Abstract
Objectives:
Treatment options for patients with unresectable or metastatic salivary gland carcinoma (SGC) are limited. Safety and efficacy of pembrolizumab for SGC expressing programmed death ligand 1 (PD-L1) were explored.
Materials and Methods:
A cohort of patients with advanced, PD-L1-positive SGC was enrolled in the nonrandomized, multicohort, phase Ib trial of pembrolizumab in patients with PD-L1-positive advanced solid tumors (KEYNOTE-028; NCT02054806). Key inclusion criteria included recurrent or metastatic disease, failure of prior systemic therapy, and PD-L1 expression on ≥1% of tumor or stroma cells (per a prototype immunohistochemistry assay). Patients received pembrolizumab 10 mg/kg every 2 weeks for ≥2 years or until confirmed disease progression or unacceptable toxicity. Primary end point was objective response rate per Response Evaluation Criteria in Solid Tumors version 1.1 by investigator review.
Results:
Twenty-six patients with PD-L1-positive SGC were enrolled and treated; median age was 57 years, 88% were men, and 74% had received prior therapy for recurrent/metastatic disease. Confirmed objective response rate after median follow-up of 20 months was 12% (95% confidence interval, 2%-30%), with 3 patients achieving partial response; there were no complete responses. Median duration of response was 4 months (range, 4 to 21 mo). Treatment-related adverse events occurred in 22 patients (85%), resulting in discontinuation in 2 patients and death in 1 (interstitial lung disease); those occurring in ≥15% of patients were diarrhea, decreased appetite, pruritus, and fatigue.
Conclusions:
Pembrolizumab demonstrated promising antitumor activity and a manageable safety profile in patients with advanced, PD-L1-positive SGC.
Key Words: salivary gland cancer, immunotherapy, pembrolizumab, anti-PD-1
Salivary gland carcinomas (SGCs) are rare, accounting for <5% of head and neck cancers, and are histologically diverse, with 24 different types identified.1,2 SGCs are generally slow growing and characterized by a prolonged clinical course, frequent local recurrence, and latent distant metastases.3
Treatment options for patients with unresectable or metastatic (advanced) SGC are limited to chemotherapy or participation in a clinical trial.2,4 Response rates to single agents (cisplatin, carboplatin, paclitaxel, docetaxel, 5-fluorouracil, methotrexate, capecitabine, cetuximab, gemcitabine, or vinorelbine) range from 15% to 35%, whereas rates are usually double with combination regimens (cisplatin or carboplatin plus 5-fluorouracil with cetuximab, cisplatin or carboplatin plus a taxane, cisplatin with cetuximab, or cisplatin with 5-fluorouracil).2
Molecular aberrations are expressed differentially among SGC subtypes and have been identified as potential therapeutic targets.1 These include altered expressions of c-kit tyrosine kinase receptor,5 epidermal growth factor receptor,6 c-ErbB2 (HER2/neu),7 and hormone receptors.8
The programmed death 1 (PD-1) receptor is expressed on activated T cells and inhibits effector T-cell function upon binding of its ligands, PD-L1 and PD-L2.9 Upregulation of the PD-1 pathway, which is involved in the induction and maintenance of peripheral immune tolerance, leads to suppression of immune response in many tumors.10 In an analysis of 217 surgically resected SGC specimens, high PD-L1 expression was reported in high-grade SGC subtypes previously shown to be associated with aggressive behavior (eg, salivary duct carcinoma and squamous cell carcinoma).11 An association between PD-L1 positivity and inferior disease-free survival was also observed.11 In addition, PD-L2 expression has been found in several tumor types, including adenoid cystic carcinoma, one of the most lethal SGCs.12,13
Pembrolizumab is a fully humanized immunoglobulin G4/κ anti-PD-1 monoclonal antibody. Pembrolizumab has demonstrated robust antitumor activity and a favorable safety profile in multiple tumor types and is currently approved in >60 countries for 1 or more advanced malignancies, including in the United States for recurrent or metastatic head and neck squamous cell carcinoma that progressed on or after platinum-containing chemotherapy.14–16 The safety and antitumor activity of pembrolizumab in patients with advanced, PD-L1-positive SGC enrolled in the phase 1b, multicohort KEYNOTE-028 trial (NCT02054806) are reported here.
