Supplemental Digital Content is available in the text.
Key Words: intrathecal catheter, nusinersen, SMN1, spinal muscular atrophy
Abstract
Background:
Many patients with spinal muscular atrophy (SMA) who might benefit from intrathecal antisense oligonucleotide (nusinersen) therapy have scoliosis or spinal fusion that precludes safe drug delivery. To circumvent spinal pathology, we designed a novel subcutaneous intrathecal catheter (SIC) system by connecting an intrathecal catheter to an implantable infusion port.
Methods:
Device safety and tolerability were tested in 10 SMA patients (age, 5.4 to 30.5 y; 80% with 3 copies of SMN2); each received 3 sequential doses of nusinersen (n=30 doses). Pretreatment disease burden was evaluated using the Revised Hammersmith Scale, dynamometry, National Institutes of Health pegboard, pulmonary function testing, electromyography, and 2 health-related quality of life tools.
Results:
Device implantation took ≤2 hours and was well tolerated. All outpatient nusinersen doses were successfully administered via SIC within 20 minutes on the first attempt, and required no regional or systemic analgesia, cognitive distraction, ultrasound guidance, respiratory precautions, or sedation. Cerebrospinal fluid withdrawn from the SIC had normal levels of glucose and protein; cerebrospinal fluid white blood cells were slightly elevated in 2 (22%) of 9 specimens (median, 1 cell/µL; range, 0 to 12 cells/µL) and red blood cells were detected in 7 (78%) specimens (median, 4; range, 0 to 2930 cells/µL).
Discussion:
Preliminary observations reveal the SIC to be relatively safe and well tolerated in SMA patients with advanced disease and spinal fusion. The SIC warrants further study and, if proven effective in larger trials of longer duration, could double the number of patients able to receive nusinersen worldwide while reducing administration costs 5- to 10-fold.
Spinal muscular atrophy (SMA; MIM# 253300) is a common monogenic cause of spinal motor neuron (SMN) degeneration caused by biallelic deletions of SMN1, which encodes SMN protein.1–4 Within the SMN1 locus on chromosome 5q13, humans have a second SMN-encoding gene (SMN2) that can be present in multiple copies. SMN2 contains a base difference (c.850C>T) that excludes exon 7 from ~90% of mRNA transcripts to produce an unstable protein fragment (SMN∆7) that is rapidly degraded.5,6 Residual intact SMN translated from each SMN2 copy partially compensates for SMN1 deficiency such that genomic SMN2 copy number correlates inversely with disease timing and severity.7–9
Functional overlap between SMN1 and SMN2 inspired the design of nusinersen, an antisense oligonucleotide engineered to alter splicing of SMN2 pre-mRNA and thereby increase expression of stable SMN protein.10 Repeated intrathecal injections of nusinersen improve survival and motor development among infants with severe SMA (2 copies of SMN2) treated between 30 and 262 days of age, and also benefit patients started on therapy between 2 and 9 years of age as observed in CHERISH, a study to assess the efficacy and safety of nusinersen in participants with later-onset SMA (NCT02292537).11
The CHERISH trial excluded older symptomatic patients with joint contractures, severe scoliosis (radiographic Cobb angle >40 degrees), gastrostomy, or dependence on mechanical ventilatory support.11 Unfortunately, such complications are common among surviving SMA patients, many of whom have skeletal deformity or instrumentation that hinders repeated interlaminar nusinersen dosing.12,13 This has prompted a search for alternative nusinersen dosing strategies14 and engenders related questions about how to structure functional assessments for patients who have advanced neuromuscular disability and its attendant skeletal and respiratory complications.12,13,15–17 We were confronted with this problem in March 2017, when an 11-year-old Mennonite boy with advanced SMA was denied intrathecal nusinersen at 2 different academic medical centers because of spinal fusion.
To circumvent spinal pathology, we devised a novel subcutaneous intrathecal catheter (SIC) system using an off-label configuration of 2 Food and Drug Administration (FDA)-approved devices: an intrathecal catheter and power injectable implantable infusion port. This allowed for repeated nusinersen dosing via subcutaneous port (Fig. 1). Following successful implantation in the sentinel patient, we designed a prospective study to evaluate 20 SMA participants treated via SIC for 14 months (through 4 loading and 3 maintenance doses of nusinersen) and tailored functional assessments to older individuals with moderate to severe neuromuscular disease.11,18 Here we report preliminary safety and tolerability of the SIC for the first 10 participants, each of whom received 3 loading doses over 4 weeks, and discuss the implications for future studies.
