Abstract
Purpose
To develop contouring guidelines to be used in the Radiation Therapy Oncology Group protocol 0848, a Phase III randomized trial evaluating the benefit of adjuvant chemoradiation in patients with resected head of pancreas cancer.
Methods and Materials
A consensus committee of six radiation oncologists with expertise in gastrointestinal radiotherapy developed stepwise contouring guidelines and an atlas for the delineation of the clinical target volume (CTV) in the postoperative treatment of pancreas cancer, based on identifiable regions of interest and margin expansions. Areas at risk for subclinical disease to be included in the CTV were defined, including nodal regions, anastomoses, and the preoperative primary tumor location. Regions of interest that could be reproducibly contoured on postoperative imaging after a pancreaticoduodenectomy were identified. Standardized expansion margins to encompass areas at risk were developed after multiple iterations to determine the optimal margin expansions.
Results
New contouring recommendations based on CT anatomy were established. Written guidelines for the delineation of the postoperative CTV and normal tissues, as well as a Web-based atlas, were developed.
Conclusions
The postoperative abdomen has been a difficult area for effective radiotherapy. These new guidelines will help physicians create fields that better encompass areas at risk and minimize dose to normal tissues.
Keywords: Pancreatic cancer, Adjuvant radiotherapy, Abdominal anatomy, Intensity-modulated radiotherapy, Consensus statement
Introduction
The benefit of adjuvant radiotherapy for resected pancreatic cancer remains unclear, according to conflicting data in the literature (1–4). The largest Phase III randomized trial of adjuvant therapies for pancreatic cancer called into question the benefit of adjuvant radiotherapy, even implicating adjuvant radiotherapy as having “a deleterious effect on survival” (1). However, antiquated radiation protocols and lack of quality control limit the interpretation of older trial results. Nevertheless, the concerns raised by the lack of quality control highlight the importance of strict quality assurance in trials using more modern radiotherapeutic regimens. In the recent Radiation Therapy Oncology Group (RTOG) 97-04 study, which randomized patients who underwent a gross total resection of pancreatic adenocarcinoma to adjuvant 5-fluorouracil (5-FU) vs. gemcitabine before and after chemoradiation, quality assurance for radiation planning was mandated (2). Radiotherapy fields were prospectively reviewed before the trial analysis; 48% of treatment plans did not fully adhere to protocol requirements. Comparing “per-protocol” vs. “less than per-protocol” radiation delivery scores showed a trend of less toxicity for gemcitabine patients who received “per-protocol” therapy (median survival: “per-protocol,” 1.74; “less than per-protocol,” 1.46 years). “Per-protocol” vs. “less than per-protocol” score correlated more strongly with median survival than treatment arm (5).
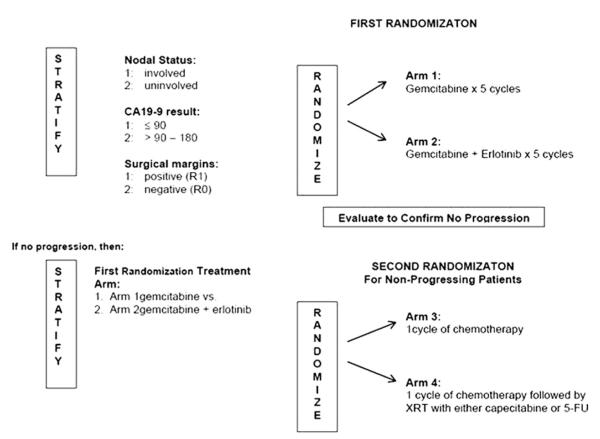
Radiation Therapy Oncology Group protocol 0848, a Phase III, randomized trial of adjuvant therapy of pancreatic cancer (Fig. 1), is currently evaluating adjuvant chemoradiotherapy for patients with resected head of pancreatic cancer. Prospective radiation quality control is mandated, with all treatment plans reviewed centrally before treatment. Computed tomography (CT)-based planning is required; radiotherapy can be delivered using either three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) planning. The dose-limiting normal tissues must be delineated and a clinical target volume (CTV) defined. To ensure adequacy of the CTV postoperatively and to develop standardized contouring guidelines for RTOG 0848, a consensus committee of six radiation oncologists, with expertise in gastrointestinal (GI) radiotherapy, developed a stepwise contouring approach based on identifiable regions of interest (ROIs) and margin expansions. Using these, reproducible CTVs can be created that cover the postoperative bed and nodal regions at risk while minimizing inclusion of the highly radiosensitive abdominal organs at risk (OARs).
