Abstract
Electrically conducting polymers such as polyaniline, polypyrrole, polythiophene and their derivatives (mainly aniline oligomer and poly(3,4-ethylenedioxythiophene)) with good biocompatibility find wide applications in biomedical fields including bioactuators, biosensors, neural implants, drug delivery systems and tissue engineering scaffolds. This review focuses on these conductive polymers for tissue engineering application. Conductive polymers exhibited promising conductivity as bioactive scaffolds for tissue regeneration and their conductive nature allows cells or tissue cultured on them to be stimulated by electrical signals. However, their mechanical brittleness and poor processability restrict their application. Therefore, conductive polymeric composites based on conductive polymers and biocompatible biodegradable polymers (natural or synthetic) were developed. The major objective of this review is to summarize the conductive biomaterials used in tissue engineering including conductive composite films, conductive nanofibers, conductive hydrogels, and conductive composite scaffolds fabricated by various methods such as electrospinning, coating or deposition by in situ polymerization. Furthermore, recent progress in tissue engineering applications by using these conductive biomaterials including bone tissue engineering, muscle tissue engineering, nerve tissue engineering, cardiac tissue engineering, and wound healing application are discussed in detail.
Keywords: conducting polymers, tissue engineering, regenerative medicine, scaffolds, electrical stimulations, conductive biomaterials, bioactive scaffolds
Graphical Abstract
1. Introduction
Organ failure and tissue loss account for roughly half of the medical cost in the United States, resulting in about 8,000,000 surgical procedures and 40–90 million hospital days/year for treatment.1, 2 Tissue engineering or regenerative medicine is a multidisciplinary and interdisciplinary field that aims to develop functional biological substitutes that restore, maintain or improve tissue function by combining a scaffold, cells, and biological molecules.3, 4 Scaffolds combine several functions including biocompatibility with host tissues, tunable biodegradation rate and non-toxic degradation products, and suitable porosity for the transportation of nutrients and wastes, mechanical strength and sterilization.5-9 Polymers possess great processing flexibility, biocompatibility and biodegradability are one of the most widely used scaffolding biomaterials. Natural polymers such as chitosan, gelatin, collage, alginate and so on and synthetic polymers including polylactide (PLA), poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), poly(glycerol sebacate), and polyurethane (PU) are the dominant biomaterials as scaffolds for tissue engineering.9−15 Biomaterials play a pivotal role during tissue repair. They not only serve as matrices for cellular adhesion, but also should improve the interactions between the biomaterials and the seeding cells, and also further control the cellular activities, such as cell proliferation and differentiation, as well as neo-tissue genesis.16, 17 It is nevertheless still a challenge to develop bioactive biomaterials which can enhance cell proliferation and guide differentiation of cells.
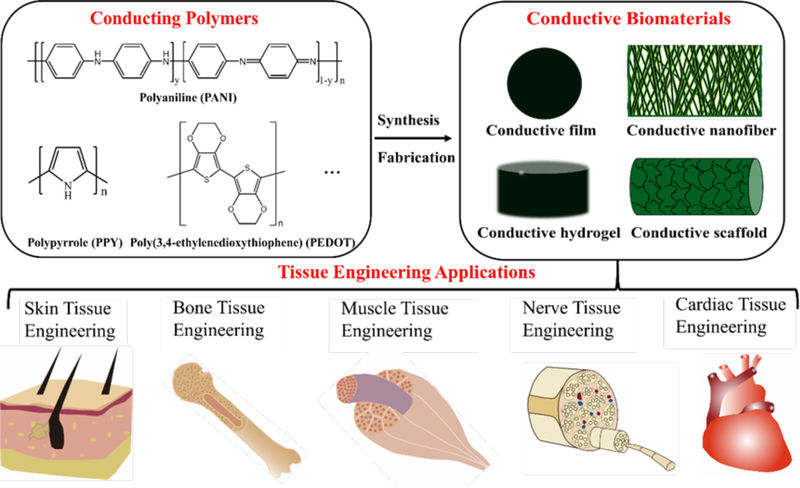
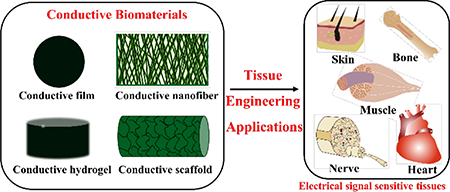
Conducting biomaterials based on carbon nanotubes, carbon nanowires, graphene, and metallic particle (e.g. gold nanoparticle) have been widely investigated in biosensor and bone tissue engineering applications due to their high electrical conductivity and tensile strength in recent years.18−25 However, drawbacks including the non-biodegradability, issues of uncertain long-term in vivo toxicity, and the inhomogeneous distribution of the conducting particles in composite system restricted their widespread and effective use. Conducting polymers (CPs) as a new generation of organic materials exhibited electrical and optical properties resembling metals and inorganic semiconductors, but also show properties including ease of synthesis and flexibility in processing.26−30 The soft nature of organic conductive polymers provides better mechanical compatibility and structural tunability with cells and organs than conventional electronic inorganic and metal materials. CPs such as polyaniline (PANI), polypyrrole (PPY), and polythiophene and their derivatives and composites are attractive biomaterials due to their biocompatibility, facile synthesis and simple modification, and their ability to electronically control a range of physical and chemical properties by (i) surface functionalization techniques and (ii) the use of a wide range of molecules that can be entrapped or used as dopants.31, 32 These advantageous properties make them attractive in many biomedical applications, including drug-delivery systems, artificial muscles, bioactuators, biosensors, neural recording, and tissue-engineering.33, 34 CPs are not only biocompatible, but also can promote cellular activities, including cell adhesion, migration, proliferation, differentiation and protein secretion at the polymer–tissue interface with or without electrical stimulations.35−38 Biomaterials based on CPs are especially useful in the engineering of electrical sensitive tissues such as skeletal muscles, cardiac muscles, nerves, skins, and bones.39, 40 It was found that biomaterials containing CPs can significantly enhance cell adhesion and proliferation of a series of cells such as L929 fibroblasts, C2C12 myoblasts, PC12 cells, RSC96 Schwann cells, H9c2 cardiac cells, primary cardiomyocytes, MC3T3-E1 cells, and mesenchymal stem cells.41−43 This review will discuss CPs as bioactive biomaterials for tissue engineering applications. In the first part, we focus on various kinds of conducting biomaterials fabricated by different techniques, and then we summarize the application of these conductive biomaterials in tissue engineering including bone, skeletal muscle, nerve, cardiac, and wound healing as depicted in Figure 1.
Figure 1.
Conducting polymers and conductive biomaterials and their tissue engineering applications.
2. Fabrication of conductive biomaterials for tissue engineering
2.1. Pure conducting polymer films for tissue engineering
Conducting polymers (e.g. PANI, PPY and polythiophene) support the in vitro adhesion, proliferation and differentiation of a large variety of cell types,41−43 indicating that they are cytocompatible.44 Furthermore, CPs’ good biocompatibility was also confirmed in animal models. Several results suggested that CPs showed no significant long-term effect in vivo, or induced only a minimal tissue response.45, 46 CPs can be chemically or electrochemically synthesized. Electrochemically synthesized conducting polymers are usually in the form of films on the electrode, which can be used for cell culture.32 For example, PPY membrane at nanoscale was electrically deposited on indium-tin oxide glass slide. The nanoscaled PPY particles with an average diameter of 62 nm were distributed uniformly. Pre-osteoblasts MC3T3-E1 cells were cultured on PPY membrane under both electrical and mechanical stimulation, and the results in terms of cell proliferation and gene expression of collagen-I demonstrated that combined electrical or mechanical stimulus greatly promoted the proliferation and differentiation of MC3T3-E1 cells compared to single stimulation, indicating that nano-PPY membrane may offer a way to stimulate for bone tissue repair.47 In situ polymerization was used to prepare a layer-by-layer self-doped sulfonated PANI copolymers on a polyethylene teraphthalate film. The cultures of bone marrow stromal cells (BMSCs) and pre-osteoblast cells (MC3T3-E1) indicated that the cell attachment and cell growth increased with increasing degree of sulfonation. The von Kossa staining of both the cells revealed that mineralization of the cells under the electrical stimulation was dramatically increased compared to their respective controls without electrical stimulation.48
2.2. Conducting blends or composite films for tissue engineering
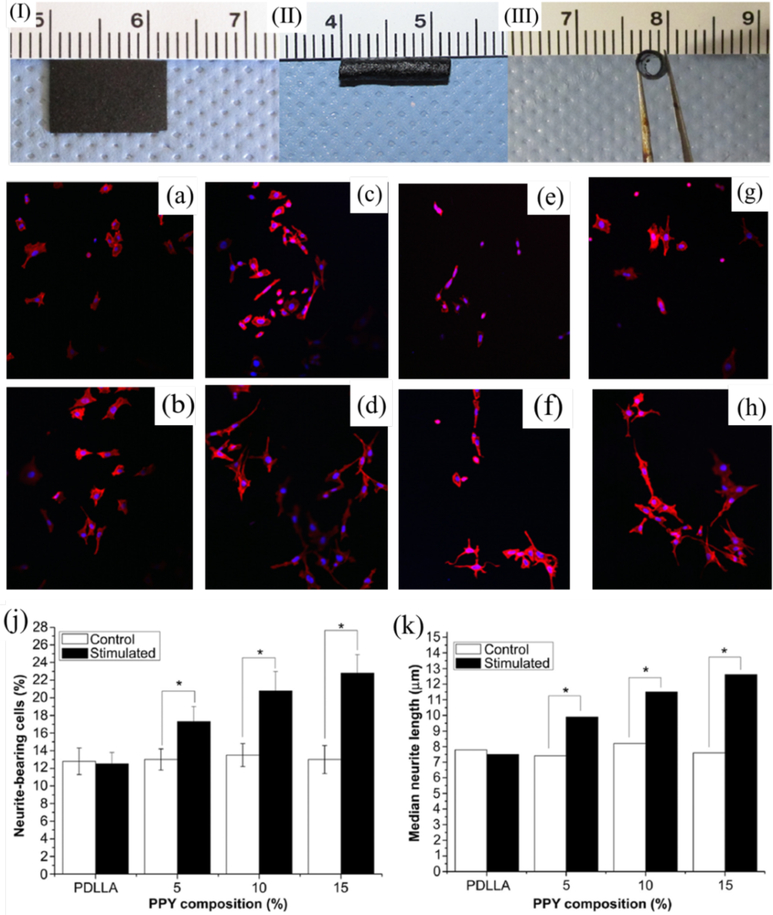
CPs are very brittle, and it is very difficult to fabricate pure conducting polymer film from CPs.49 Therefore, blending CPS with other degradable polymers is widely used as conductive biomaterials for tissue engineering. Synthetic and natural polymers including PLA, PLGA, PCL, chitosan, and silk fibroin were used to blend with CPs such as PANI and PPY.37, 50, 51 For example, a conductive PPY/PCL film was developed by first immersing PCL film into polystyrene sulfonic acid and pyrrole DI water mixture. Ferric chloride was then added to the mixture as oxidant resulting in the interpenetrating network of PPY coated PCL conductive film. The film showed a resistivity of 1.0 ± 0.4 kΩ cm similar to the native cardiac tissue.52 Conductive PPY/poly(D, L-lactic acid) (PDLLA) composite films and nerve conduits were fabricated using emulsion polymerization and dip coating methods.53 The photographic micrographs and scanning electron images of the PPY/PDLLA films and conduits were shown in Figure 2 (I, II, and III).