PATIENTS AND METHODS
Study Design and Patients
KEYNOTE-028 is an ongoing multicenter, open-label, phase 1b trial of pembrolizumab in patients with PD-L1-positive advanced solid tumors. Patient eligibility criteria for the SGC cohort included age 18 years and older; histologically or cytologically documented locally advanced or metastatic SGC of any subtype (except sarcomas or mesenchymal tumors) for which standard therapy was ineffective, does not exist, or is not considered appropriate; PD-L1-positive disease; measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1; and adequate organ function.
Exclusion criteria included a diagnosis of immunodeficiency or received systemic steroid therapy within 7 days, prior monoclonal antibody anticancer therapy within 4 weeks, and chemotherapy, targeted small-molecule therapy, or radiation therapy within 2 weeks of first pembrolizumab dose; active autoimmune disease; interstitial lung disease; known additional progressing malignancy; and active brain metastases. Previous treatment with an immune checkpoint inhibitor was not allowed. The study protocol was approved by institutional review boards or ethics committees of all participating sites. All enrolled patients provided written informed consent. This article describes off-label use of pembrolizumab for the treatment of PD-L1-positive SGC.
Treatment and Assessments
Pembrolizumab 10 mg/kg was administered through intravenous infusion every 2 weeks for 24 months or until confirmed disease progression (PD), unacceptable adverse events (AEs), or investigator/patient decision to withdraw. Treatment was withheld in cases of unacceptable toxicity but could be resumed after resolution of toxicity to grade 0/1 within 12 weeks of the last infusion; otherwise, treatment was discontinued. Response was assessed by computed tomography or magnetic resonance imaging every 8 weeks for the first 6 months and every 12 weeks thereafter. Clinically stable patients with radiographic progression who had no further increase in tumor dimensions on subsequent scans could continue treatment at the investigator’s discretion. Patients were considered clinically stable if they met the following criteria: absence of signs and symptoms indicating disease progression, no decrease in ECOG PS, absence of rapid disease progression, and absence of a progressive tumor at a critical anatomic site that would necessitate urgent alternative medical intervention. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and monitored throughout and for 30 days after treatment discontinuation (90 days for serious AEs). Immune-mediated AEs were defined as events with potentially drug-related immunologic causes that were consistent with an immune phenomenon, regardless of attribution by the investigator.
Tumor PD-L1 positivity was determined at a central laboratory using formalin-fixed archived or newly obtained biopsy samples and a prototype immunohistochemical assay (QualTek Molecular Laboratories, Goleta, CA),17 based on the 22C3 antibody (Merck & Co. Inc., Kenilworth, NJ). Positivity was defined as membranous staining on ≥1% modified proportion score or interface pattern, as described elsewhere.17,18
End Points
The primary efficacy end point was objective response rate (ORR) by investigator review, defined as the proportion of patients having complete response (CR) or partial response (PR) per RECIST version 1.1. Secondary end points were duration of response (time from first RECIST response to PD), progression-free survival (PFS; time from enrollment to first documented PD or death from any cause, whichever occurred first), overall survival (OS), and safety and tolerability.
Statistical Analyses
A sequential monitoring approach was used to evaluate ORR within each cohort of KEYNOTE-028 after a minimum of 6 patients underwent ≥1 postbaseline scan. The type I error rate over multiple evaluations was controlled using the truncated sequential probability ratio test procedure at 0.08 (1-sided),19 allowing simultaneous evaluation of efficacy and futility based on the number of patients with confirmed or unconfirmed response. Cohorts not closed for futility enrolled approximately 22 patients each. A sample of 22 evaluable patients would provide 80% power to demonstrate that the ORR exceeded 10% at an overall 1-sided 8% α level if the true best ORR for a cohort was 35%. PFS, OS, and duration of response were analyzed using the Kaplan-Meier method.
The efficacy population was composed of patients who received ≥1 dose of pembrolizumab and had measurable disease at baseline per RECIST. The safety population included all patients who received ≥1 dose of pembrolizumab. The data cutoff for this analysis was February 20, 2017.