Fig. 1.
Radiation Therapy Oncology Group protocol 0848 study schema.
Methodology
The contouring guidelines and atlas for the delineation of the CTV in the postoperative treatment of pancreas cancer were produced by a consensus committee of six radiation oncologists specializing in GI cancers and representatives from several physics departments and the Advanced Technology Consortium through conference calls, web-based contouring sessions, and meetings. The physicians defined the abdominal OAR, areas at risk to be included in the CTV, and ROIs that could be easily identified on postoperative CT axial imaging. These ROIs could be used to define the nodal regions and the primary areas at risk for subclinical disease. Appropriate standardized expansion margins were then established on the basis of multiple iterations to achieve adequate coverage of the nodal regions. The radiation oncologists were then asked to contour the CTV for three model cases, according to the written guidelines. The imaging files were shared via the Advanced Technology Consortium, with each participant using his/her own treatment-planning system. Final consensus guidelines were further refined by conference calls. Two of the three cases were included on the web-based atlas as examples of postoperative anatomy, ROIs, and subsequent CTV definition using the margin expansions. The OARs are also shown for these cases.
Results
Guideline development
Contouring guidelines were initially developed for RTOG 0848 on the basis of field-border and treatment-field specifications from RTOG 9704. Building from these guidelines, based on bony anatomy landmarks, new contouring recommendations were developed on the basis of CT anatomy, including lymph node regions defined according to patterns of spread from surgical series and analyses of patterns of spread (6–8), anastomoses, and preoperative tumor location. An initial version of the CT-based guidelines was developed, and three representative CT datasets of patients with associated clinical information, including pathology reports, operative reports, and initial imaging information, from three different institutions were initially used as test cases to evaluate the consistency in CTV definitions among consensus panel members using the initial guidelines. Even among radiation oncologists specializing in GI cancers, there were discrepancies in volumes, particularly given the complicated abdomen anatomy after a Whipple procedure. Therefore, the consensus panel pursued identifying anatomic ROIs that could be reproducibly contoured by radiation oncologists and were easily distinguished on postoperative abdominal imaging. Thus, focus was changed to a step-by-step approach of identifying vascular structures, one anastomosis (pancreaticojejunostomy [PJ]), and the preoperative tumor volume, and subsequently defining appropriate margin expansions to create a CTV that encompassed the regional nodes and areas at risk for subclinical disease. Regions of interest were generally chosen on the basis of ease of identification, reproducibility on imaging studies, and their proximity to nodal regions and/or anastomoses at risk for micrometastatic disease. The vascular structures chosen were the celiac axis (to cover the celiac nodes), superior mesenteric artery (peripancreatic nodes and those in the superior mesenteric artery/vein distribution), the portal vein (porta hepatis nodes and the choledochojejunostomy), and the aorta (para-aortic, interaortocaval, and paracaval nodes). The additional structures, including the PJ and tumor bed, are areas at risk of harboring subclinical disease and can be identified using preoperative and postoperative cross-sectional imaging, aided by intravenous contrast and placement of clips by the surgeon. New written guidelines were developed and, after multiple iterations to determine the optimal margin expansions, the consensus panel contouring guidelines were developed for both 3D-CRT and IMRT target volumes and OARs.
Treatment volume and normal tissue guidelines
Gross tumor volume
By definition there is no gross tumor volume (GTV) at time of radiotherapy because the tumor has been resected (see description of preoperative tumor ROI below).
Clinical target volume
The postoperative CTV is the region at highest risk for residual subclinical tumor that can be encompassed in a reasonable radiotherapy field. It is necessary to review the surgical, pathologic, and preoperative axial imaging information at the time of treatment planning. The stepwise process for defining the CTV is described here.
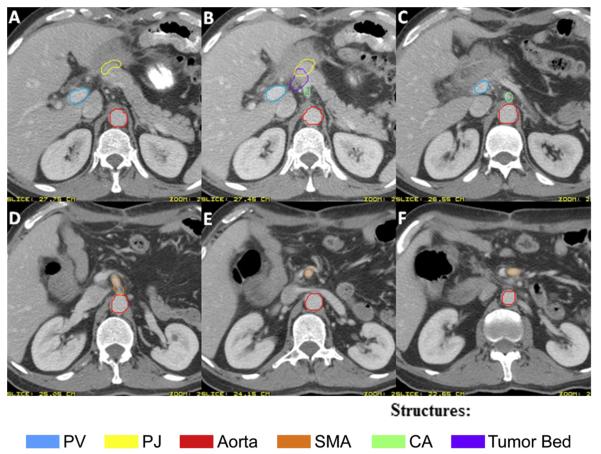
The following should be identified and targeted as specific ROIs (Fig. 2):
Celiac artery (CA): The most proximal 1.0–1.5 cm of the CA from the take-off from the aorta and should include up to the first branching.