Figure 2.
Image of the PPY/PDLLA film (I) and the PPY/PDLLA nerve conduit (II, III). Fluorescent images of PC12 cells labeled for actin (red) and nuclei (blue). (a), (c), (e), and (g) are PDLLA, 5% PPY/PDLLA, 10% PPY/PDLLA, and 15% PPY/PDLLA without electrical stimulations. (b), (d), (f), and (h) are PDLLA, 5% PPY/PDLLA, 10% PPY/PDLLA, and 15% PPY/PDLLA with electrical stimulations of 100 mV for 2 h. Scale bar: 200 mm. (j) Percentage of neurite-bearing PC12 cells on conductive composite films with different PPY content (n= 4, *p < 0.05). (k) Median neurite length on PPY/PDLLA composite films with varying PPY composition. Reprinted from reference 53, Copyright (2013), with permission from Elsevier.
Due to the segmented-block structure, polyurethane (PU) usually exhibited tunable mechanical properties, physical properties, biological properties, and good biocompatibility.54 An electrically conducting composite based on polyurethane and PPY was developed. The conducting composite film was prepared by in situ polymerization of pyrrole in the PU emulsion mixture, and then the obtained composite was pressed into film and dried in a vacuum chamber. Increasing the mass ratio of PPY to PU, the stiffness increased while the maximum elongation length decreased.55
Surface property and morphology play an important role in modulating cell behavior. 56−58 Electrically conductive silk film-based nerve tissue scaffolds with micrometer-scale grooves were prepared. The silk solution was cast on polydimethylsiloxane substrates with micrometer-scale grooves on the surfaces, and then dried to impart β-sheets formation of silk. Then the obtained surfaces were immersed in an aqueous solution of pyrrole, polystyrenesulfonate and iron chloride to generate an interpenetrating network of PPY and polystyrenesulfonate in the silk matrix. The formed conductive silk sheet had a resistance of 124 ± 23 kΩ square−1. The micrometer-scale grooves on the film surface are similar to the natural topography in the extracellular matrix. 59
2.3. Conducting copolymer films for tissue engineering
CPs were widely used as biomaterials in tissue engineering, but their non-degradability limits the in vivo applications.60 Although CPs are not inherently biodegradable, the use of aniline/pyrrole based copolymers functionalized with hydrolysable groups endowed the resulting materials with similar electroactivity as CPs and the additional benefit of being erodible (biodegradable).61, 62 Aniline/thiophene oligomers such as aniline trimer, aniline tetramer and aniline pentamer with well-defined structure, good electroactivity, and renal clearance property were used to synthesize conductive copolymers as degradable conductive biomaterials.17, 63−68 For example, electroactive polyurethane/siloxane/ aniline tetramer composite film was developed via sol-gel reaction of methoxysilane end-functionalized urethane prepolymers composed of castor oil, ricinoleic methyl ester and methoxysilane functional aniline tetramer moieties. The introduction of inorganic siloxane domain into the polyurethane matrices could enhance the film’s mechanical properties while aniline tetramer could give the material electroactivity and antioxidant property. The obtained films presented appropriate tensile strength at both dry and hydrated states, proper water absorption and water vapor transmission rate for wound dressing application.69 Our group developed a series of conductive and degradable films based on aniline oligomers and other degradable polymers for tissue engineering applications.70, 71 A series of stretchable electroactive polyurethane-urea (PUU) elastomers were synthesized based on polylactide, poly(ethylene glycol), and aniline trimer. The films showed a strain at break higher than 1600% and a modulus close to soft tissues. The sphere-like hard domains self-assembled from aniline trimer segments provided the crucial physical interactions to endow the films with novel super elastic properties. After blending conductive fillers such as PANI nanofibers and nanosized carbon black with the copolymers, the films could achieve a high electric conductivity of 0.1 S/cm.71
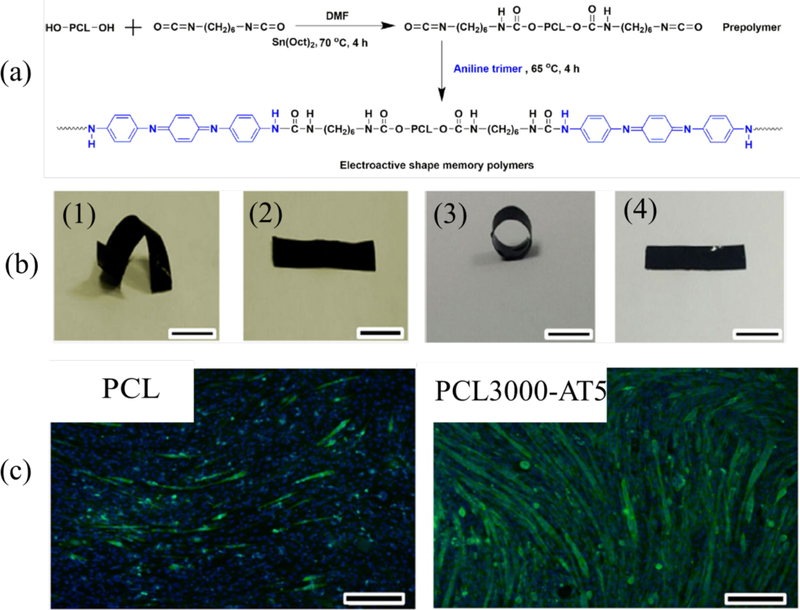
Conductive biomaterials made from shape memory polymer (ESMP) offer new possibility to minimize invasive surgery in implanting these biomaterials into the human body.73, 74 We synthesized a series of flexible degradable electroactive shape memory polymer (ESMP) films with tunable switching temperature by using hexamethylene diisocyanate as crosslinker to crosslink PCL segment and electroactive amino capped aniline trimer segment as shown in Figure 3a.72 The biodegradable ESMP film exhibited high elasticity, tunable recovery temperature around body temperature, and good shape memory properties (Figure 3b). Furthermore, dual bioactive ESMP was synthesized.75 We also prepared a series of dopamine-incorporated polyurethane based ESMPs for skeletal muscle repair. The poly(citric acid-co-polycaprolactone-co-dopamine) (CA-PCL-DA) pre-polymer was synthesized by melting polymerization of citric acid, polycaprolactone, and dopamine. The HDI-aniline hexamer was introduced into the CA-PCL-DA pre-polymer by using HDI as crosslinker. The obtained elastomers showed good electroactivity, elongation and body temperature triggered shape memory property. 75
Figure 3.
(a) Synthesis scheme of PCL3000-AT (the molecular weight of PCL is 3000, aniline trimer: AT) electroactive copolymers. (b) Shape memory properties of the degradable conductive copolymers. (1) The spiral shape of PCL3000-AT5 film after fixation; (2) The recovered shape of the spiral film; (3) The circle shape of PCL3000-AT5 film after fixation; (4) The recovered shape of the circle film. (c) myogenic differentiation of C2C12 myoblasts. Tubulin (green) and nuclei (blue) immunofluorescence staining of C2C12 cells at day 7 cultured on PCL80000 (a) and PCL3000-AT5 (the AT content in the copolymer is 5wt%). Scale bar: 200 μm. Reprinted from reference 72, Copyright (2016) Acta Materialia Inc, with permission from Elsevier.
