Abstract
Deficiency of ZNF341, a transcription factor featuring 12 Cys2His2 zinc fingers that regulates the expression and autoinduction of STAT3 (signal transducer and activator of transcription 3), results in hyper–immunoglobulin E syndrome and defective T helper 17 cell differentiation in humans.
Introduction
By investigating patients from 10 families who present with symptoms resembling monogenic autosomal-dominant hyper-IgE syndrome (AD-HIES) but lack mutations in STAT3, two groups, Beziat et al1, and Frey-Jacobs et al2, identify a novel player in the pathway that regulates Th17 differentiation, and levels of IgE. The identification of the STAT3 mutations in humans with monogenic AD-HIES3, has emphasized the importance of this transcription factor in regulating Th17 cells, as well as the function of these cells in human health and disease. In humans, AD-HIES (also referred to as Job’s Syndrome) is a rare autosomal dominant genetic disorder affecting <1 in 1 million individuals. Major symptoms include immunodeficiency along with elevated IgE, and patients suffer from recurrent bacterial and fungal infections, particularly candida infections in the lung, along with eczema3. Patients with AD-HIES also exhibit skeletal abnormalities. Greater than 80% of cases of AD-HIES, are a result of mutations in the gene for STAT3, a transcription factor that is a major regulator of T cell responses, particularly Th17 responses3. These mutations in STAT3 usually generate dominant acting negative mutants that affect the function of WT STAT3. The critical role for STAT3 in negatively regulating Th2 responses and accompanying elevated IgE may also underlie the Th2 bias observed in AD-HIES patients.
Cases of syndromes with similarity to AD-HIES have been described where the genetic alteration is unknown, but that are likely act in the STAT3 pathway. Indeed, similar symptoms, such as those observed outside the immune system, are observed in individuals carrying mutants in genes that act upstream of STAT3, such as cytokines and cytokine receptors that utilize STAT3 for signals (e.g. leukemia inhibitory factor receptor (LIFR), IL-11R, and the gp130 common subunit of the IL-6 receptor family)4. Other mutations in genes such as DOCK82 can also lead to similar symptoms as AD-HIES (e.g. hyper-IgE syndrome or HIES), although these are autosomal recessive (AR-HIES). Notably DOCK8 regulates STAT3 phosphorylation and Th17 differentiation5, further highlighting the importance of STAT3 as a central regulator of this syndrome.
The importance of STAT3 in regulating Th17 differentiation underlies the phenotypes and symptoms of the STAT3 mutants. Differentiation of naïve T cells to Th17 cells depends on signaling via the T cell receptor (driven by antigen) and accompanying cytokine signals, usually IL6 and TGFβ, plus or minus IL1β6 (Fig. 1). The critical role for STAT3 downstream of IL6 makes this pathway particularly sensitive to disrupting Th17 differentiation by STAT3 mutations. Most work examining the role of STAT3 in regulating Th17 differentiation focus on the induction of phosphorylation of STAT3 by upstream JAK kinases triggered by the IL6 receptor. These JAKs, JAKs1 and 2, and TYK2, interact with the IL6 receptor common chain, gp130, and when triggered by IL6, are activated and phosphorylate STAT3 on tyrosine 705. This phosphorylated tyrosine allows STAT3 to dimerize via its SH2 domain and translocate to the nucleus, where it can regulate genes involved in Th17 differentiation, including the critical regulator of Th17 cells, Rorγt6(Fig. 1). While this is a major regulatory pathway for STAT3, this signaling can also be potentially regulated by the expression of STAT3. Indeed, STAT3 is involved in a positive feed forward loop in cooperation with CREB, that autoregulates its own expression7. However, despite its importance, few studies have examined how the expression of STAT3 is actually regulated. ZNF382, a zinc finger containing transcription factor, has been reported to down regulate STAT3, possibly via heterochromatin silencing8. In addition, SMAD3 and its adaptor SPTBN1 are reported to downregulate STAT3 expression9.