Superior mesenteric artery (SMA): The most proximal 2.5–3.0 cm of SMA from the take-off from the aorta.
Portal vein (PV): The portion of the PV slightly to the right of, anterior to, and anteromedial to the inferior vena cava (IVC) should be contoured to cover the choledochal- or hepaticojejunostomy and porta hepatis nodes. Starting from below, contour the PV from just above its junction with the superior mesenteric vein (or splenic vein, whichever is more cephalad) and proceed cephalad crossing anterior to the IVC and up to, but not including, the slices where it starts to bifurcate into right and left branches. There is substantial anatomic variability: the PV bifurcation can occur extrahepatically or intrahepatically and may merge first with the superior mesenteric vein or with the splenic vein.
Preoperative tumor volume: Include surgical clips, if any, that were placed to delineate areas of concern intraoperatively, such as close margins or uncinate margin. The significance of surgically placed clips can vary and may be irrelevant for treatment planning unless specifically documented in the operative note-writing that clips were for specific tumor-related or radiotherapy planning-related purposes. Using preoperative imaging as a guide to identify the primary pancreatic tumor, contour the preoperative primary tumor volume onto the postoperative planning CT. The preoperative diagnostic or planning CT scan can be fused with the postoperative CT to facilitate identifying tumor bed location. The postoperative anatomy varies considerably from preoperative anatomy: the preoperative imaging was not obtained on a flat table or with appropriate patient positioning, the imaging sets may have been obtained in different phases of respiration, and there may be significant change in weight and body habitus.
Pancreaticojejunostomy (PJ): The PJ usually is identified by following the pancreatic remnant medially and anteriorly until the junction with the jejunal loop is noted. Alternatively, there may be a pancreaticogastrostomy (PG). If there is a PG instead of a PJ, the PG is not included because this would lead to more radiotherapy toxicity.
Aorta: Contour the aorta from the most cephalad contour of the CA, PV, or PJ (whichever is most cephalad) to the bottom of the L2 vertebral body. If the preoperative GTV contour extends to or below the bottom of L2, then contour the aorta toward the bottom of the L3 vertebral body as needed to cover the region of the preoperative GTV location.
Fig. 2.
Axial computed tomographic imaging of a patient after pancreaticoduodenectomy, demonstrating the regions of interest outlined in the contouring guidelines: portal vein (PV), pancreaticojejunostomy (PJ), aorta, superior mesenteric artery (SMA), celiac artery (CA), and the tumor bed.
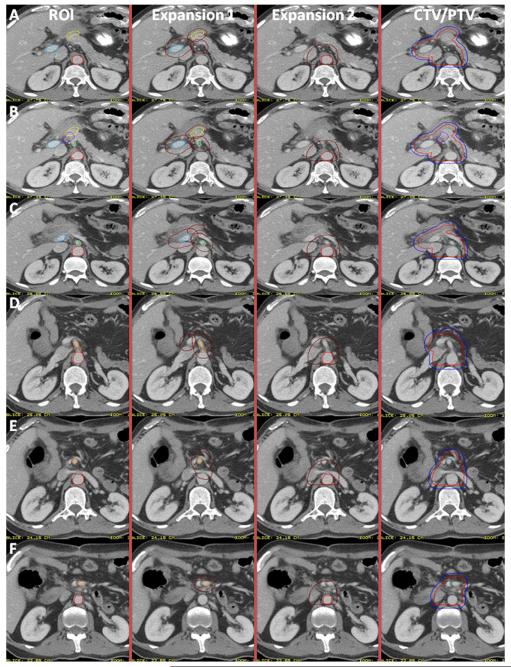
Steps taken with the above ROIs to generate the CTV (Figs. 3 and 4) are as follows:
The CA, SMA, and PV ROIs should be expanded by 1.0–1.5 cm in all directions. In most cases, 1.0-cm expansions will be sufficient.
The PJ should be expanded 0.5–1.0 cm in all directions.
The preoperative TV may be expanded by 0.5–1.0 cm in all directions or used without expansion.