FIGURE 1.
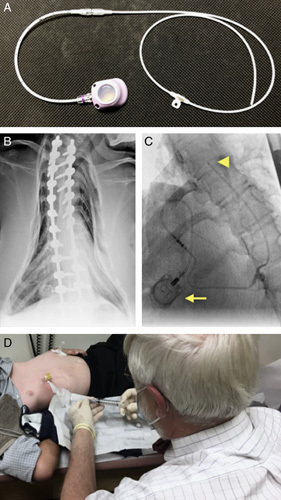
SIC system. A, The interlocked intrathecal catheter and subcutaneous infusion port used to construct the hybrid nusinersen delivery system. B, Distortions of spinal and pulmonary anatomy, commonly observed among older SMA patients, that inspired design of the SIC system. C, The SIC system in situ, with the infusion port and intrathecal catheter tip marked by a yellow arrow and arrowhead, respectively. D, The SIC allows for safe, low-cost, repeated outpatient nusinersen dosing. SIC indicates subcutaneous intrathecal catheter; SMA, spinal muscular atrophy.
METHODS
Patients
Ten study participants born between 1987 and 2012 shared homozygous exon 7 deletions of SMN1 that traced to common ancestral founders across 11 generations and segregated into individuals with 2 (n=1), 3 (n=8), or 4 (n=1) copies of SMN2 (Table 1). The 6 eldest patients (age, 12.1 to 30.5 y) had spinal fusion that precluded repeated lumbar puncture; the 4 youngest (age, 5.4 to 10.4 y) had no spinal pathology, but parents elected the SIC based on its perceived administration safety, convenience, and cost. The study was approved by Penn Medicine-Lancaster General Hospital Institutional Review Board. Adults consented in writing to participate and parents consented on behalf of their children. In accordance with journal policy, a separate signed consent was obtained for reproduction of the photograph in Figure 1. This interim analysis, focused upon initial safety and tolerability of the SIC, was conducted between June 2017 and January 2018.
TABLE 1.
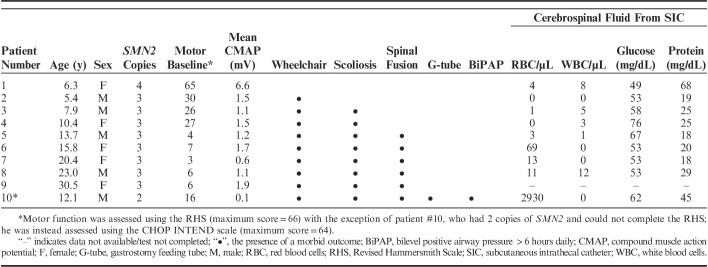
Patient Characteristics (n=10), Comorbidities, and Cerebrospinal Fluid Indices
Surgical Procedure and Nusinersen Dosing
We constructed a hybrid infusion system using 2 FDA-approved devices: a catheter commonly utilized for continuous or repeated intrathecal infusion (Medtronic) and a power injectable implantable infusion port (MedComp) designed for repetitive blood sampling or chemotherapy (Fig. 1). The infusion port locked firmly into the intrathecal catheter, nonleakage was verified ex vivo, and the implantation procedure was performed under general anesthesia.
We exposed the spine through a 3 to 4 cm incision and drilled a hole (under fluoroscopic guidance) through the spinal fusion to access the epidural space. The catheter was threaded into the midthoracic intrathecal space and the threading needle withdrawn to document free backflow of cerebrospinal fluid (CSF). Although nusinersen is typically administered in the lumbar region via interlaminar puncture, SMA affects lower motor neurons along the entire neuraxis, and lumbar administration is based on tactical rather than biological considerations.19 Unlike infants with SMA who spend much of their time supine,18 most of our patients are upright (ie, on a wheelchair) during waking hours. We therefore chose a higher delivery site to expose thoracic and potentially cervical motor neurons to nusinersen.
Once positioned, an anchor was placed over the catheter and sutured to deep fascia, just a top bone. The infusion port was then placed through a 2 cm incision of the chest wall and implanted subcutaneously, where it was anchored to hard fascia of the chest, flank, or lower back, depending on anatomic considerations and patient preference. The port catheter was tunneled under the fascia to the posterior wound and connected firmly to the intrathecal catheter. Before wound closure, the port was accessed to document CSF flow through the complete hybrid system.
