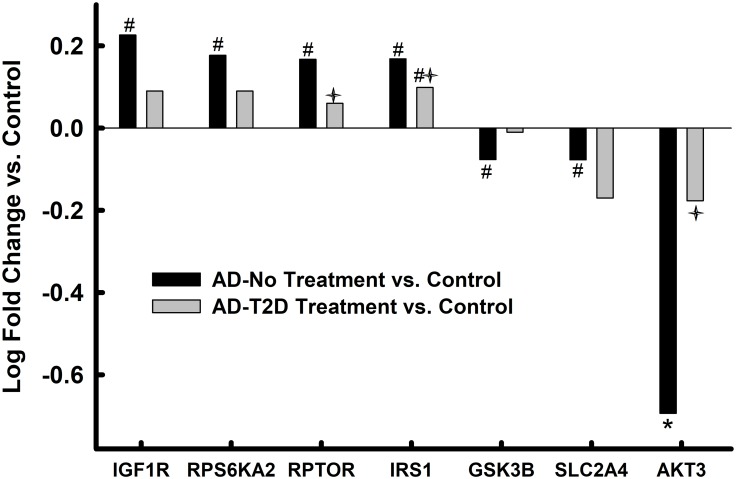
Compared to controls, 12 of the 18-endothelial cell and IRSP-associated genes assayed were significantly (12 of 18 unadjusted ps<0.05; 6 of 12 after FDR correction) altered in AD (Figs 2 and 3). One third of the altered genes (unadjusted ps<0.05), or 44% after FDR correction, were genes with preponderant expression in endothelial cells[74]. In AD donors who had a history of receiving antidiabetic treatment, only 4 of these 12 genes remained significant before FDR correction and only 1 (ANGPT1) after FDR correction.
Fig 2. Endothelial cell markers in parahippocampal gyrus bulk tissue.
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDR correction; # = p<0.05 without FDR correction; ✦ = p<0.05 AD-No Treatment vs. AD-T2D Treatment.
Fig 3. IRSP-associated markers in parahippocampal gyrus bulk tissue.
Values represent relative log fold change in persons with AD relative to controls and log fold change in in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDR correction; # = p<0.05 without FDR correction; ✦ = p<0.05 AD-No Treatment vs. AD-T2D Treatment.
Altered mRNA expression in endothelial cells of the parahippocampal gyrus in AD and anti-T2D medicated AD
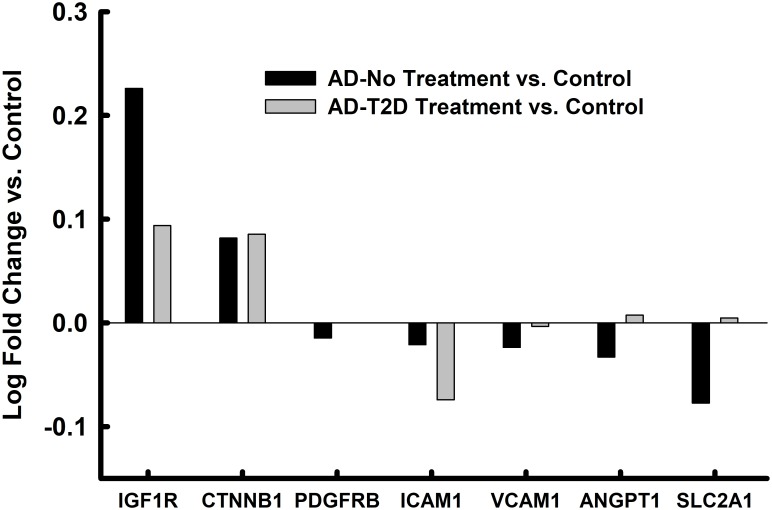
Compared to EC enriched fraction of controls, 5 (unadjusted for FDR) endothelial and IRSP-related genes were abnormally expressed in untreated persons with AD (Figs 4 and 5). None of the IRSP/endothelial cell associated genes, except for AKT3, met the FDR corrected threshold. Only one of these 5 genes (IRS1) remained significantly different in expression in the endothelial cell fraction of treated persons with AD, and even the expression levels of this gene approached that of the controls, nominally. Interestingly, the expression level of IRS1 was not changed in the endothelial fractions derived from persons with AD, but its expression increased significantly (corrected p = 0.05) in those AD donors who had been treated with antidiabetic medications.