The aorta ROI should be expanded asymmetrically to include the prevertebral nodal regions from the top of the PJ, PV, or CA (whichever is most superior) to the bottom of L2 (or L3 if GTV location low, see above section). Suggested approximate expansions for the aortic ROI are 2.5–3.0 cm to the right, 1.0 cm to the left, 2.0–2.5 cm anteriorly, and 0.2 cm posteriorly toward the anterior edge of the vertebral body. The lateral margins should cover the paravertebral nodes laterally but not include either kidney. These expansions will require clinical judgment. Occasionally, the PJ or PV expansion may extend cephalad to above the level of the CA. In that case the aorta expansion should be extended cephalad to the same level as the highest level (CT slice) of the PV or PJ expansion (whichever is more cephalad).
- The CTV should then be created by merging the above ROI/ROI expansions (CA, SMA, PV, GTV, aortic, PJ, preoperative TV) with the following:
- The posterior margin should follow the contour of the anterior aspect of the vertebral body without including more than 0.5 cm of the anterior vertebral body anterior edge.
- If the PJ cannot be identified, the CTV should be generated without it.
- If the CTV with the noted expansions protrudes into a dose-limited normal organ such as the liver or stomach, the CTV should be edited to be adjacent to the relevant structure.
Fig. 3.
Axial computed tomographic imaging demonstrating the margin expansions on the regions of interest (ROI) and the final clinical target volume/planning target volume (CTV/PTV).
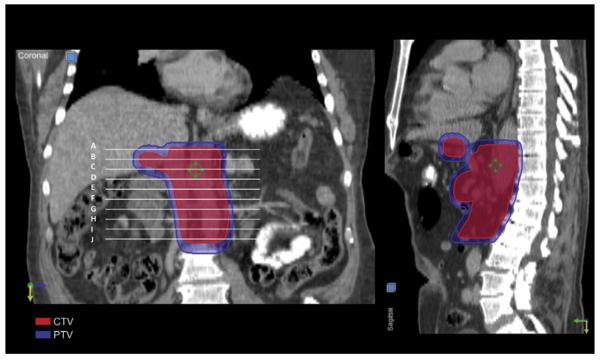
Fig. 4.
Coronal and sagittal views of the clinical target volume and planning target volume.
Normal organ dose–volume considerations
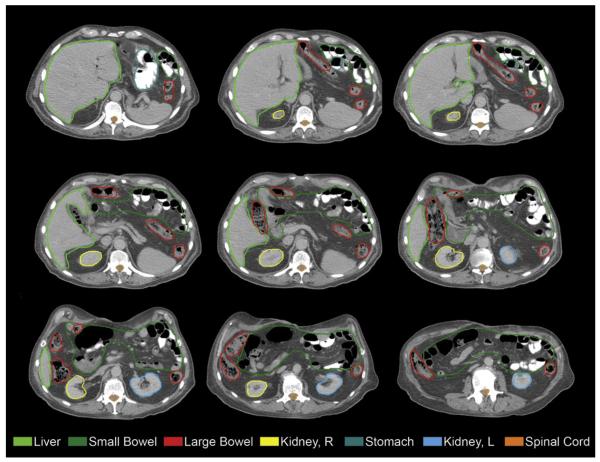
Definitions and dose constraints were also discussed by the consensus panel. The following normal structures were required to be contoured: left and right kidneys, liver, stomach, small intestine, large bowel, and spinal canal (Fig. 5). The kidneys, liver, and stomach should be contoured entirely to calculate a dose–volume histogram (DVH). The renal hilum should be excluded from the kidney contours to avoid overestimating the renal parenchymal volume. The small intestine from the jejunum to 2 cm below the lower extent of the CTV should also be contoured and should not include the entire abdominal cavity. Large bowel can be contoured separately. The spinal canal can be defined within the cranial–caudal extent of the CTV extending as necessary to calculate dose to the spinal cord resulting from entrance dose or exit dose of any treatment beam. Dose–volume constraints, based on a single-phase prescription dose of 5040 cGy for RTOG 0848, are outlined in Table.
Fig. 5.
Axial computed tomographic imaging of delineation of normal organs at risk.
Table.