The posterior bone hole was closed with a pressure injection of surgical sealant-coagulant to prevent CSF leak and fascia were closed tightly to further safeguard against leakage. Following complete posterior wound closure, the exposed anterior infusion port was again aspirated to document free flow. A CSF volume of 5 mL was withdrawn from the port followed by injection of 12 mg (5 mL) of nusinersen (loading dose 1; LD1), which was then cleared from the catheter using 0.5 mL of normal saline. The anterior wound was then completely closed.
Patients remained supine for 48 hours postoperatively, after which they were placed in seated position and, if asymptomatic when upright for 12 hours, discharged home. Surgical wound inspection was performed on postoperative day 14 to insure the access port was completely under the skin, readily palpable, and easily accessed via a noncoring needle (Fig. 1). No imaging, sedation, or regional anesthesia was required to access the port or administer drug thereafter.
All subsequent nusinersen doses were given in the outpatient setting by standard procedure: (1) topical lidocaine 2.5%/prilocaine 2.5% was applied over the infusion port at least 30 minutes before dosing; (2) skin overlying the access port was then prepped and draped using sterile technique (Fig. 1); (3) the port reservoir was accessed via noncoring needle and flushed with 0.2 mL saline to float the intrathecal catheter tip free from surrounding pia and arachnoid mater; (4) 3 mL CSF was withdrawn and discarded and an additional 4 mL withdrawn for analysis; and (5) nusinersen 12 mg (5 mL) was infused through the port and the catheter was flushed with 2 mL saline.
Assessments
Each participant received 3 loading doses of nusinersen via SIC (baseline, LD1; week 2, LD2; week 4, LD3). At scheduled doses, we obtained laboratory indices of safety and solicited information about adverse events and concomitant medications. Before administration of LD3, CSF was collected through the SIC and divided into aliquots for measurement of cell counts, protein, and glucose. A structured battery of assessments was conducted at the preimplantation visit and 4 weeks after implantation, before administration of LD3. We did not expect to observe any significant changes in motor function, power, or respiratory performance after just 2 doses of nusinersen, which for infants only improves event-free survival beyond the loading phase.18
Motor assessments were performed at ~09:00 hours by the same examiner and included tests of neuromuscular function [Revised Hammersmith Scale (RHS)20 or Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders,21 as appropriate to pretreatment functional level]. Nine (90%) participants could be studied using the RHS, for which scores range between 0 and 66 (with higher numbers indicating better motor function). Motor speed and dexterity were assessed using the National Institutes of Health (NIH) Toolbox pegboard,22 and power was measured by 12 dynamometry maneuvers (JTech Medical, Commander Echo MMT Dynamometry). A novel dynamometric parameter, the SMA Force Index (SFI) was devised to capture the distribution of weakness characteristic of SMA (see Supplemental Materials for details, Supplemental Digital Content 1, http://links.lww.com/BPO/A180).
Baseline compound muscle action potential (CMAP; mV) was recorded from the distribution of ulnar (abductor digiti minimus), radial (extensor carpi radialis longus), and deep peroneal (extensor digitorum brevis, tibialis anterior) nerves—and used to calculate an average CMAP amplitude for each patient.23 Pulmonary function testing was performed in the seated position in accordance with American Thoracic Society standards using an EasyOne Pro Lab (NDD). Maximal inspiratory (MIP) and expiratory (MEP) pressures were measured after full exhalation and inhalation, respectively, using a Micro respiratory pressure monitor (RPM; Micro Direct); these are reported as z-scores calculated from age-specific and sex-specific reference values,24 where z=(patient value/reference mean)/(reference SD) and normal z-scores range between −2 and +2. To assess well-being, we administered age-appropriate Pediatric Quality of Life (PedsQL) Acute and Family Impact Modules (www.pedsql.org; parent and child forms)25 and the NIH Toolbox Emotion Domain.22
Statistics
Functional measures, laboratory indices, and measures of HRQOL at preimplantation and week 4 were compared using a paired t test. Relationships of RHS, dynamometer measures, and pulmonary function to patient age were studied with Pearson (r) correlations (Prism 7, GraphPad).