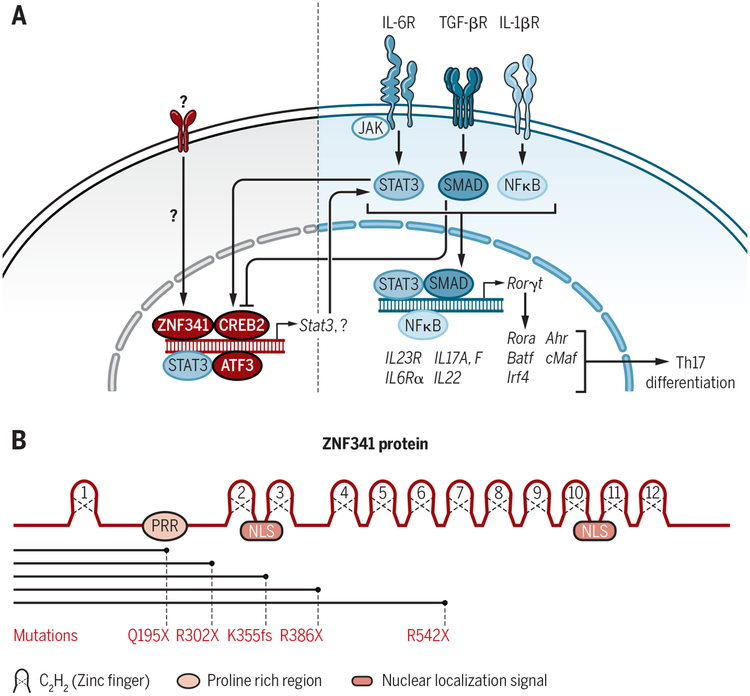
Figure 1. ZNF341, STAT3 and Th17 differentiation, a more complex song.
A) Th17 differentiation requires TCR signals (not shown) along with cytokines IL-6, TGF-β, and/or IL-1β. Signaling through the IL-6 receptor triggers activation of JAK2, which phosphorylates STAT3, driving its dimerization and nuclear localization. STAT3 cooperates with other transcription factors such as SMADs to drive expression of critical genes required for Th17 differentiation. ZNF341 regulates STAT3 expression and its autoinduction during Th17 differentiation. (B) Key structural motifs within ZNF341 and the location of mutations in HIES patients identified by Beziat et al and Frey-Jacobs et al1,2.
Enter Beziat et al and Frey-Jacobs et al, who analyzed 10 families with reported phenotypes resembling AD-HIES1,2. The probands from these 10 families are largely from the middle east (Israel by way of Sudan, Turkey, Iran, and Lebanon) with one Afro-Caribbean, and all carried mutations in ZNF341, a putative transcription factor containing 12 C2H2 zinc finger domains with previously unknown function. The mutations identified in these studies in ZNF341 are homozygous nonsense mutations that reduce expression partially or completely of ZNF341, and which affect the levels of STAT3 in the lymphoid populations. As a result the patients reported higher proportions of naive CD4+ T cells, and lower central memory CD4+ and CD8+ T cells, as well as MAIT cells, very closely resembling what is seen in AD-HIES caused by STAT3 dominant negative mutations.
While the function of ZNF341 is unknown, it is likely acting as a transcriptional regulator of at least STAT3, and a number of other genes including STAT1, since it can bind to the STAT3 promoter, as well as activate transcription of a STAT3 reporter plasmid. Furthermore, ectopic expression of ZNF341 in ZNF341 deficient cell line from one of the carriers was able to rescue expression of STAT1, but surprisingly not STAT3, suggesting more complex relationship. It is possible that ZNF341 acts along with STAT3 to allow autoregulation of STAT3, since a major phenotype in these patients is a lack of Th17 cells, defective Th17 differentiation and IL22 producers from naïve CD4+ T cell, accompanied by reduced STAT3 phosphorylation secondary to reduced STAT3 expression and lack of induction of STAT3. Furthermore, while WT ZNF341 is able to enter the nucleus and bind to the STAT3 promoter, mutants that delete the first nuclear localization sequence (i.e. R302*), is either unable to enter the nucleus are unable to bind to chromatin or the STAT3 promoter (e.g. R386*). The reported mutations result in deletion of 9–12 of the C2H2 zinc finger domains, as well as disrupting one of the two nuclear localization sequences, suggesting that both of these domains are critical for this function of ZNF341.
While ZNF341 was also found to interact with the STAT1 promoter, and STAT1 expression was enhanced by overexpression of STAT1 in ZNF341 deficient cells, less effect was seen on the expression of STAT1 in the patients with the mutations. It is however, likely that given its role in regulating the expression of STAT3, ZNF341 may be found to regulate STAT1 under specific circumstances. This relationship between ZNF341 and STAT1 may be interesting in light of the observation that patients with STAT1 gain of function mutants have similar decreased Th17 cells and defects in anti-fungal immune responses as well in AD-HIES10.
The identification of ZNF341 adds a new play and twist to the regulation of STAT3 signaling, and of Th17, and presumably other immunological responses that depend on STAT3 expression, or autoinduction. More work needs to be done really understand the role of ZNF341 in this pathway, since there is no clear induction of STAT3 when ZNF341 is overexpressed, and it is likely that the regulation of more complex, perhaps in cooperation with STAT3, or other upstream signals. On the basis of on the behavior of the mutant, the C2H2 zinc fingers, particular the 4–12 fingers, cooperatively regulate transcription since mutants R386* and Y542* exhibit partial activity when overexpressed (although the proteins may be unstable given the lack of expression in patients). It is also not clear what signals regulate ZNF341 expression and or nuclear translocation and transcriptional activity. It would also be quite informative to examine whether ZNF341 regulates other biological pathways regulated by other cytokines that utilize STAT3, including IL10, IL11, IL17, IL21 and IL22. The identification of a new form of HIES caused by ZNF341 mutations should catalyze further work to better understand how ZNF341 regulates immune responses.