2.4. Conducting nanofibers for tissue engineering
Biomaterials should mimic the structure of extracellular matrix (ECM). Collagen as the major protein of ECM takes the form of nanofibers. To imitate the natural ECM, nanofibrous polymeric scaffolds have been developed using several different techniques, such as electrospinning, phase separation, and molecular assembly.76−78 Electrospinning is the most widely used technique to fabricate nanofibers. A variety of natural and synthetic biodegradable polymers was blended with CPs and was further electrospun into nanofibers for tissue engineering. Fiber fabricated from electrospinning shows high surface area and porosity, and its diameter is tunable between several nanometers and several microns.79−81 Conductive nanofiber composite based on gelatin and PANI was fabricated via electrospinning using N, N-dimethylformamide/water mixture as solvent. After that, the conductive nanofibers were stabilized by using glutaraldehyde as the crosslinker. The gelatin and gelatin + CSA (camphorsulfonic acid) groups were poorly conductive, with electrical conductivity of 5.1 × 10−7 S/cm and 9.1 × 10−7 S/cm, respectively. However, gelatin + CSA 5% +PANI 5% and gelatin + CSA 10% + PANI 10% showed substantial electrical conductivity of 4.2 × 10−3 S/cm and 2.2 × 10−3 S/cm, respectively.82 Electrospun conductive nanofibrous scaffolds based on PANI and PLA were fabricated by our group. The scaffolds were prepared by electrospinning the blend of PANI/CSA and PLA in hexafluoroisopropanol. The scaffolds presented similar fiber diameter but gradually enhanced conductivity when changing the PANI content from 0 wt% to 3 wt% in the PLA polymer, which could be used for cardiac tissue engineering.50
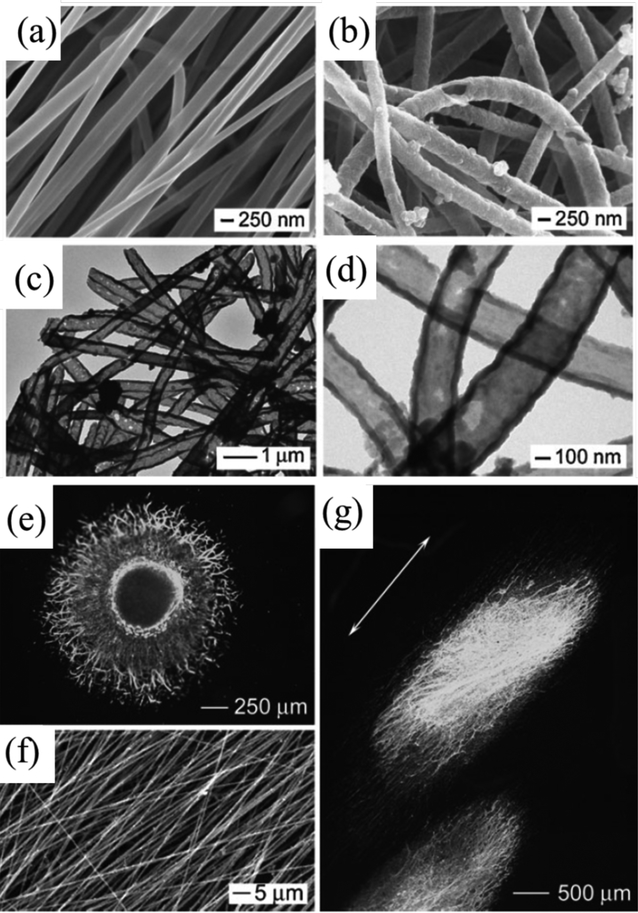
Core–shell structured nanofibers were also fabricated by combining electrospinning and in situ polymerization technique.83, 84 Conductive core–sheath nanofibers based on PLA or PCL were prepared by a typical electrospinning procedure as the templates. PPY was then coated on the nanofibers by in-situ polymerization of pyrrole with Fe3+ as an oxidant and Cl−1 as a dopant as shown in Figure 4a-d. After that, the PCL or PLA template was removed by soaking the obtained nanofibers in dichloromethane for 24 h to dissolve the cores, resulting in the conductive core–sheath nanofibers. The PPY tubes showed an outer diameter of approximately 320 ± 44 nm, and the PPY coating was about 50 ± 3 nm.83 Electroactive nanofibers were prepared by using PCL and conducting PPY, which combined electrospinning and vapor phase polymerization. First, PCL nanofiber was produced by electrospinning and then oxidant was further electrosprayed onto the PCL nanofiber. For the second step, immediately after electrospraying the oxidant on electrosprayed PCL nanofiber, vapor-phase polymerization of pyrrole was conducted. The as-prepared conductive nanofibers showed a porosity of approximately 92%, high conductivity (1.3–1.9 S/cm), dimensional stability, and good mechanical strength. 84
Figure 4.
SEM images of PCL nanofibers (a) and PPY nanotubes (b), respectively. TEM images of the PPY nanotubes (c, d). The nanotubes were prepared by soaking PCL/PPY core–sheath nanofibers in dichloromethane to selectively remove the PCL cores. Fluorescence micrograph showing the dorsal root ganglia (DRG) neurite field on random PCL/PPY core–sheath nanofibers (e), and aligned PCL/PPY core–sheath nanofibers (f). Fluorescence micrograph showing the DRG neurite field on aligned PCL/PPY core–sheath nanofibers (g). Reprinted with permission from reference 83. Copyright 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Myoblasts and neurons were shown to be guided by designed fibrous structures. 85 It was also found that aligned structures can enhance the proliferation and guide the differentiation of these cells. Therefore, the aligned conductive nanofibers were further fabricated. For example, well-ordered conductive PCL/PANI nanofibers were prepared. The PCL and camphor sulfonic acid doped PANI was mixed in 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) and then electrospun by using the magnetic field-assisted electrospinning process, resulting in highly aligned conductive nanofibers. The pure PCL fiber showed non-detectable electrical conductivity while PCL fibers with 3 wt% PANI showed a conductivity of 63.6 ± 6.6 mS/cm.78, 86 Aligned, porous and conductive fibers were prepared based on poly(L-lactide) (PLLA) and PPY coating. When using a 7.5 wt% PLLA solution for electrospinning, the aligned fibers showed abundant pores, but no pores were found on the aligned PLLA fiber electrospun at a 4.5% concentration. PPY could be uniformly coated onto the aligned and porous fibers by in situ polymerization of pyrrole on the fibers surfaces for 8 h. 87
The degradable conductive nanofibers were developed by electrospinning by our group.88 The degradable electroactive chitosan-aniline tetramer was synthesized for preparing electroactive nanofibers for tissue engineering. Chitosan-aniline tetramer was dissolved in the mixture of trifluoroacetic acid and dichloromethane, and then electrospun into electroactive nanofibers. The diameter of the nanofiber scaffolds could be controlled by changing the polymer concentration and the aniline tetramer content.89 In another example, fibrous non-woven scaffolds were fabricated by electrospinning a block copolymer of aniline tetramer and PCL. The copolymer presented good processability and biodegradability than the PANI conjugated polymer. The fibrous scaffold under oxidative state showed fibronectin (Fn) adhesion and the doped fibrous scaffold presented reduced Fn unfolding length on the membranes.90 Blends of aniline pentamer-graft-gelatin and PLA were electrospun into uniform nanofibers as biomimetic scaffolds. The nanofibers showed good electroactivity, thermal stability and biodegradability. The elongation of MC3T3-E1 cells was significantly higher on electroactive nanofibers than on PLA nanofibers. Moreover, the electroactive nanofibers stimulated by an electrical pulsed signal promoted the differentiation of MC3T3-E1 cells compared with pure PLA nanofibers.91
2.5. Conducting hydrogels for tissue engineering
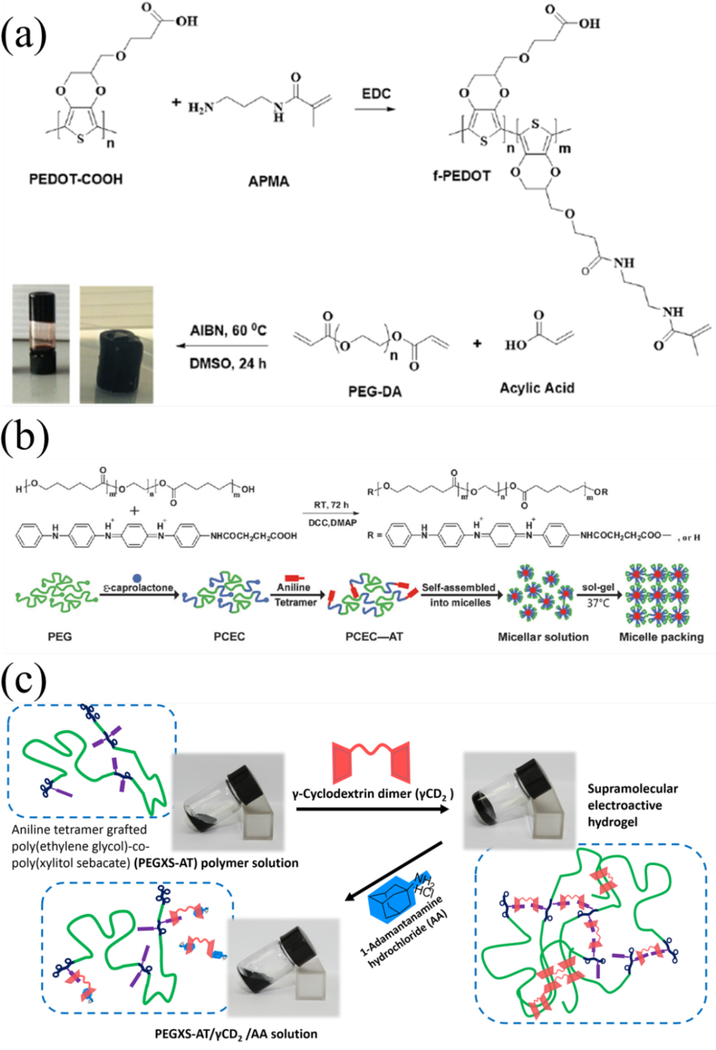
Hydrogels are an important class of biomaterials due to their rubbery nature similar to soft tissues, tunable properties, and their excellent biocompatibility.92−95 Conductive hydrogels with different structures and compositions have been fabricated.96−104 Electroactive poly(ethylene dioxythiophene) (PEDOT)/poly(acrylic acid) (PAA) hydrogel was developed by free radical polymerization of acrylic acid, double bond modified PEDOT and poly(ethylene glycol) diacrylate. The double bond functionalized PEDOT was covalently bonded to a hydrophilic PAA network as shown in Figure 5a. In addition, the covalently crosslinked PEDOT is stable within the hydrophilic polymer network and such conductive hydrogel endows the scaffolds with tailored properties, such as notable swelling ratio, appropriate mechanical properties, and electroactivity.105 Furthermore, flexible and biologically derived heparins-based conductive scaffold materials were developed. Photo-crosslinkable heparin-methacrylate hydrogels was firstly formed. Then the hydrogel was used as template to control the microstructure and doping of in situ polymerized PANI structures. The hydrogels showed hybrid electronic/ionic conductivity and ultra-compliant mechanical properties, and showed impedances as low as Z = 4.17 Ω at 1 kHz. 106
Figure 5.