RESULTS
Baseline Assessments
Motor function, dexterity, power, mean CMAP, pulmonary function, and HRQOL among 10 study participants are represented in Tables 1 and 2. For 8 patients with 3 copies of SMN2, the RHS, weigt-adjusted SFI, and select dynamometry maneuvers correlated inversely with age in children below 16 years (Pearson r values −0.94 to −0.97). These correlations broke down thereafter, revealing their limits for patients with advanced neuromuscular disease (Fig. 2). Measured parameters did not change significantly after 2 loading doses of nusinersen (Table 2). Baseline mean CMAP varied by SMN2 copy number: values for patients with 4 (n=1), 3 (n=8), and 2 (n=1) copies of SMN2 were 6.6±0.8, 1.3±0.7, and 0.2±0.1 mV, respectively. Motor nerve amplitude did not vary as a function of age among patients with 3 copies of SMN2 (Fig. 2).
TABLE 2.
Clinical Assessments Pretreatment and After 2 Loading Doses of Nusinersen (n=10)
FIGURE 2.
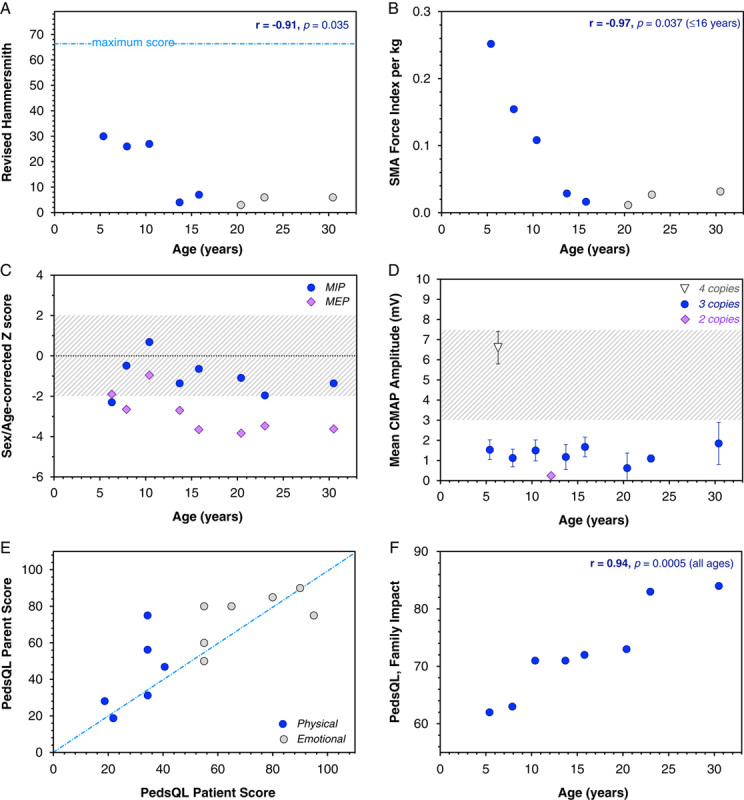
Multidomain baseline assessments. Among SMA patients with 3 copies of SMN2 and below 16 years of age (blue circles), we found strong inverse Pearson correlations between age and Revised Hammersmith Functional Motor Scale (A), weight-adjusted SFI (B), and forced vital capacity. This association broke down after age 16 (gray circles). C, MEP as compared with inspiratory pressure (corrected for age and sex) was low for all patients, reflecting weakness of intercostal muscles relative to the diaphragm. Gray shaded area indicates normal z-scores ranging from −2 to +2. D, Baseline mean CMAP, averaged from 4 separate recordings, varied by SMN2 copy number (white triangle, 4 copies; blue circles, 3 copies; purple diamond, 2 copies) but did not vary as a function of age among patients with 3 SMN2 copies. Gray shaded area represents the range of CMAP amplitude observed in ambulatory (as compared with nonambulatory) SMA patients (Lewelt et al23). E, Independent PedsQL responses of participants and their caregivers were generally in good agreement (blue-dashed line represents unity) but there was a tendency for parents to overrate their child’s well-being. F, The overall burden of disease on family functioning (PedsQL family impact module) was strongly associated with patient age. CMAP indicates compound muscle action potential; SMA, spinal muscular atrophy; PedsQL, Pediatric Quality of Life.