Fig 4. Endothelial cell markers in microvascular enriched isolates from the parahippocampal gyrus.
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDR correction; # = p<0.05 without FDR correction; ✦ = p<0.05 AD-No Treatment vs. AD-T2D Treatment.
Fig 5. IRSP-associated markers in microvascular enriched isolates from the parahippocampal gyrus.
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDR correction; # = p<0.05 without FDR correction; ✦ = p<0.05 AD-No Treatment vs. AD-T2D Treatment.
Non-IRSP and/or non-endothelial cell mRNAs in the parahippocampal gyrus in AD and anti-T2D medicated AD
Twenty-three mRNAs representing neuronal, oligodendroglial, astrocytic, microglial cell types, and cell-cell adhesion, inflammation/immune, cell fate markers were also selected (S1 File) to study the consequences of antidiabetic treatment on some of the more non-IRSP/endothelial cell functions of the parahippocampal gyrus. Not surprisingly, the expression of 8 of the 23 mRNAs studied was affected in the whole tissue homogenates of untreated persons with AD and included mRNAs encoding for proteins associated with synaptic function, astrocytes, cell adhesion and immune/inflammation responses. Treatment with antidiabetic medications reduced the number of adversely affected mRNAs to 3 that included markers of immune/inflammation and cell-cell adhesion. Significantly fewer of these markers were affected in the endothelial cell enriched fraction, where 2 (oligodendroglial and nuclear RNA retention) of the 23 markers were affected in untreated AD samples and altered expression remained significant, albeit nominally moderated in the treatment group.
Discussion
Numerous clinical, animal model, and postmortem studies have suggested that treatment of persons suffering from dementia with antidiabetic medications may have beneficial effects on cognitive function and on AD-related neuropathology[19, 26]. A number of recent studies and reviews have drawn attention to the role(s) of the brain microvasculature in dementia[56] and especially in persons with diabetes[55, 56, 81]. The current study was designed to uncover some of the transcriptomic substrates for microvascular abnormalities in AD and to delineate whether any beneficial effects of T2D medications could be attributed to the restoration of microvascular and endothelial cell attributes affected in AD.
The results indicated that there are considerable abnormalities and reductions in gene expression (whole tissue homogenates and endothelial cell isolates) in the parahippocampal gyrus of persons with AD that map directly to genes associated with the microvasculature and the IRSP. Whether these endothelial and IRSP abnormalities contribute to the genesis of AD neuropathology, or whether they result from other neuropathological changes cannot be determined in postmortem studies. The findings also showed that the numbers of abnormally expressed microvasculature and IRSP associated genes in diabetic AD donors who had been treated with anti-diabetic agents were reduced significantly. Of course, it can be argued that in the absence of untreated studied cases with AD and T2D, the “normalization” of microvasculature and the IRSP gene expression could be due to T2D and not its treatment. Although this argument cannot be countered by the results of this study, we find this alternative explanation to be less biologically plausible given knowledge of the well-documented detrimental effects of T2D on brain parenchyma and especially on the brain microvasculature. The interpretation that treatment of T2D “normalized” multiple transcriptional abnormalities associated with AD is, however, consistent with the results of some of the clinical trials reported to date[24, 82] as well as our earlier studies of the effects of anti-diabetic treatments on AD neuropathology[26, 27]. That AKT3 expression was dramatically and robustly reduced in endothelial cells, and to a lesser extent in whole tissue homogenates suggests that tissue level expression was driven by changes in the endothelial transcriptome and that the disruption of components of the IRSP in endothelial cells is a significant participant in AD neuropathology.