RESULTS
Patient Characteristics
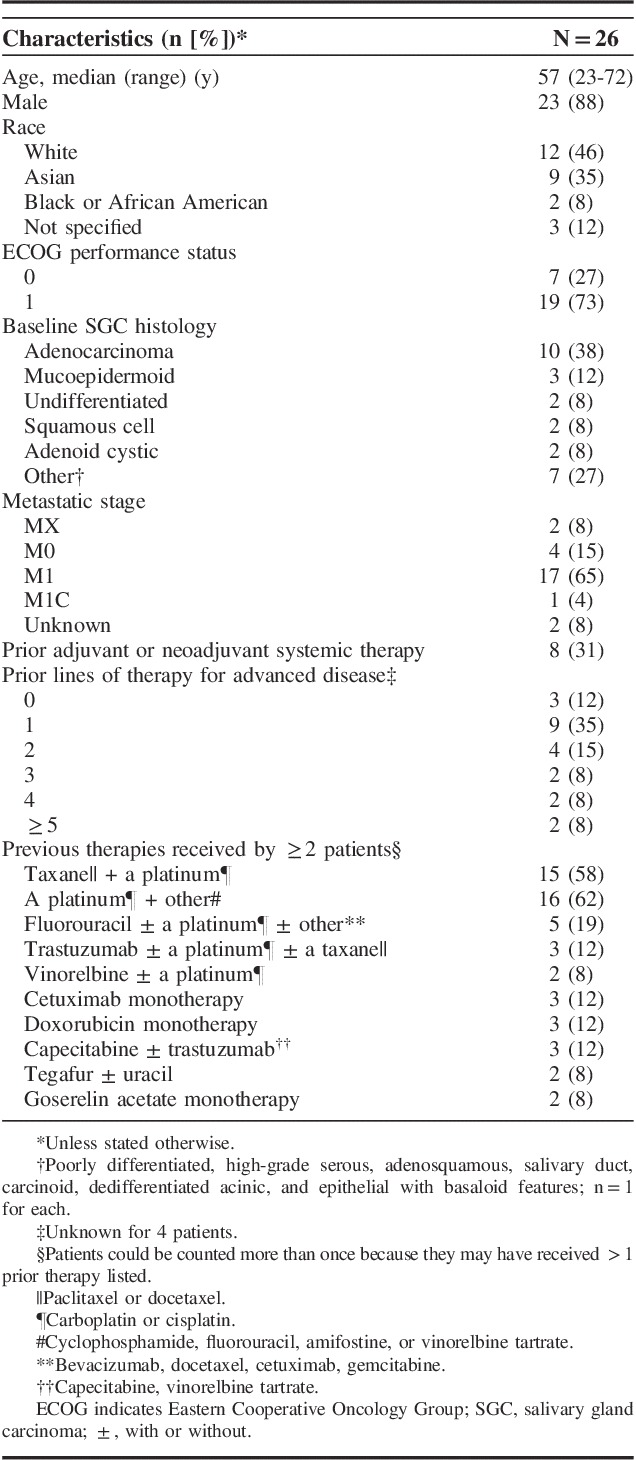
Overall, 142 patients with advanced SGC were screened, 102 patients did not pass the screening stage because they were PD-L1 negative, and 7 did not pass because they did not provide adequate tissue for biomarker analysis. Thirty-three (23%) patients had PD-L1-positive tumors, 26 of these patients were enrolled, and all received ≥1 dose of pembrolizumab (safety population). The median age was 57 years (range, 23 to 72 y). The majority were men (88%) and had ECOG PS 1 (73%) (Table 1). The most common subtype of SGC was adenocarcinoma (38%), followed by mucoepidermoid (12%), undifferentiated (8%), squamous cell (8%), and adenoid cystic (8%) carcinoma. Six patients (23%) received ≥3 prior lines of therapy for advanced disease. At the cutoff date, 24 patients had discontinued treatment, most commonly because of PD (n=15) or AEs (n=5); 2 patients remained on treatment. Among the 26 PD-L1-positive patients enrolled in this cohort, 12 (46%) were PD-L1 positive in the tumor only, 10 (38%) were positive in the stroma only, and 6 (23%) were positive in the tumor and the stroma.
TABLE 1.