Conductive hydrogels synthesized via chemical or physical crosslinking. (a) PEDOT hydrogel was synthesized via chemical crosslinking by free radical polymerizations. Reprinted with permission from reference 105. Copyright 2016 American Chemical Society. (b) A single component hydrogel based on PCL–poly(ethylene glycol)–PCL grafted aniline tetramer copolymers via thermal gelation process. Reproduced from reference 107 with permission of The Royal Society of Chemistry. http://dx.doi.org/10.1039/C5TB01658D (c) Formation of injectable conductive hydrogels via host−guest interaction between γ-cyclodextrin dimer host (γCD2) and poly(polyethylene glycol-co-sebacate-co-glycerol-co-xylitol)-g-aniline tetramer (PEGXS-AT) guest copolymer. The reversible sol−gel transition was observed by subsequent alternation addition of 1-adamantanamine hydrochloride (AA) and γCD2 into the mixture. Reprinted with permission from reference 108. Copyright 2014 American Chemical Society.
Due to the ability to effectively encapsulate cells, injectable hydrogels are promising biomaterials for cell encapsulation and tissue engineering applications.109, 110 Injectable conductive and degradable hydrogels based on gelatin and PANI were developed. The water soluble and electroactive copolymer of gelatin-g-PANI (GP) was synthesized by grafting PANI onto the gelatin backbone. Then genipin was used as crosslinker to crosslink GP at body temperature resulting in the hydrogel. The hydrogels’ conductivity gradually increased with the increase of PANI in the GP copolymers.111 Schiff base reaction between amine group and aldehyde group can occur under physiological condition; therefore, it is widely used to prepare injectable conductive hydrogels. We synthesized a water soluble and conductive copolymer of quaternized chitosan-g-PANI (QCSP) by in situ grafting PANI onto the QCS backbone. Then oxidized dextran (Odex) was used as crosslinker to crosslink the QCSP via Schiff base reaction resulting in injectable conductive hydrogels. Self-healing conductive hydrogel was also prepared based on chitosan-g-aniline tetramer and benzaldehyde capped PEG2000 via Schiff base reaction. The hydrogel showed rapid self-healing capacity, good adhesiveness to host tissue and antibacterial property. The conductivity of the hydrogels is about 10−3 S·cm−1 close to native cardiac tissue.112
Although chemical gelation of injectable conducting hydrogels are widely used as biomaterials, they often contain residual monomers, initiators or crosslinker in the hydrogel system. Some residual agents such as glutaraldehyde and isocyanate are toxic to cells.36 In contrast, physically crosslinked hydrogels show advantages over chemically cross-linked hydrogels because they usually undergo mild gelation processing, and do not need crosslinking agents.113, 114 Different non-covalent interactions were used to synthesize physical conductive hydrogels. Thermosensitive gelation is mostly widely used to synthesize injectable conductive hydrogels.115, 116 An injectable electroactive hydrogel was synthesized by chemically grafting electroactive tetraaniline segment onto the chain end of thermo-sensitive Pluronic® F127 copolymer. The hydrogel showed good electroactivity and good cytocompatibility. The PF127-tetraaniline hydrogel also presented enhanced mechanical properties for the hydrophobic interactions, π–π stacking and hydrogen bonding between the tetraaniline molecules.114 An injectable degradable electroactive hydrogel based on a single component copolymer poly(caprolactone)–poly(ethylene glycol)–poly(caprolactone) grafted aniline tetramer via a thermo-gelling approach was prepared as shown in Figure 5b. The copolymer showed good electroactivity and injectability. Besides, the hydrogel presented a revisable sol-gel-sol transition, attributed to the amphipathy of the conductive copolymer.107 Host-guest interactions were used to fabricate injectable electroactive hydrogel by our group. PEGXS-AT was synthesized by melting polymerization of PEG, sebacic acid and xylitol and then grafting carboxyl-capped aniline tetramer onto PEGXS copolymer as shown in Figure 5c. The AT and PEG from the PEGXS-AT served as the guest molecule while γ-cyclodextrin dimer was used as the host molecule, mixing the two component resulted in the supramolecular conductive hydrogel. 108
2.6. Conducting composite 3D scaffolds for tissue engineering
Except hydrogels, the above conductive blends, composites or nanofibers are usually in the form of a film or a membrane, which do not exhibit the 3D structures important for tissue regeneration.117 Therefore, 3D conductive composite scaffolds were fabricated for tissue engineering application. Advanced polymer-based nanocomposite materials have gained popularity for wide engineering applications. A 3D conductive scaffold was developed by introducing poly(3,4-ethylenedioxythiophene) poly(4-styrene sulfonate) (PEDOT: PSS) into nanocomposite of gelatin and bioactive glass. The PEDOT: PSS was physically crosslinked into the gelatin and bioactive glass matrix, while gelatin network was chemically crosslinked by N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide and N-hydroxysuccinimide. The PEDOT/PSS component could enhance the thermal stability, resistive to enzymic degradation and mechanical properties of the conductive scaffold.118 An electroactive 3D scaffold was developed by using silk fibroin (SF) and water-soluble conductive poly(aniline-co-N-(4-sulfophenyl) aniline) (PASA) conductive polymer. When PASA solution was mixed into the SF solution, the PASA enhanced the intermolecular self-assembly of SF. Thus, the PASA not only stably crosslinked the SF/PASA scaffold, but also enhanced the compression modulus and decreased degradation rate of the conductive scaffold. The scaffold had appropriate porosity (≈90%) and sufficient mechanical property for cell culture (200–450 kPa).
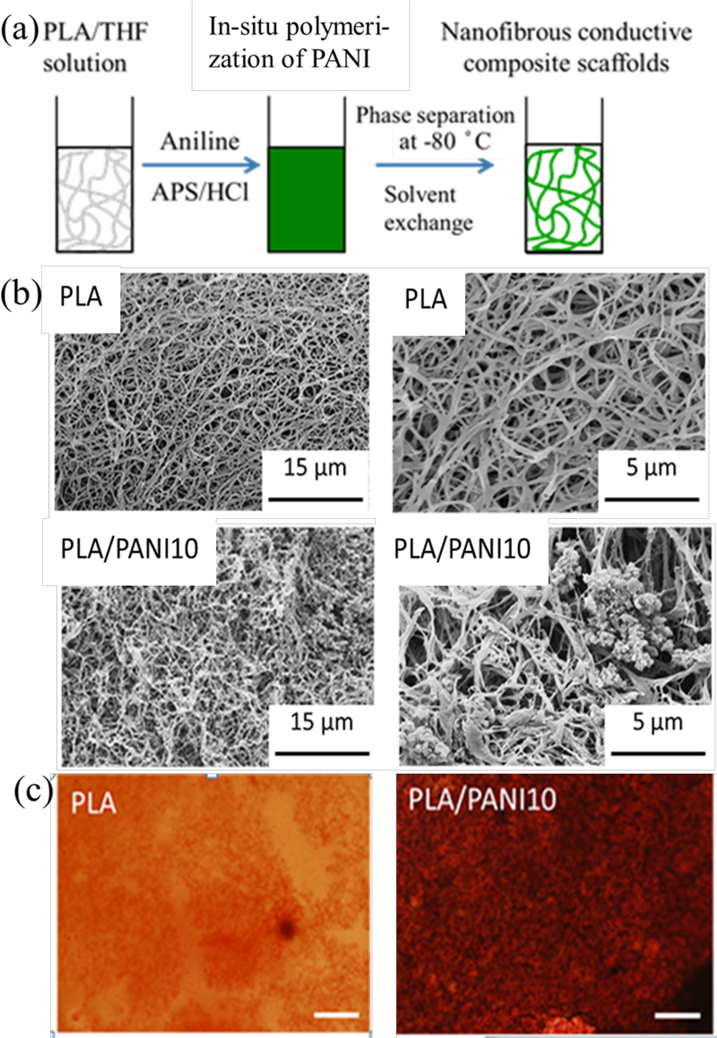
With great processing flexibility, thermal induced phase separation (TIPS) is a promising technique to fabricate nanofibrous scaffolds for tissue regeneration.77, 119 To overcome the limitation of poor solubility of conductive polymers, we successfully developed conducting and biodegradable PLA/PANI nanocomposite nanofibrous scaffolds via in-situ polymerization of aniline in a PLA/THF solution and then through a TIPS procedure as depicted in Figure 6a. The nano-structured PANI was well-distributed in the scaffold as shown in Figure 6b, and this method showed the possibility to scale-up production of the conductive nanofibrous composite scaffolds. 120 The scaffolds enhanced the osteogenic differentiation of BMSCs in term of increased mineralization of BMSCs (Figure 6c).