The expression levels of GLUT4 (SLC2A4), which encodes for an insulin-regulated glucose transporter, were significantly upregulated in bulk tissue from the parahippocampal gyrus of AD donors (Fig 3), while the expression levels of this same transcript were significantly reduced in the endothelial cell enriched isolates derived from the same donors and brain region. On the other hand, the expression levels of GLUT4 in persons with AD and T2D who had been treated with anti-diabetic medications were similar to the levels detected in controls. This suggests that anti-diabetic medications restored homeostasis to this critical glucose transporter and that dis-homeostasis of glucose transport in brain endothelial and non-endothelial cells may be a critical abnormality in AD that is restored by anti-diabetes medications. Animal model and in vitro studies will need to be conducted to determine whether dysregulation of GLUT4 may be a key contributor to at least some of the other IRSP and endothelial cell abnormalities observed in the current study. Zlokovic and colleagues have independently implicated GLUT1, another glucose transporter, as a key player in the microvascular pathology associated with AD[83].
“Normalization” of abnormally expressed genes in AD by anti-T2D treatment was not limited to transcripts associated with the IRSP and endothelial cells, but as shown in Figs 1 and 2 of the S1 File carried over to genes associated with immune-inflammation, microglia, cell-adhesion and synaptic function. These findings are not surprising if it is assumed that treatment with anti-T2D medications results in decreased overall inflammation and improved insulin signaling.
The fact that no brains from AD+T2D diabetic donors that had not been treated with antidiabetic agents were included in this study is a distinct weakness and detracts from interpretative power. However, in developed countries, the vast majority of persons diagnosed with T2D receive insulin or oral anti-diabetes medications, making it difficult to include such a group in clinical and postmortem studies. In addition, interpretation of results, even if such a group had been included, would have been hampered since, almost by definition, the severity of T2D would have been significantly less than that of the treated subjects. Despite the imperfect nature of mouse models, these variables will be easier to control in such a system, and we plan to attempt to address some of these questions left open in our postmortem human studies by employing mouse models of amyloidosis or tauopathy, each of which will be studied without or with diabetes, and each of those groups, in turn, can be studied with or without antidiabetic medication. Choice of the adequate mouse model of diabetes will be key since we have observed some unexpected effects in some transgenic mouse models for amyloidosis[84] and the effect of a deficiency of the T2D gene, Sorcs1[85]. Finally, we might also need to examine the effects of young ages vs older as additional parameters in this highly ambitious study.
As noted in Table 1, the great majority of the donors (79+%) who were treated with oral anti-diabetes agents were treated with sulfonylureas. Although this makes it easier to attribute any oral medication effects to sulfonylureas, it detracts from our ability to distinguish between the effects of the myriad of different agents with different mechanisms of action. The inability to discern the specific neuro/vascular effects of different anti-diabetic agents in this study also precluded our ability to assess how the reported interactions of metformin and APP/Aβ influence IRSP and endothelial cell markers[86–88]. In addition, it would have been of significant interest to stratify the studied cohort according to whether they received insulin only, oral agents only, or insulin plus oral agents as well as the efficacy and duration of treatment. Unfortunately, extended medical histories (e.g., since mid-life) were not available since the study cohort was nursing home based and the sample size was not large enough for adequate statistical power to analyze the results with medication type granularity.
Challenge notwithstanding, about half of diabetics develop dementia and that roughly doubles the cost of caring for each demented diabetic patient especially since their cognitive decline makes it impossible for the patient to participate in the monitoring and modulation of his/her diabetic status. Given the dual epidemics of T2D and dementia in the most rapidly growing segment of our population, there is enormous importance in elucidating the basis for cognitive impairment in T2D so that potentially meaningful interventions can be evaluated.
Supporting information
(DOCX)
(XLSX)
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDC correction; # = p<0.05 without FDR correction.
(TIF)
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDC correction; # = p<0.05 without FDR correction.