Consensus panel normal tissue contraints
| Structure | Constraints |
|---|---|
| Kidney (left and right) | For three-dimensional conformal plans in patients with two normally functioning kidneys, the dose to 50% of the right kidney and 65% of the left kidney must be <18 Gy. For IMRT planning, mean dose to bilateral kidneys must be <18 Gy. If only one kidney is present, 15% of the volume of that kidney must receive <18 Gy and 30% must receive <14 Gy. |
| Liver | Mean liver dose must be ≤30 Gy. |
| Stomach and small intestine | Maximum dose ≤58 Gy; 10% of each organ volume must receive <56.0 Gy, 15% of the volume of each organ must receive <52.0 Gy. |
| Spinal canal | Maximum dose to a point that is 0.03 cm3 must be <50 Gy. |
Abbreviation: IMRT = intensity-modulated radiotherapy.
Conclusions
Local failure continues to be a major challenge in the management of pancreatic cancer. Adjuvant chemoradiation with concurrent 5-FU integrated with gemcitabine has been established as a standard therapy for resectable pancreatic cancer on the basis of a Phase III randomized trial (2). However, subsequent randomized trials have not shown a survival benefit to adjuvant radiotherapy for resected pancreatic cancer (1, 4). These studies have primarily used older treatment techniques with large field sizes and two-dimensional treatment planning. The results are therefore subject to scrutiny because of the poor radiotherapy quality. Moreover, suboptimal radiation doses using split courses were prescribed in these studies to reduce toxicity.
Modern radiotherapy has increasingly used conformal fields and dose escalation to enhance tumor control. Three-dimensional conformal radiotherapy or IMRT may overcome some of these restrictions. Recent publications on the use of IMRT for pancreatic cancer have demonstrated that there were no treatment breaks attributable to GI or skin toxicity (9). Compared with conventional radiotherapy, IMRT reduced the mean dose to the liver, kidneys, stomach, and small bowel. Intensity-modulated radiotherapy was well tolerated, with 80% experiencing Grade ≤2 acute upper GI toxicity (10). Further dosimetric studies have shown a benefit to IMRT in reducing doses to abdominal organs over standard 3D-CRT (11, 12). Moreover, in the adjuvant setting, IMRT has been shown to reduce GI toxicity compared with conventional 3D-CRT planning (13). Among 46 patients treated with adjuvant chemoradiation using IMRT, the rates of Grade 3–4 diarrhea, nausea/vomiting, and anorexia were significantly less than on RTOG 97-04, confirming the benefit of more focal radiotherapy fields that spare the normal abdominal tissues. These publications notwithstanding, it is often possible and appropriate to treat the desired CTVs and PTVs with 3D-CRT to 5040 cGy without incurring unacceptable acute toxicity or risk of late toxicity based on standard DVH considerations. Thus, RTOG 0848 allows both 3D-CRT and IMRT planning of the adjuvant radiotherapy among the chemoradiation arm.
The primary endpoint of RTOG 0848 is whether the use of concurrent 5-FU and radiotherapy further enhances survival in the adjuvant setting for patients without disease progression after five cycles of gemcitabine-based chemotherapy. More than 900 patients will be enrolled on RTOG 0848 to determine whether chemotherapy followed by chemoradiation will improve overall survival with an improvement in median survival time from 17 months to 22.5 months. There will be variability in the radiation fields because patients will be accrued from a wide range of radiation oncology centers, academic and nonacademic, large and small, where practices vary significantly. Variations in radiation treatment planning and execution have been documented in previous adjuvant trials for GI cancers. In Intergroup 0116, a large gastric cancer adjuvant therapy trial that enrolled 556 patients who were randomized to surgery with or without adjuvant chemo-radiation (14), radiotherapy plans also required approval as part of the quality assurance before initiating therapy. Of the initially submitted radiation plans, 35% had major or minor errors, and 6.5% contained major deviations in a second review after radiotherapy delivery. The poor compliance with the protocol treatment-planning recommendations may have reflected the unfamiliarity with the postoperative abdominal anatomy but may have also been in part due to concerns about potential toxicity associated with large fields (15).
In RTOG 0848, familiarity with postoperative abdominal anatomy is also essential because the treatment planning is CT based. To overcome some of the anticipated variability in knowledge of abdominal anatomy, particularly postoperatively, the consensus guidelines specify anatomic structures (ROIs) that can be easily identified on axial imaging. Examples are also provided through a web-based atlas that allows clinicians to follow the stepwise ROI delineation, margin expansion, and normal tissue contouring (http://www.rtog.org/CoreLab/ContouringAtlases/PancreasAtlas.aspx).