Figure 6.
(a) Schematic fabrication of conductive nanofibrous scaffolds. (b) SEM images of nanofibrous conductive scaffolds, PLA/PANI10: 10%wt PANI in the composite scaffolds, and the magnified images of scaffolds showing the nanofiber and PANI nanoparticles. (c) Alizarin red staining of BMSCs on different substrates for two weeks. Scale bar = 200 μm. Much larger area of positive alizarin red aggregates was present in the BMSCs of conductive composite scaffolds than that on PLA at day 14. Reprinted from reference 120, Copyright (2017), with permission from Elsevier.
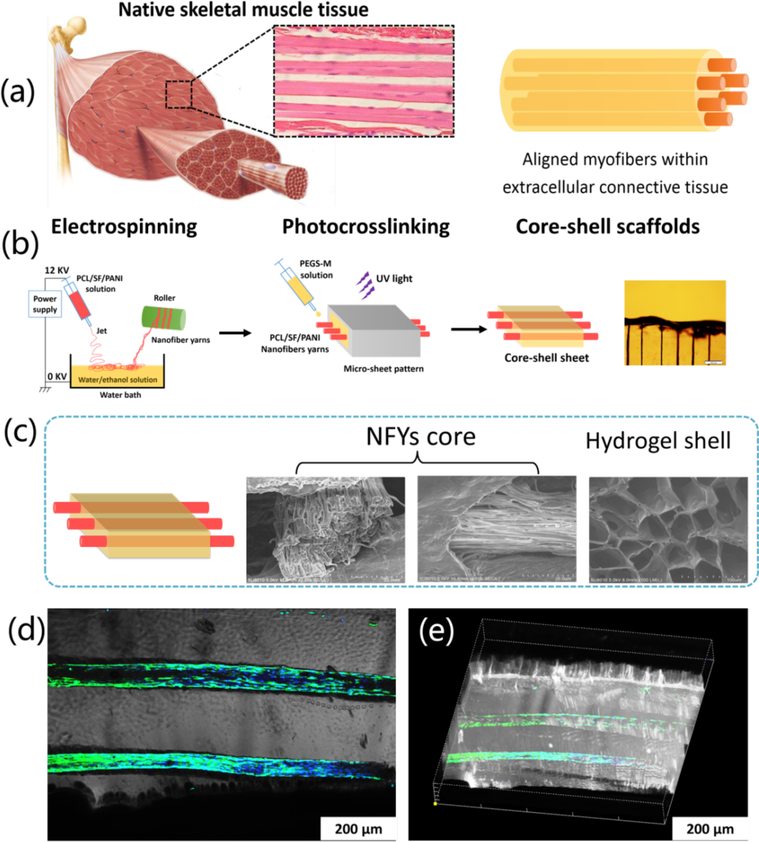
Designing scaffolds that can mimic structure of native tissues and guide 3D cellular alignment remains an ongoing challenge for skeletal muscle or cardiac muscle with an aligned structure. Our group fabricated core-shell composite scaffolds to mimic native skeletal muscle structure, and the scaffolds were composed of aligned nanofiber yarn (NFY) core and photocurable hydrogel shell. The NFYs were fabricated by dry-wet electrospinning method based on a composite of PCL, silk fibroin and PANI. A series of composite scaffolds are fabricated by encapsulating the aligned NFY cores within the hydrogel shell via photo-crosslinking. These core-shell scaffolds combine the aligned NFY core and the hydrogel shell which can mimic the structure of native skeletal muscle and have a practical application for skeletal muscle repair.
3. Conductive biomaterials for various tissue engineering applications
It becomes increasing important for biomaterials to be designed to physically enhance tissue growth and improve specific cell functions. Conductive matrix material can stimulate electrically responsive cell types including bone, muscle, nerve, cardiac, and skin, and they have been widely used in the regeneration of these electrical signal sensitive tissues.51
3.1. Bone tissue engineering
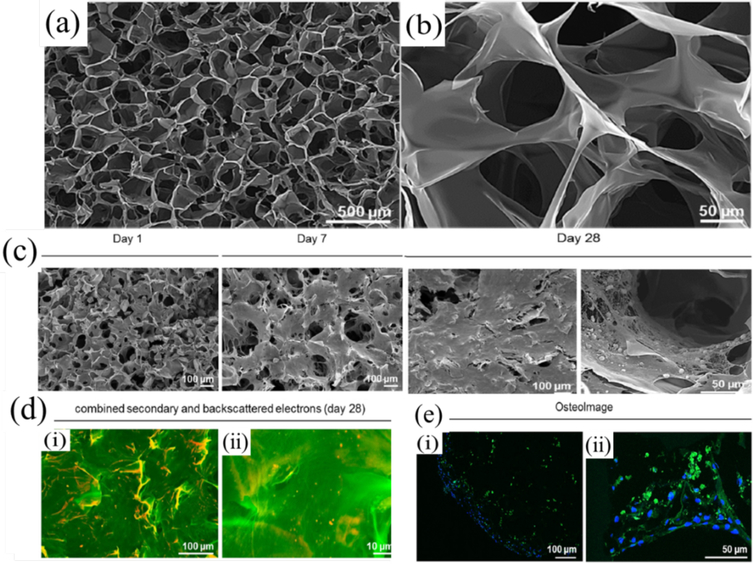
Conducting biomaterials can enhance the adhesion and proliferation of MC3T3-E1 cells, osteoblast-like SaOS-2 cells, C2C12 cells and mesenchymal stem cells.60, 121 Ice-templated porous and conductive PEDOT: PSS scaffold with median pore diameter above 50 μm allowed MC3T3-E1 cell infiltration and matrix deposition within the void space as shown in Figure 7a-c. Furthermore, the scaffold also could enhance the gene expression levels of ALP, COL1 and Runx2, increase extracellular matrix mineralization, and promote osteocalcin deposition of MC3T3-E1 cells as shown in Figure 7c. These results demonstrate that the conductive PEDOT: PSS scaffold can induce the differentiation of osteogenic precursor cells (MC3T3-E1) into osteocalcin positively stained osteoblasts (Figure 7d-e). The underlying mechanisms of PEDOT: PSS scaffold’s increasing matrix mineralization and gene expression of osteogenic markers is not clear in the present study.122 The 3D conductive composite scaffolds based on PEDOT: PSS, gelatin, and bioactive glass nanoparticles showed approximately 60% porosity and the pore size varied from 110 μm to 160 μm by changing the PEDOT:PSS content. The scaffolds supported human mesenchymal stem cells adhesion, and the cell viability increased with the increase of the concentration of the conductive polymer in the scaffold, which might be caused by the improved microstructure of the scaffolds or boosted electrical signaling among cells. 118
Figure 7.
Scanning electron microscopy (SEM) images of PEDOT: PSS scaffolds (a, b). The highly interconnected, porous structure was achieved through ice template and subsequent sublimation. (c) SEM images of MC3T3-E1 cells cultured on PEDOT: PSS scaffolds. Samples were harvested on day 1, 7 and 28 post-seeding. Cell number and matrix deposition was increased over time, with a densely formed tissue-construct, after 28 days in differentiation media. (d) False colored SEM image of back scattered and secondary electrons (i, ii). Calcified particles are depicted in red, organic material in green. (e) Confocal microscopy images of OsteoImage stained MC3T3-E1 cells on day 28 (i, ii). Mineralized bone nodules appeared green, while nuclei were counterstained with DAPI (blue). Reprinted from reference 122. (http://dx.doi.org/10.1016/j.actbio.2017.08.045) under a Creative Commons Attribution 4 International License. http://creativecommons.org/licenses/by/4.0/. Copyright 2017 Acta Materialia Inc. Published by Elsevier Ltd.
We synthesized electroactive shape memory polymeric (SMP) films based on six-armed branched polylactide and aniline trimer and found that they could significantly enhance the proliferation of C2C12 cells when compared to aniline trimer-free SMP films. Besides, electroactive SMP films improved the osteogenic differentiation of C2C12 myoblast cells based on the ALP enzyme activity, immunofluorescence staining, and relative gene expression, showing their great potential for bone regeneration.123 3D conductive PLA/PANI composite nanofibrous scaffolds presented good cytocompatibility for bone marrow derived mesenchymal stem cells (BMSCs). The conductive nanofibrous scaffolds enhanced the osteogenic differentiation of BMSCs which increased the expression levels of alkaline phosphatase (ALP), osteocalcin (Ocn) and runt-related transcription factor 2 (Runx2), as well as mineralization of BMSCs (Figure 6c), indicating that moderate content of PANI in the conductive scaffolds could promote osteogenic differentiation of BMSCs for bone tissue engineering application.120 These results provided a new biomaterial approach to induce osteogenic differentiation from BMSCs.