Total lung capacity and forced vital capacity were 32% to 78% and 16% to 97% predicted, respectively (Tables 1, 2, and Fig. 2). MIP and MEP pressures decreased with age and MEPs corrected for age and sex were low relative to MIPs (Fig. 2). This reflects a reversal of normal physiology characteristic of SMA; the diaphragm is less impaired than intercostal muscles and results in development of a bell-shaped chest (Fig. 1).
PedsQL responses of participants and their caregivers, elicited independently, were generally in good agreement (r2=0.77, P<0.0001). Participants and parents alike consistently rated physical function lower than emotional function and parents tended to overrate their child’s physical well-being (Fig. 2). Within our cohort of predominantly Old Order Mennonite participants harboring 3 copies of SMN2, baseline indices of emotional health were similar to values from healthy pediatric reference populations, 26,27 whereas physical functioning scores were 60% to 70% lower (Table 2).
Safety and Tolerability of the SIC
For all 10 participants, the SIC was successfully implanted during a single procedure lasting an average 1.4 (range, 1.1 to 2.0) hours followed by a hospital stay of 50 to 55 hours. There were no perioperative or postoperative complications and all wounds were clean and dry by postoperative week 2. Each participant received 3 nusinersen doses over a 4 week postimplantation period (30 doses for the cohort). All outpatient nusinersen doses were successfully administered via SIC with a single attempt through topical anesthetic, and required no regional or systemic analgesia, cognitive distraction, ultrasound guidance, respiratory precautions, or sedation.28 No patient required oropharyngeal suctioning, supplemental oxygen, or noninvasive ventilation before or during receipt of an outpatient SIC dose.
During the interim study period, 3 adverse events occurred among 3 participants. These included: (1) urinary tract infection in a male child, (2) back pain secondary to a protruding spinal rod in an adult female, and (3) inability to withdraw CSF from the SIC port during LD2 in another adult female; nusinersen could, however, be freely administered through this same port, implicating dynamic suction-induced catheter obstruction. None of these events were serious and only the latter was definitely related to the device.
Nine study participants had CSF withdrawn from the SIC. CSF glucose (median, 53 mg/dL; range, 49 to 76 mg/dL) and protein (median, 25 mg/dL; range, 18 to 68 mg/dL) levels were normal. White blood cells were slightly elevated in 2 (22%) of 9 specimens (median, 1 cell/µL; range, 0 to 12 cells/µL) and red blood cells were detected in 7 (78%) of 9 specimens (median, 4; range, 0 to 2930 cells/µL) (Table 1). There were no serum or urine laboratory abnormalities associated with SIC nusinersen administration.
DISCUSSION
Barriers to Nusinersen Administration
Nusinersen has well-documented efficacy for infants and children with SMA,11,18 but clinical studies to date do not adequately address older patients who have more advanced neuromuscular disease and its attendant skeletal and pulmonary morbidities. Specifically, scoliosis is observed in as many as 80% of SMA patients with 2 or 3 copies of SMN2 who survive into late childhood12 and can preclude safe, repeated interlaminar dosing of nusinersen. These same patients often require sustained mechanical ventilatory support or gastrostomy tube feeding, which were exclusion criteria for CHERISH.11
Absolute anatomic and relative pulmonary barriers to repeated interlaminar dosing have prompted efforts to administer nusinersen by alternative routes. As one recent example, 4 SMA patients with spinal fusion received 15 consecutive nusinersen injections via a transforaminal route guided by cone-beam computed tomography with fluoroscopic navigational overlay.14 Although the complication rate was low (6.7%), each procedure was technically complex, required sedation, and incurred radiation exposure. Lower complication rates from lumbar puncture (ie, 6% to 17%) might be achieved with conscious sedation16,29 but consistently safe, controlled interlaminar access in young people often requires a high degree of technical expertise and/or general anesthesia.29–31
Even among SMA patients without advanced neuromuscular disease or spinal pathology, repeated interlaminar dosing of nusinersen poses challenges. The overall incidence of traumatic lumbar puncture in children ranges from 20% to 50% and is highest among newborns.32–34 In one series of 73 lumbar punctures for 28 SMA patients (2 to 14 y), headache, back pain, or CSF leak complicated 32% of nusinersen doses.35 In a second series of 84 interlaminar nusinersen doses at an academic neuromuscular center (20 patients, ages 2 to 50 mo),28 the first lumbar puncture attempt failed 33% of the time (mean, 1.5±1.0 attempts/child/dose) and 2 (10%) children below 6 months of age required ultrasound guidance. Oropharyngeal suctioning was regularly performed, and 7 (35%) children received noninvasive ventilation during lumbar puncture. Procedural sedation and postprocedural analgesia were required for 30% and 40% of children, respectively.28
Preliminary Device Safety and Tolerability
In contrast to repeated lumbar puncture, dosing via SIC required only topical anesthetic and took <20 minutes during the course of routine outpatient visit. All doses were administered on the first attempt and patients experienced no significant pain, distress, or clinical instability from the procedure. Accordingly, we found no need to use special respiratory maneuvers, systemic analgesia, conscious sedation, or cognitive distraction. Parents of the four youngest study participants who had no anatomic or pulmonary contraindications to interlaminar dosing—elected SIC implantation as potentially safer, more convenient, and less costly than repeated lumbar puncture.