Baseline Demographics and Clinical Characteristics
Safety
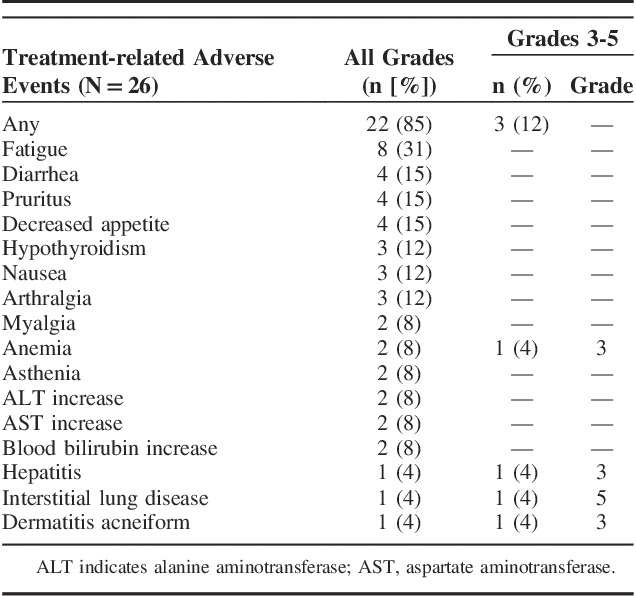
Treatment-related AEs occurred in 22 patients (85%); the most frequently occurring were fatigue (n=8), diarrhea (n=4), pruritus (n=4), and decreased appetite (n=4) (Table 2). Grade ≥3 treatment-related AEs were experienced by 3 patients (12%): anemia (grade 3) in 1, hepatitis (grade 3) in 1, and dermatitis acneiform (grade 3) and interstitial lung disease (grade 5) in 1. Six patients (23%) experienced immune-mediated AEs, including 4 cases of hypothyroidism (3 grade 2, one grade 1), 1 case of hepatitis (grade 3), and 1 case of interstitial lung disease (grade 5). There was 1 treatment-related death from interstitial lung disease in a 66-year-old man; however, a history of tuberculosis, malignant pleural effusions, and possible pulmonary infection were noted as possible confounding factors. Other treatment-related AEs that led to discontinuation were grade 2 arthritis and grade 3 hepatitis (n=1 each).
TABLE 2.
Treatment-related Adverse Events, All Grades (Occurring in ≥2 Patients) and Grades 3 to 5 (Occurring in ≥1 Patient)
Antitumor Activity
Three patients achieved PR (there were no CRs) for an ORR of 12% (Table 3). An additional 12 (46%) patients experienced stable disease (SD; defined as tumor shrinkage or growth insufficient to qualify as PR or PD, respectively) for a disease control rate (CR+PR+SD) of 58%. PRs were observed in patients with adenocarcinoma (n=2) and high-grade serous carcinoma (n=1). Two of these patients had PD-L1 expression in the stroma only (1 with adenocarcinoma and 1 with high-grade serous) and 1 patient with adenocarcinoma had PD-L1 expression in the tumor only. As of the data cutoff, no responses were ongoing (Fig. 1). A reduction in tumor size (sum of the longest tumor diameters) was documented for 35% (n=9) of evaluable patients (N=26) and was generally maintained over time (Fig. 2); 5 of these patients had adenocarcinoma, whereas the remaining 4 had high-grade serous, dedifferentiated acinic cell, adenoid cystic, and undifferentiated carcinoma. Over a median follow-up of 20 months (range, 2 to 35 mo), median time to response and duration of response were 2 and 4 months, respectively (Table 3). A best response of PD occurred in 11 patients (42%).
TABLE 3.
Antitumor Activity of Pembrolizumab (10 mg/kg every 2 wk) in Patients With Advanced Salivary Gland Cancer Over a Median Follow-up of 20 Months (Range, 2 to 35 mo)
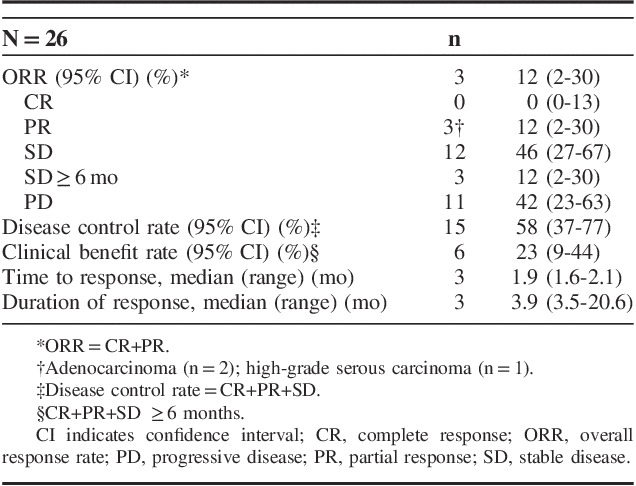
FIGURE 1.