Real-time assessment of radiotherapy quality is also necessary to improve the actual delivery of radiation therapy on protocol. In the RTOG 9704 trial of adjuvant chemoradiotherapy for resected pancreatic cancer, submission of materials for prospective quality assurance of radiotherapy was required, including pathology report, operative note, and preoperative imaging in conjunction with treatment-planning images showing isocenter, field edges, and blocking. These data were requested to be submitted to RTOG headquarters for pretreatment review and possible correction; however, although treatment-planning films were submitted before treatment, other necessary documentation did not arrive in time. Thus, the attempted prospective review and recommendations for changes in the treatment fields, which were not absolutely required for eligibility, often did not occur (2). Scoring of the adherence to protocol specifications occurred after therapy but before trial analysis and without knowledge of individual patient treatment outcomes. Among 416 analyzable patients, 48% were scored as having received “less than per-protocol requirements.” The most common variation from protocol requirements was in either field placement or field size. On the basis of this analysis, it was clear that rapid review of radiotherapy fields would be necessary on RTOG 0848, particularly because this study had moved toward CT-based planning and allowed for more conformal fields using 3D-CRT and IMRT. The potential for variation in target coverage is greater without the stringent field borders set by bony anatomy. Thus, RTOG 0848 requires evaluation of preoperative imaging, operative and pathology reports, treatment-planning CT scans, all contours including ROIs, normal structures, CTV, and PTV, as well as dosimetric data before initiating radiotherapy. These data are reviewed by the radiation oncology principal investigators, and feedback is given to the treating sites, if necessary, in an attempt to achieve closer to 100% adherence to protocol radiotherapy guidelines. Normal tissue OARs were also defined for the RTOG 0848 study and presented in the atlas to standardize the contouring for these critical normal structures. This will help to standardize the calculation of DVHs for OARs and allow better assessment of any deviations from protocol-specified dose constraints.
As with any recommendations, they must be individualized to the particular patient. Both the CTV and OAR contours should be adjusted according to the patient’s anatomy and physician discretion. However, these guidelines serve as a starting point to help develop appropriate fields in the setting of very difficult anatomy in the postoperative abdomen and to ensure that areas at risk are included in the field while normal tissues are spared. Real-time central review for patients enrolled on RTOG 0848 will also help to identify deficiencies in contouring and treatment planning and, by reporting back to individual practitioners regarding modifications in contouring, to further educate the radiation oncology community on the appropriate definition of the CTV after a Whipple procedure for pancreatic cancer.
The benefit of adjuvant chemoradiotherapy over chemotherapy will be evaluated in RTOG 0848, and this study will define the role of radiotherapy in resectable pancreatic cancer for future generations of radiation oncologists. Thus, it is imperative that the quality of the radiotherapy is optimal and that the outcomes of the study will not be called into question by variability in the radiotherapy itself. Moreover, the improvements in the quality of radiotherapy, by using CT-based planning, 3D-CRT, or IMRT treatment planning, and more standardized contouring guidelines, may tip the balance in favor of adjuvant radiotherapy by allowing more conformal treatments that have reduced toxicity and improved targeting of the areas at risk.
Footnotes
Conflict of interest: none.
References
- 1.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 2.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 3.Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 5.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704—a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2012;82:809–816. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Brunner TB, Merkel S, Grabenbauer GG, et al. Definition of elective lymphatic target volume in ductal carcinoma of the pancreatic head based on histopathologic analysis. Int J Radiat Oncol Biol Phys. 2005;62:1021–1029. doi: 10.1016/j.ijrobp.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Brown MW, Ning H, Arora B, et al. A dosimetric analysis of dose escalation using two intensity-modulated radiation therapy techniques in locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:274–283. doi: 10.1016/j.ijrobp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Spalding AC, Jee KW, Vineberg K, et al. Potential for dose-escalation and reduction of risk in pancreatic cancer using IMRT optimization with lexicographic ordering and gEUD-based cost functions. Med Phys. 2007;34:521–529. doi: 10.1118/1.2426403. [DOI] [PubMed] [Google Scholar]
- 13.Yovino S, Poppe M, Jabbour S, et al. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys. 2011;79:158–162. doi: 10.1016/j.ijrobp.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalley SR, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report: Rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:283–293. doi: 10.1016/s0360-3016(01)02646-3. [DOI] [PubMed] [Google Scholar]
- 15.Chung HT, Shakespeare TP, Wynne CJ, et al. Evaluation of a radiotherapy protocol based on INT0116 for completely resected gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1446–1453. doi: 10.1016/j.ijrobp.2004.01.001. [DOI] [PubMed] [Google Scholar]