Efficacy was not an endpoint of the present study. However, consistent with best clinical practice, we used a multidimensional battery to assess patients at pretreatment baseline and at various intervals until study completion (ie 14 mo, after 4 loading and 3 maintenance doses of nusinersen). We expect therapeutic effects of nusinersen in this cohort will be relatively modest, slow to emerge, and observed primarily as an arrest of disease progression.11 Unfortunately, as shown in Figure 2, many conventional endpoints of motor function used for infants and young children lose their informative value after age 16 years. Thus, different assessment tools might be needed to gauge efficacy of disease-modifying therapies in adolescents and adults with SMA.
Laboratory monitoring supported the safety of the SIC but, on a cautionary note, we detected red blood cells (median, 4; range, 0 to 2930 cells/µL) in 7 (78%) of 9 specimens withdrawn from the system (Table 1). A similar phenomenon was observed among newborns with an intrathecal reservoir or ventriculoperitoneal shunt,36 raising the possibility that indwelling CSF catheter tips, when not continuously infusing, can adhere to pial or arachnoid membranes to become dynamically obstructed and/or cause microvascular injury when suction is applied. To safeguard against this, we added an initial 0.2 mL saline flush to the administration protocol to gently dislodge the catheter tip from surrounding thecal membranes before withdrawal of CSF. It is worth noting that CSF withdrawal and examination were a means by which to assess SIC patency and safety for the purpose of this trial, but need not be a routine component of long-term administration protocols.
Clinical and Economic Implications
Since the FDA approved nusinersen for treatment of all forms of SMA in December 2016, there is growing demand for delivery methods adapted to older patients with respiratory and spinal comorbidities. The publication of CHERISH,11 which demonstrated clinical efficacy in an older SMA patient population with later-onset disease, brought the need into sharp relief. In recognition of this fact and its time-sensitivity for many patients with SMA, we chose a relatively short interval to describe device implantation and preliminary safety with the intention to prompt additional research in this area. If the SIC or similar device proves safe and well tolerated in multicenter trials involving more patients over longer intervals, indwelling catheter systems might become a preferred route of nusinersen dosing for patients across the SMA spectrum. This could not only increase the proportion of patients who receive drug, but also reduce its overall administration cost (Fig. 3).
FIGURE 3.
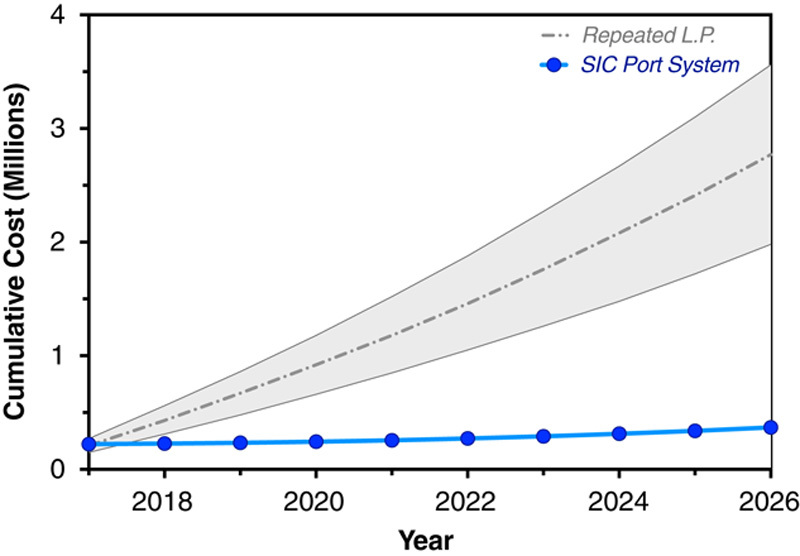
Administration economics. Projected nusinersen administration costs (not including drug) for 10 participants were plotted over 10 years, assuming a 1-time SIC implantation cost of $22,000 and an annual medical inflation rate of 6%. The SIC (blue line) yields average savings of $24,000 per child per year (2.4 million dollars for the cohort over 10 y) as compared with repeated lumbar puncture (gray shaded area, bounded by upper and lower limits of projected interlaminar administration costs). SIC indicates subcutaneous intrathecal catheter.