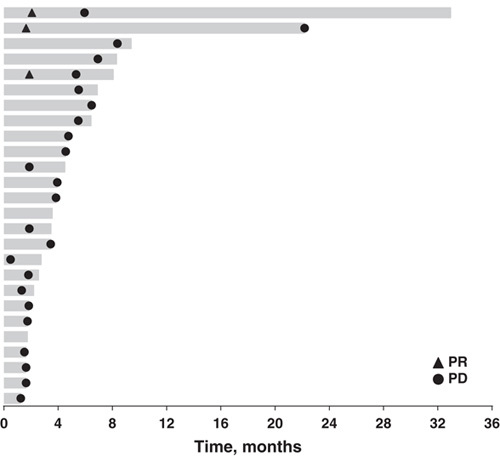
Treatment exposure to pembrolizumab and summary of best overall response assessed per RECIST by investigator review (N=26). Patients with radiographic progression who were clinically stable or clinically improved, with no further evidence of RECIST progression on subsequent scans could continue treatment after consultation with the sponsor and at the investigator’s discretion. PD indicates progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors version 1.1.
FIGURE 2.
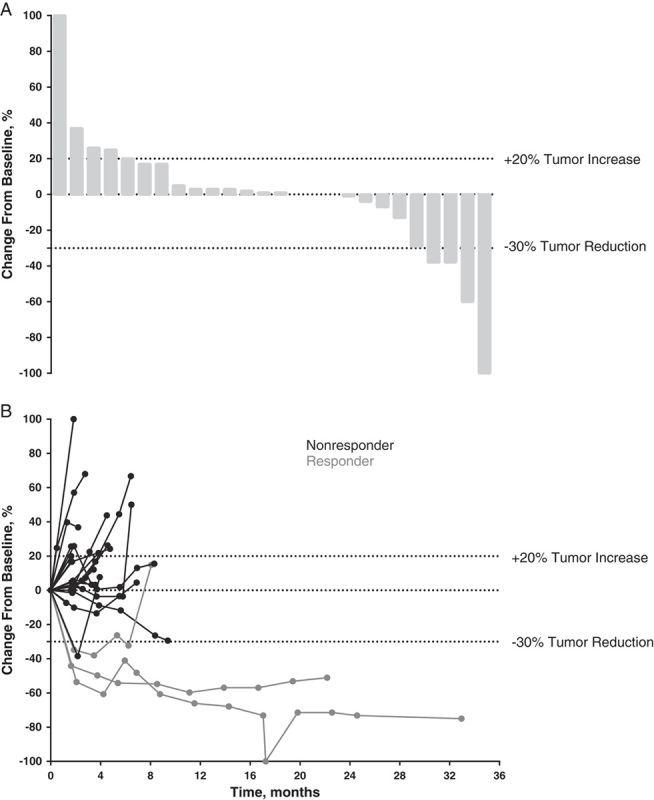
Waterfall plot of best percentage change from baseline (A) and percentage change from baseline in the sum of the longest diameters of target lesions (B) as assessed per Response Evaluation Criteria in Solid Tumors by investigator review (N=26).
Median PFS was 4 months (95% confidence interval, 2 to 5); 6- and 12-month PFS rates were 17% and 4%, respectively (Fig. 3A). Median OS was 13 months (95% confidence interval, 6 mo to not reached); 6- and 12-month OS rates were 76% and 53%, respectively (Fig. 3B).
FIGURE 3.