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Awards 1 I21 RX002069-01 and 1 I21 RX002876-01 (MAGS); VA Merit BX002267, NIH- N01HHSN 271201300031, P50 AG005138-31 (VH); R01 AG034087, R01 AG053446, R01 AG051545 (MSB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ravona-Springer R, Moshier E, Schmeidler J, Godbold J, Akrivos J, Rapp M, et al. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis. 2012;30(2):299–309. 10.3233/JAD-2012-120106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravona-Springer R, Luo X, Schmeidler J, Wysocki M, Lesser G, Rapp M, et al. Diabetes is associated with increased rate of cognitive decline in questionably demented elderly. Dement Geriatr Cogn Disord. 2010;29(1):68–74. 10.1159/000265552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–81. [DOI] [PubMed] [Google Scholar]
- 4.Salameh TS, Bullock KM, Hujoel IA, Niehoff ML, Wolden-Hanson T, Kim J, et al. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. J Alzheimers Dis. 2015;47(3):715–28. 10.3233/JAD-150307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56(1):211–6. 10.2337/db06-0879 [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Caracciolo B, Wang HX, Winblad B, Backman L, Qiu C, et al. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59(11):2928–35. 10.2337/db10-0539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neth BJ, Craft S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Frontiers in aging neuroscience. 2017;9:345 10.3389/fnagi.2017.00345 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Bussel FC, Backes WH, Hofman PA, van Oostenbrugge RJ, Kessels AG, van Boxtel MP, et al. On the interplay of microvasculature, parenchyma, and memory in type 2 diabetes. Diabetes Care. 2015;38(5):876–82. 10.2337/dc14-2043 . [DOI] [PubMed] [Google Scholar]
- 9.Kerti L, Witte AV, Winkler A, Grittner U, Rujescu D, Floel A. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology. 2013;81(20):1746–52. 10.1212/01.wnl.0000435561.00234.ee . [DOI] [PubMed] [Google Scholar]
- 10.Hayashi K, Kurioka S, Yamaguchi T, Morita M, Kanazawa I, Takase H, et al. Association of cognitive dysfunction with hippocampal atrophy in elderly Japanese people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94(2):180–5. 10.1016/j.diabres.2011.07.002 . [DOI] [PubMed] [Google Scholar]
- 11.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22(1):246–60. 10.1096/fj.06-7703com [DOI] [PubMed] [Google Scholar]
- 12.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–72. 10.2337/db13-1954 . [DOI] [PubMed] [Google Scholar]
- 13.Ruiz HH, Chi T, Shin AC, Lindtner C, Hsieh W, Ehrlich M, et al. Increased susceptibility to metabolic dysregulation in a mouse model of Alzheimer’s disease is associated with impaired hypothalamic insulin signaling and elevated BCAA levels. Alzheimers Dement. 2016;12(8):851–61. 10.1016/j.jalz.2016.01.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809–22. [DOI] [PubMed] [Google Scholar]
- 15.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. PharmacogenomicsJ. 2006;6(4):246–54. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29(2):345–51. [DOI] [PubMed] [Google Scholar]
- 17.Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc. 2012;60(5):916–21. 10.1111/j.1532-5415.2012.03916.x . [DOI] [PubMed] [Google Scholar]
- 18.Saxena R, Elbers CC, Guo Y, Peter I, Gaunt TR, Mega JL, et al. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. Am J Hum Genet. 2012;90(3):410–25. 10.1016/j.ajhg.2011.12.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nature reviews Neurology. 2018;14(3):168–81. 10.1038/nrneurol.2017.185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, et al. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. JBiolChem. 1999;274(49):34893–902. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman RS, Hobbs TM, Wells BJ, Kong SX, Kattan MW, Bouchard J, et al. Association of glucagon-like peptide-1 receptor agonist use and rates of acute myocardial infarction, stroke and overall mortality in patients with type 2 diabetes mellitus in a large integrated health system. Diabetes Obes Metab. 2017;19(11):1555–61. 10.1111/dom.12969 . [DOI] [PubMed] [Google Scholar]