In our office, nusinersen via SIC cost US$75 per administration. By comparison, 4 Mennonite children (age, 0.7 to 2.9 y) treated contemporaneously by standard lumbar puncture incurred hospital charges of $5000 and $9000 per administration, depending on the type and duration of sedation used. On the basis of these data, Figure 3 depicts comparative administration costs (not including drug) for 10 participants over a period of 10 years, assuming an SIC implantation cost of $22,000 and an annual medical inflation rate of 6%. The SIC yields average savings of $24,000 per child per year (2.4 million dollars for the cohort over 10 y). Although not a primary endpoint of this study, this simple economic heuristic has unambiguous implications for the overall cost of nusinersen administration worldwide.
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, nusinersen via repeated intrathecal injection is effective therapy for all types of SMA,11,18 but its standard method of interlaminar delivery poses both absolute and relative challenges for a large proportion of patients. More data are needed to determine if nusinersen has comparable efficacy when delivered by subcutaneous port as compared with the standard interlaminar route. However, our initial observations are promising, and long-term administration of nusinersen via the SIC or similar device has the potential to double the number of children worldwide who can safely receive the drug12,14 while simultaneously lowering its long-term administration cost 5- to 10-fold.
Although the SIC was designed for SMA patients with advanced disease and attendant spinal pathology, our preliminary observations have implications for younger, less severely affected patients. As private and government insurers adapt to the extraordinary costs associated with new disease-modifying precision therapies, they will likely seek practical innovations like the SIC, which have the potential to safely control administration costs while preserving therapeutic value.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.pedorthopaedics.com.
ACKNOWLEDGMENTS
This work was supported in part by charitable contributions from the communities we serve. We thank SMA patients and their families for their creativity, courage, and partnership in this endeavor.
Footnotes
This study was funded in part by a grant from Biogen, the manufacturer of nusinersen. The authors received no direct or indirect compensation from Biogen and have no personal financial interests in the company. Author Robert M. Reed reports grants from Flight Attendants Medical Research Institute (FAMRI) during the conduct of the study. Funding from FAMRI did not influence the collection or analysis of data.
The authors declare no conflicts of interest.
REFERENCES
- 1.Govoni A, Gagliardi D, Comi GP, et al. Time is motor neuron: therapeutic window and its correlation with pathogenetic mechanisms in spinal muscular atrophy. Mol Neurobiol. 2018;55:6307–6318. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. [DOI] [PubMed] [Google Scholar]
- 3.Burglen L, Spiegel R, Ignatius J, et al. SMN gene deletion in variant of infantile spinal muscular atrophy. Lancet. 1995;346:316–317. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265–269. [DOI] [PubMed] [Google Scholar]
- 5.Butchbach ME. Copy number variations in the survival motor neuron genes: implications for spinal muscular atrophy and other neurodegenerative diseases. Front Mol Biosci. 2016;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han KJ, Foster DG, Zhang NY, et al. Ubiquitin-specific protease 9x deubiquitinates and stabilizes the spinal muscular atrophy protein-survival motor neuron. J Biol Chem. 2012;287:43741–43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb SJ, Coffey CS, Yankey JW, et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol. 2016;3:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb SJ, Coffey CS, Yankey JW, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coovert DD, Le TT, McAndrew PE, et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6:1205–1214. [DOI] [PubMed] [Google Scholar]
- 10.Khorkova O, Wahlestedt C. Oligonucleotide therapies for disorders of the nervous system. Nat Biotechnol. 2017;35:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. [DOI] [PubMed] [Google Scholar]
- 12.Carter GT, Abresch RT, Fowler WM, Jr, et al. Profiles of neuromuscular diseases. Spinal muscular atrophy. Am J Phys Med Rehabil. 1995;74:S150–S159. [DOI] [PubMed] [Google Scholar]