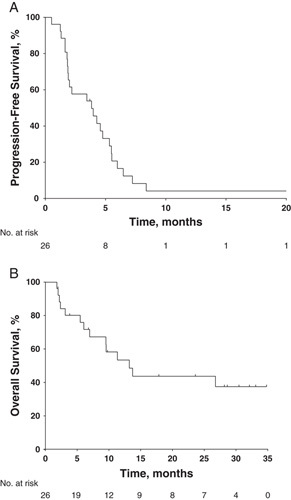
Kaplan-Meier estimates of (A) progression-free survival per Response Evaluation Criteria in Solid Tumors by investigator review and (B) overall survival (N=26).
DISCUSSION
Pembrolizumab monotherapy was generally well tolerated in advanced SGC, with a safety profile that reflects previous experience of pembrolizumab in patients with advanced cancers. Promising antitumor activity was observed (disease control rate, 58%); durable responses were reported in patients with heavily pretreated, PD-L1-positive advanced SGC (median duration of response, 4 mo). Three patients experienced PR and 12 patients had SD; 3 patients had SD ≥6 months. PR occurred in 2 patients with adenocarcinoma and 1 with high-grade serous carcinoma; tumor shrinkage was observed primarily in patients with adenocarcinoma (n=5), the most prevalent histologic type in this cohort (10 of 26 patients).
There is no gold standard for management of advanced SGC, and existing therapies generally lack significant clinical benefit. Available data supporting use of traditional cytotoxic chemotherapy are based on small studies, many of which were conducted before contemporary analytical concepts (eg, RECIST).1 SGC tumor heterogeneity further complicates the assessment of treatments because different subtypes seem to respond differently to different chemotherapies.20 Furthermore, objective responses with other targeted therapies are rarely reported.4 Nonetheless, the ORR (12%) and OS (13 mo) with pembrolizumab lie within the reported ranges for response rates and OS seen with chemotherapies. In addition, the ORR is higher than the ORR reported with sorafenib (9%) and dovitinib (11%) in adenoid cystic carcinoma.21,22 Median PFS (4 mo) and OS in the current study are lower than those reported with sorafenib (11 and 20 mo, respectively), and median PFS is lower than that reported for dovitinib (8 mo).21,22 These disparate findings could be, in part, attributable to differences in follow-up time and treatment durations, as well as the mixed-subtype population studied here. Clinical trials with larger and more homogeneous populations with respect to SGC subtype are warranted to elucidate the relative effectiveness of treatment strategies for this disease.
In conclusion, pembrolizumab monotherapy has antitumor activity in patients with PD-L1-positive SGC. The safety profile of pembrolizumab was acceptable, although 1 death occurred owing to an occurrence of immune-related toxic effect interstitial lung disease. Because there is a lack of effective standard treatments for patients with SGC, the antitumor activity of pembrolizumab is a notable finding and justifies further investigation of immune checkpoint inhibitors in SGC. The clinical activity of a fixed dose of pembrolizumab in SGC (all histologies except sarcomas and mesenchymal tumors) is being investigated further in the multicohort, phase 2 KEYNOTE-158 trial (NCT02628067). Future trials in SGC must determine which patients and disease subtypes are likely to benefit from therapy with pembrolizumab.
ACKNOWLEDGMENTS
The authors thank the patients and their families and caregivers for participating in the study. The authors thank Roger Dansey, MD (Merck & Co. Inc., Kenilworth, NJ), for critical manuscript review and Guoqing Zhao for his contribution to the data analyses. Medical writing and/or editorial assistance was provided by Jacqueline Kolston, PhD, and Matthew Grzywacz, PhD, of the ApotheCom pembrolizumab team (Yardley, PA). This assistance was funded by Merck & Co. Inc., Kenilworth, NJ.
Footnotes
Presented in part at the American Society of Clinical Oncology (ASCO) Annual Meeting, June 3 to 7, 2016, Chicago, IL.
Supported by Merck & Co. Inc., Kenilworth, NJ.
R.B.C., S.V.L., P.T.: analysis and acquisition of the data and interpretation of the results. J.P.D., J.G., S.D., C.L., E.E., B.K.: acquisition of the data and interpretation of the results. T.D., S.A.P., A.P.A., R-L.H., D.D., A.V., J.W.: acquisition of the data. S.S.: analysis of the data and interpretation of the results. J.C.: conception, design or planning of the study, analysis of the data and interpretation of the results. All authors: critically reviewed or revised the manuscript for important intellectual content and approved the final manuscript for submission.