One of the benefits of conducting polymers used as scaffolds is the application of electrical stimulation on the substrates. Heparin-doped PPY/PLLA conductive film could support osteoblast-like SaOS-2 cells adhesion, proliferation and osteogenic differentiation. Electrical stimulation (ES) enhanced osteoblast adhesion and growth, resulting in significantly higher calcium and phosphate contents in the mineral deposition than the control group. Besides, the ES also significantly upregulated the osteoblast-specific markers’ (ALP, BMP2, and Runx2) expression levels.124
3.2. Skeletal muscle tissue engineering
Skeletal muscles show a robust ability to regenerate, but under severe conditions, such as trauma, the loss of muscle function is inevitable. Skeletal muscle is a highly organized, composite structure consisting of myofibers, blood vessels, and nerves, as well as extracellular connective tissue.86, 125, 126 Skeletal muscle tissue engineering often relies on the prefabrication of muscle tissue in vitro via differentiation and maturation of muscle precursor cells or stem cells on a functional scaffold.87, 115, 127 Poly(l-lactide-co-ɛ-caprolactone)(PLCL)/PANI fibers were cytocompatible and they showed the enhanced number of cells positive for sarcomeric myosin than PLCL fiber group. Besides, PLCL/PANI fibers also improved myogenin expression over the PLCL fibers. The expression of troponin T and myosin heavy chain gene were also enhanced by the conductive PLCL/PANI fibers, indicating that electrically conductive substrates modulated the induction of myoblasts into myotube formation without additional electrical stimulation.128 Gelatin+CSA+PANI nanofibers could improve the C2C12 cell myogenic differentiation than gelatin or gelatin+CSA nanofibers. Besides, the conductive nanofiber group also showed increased intracellular organization, colocalization of the dihydropyridine receptor and ryanodine receptor, expression of genes related to excitation−contraction coupling apparatus, calcium transients, and myotube contractibility. The functionality of the formed myotubes could be further enhanced when myotubes were electrically stimulated, which showed more calcium transients and contractions with higher amplitude and regularity. 82
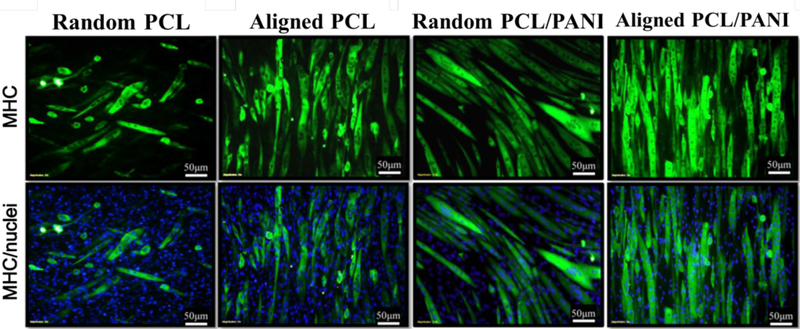
Aligned structure of the scaffolds can mimic the anisotropic structure of elongated myofibers in skeletal muscle. It was found that electrospun scaffolds composed of aligned nanofibers can serve as topographical cues that enhance the alignment and elongation of skeletal muscle cells.55 Therefore, aligned conductive nanofibers were fabricated for skeletal muscle tissue engineering. For example, conductive PCL/PANI nanofibers with highly aligned structure could guide myoblast orientation and promote myotube formation than random fibers as shown in Figure 8. Besides, the conductive and aligned PCL/PANI nanofibers showed further enhanced myotube maturation than non-conductive and aligned PCL fibers and random conductive PCL/PANI fibers (Figure 8), demonstrating that the combined effect of both guidance cues were more effective than an individual cue.86 We combined electrospinning and electrospray technique together, and prepared nanofibrous electroactive composite scaffolds with both the aligned morphology of the scaffold and electroactivity from polyurethane-urea (PUU) nanoparticles, and they could enhance the C2C12 myoblast proliferation and differentiation when compared the random scaffold and non-conductive aligned scaffold, demonstrating the synergetic effect of topographical cue and electroactive cue for differentiation of C2C12 myoblasts. Thus, the electroactive nanofibrous composite scaffolds presented great potential for muscle regeneration. 88
Figure 8.
Representative immunofluorescent images of myotubes differentiated for 5 days on random PCL fiber, aligned PCL fiber, random PCL/PANI3 fiber and aligned PCL/PANI3 fibers. MHC was immunostained green and nucleus blue. Reprinted from reference 86, Copyright (2012) Acta Materialia Inc., with permission from Elsevier.
To enhance the efficiency of skeletal muscle engineering, a number of key properties of the scaffolds need to be optimized, including conductivity, degradability, and elasticity. We designed and synthesized a series of degradable conductive copolymers with great elasticity to enhance C2C12 myoblast differentiation. The synthesized electroactive PUU copolymer films with elongation at break higher than 1600% enhanced C2C12 myoblast cells proliferation than PLA film and non-conductive PU film. Besides, they also could improve the myogenic differentiation of C2C12 myoblasts, which might be caused by the wettability and electroactivity of the doped electroactive PUU films.129 We further synthesized conductive and shape memory PCL-aniline tetramer films which also showed good electroactivity and elasticity, and they enhanced the proliferation, myotube formation and related myogenic differentiation genes expression of C2C12 myoblasts compared to the pure PCL films (Figure 3c), suggesting their great potential in skeletal muscle tissue engineering application.72
The basic structural unit of skeletal muscles is the myofiber. Myofibers are formed when myoblasts fused together to form elongated, multinucleated myotubes. Therefore, we designed core-shell composite scaffolds to induce 3D cellular alignment and elongated myotube formation as depicted in Figure 9a-c.38 Cultivation of C2C12 myoblasts on the aligned NFYs c38onfirmed the good biocompatibility of these aligned NFYs and aligned NFYs in the hydrogels induced C2C12 cellular alignment and elongation. The formation of 3D elongated myotubes within core-shell scaffolds is also observed after long-term cultivation (Figure 9d-e). Therefore, these core-shell scaffolds can mimic the native structure of skeletal muscle showed a great potential for skeletal muscle regeneration.
Figure 9.
(a) The composite core-shell scaffolds resemble the native structure of skeletal muscle tissue, consisting of aligned myofibers surrounded within extracellular connective tissue. (b) Preparation scheme of core-shell sheet scaffolds that mimic the native skeletal muscle tissue by the combination of aligned nanofiber yarns (NFYs) and hydrogel shell of poly(ethylene glycol)-co-poly(glycerol sebacate) (PEGS-M) solution. SEM images (c) of aligned NFYs core and hydrogel shell of the core-shell sheet scaffold after lyophilization. (d) The merged image of aligned NFYs cores exhibited overspreading C2C12 myotubes within the hydrogel sheet. (e) The 3D side view of the core-shell scaffolds containing highly organized myotubes. Reprinted with permission from reference 51. Copyright 2015 American Chemical Society.
3.3. Nerve tissue engineering
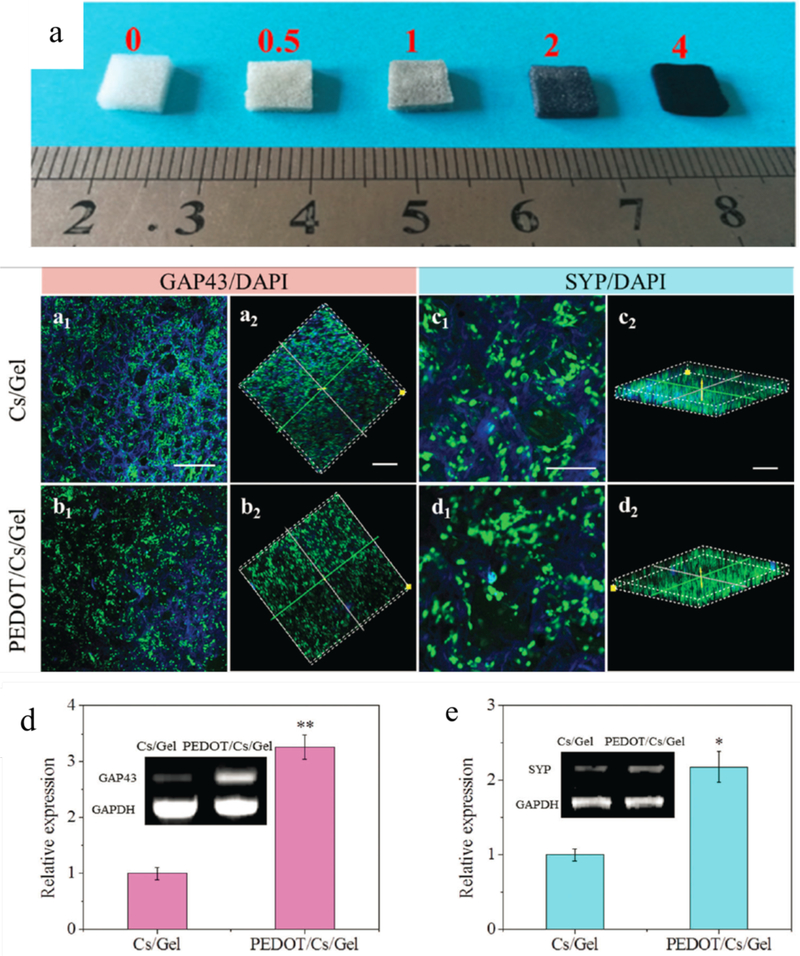
Neurons in nervous system are electrically excitable cells which transmit signals at a quick pace.130 Researchers have developed conducting polymers including PPY and PANI as conductive scaffolds to enhance the growth and regeneration of nerve tissue.131-134 The conductive PCL/PPY conductive nanofibers with good cytocompatibility supported PC12 cell differentiation for the presence of neurite outgrowth from PC12 cells on the conductive nanofibers. Furthermore, the PC12 cells spread significantly larger areas than those of on PCL without PPY coating. These results suggested the potential applications of the conductive nanofibers scaffolds for nerve tissue engineering.84 Conductive PEDOT/chitosan/gelatin scaffolds as shown in Figure 10a exhibited good biocompatibility and significantly improved neuron-like rat PC12 cell adhesion and proliferation. Besides, the conductive scaffold could maintain cells in more active proliferation and neurite growth, as well as upregulating GAP43 and SYP protein and gene expression level (Figure 10), which suggested that it may be a promising conductive scaffold for neural tissue engineering.135 Electroactive tobacco mosaic virus (TMV)/PANI/PSS nanofibers showed growth of neuronal cells and augmentation of the length of neurites. The number of cells with neurites was larger than that of cells cultured on the TMV-derived non-conductive nanofibers. Besides, the aligned electroactive TMV/PANI/PSS nanofibers could guide the outgrowth direction of neurites, improve the percentage of cells with neurites, and cause a bipolar cellular morphology. The results revealed that both the electroactivity and topographical cues from TMV/PANI/PSS nanofibers could promote neural cells differentiation and neurites outgrowth. 136
Figure 10.