- 13.Mayer OH. Scoliosis and the impact in neuromuscular disease. Paediatr Respir Rev. 2015;16:35–42. [DOI] [PubMed] [Google Scholar]
- 14.Weaver JJ, Natarajan N, Shaw DWW, et al. Transforaminal intrathecal delivery of nusinersen using cone-beam computed tomography for children with spinal muscular atrophy and extensive surgical instrumentation: early results of technical success and safety. Pediatr Radiol. 2018;48:392–397. [DOI] [PubMed] [Google Scholar]
- 15.van der Ploeg AT. The dilemma of two innovative therapies for spinal muscular atrophy. N Engl J Med. 2017;377:1786–1787. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt M, Johnson DW, Chan J, et al. Risk factors for adverse events in emergency department procedural sedation for children. JAMA Pediatr. 2017;171:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham RJ, Athiraman U, Laubach AE, et al. Anesthesia and perioperative medical management of children with spinal muscular atrophy. Paediatr Anaesth. 2009;19:1054–1063. [DOI] [PubMed] [Google Scholar]
- 18.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–1732. [DOI] [PubMed] [Google Scholar]
- 19.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–3026. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey D, Scoto M, Mayhew A, et al. Revised Hammersmith Scale for spinal muscular atrophy: A SMA specific clinical outcome assessment tool. PLoS One. 2017;12:e0172346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia infant test of neuromuscular disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon RC, Wagster MV, Hendrie HC, et al. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewelt A, Krosschell KJ, Scott C, et al. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle Nerve. 2010;42:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson SH, Cooke NT, Edwards RH, et al. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax. 1984;39:535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunaway S, Montes J, Montgomery M, et al. Reliability of telephone administration of the PedsQL generic quality of life inventory and neuromuscular module in spinal muscular atrophy (SMA). Neuromuscul Disord. 2010;20:162–165. [DOI] [PubMed] [Google Scholar]
- 26.Panepinto JA, Pajewski NM, Foerster LM, et al. The performance of the PedsQL generic core scales in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam KC, Valier AR, Bay RC, et al. A unique patient population? Health-related quality of life in adolescent athletes versus general, healthy adolescent individuals. J Athl Train. 2013;48:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pechmann A, Langer T, Wider S, et al. Single-center experience with intrathecal administration of Nusinersen in children with spinal muscular atrophy type 1. Eur J Paediatr Neurol. 2018;22:122–127. [DOI] [PubMed] [Google Scholar]
- 29.Iannalfi A, Bernini G, Caprilli S, et al. Painful procedures in children with cancer: comparison of moderate sedation and general anesthesia for lumbar puncture and bone marrow aspiration. Pediatr Blood Cancer. 2005;45:933–938. [DOI] [PubMed] [Google Scholar]
- 30.Chiaretti A, Benini F, Pierri F, et al. Safety and efficacy of propofol administered by paediatricians during procedural sedation in children. Acta Paediatr. 2014;103:182–187. [DOI] [PubMed] [Google Scholar]
- 31.Crock C, Olsson C, Phillips R, et al. General anaesthesia or conscious sedation for painful procedures in childhood cancer: the family’s perspective. Arch Dis Child. 2003;88:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glatstein MM, Zucker-Toledano M, Arik A, et al. Incidence of traumatic lumbar puncture: experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila). 2011;50:1005–1009. [DOI] [PubMed] [Google Scholar]
- 33.Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and bloody lumbar puncture in children with acute lymphoblastic leukemia. JAMA. 2002;288:2001–2007. [DOI] [PubMed] [Google Scholar]
- 34.Bonadio W. Pediatric lumbar puncture and cerebrospinal fluid analysis. J Emerg Med. 2014;46:141–150. [DOI] [PubMed] [Google Scholar]
- 35.Hache M, Swoboda KJ, Sethna N, et al. Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. J Child Neurol. 2016;31:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenfestey RW, Smith PB, Moody MA, et al. Predictive value of cerebrospinal fluid parameters in neonates with intraventricular drainage devices. J Neurosurg. 2007;107:209–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.pedorthopaedics.com.