Acknowledgements
A.A is supported by grants from the National Institutes of Health (AI120701, AI126814, AI129422) and Howard Hughes Medical Institute (HHMI Professorship to A.A.).
Footnotes
Conflict of Interest Disclosure
The author declares no competing financial interests.
References:
- 1.Béziat V, Li J, Lin J-X, Ma CS, Li P, Bousfiha A, Pellier I, Zoghi S, Baris S, Keles S, Gray P, Du N, Wang Y, Zerbib Y, Lévy R, Leclercq T, About F, Ing Lim A, Rao G, Payne K, Pelham SJ, Avery DT, Deenick EK, Pillay B, Chou J, Guery R, Belkadi A, Guérin A, Migaud M, Rattina V, Ailal F, Benhsaien I, Bouaziz M, Tanwir H, Chaussabel D, Marr N, El Benna J, Grimbacher B, Wargon O, Bustamante JC, Boisson B, Müller-Fleckenstein I, Fleckenstein B, Chandesris M-O, Titeux M, Fraitag S, Alyanakian M-A, Leruez-Ville M, Picard C, Meyts I, Di Santo JP, Hovnanian A, Somer A, Ozen A, Rezaei N, Chatila T, Abel L, Leonard WJ, Tangye SG, Puel A, Casanova J-L, A recessive form of Hyper IgE Syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci. Immunol 3, eaat4956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey-Jakobs S, Hartberger JM, Fliegauf M, Bossen C, Wehmeyer ML, Neubauer JC, Bulashevska A, Proietti M, Fröbel P, Nöltner C, Yang L, Rojas-Restrepo J, Langer N, Winzer S, Engelhardt KR, Glocker C, Pfeifer D, Klein A, Schäffer AA, Lagovsky I, Lachover-Roth I, Béziat V, Puel A, Casanova J-L, Fleckenstein B, Weidinger S, Kilic SS, Garty B-Z, Etzioni A, Grimbacher B, ZNF341 controls STAT3 expression and thereby immunocompetence. Sci. Immunol 3, eaat4941 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mogensen TH “Primary Immunodeficiencies with Elevated IgE”. Int Rev Immunol 35: 39–56, (2016) [DOI] [PubMed] [Google Scholar]
- 4.Hillmer EJ, Zhang H, Li HS, Watowich SS “STAT3 signaling in immunity”. Cytokine Growth Factor Rev 31:1–15 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keles S, Charbonnier LM, Kabaleeswaran V, Reisli I, Genel F, Gulez N, Al-Herz W, Ramesh N, Perez-Atayde A, Karaca NE, Kutukculer N, Wu H, Geha RS, Chatila TA “Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes TH17 cell differentiation” J Allergy Clin Immunol 138:1384–1394.e2 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockinger B, Veldhoen M “Differentiation and function of Th17 T cells” Curr Opin Immunol 19: 281–6 (2007) [DOI] [PubMed] [Google Scholar]
- 7.Narimatsu M, Maeda H, Itoh S, Atsumi T, Ohtani T, Nishida K, Itoh M, Kamimura D, Park SJ, Mizuno K, Miyazaki J, Hibi M, Ishihara K, Nakajima K, Hirano T “Tissue-specific autoregulation of the stat3 gene and its role in interleukin-6-induced survival signals in T cells” Mol Cell Biol 21:6615–25 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Geng H, Cheng SH, Liang P, Bai Y, Li J, Srivastava G, Ng MH, Fukagawa T, Wu X, Chan AT, Tao Q, “KRAB zinc finger protein ZNF382 is a proapoptotic tumor suppressor that represses multiple oncogenes and is commonly silenced in multiple carcinomas “Cancer Res 70: 6516–26 (2010) [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Yao Z, Bhuvaneshwar K, Gusev Y, Kallakury B, Yang S, Shetty K, He AR, “Transcriptional regulation of STAT3 by SPTBN1 and SMAD3 in HCC through cAMP-response element-binding proteins ATF3 and CREB2” Carcinogenesis 35: 2393–403 (2014) [DOI] [PubMed] [Google Scholar]
- 10.Okada S, Puel A, Casanova JL, Kobayashi M “Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity” Clin Transl Immunology 5: e114 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]