(a) Images of the polymerized PEDOT/chitosan/gelatin (PEDOT/Cs/Gel) scaffolds with different molar ratios of ammonium persulfate (APS) to EDOT. Neurite growth protein (a1-d2) and gene expression of PC12 cells (d, e) in the scaffolds after 5 days of culture. 2D and 3D confocal fluorescence micrographs of immunostained cells on the chitosan/gelatin (Cs/Gel) scaffold (a and c) and the PEDOT/Cs/Gel scaffold (b and d). GAP43 and SYP are shown in green, and the nuclei are stained in blue (DAPI). (a, b, c2 and d2) scale bar = 500 mm, (c1 and d1) scale bar = 200 mm. qPCR analysis of the gene expression levels of GAP43 (d) and SYP (e) on the Cs/Gel and PEDOT/Cs/Gel scaffold. *P<0.05, **P<0.01 are statistically different from the Cs/Gel control group. Reproduced from reference 135 with permission of The Royal Society of Chemistry. http://dx.doi.org/10.1039/C7TB00608J
Electrical stimulation has been proven to be an effective approach for neuronal function and nerve regeneration.131, 137, 138 When seeding PC12 cells onto conductive PPY/poly(DL-lactic acid) (PDLLA) conduits and applying electrical stimulation with 100 mV for 2 h, the cells showed gradually increased percentage of neurite-bearing cells and median neurite length with the increase of the PPY content (Figure 2a-k). The PPY/PDLLA nerve conduit with 5% PPY was used to bridge a 10 mm defect in the sciatic nerve of a rat, and it presented functional recovery similar to autologous nerve graft and significant improvement compared to that of the PDLLA conduits, showing its great potential for nerve tissue regeneration.53 The aligned conductive PPY nanofiber presented oriented neurites along one direction and showed longer maximum length than those cultured on the random counterparts (Figure 4e-g). When applying electrical stimulation on both the random and aligned conductive core–sheath nanofibers, they all showed further increased maximum length of neurites compared to the controls without electrical stimulation, revealing their potential use as scaffolds for neural tissue engineering. 83
Stem cells also show great potential for neural tissue repair without the addition of nerve growth factors. The 3D electroactive PPY/collagen fiber scaffolds supported the human bone marrow-derived mesenchymal stem cells (hMSCs) adhesion, and the hMSCs on the scaffold presented upregulation of neural markers that are characteristic of neural lineage after stimulated with an external electrical pulse generator for 5 days and 10 days. However, hMSC viability was affected after prolonged stimulation of the culture system.139 Cross-linked PEDOT substrate showed good cytocompatibility on L929 fibroblasts and improved population of neurons and longer neurites for neural stem cells when applying electrical stimulus. 140
CPs are attractive candidates for neural probes and implantable electrodes because they can achieve high surface area, and enhance effective ion exchange between recording sites and the surrounding tissue.141 PANI was used as electrode coating material to improve the function of neural probes. A PANI-coated platinum (Pt) electrode was prepared by in situ polymerization on Pt electrode. Compared to the uncoated Pt electrode, the PANI-coated Pt electrode surface tended to aggregate retinal fragments rather than spread them, showing great potential to reduce inflammation and scar formation in long-term implantation. The PANI coating exhibited long-term stability for 6 months under electrical stimulation.142 Lacour’s group reported the development of a microfabricated conformable electrode array with small (100 μm diameter) electrode sites which were coated with PEDOT: PSS, to offer high charge injection properties and to safely stimulate the auditory system with small stimulation sites. These studies demonstrated the potential of using conducting PEDOT: PSS coatings on small electrode sites for electrochemically safe and efficient stimulation of the central auditory system.143
3.4. Cardiac tissue engineering
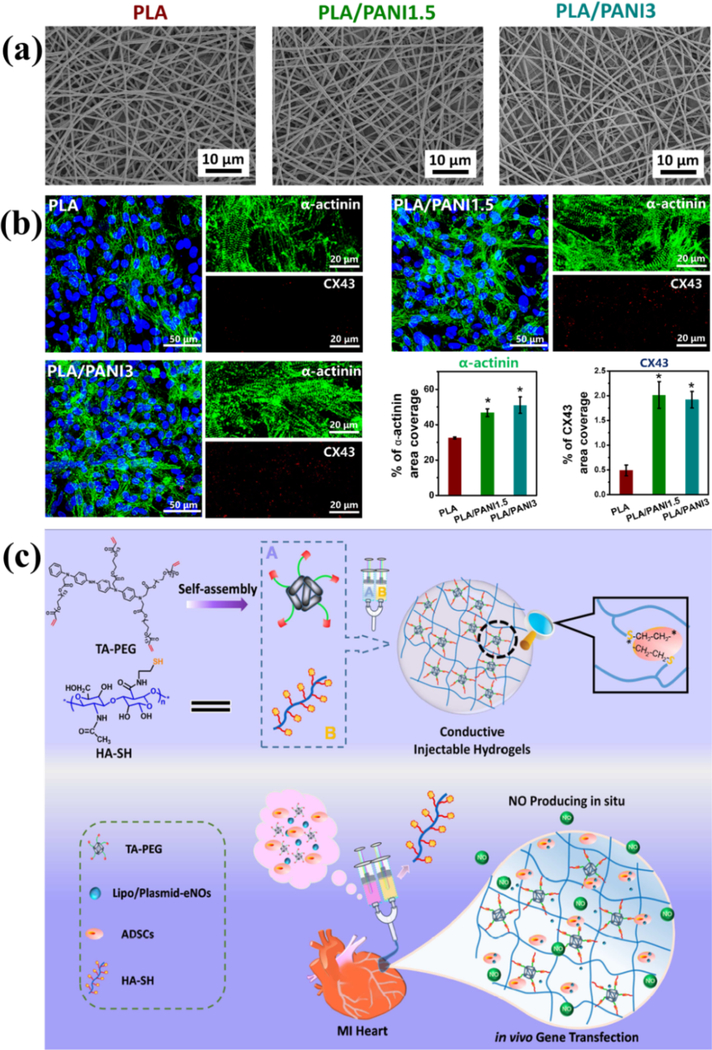
The well-known behavior of excitation-contraction coupling of the heart is caused by the propagation of electrical signals via the cardiac cells in a synchronized fashion.144 PPY/PCL conductive film could improve the velocity of calcium wave propagation and shorten the calcium transient duration of the cardiomyocyte monolayers when compared to PCL film, and they also supported cardiomyocyte adhesion, and compared to pure PCL, more cardiomyocytes on PPY/PCL film presented peripheral localization of the gap junction protein connexin-43 (CX43). However, the gene expression level of CX43 showed no difference between two materials. 52 Our group prepared PLA/PANI conductive nanofibrous sheets by electrospinning with the same diameter of the nanofibers (Figure 11a) and they showed high cell viability and could promote the differentiation of H9c2 cardio-myoblasts in terms of maturation index and fusion index. Moreover, PLA/PANI nanofibrous sheets also improved the cell-cell interaction, maturation with more secreted proteins including α-actinin and connexin-43 and spontaneous beating of primary cardiomyocytes (Figure 11b), suggesting PLA/PANI conductive nanofibrous sheets’ potential in cardiac tissue engineering application.50 The doped conductive poly(lactic-co-glycolic acid) (PLGA)/PANI nanofiber meshes could attract negatively charged adhesive proteins and enhance cell adhesion. Cardiomyocytes on the nanofiber meshes could associate with each other and formed isolated cell clusters, and the cluster presented elongated and aligned morphology along the major axis of the fibrous mesh. The expression of the gap-junction protein connexin-43 was also shown in the isolated clusters, and the cells in each cluster presented synchronous beating. The beating rate could also be synchronized using a native heart mimicking electrical stimulation. 145
Figure 11.
(a) SEM images of PLA/PANI nanofibrous sheet with different PANI concentration including 0 wt% (PLA), 1.5 wt% (PLA/PANI1.5), and 3 wt% (PLA/PANI3). All the nanofibers showed similar nanofiber size. (b) Representative fluorescence images of primary cardiomyocytes immunostained for sarcomeric α-actinin (green) and connexin-43 (red) on different nanofibrous sheets after culturing for 8 days. The area fraction of α-actinin and CX43 were also analyzed. *P < 0.05. Reprinted from reference 50, Copyright (2017) Acta Materialia Inc., with permission from Elsevier. (c) Scheme demonstrates a new conception to construct an injectable conductive hydrogel, which is used to encapsulate plasmid DNA-eNOs nanoparticles and ADSCs to treat MI. The conductive hydrogels promoted the electrical communications to restore the functions of heart. ADSCs loaded in hydrogels were injected into the infarcted myocardium to compensate the cell loss after MI, and the upregulated eNOs expression enhanced neo-vascularization and the maturation of myocardium. Reprinted from reference 146, Copyright (2018), with permission from Elsevier.