R.B.C. received a research grant from Merck & Co. Inc., paid to the University of Pennsylvania and was a member of an advisory board for Bristol-Myers Squibb. T.D. received research funding from Taiho, Novartis, Merck Serono, Astellas Pharma, Merck & Co. Inc., Janssen, Boehringer Ingelheim, Takeda, Pfizer, Eli Lilly Sumitomo Group, Chugai Pharma, Bayer, Kyowa Hakko Kirin, Daiichi Sankyo, Celgene, and Amgen. S.A.P. received research funding to the institution from the National Institutes of Health (grant number P30CA016672). S.V.L. was a member of an advisory board for Genentech, Boehringer Ingelheim, Celgene, Ariad, and Pfizer. J.G. received a research grant paid to the institution from Merck & Co. Inc., AstraZeneca, Novartis, Bristol-Myers Squibb, Karyopharm, and Pfizer. A.P.A. received a research grant from Merck & Co. Inc., paid to the institution. C.L. was a member of an advisory board for Merck & Co. Inc. S.S., J.C. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ. P.T. is currently an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, and was previously an employee of Boehringer Ingelheim, from May 2012 to August 2014, and owned stock in Gilead from June 2014 to June 2016. J.P.D., S.D., R-L.H., D.D., A.V., E.E., J.W., B.K. declare no conflicts of interest.
Reprints: Roger B. Cohen, MD, Department of Medicine, Division of Hematology-Oncology, University of Pennsylvania School of Medicine, 3400 Spruce Street, Philadelphia, PA 19104. E-mail: roger.cohen@uphs.upenn.edu.
REFERENCES
- 1.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): head and neck cancers (Version 2.2017). 2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed January 23, 2018.
- 3.Sung MW, Kim KH, Kim JW, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129:1193–1197. [DOI] [PubMed] [Google Scholar]
- 4.Lagha A, Chraiet N, Ayadi M, et al. Systemic therapy in the management of metastatic or advanced salivary gland cancers. Head Neck Oncol. 2012;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett. 2000;154:107–111. [DOI] [PubMed] [Google Scholar]
- 6.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–769. [DOI] [PubMed] [Google Scholar]
- 7.Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10:944–946. [DOI] [PubMed] [Google Scholar]
- 8.Jeannon JP, Soames JV, Bell H, et al. Immunohistochemical detection of oestrogen and progesterone receptors in salivary tumours. Clin Otolaryngol Allied Sci. 1999;24:52–54. [DOI] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukaigawa T, Hayashi R, Hashimoto K, et al. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol. 2016;114:36–43. [DOI] [PubMed] [Google Scholar]
- 12.Yearley J, Gibson G, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23:3158–3167. [DOI] [PubMed] [Google Scholar]
- 13.Sridharan V, Gjini E, Liao X, et al. Immune profiling of adenoid cystic carcinoma: PD-L2 expression and associations with tumor infiltrating lymphocytes. Cancer Immunol Res. 2016;4:679–687. [DOI] [PubMed] [Google Scholar]
- 14.Merck Sharp & Dohme Corp Keytruda® (Pembrolizumab) injection, for Intravenous Use Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017. [Google Scholar]
- 15.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. [DOI] [PubMed] [Google Scholar]
- 17.Dolled-Filhart M, Roach C, Toland G, et al. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med. 2016;140:1243–1249. [DOI] [PubMed] [Google Scholar]
- 18.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. [DOI] [PubMed] [Google Scholar]
- 19.Romeu JL. Understanding binomial sequential testing. Statistical Confidence, Reliability Information Analysis Center (RIAC START). START. Selected Topics in Assurance Related Technologies. 2016;12:1–8. [Google Scholar]
- 20.Gilbert J, Li Y, Pinto HA, et al. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2006;28:197–204. [DOI] [PubMed] [Google Scholar]
- 21.Thomson DJ, Silva P, Denton K, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37:182–187. [DOI] [PubMed] [Google Scholar]
- 22.Keam B, Kim SB, Shin SH, et al. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer. 2015;121:2612–2617. [DOI] [PubMed] [Google Scholar]