- 22.Gough NR. Placing the nuclear pore in the metformin mechanism of action. Sci Signal. 2017;10(460). 10.1126/scisignal.aam6836 . [DOI] [PubMed] [Google Scholar]
- 23.Sanahuja J, Alonso N, Diez J, Ortega E, Rubinat E, Traveset A, et al. Increased Burden of Cerebral Small Vessel Disease in Patients With Type 2 Diabetes and Retinopathy. Diabetes Care. 2016;39(9):1614–20. 10.2337/dc15-2671 . [DOI] [PubMed] [Google Scholar]
- 24.Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J Alzheimers Dis. 2017;57(4):1325–34. 10.3233/JAD-161256 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–78. 10.1016/S1474-4422(04)00681-7 [DOI] [PubMed] [Google Scholar]
- 26.Beeri MS, Schmeidler J, Silverman JM, Gandy S, Wysocki M, Hannigan CM, et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008;71(10):750–7. 10.1212/01.wnl.0000324925.95210.6d . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, et al. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2005;60(4):471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11(5):504–10 e1. 10.1016/j.jalz.2014.03.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CH, Shaikenov T, Peterson TR, Aimbetov R, Bissenbaev AK, Lee SW, et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. SciSignal. 2011;4(161):ra10 10.1126/scisignal.2001731 [DOI] [PubMed] [Google Scholar]
- 30.Anderton BH, Dayanandan R, Killick R, Lovestone S. Does dysregulation of the Notch and wingless/Wnt pathways underlie the pathogenesis of Alzheimer’s disease? MolMedToday. 2000;6(2):54–9. [DOI] [PubMed] [Google Scholar]
- 31.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7(1):45–61. [DOI] [PubMed] [Google Scholar]
- 32.Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: Mechanisms of Age-Related Cognitive Change and Targets for Intervention: Inflammatory, Oxidative, and Metabolic Processes. JGerontolA BiolSciMedSci. 2012;67(7):754–9. 10.1093/gerona/gls112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61(4):867–79. 10.1016/j.neuropharm.2011.06.003 . [DOI] [PubMed] [Google Scholar]
- 34.Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. 10.1016/j.nbd.2014.03.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer’s disease: therapeutic implications. CNS Drugs. 2008;22(1):1–14. 10.2165/00023210-200822010-00001 . [DOI] [PubMed] [Google Scholar]
- 36.Salvado L, Serrano-Marco L, Barroso E, Palomer X, Vazquez-Carrera M. Targeting PPARbeta/delta for the treatment of type 2 diabetes mellitus. ExpertOpinTherTargets. 2012;16(2):209–23. 10.1517/14728222.2012.658370 [DOI] [PubMed] [Google Scholar]
- 37.Akram A, Schmeidler J, Katsel P, Hof PR, Haroutunian V. Increased expression of RXRalpha in dementia: an early harbinger for the cholesterol dyshomeostasis? Mol Neurodegener. 2010;5:36 10.1186/1750-1326-5-36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin WP, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, et al. PGC-1 alpha Expression Decreases in the Alzheimer Disease Brain as a Function of Dementia. Archives of Neurology. 2009;66(3):352–61. 10.1001/archneurol.2008.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. ProgNeurobiol. 2006;79(4):205–21. 10.1016/j.pneurobio.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 40.Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014;63(12):3992–7. 10.2337/db14-0340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67(11):1960–5. 10.1212/01.wnl.0000247053.45483.4e [DOI] [PubMed] [Google Scholar]
- 42.Stoeckel L, Arvanitakis Z, Gandy S, Small D, Kahn C, Pascual-Leone A, et al. "White Paper" meeting summary and catalyst for future inquiry: Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Research. 2016;5(353). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Frontiers in aging neuroscience. 2014;6:88 10.3389/fnagi.2014.00088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22(10):RC221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. ProcNatlAcad Sci US A. 2009;106(6):1971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craft S. Alzheimer disease: Insulin resistance and AD—extending the translational path. Nature reviews Neurology. 2012;8(7):360–2. 10.1038/nrneurol.2012.112 . [DOI] [PubMed] [Google Scholar]
- 47.Ho L, Yemul S, Knable L, Katsel P, Zhao R, Haroutunian V, et al. Insulin receptor expression and activity in the brains of nondiabetic sporadic Alzheimer’s disease cases. IntJAlzheimersDis. 2012;2012:321280 10.1155/2012/321280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. JClinInvest. 2012;122(4):1316–38. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res. 2009;171(3):232–41. 10.1016/j.pscychresns.2008.04.003 . [DOI] [PubMed] [Google Scholar]
- 50.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–13. 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. JCerebBlood Flow Metab. 2007;27(5):909–18. 10.1038/sj.jcbfm.9600419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28(4):202–8. 10.1016/j.tins.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 53.De Silva TM, Faraci FM. Microvascular Dysfunction and Cognitive Impairment. Cell Mol Neurobiol. 2016;36(2):241–58. 10.1007/s10571-015-0308-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–97. 10.1016/S1474-4422(13)70060-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2017;102(12):4343–410. 10.1210/jc.2017-01922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11(6):710–7. 10.1016/j.jalz.2014.10.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas T, Miners S, Love S. Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer’s disease and vascular dementia. Brain. 2015;138(Pt 4):1059–69. 10.1093/brain/awv025 . [DOI] [PubMed] [Google Scholar]
- 58.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–713. 10.1161/STR.0b013e3182299496 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119(4):421–33. 10.1007/s00401-010-0654-5 [DOI] [PubMed] [Google Scholar]
- 60.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al. Neuropathological Correlates of Dementia in Over-80-Year-Old Brain Donors from the Population-Based Cambridge City over-75s Cohort (CC75C) Study. JAlzheimers Dis. 2009. 10.3233/JAD-2009-1182 [DOI] [PubMed] [Google Scholar]
- 61.Giannakopoulos P, Kovari E, Herrmann FR, Hof PR, Bouras C. Interhemispheric Distribution of Alzheimer Disease and Vascular Pathology in Brain Aging. Stroke. 2009;40:983–6. 10.1161/STROKEAHA.108.530337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giannakopoulos P, Gold G, Kovari E, Von Gunten A, Imhof A, Bouras C, et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol. 2007;113(1):1–12. 10.1007/s00401-006-0144-y [DOI] [PubMed] [Google Scholar]
- 63.Bouras C, Kovari E, Herrmann FR, Rivara CB, Bailey TL, Von Gunten A, et al. Stereologic analysis of microvascular morphology in the elderly: Alzheimer disease pathology and cognitive status. Journal of Neuropathology and Experimental Neurology. 2006;65(3):235–44. 10.1097/01.jnen.0000203077.53080.2c [DOI] [PubMed] [Google Scholar]
- 64.Bailey TL, Rivara CB, Perl DP, Haroutunian V, Bouras C, Giannakopoulos P, et al. Hippocampal microvasculature attrition and cognitive decline in Alzheimers disease. Journal of Neuropathology and Experimental Neurology; 5/20052005. p. 467-. [Google Scholar]
- 65.Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis. 2015;82:593–606. 10.1016/j.nbd.2015.08.014 . [DOI] [PubMed] [Google Scholar]
- 66.Gama Sosa MA, Gasperi RD, Rocher AB, Wang AC, Janssen WG, Flores T, et al. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am J Pathol. 2010;176(1):353–68. 10.2353/ajpath.2010.090482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer’s disease patients and experimental models. J Cereb Blood Flow Metab. 2011;31(6):1354–70. 10.1038/jcbfm.2011.43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stirban A. Microvascular dysfunction in the context of diabetic neuropathy. Curr Diab Rep. 2014;14(11):541 10.1007/s11892-014-0541-x . [DOI] [PubMed] [Google Scholar]
- 69.Ostergaard L, Finnerup NB, Terkelsen AJ, Olesen RA, Drasbek KR, Knudsen L, et al. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia. 2015;58(4):666–77. 10.1007/s00125-014-3461-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clegg LE, Mac Gabhann F. Systems biology of the microvasculature. Integr Biol (Camb). 2015;7(5):498–512. 10.1039/c4ib00296b . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res. 2015;116(7):1231–44. 10.1161/CIRCRESAHA.116.302855 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Mol Neurobiol. 1989;3(1–2):71–100. . [DOI] [PubMed] [Google Scholar]
- 73.Katsel P, Tan W, Haroutunian V. Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS One. 2009;4(10):e7642 10.1371/journal.pone.0007642 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing Transcriptome and splicing database of Glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34 10.1523/jneurosci.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haroutunian V, Katsel P, Schmeidler J. Transcriptional vulnerability of brain regions in Alzheimer’s disease and dementia. Neurobiol Aging. 2009;30(4):561–73. 10.1016/j.neurobiolaging.2007.07.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M, Roussos P, McKenzie A, Zhou X, Kajiwara Y, Brennand KJ, et al. Integrative network analysis of nineteen brain regions identifies molecular signatures and networks underlying selective regional vulnerability to Alzheimer’s disease. Genome Med. 2016;8(1):104 10.1186/s13073-016-0355-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gama-Sosa M, Katsel P, De Gasperi R, Garcia GP, Triperson V, Searcy CJ, et al. Step-gradient isolation of the adult mammalian brain microvasculature through a layer of Ficoll-Paque PLUS. Microvascular Research. 2016;In press. [Google Scholar]
- 78.Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56(6):713–8. Epub 1999/11/24. . [DOI] [PubMed] [Google Scholar]
- 79.Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55(9):1185–91. 10.1001/archneur.55.9.1185 [DOI] [PubMed] [Google Scholar]
- 80.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–86. . [DOI] [PubMed] [Google Scholar]
- 81.Hughes TM, Craft S. The role of insulin in the vascular contributions to age-related dementia. Biochim Biophys Acta. 2016;1862(5):983–91. 10.1016/j.bbadis.2015.11.013 . [DOI] [PubMed] [Google Scholar]
- 82.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. ArchNeurol. 2012;69(1):29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18(4):521–30. 10.1038/nn.3966 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lubitz I, Haroutunian V, Katsel P, Leroith D, Landa N, Castel D, et al. Non-viability of crossing the Alzheimer mouse model Tg2576 with the type 2 diabetes mouse model ob/ob. Neurobiol Aging. 2014;35(7):e19–20. 10.1016/j.neurobiolaging.2014.01.138 [DOI] [PubMed] [Google Scholar]
- 85.Knight EM, Ruiz HH, Kim SH, Harte JC, Hsieh W, Glabe C, et al. Unexpected partial correction of metabolic and behavioral phenotypes of Alzheimer’s APP/PSEN1 mice by gene targeting of diabetes/Alzheimer’s-related Sorcs1. Acta Neuropathol Commun. 2016;4:16 10.1186/s40478-016-0282-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Picone P, Vilasi S, Librizzi F, Contardi M, Nuzzo D, Caruana L, et al. Biological and biophysics aspects of metformin-induced effects: cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging (Albany NY). 2016;8(8):1718–34. doi: 10.18632/aging.101004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Picone P, Nuzzo D, Caruana L, Messina E, Barera A, Vasto S, et al. Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-kappaB activation: Use of insulin to attenuate metformin’s effect. Biochim Biophys Acta. 2015;1853(5):1046–59. 10.1016/j.bbamcr.2015.01.017 . [DOI] [PubMed] [Google Scholar]
- 88.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106(10):3907–12. 10.1073/pnas.0807991106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDC correction; # = p<0.05 without FDR correction.
(TIF)
Values represent relative log fold change in persons with AD relative to controls and log fold change in persons with AD and T2D who had been treated with anti-diabetes agents. * = p<0.05 after FDC correction; # = p<0.05 without FDR correction.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.