Myocardial infarction (MI) remains a therapeutic challenge all around the world. 87, 114 MI usually leads to a mass death of cardiomyocytes and generation of a fibrosis scar 115. We designed a self-healing conductive chitosan-graft-aniline tetramer and dibenzaldehydeterminated poly(ethylene glycol) hydrogel with good biocompatibility as cell delivery system for treating MI. After injection of the hydrogels, the viability of C2C12 cells encapsulated in the hydrogels presented no obvious difference compared to that before injection. The cell encapsulated hydrogel showed a tunable release profile and good in vivo cell retention. The degradation ability and in vivo biocompatibility of the hydrogel was also demonstrated suggesting its potential as cell delivery vehicle for MI.112 The group of Liu constructed an injectable conductive hydrogel based on multi-armed conductive crosslinker tetraaniline-polyethylene glycol diacrylate and thiolated hyaluronic acid (Figure 11c). The obtained conductive hydrogels showed a conductivity equivalent to native myocardium, and they were loaded with plasmid DNA encoding eNOs nanocomplexes and stem cells for treating MI. After the injection of hydrogel-based holistic system into the infarcted myocardium of SD rats, an increased expression of eNOs in myocardial tissue was observed, accompanying with upregulation of proangiogenic growth factors and myocardium related mRNA. The electrocardiography, cardiogram, and histological analysis confirmed a distinct increase of ejection fraction, shortened QRS interval, and smaller infarction size, indicating that the conductive hydrogel encapsulated with stem cells and gene-encoding eNOs nanoparticles is a robust therapeutic approach for the treatment of MI. 146
3.5. Skin tissue engineering
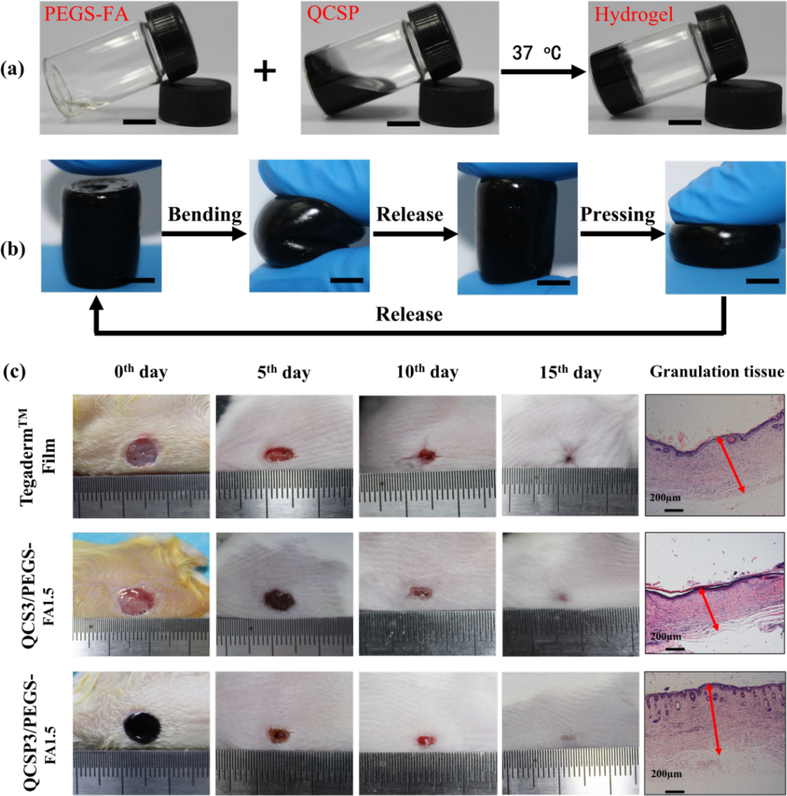
Skin protects the human body from damage and microbial invasion. About 265,000 deaths occur due to thermal burn wounds each year.147 Various biomaterials as wound dressing with good antibacterial activity have been developed.148 Conductive materials have been proven to promote cellular activities including fibroblasts and keratinocytes.149, 150 Conducting polymers such as PANI also showed anti-bacterial property.151, 152 These advantages of conducting polymers make them promising biomaterials for wound healing application. For example, the wound healing performance of conductive nanofiber composites based on poly(aniline-co-aminobenzenesulfonic acid), poly(vinyl alcohol) and chitosan oligossacaride were conducted by employing double full-thickness skin wounds model on the dorsum of SD rats. The results demonstrated that the conductive nanofiber composite dressing showed lesser provocative responses than control group throughout the test. The conductive dressing presented almost complete healing and enhanced collagen and granulation as compared to control group after 15 days’ treatment, suggesting the promising application of conductive nanofiber composite for wound healing.153 PANI/chitosan (1:3) nanofiber mat showed a synergistic effect in improving both osteoblast and fibroblast growth attributed to its proper hydrophilicity and conductivity, showing potential for wound healing application.154 We developed antibacterial and conductive injectable benzaldehyde functionalized poly(ethylene glycol)-co-poly(glycerol sebacate) (PEGS-FA)/ quaternized chitosan-g-PANI hydrogels, and they showed robust mechanical property, good electroactivity, free radical scavenging capacity, antibacterial activity, adhesiveness, conductivity, and biocompatibility (Figure 12a-b). The hydrogel with an 1.5 wt% crosslinker presented excellent in vivo blood clotting capacity, and significantly enhanced in vivo wound healing in a full-thickness skin defect model than non-conductive quaternized chitosan/PEGS-FA hydrogel and commercial dressing (Tegaderm™ film), which could upregulate the gene expression of growth factors including VEGF, EGF and TGF-β, and promote granulation tissue thickness and collagen deposition (Figure 12c).117
Figure 12.
(a) Photographs of 4-formylbenzoic acid (FA) functionalized poly(ethylene glycol)-co-poly(glycerol sebacate) (PEGS-FA) solution, quaternized chitosan-g-PANI solution and hydrogel QCSP/PEGS-FA; (b) The bending and pressing behavior of soft and flexible hydrogel QCSP/PEGS-FA. Scale bar: 5 mm (c) Photographs of wounds at 0th, 5th, 10th and 15th days and granulation tissue at 15th days for commercial film dressing (Tegaderm™), hydrogel QCS/PEGS-FA and hydrogel QCSP/PEGS-FA. Sample QCSP/PEGS-FA showed an enhanced wound healing rate and thicker granulation tissue compared to commercial film dressing and QCS/PEGS-FA. Reprinted from reference 117. Copyright (2017), with permission from Elsevier.
4. Conclusions and future prospective
This review summarizes the applications of electrically conductive polymers for tissue engineering. The many positive attributes of conducting polymers such as their biocompatibility, tunable conductivity, facile synthesis and simple modification make conducting polymers very attractive as bioactive scaffolds for tissue engineering. Different fabrication techniques have been developed for fabricating conductive biomaterials as scaffolds for tissue regeneration. The conductive biomaterials in the form of composite films, electrospun fibers or hydrogels, can improve the usefulness of conducting polymers.
The electrospinning was the most widely used method to prepare conducting fibrous scaffolds based on conducting polymers and polymers such as PCL, PLA and PLGA. And these scaffolds can mimic the structure of ECM, and could simultaneously provide electrical cue for the scaffolds. Applying an electrical stimulus on a conductive substrate can enhance the cellular activity (cell proliferation and differentiation) of the cells cultured on the scaffolds. The results of both in vitro and in vivo research demonstrated that conductive biomaterials as scaffolds are promising candidates for the repair of bone, muscle, nerve, cardiac and skin tissues.
Although conducing polymers were shown to be biocompatible in vitro, remarkable advances in the synthesis and functionalization of these conducing polymer-based biomaterials have been made, and the systematic studies of their in vivo biocompatibility and biodegradability remain needed. Extensive in vivo experiments of long-term cytotoxicity and biodegradation are necessary to ensure the non-toxicity and biodegradability of the conductive biomaterials. We believe that more research on the application of conductive polymers in tissue engineering should be conducted and they as smart materials may be used in clinic to regenerate electrical sensitive tissues in the future.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant number: 51673155), the Fundamental Research Funds for the Central Universities, and the US NIH (HL136231).
References
- (1).Karp JM; Langer R, Development and therapeutic applications of advanced biomaterials. Curr. Opin. Bio.Tech 2007, 18, 454–459. [DOI] [PubMed] [Google Scholar]
- (2).Smith LA; Liu X; Ma PX, Tissue engineering with nano-fibrous scaffolds. Soft Matter 2008, 4, 2144–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Makris EA; Gomoll AH; Malizos KN; Hu JC; Athanasiou KA, Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol 2015, 11, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Vacanti JP; Vacanti CA, The history and scope of tissue engineering In Principles of Tissue Engineering (Fourth Edition), Elsevier: 2014; pp 3–8. [Google Scholar]
- (5).Schumann D; Ekaputra AK; Lam CX; Hutmacher DW, Biomaterials/Scaffolds In Tissue Engineering, Springer: 2007; pp 101–124. [PubMed] [Google Scholar]
- (6).Guo BL; Ma PX, Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci. China Chem 2014, 57, 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Van Vlierberghe S; Dubruel P; Schacht E, Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 2011, 12, 1387–1408. [DOI] [PubMed] [Google Scholar]
- (8).Zustiak SP; Leach JB, Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 2010, 11, 1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wu Y; Wang L; Zhao X; Hou S; Guo BL; Ma PX, Self-healing supramolecular bioelastomers with shape memory property as a multifunctional platform for biomedical applications via modular assembly. Biomaterials 2016, 104, 18–31. [DOI] [PubMed] [Google Scholar]
- (10).Guo BL; Yuan JF; Gao QY, pH and ionic sensitive chitosan/carboxymethyl chitosan IPN complex films for the controlled release of coenzyme A. Colloid. Polym. Sci 2008, 286, 175–181. [Google Scholar]
- (11).Guo BL; Yuan JF; Gao QY, Preparation and characterization of temperature and pH-sensitive chitosan material and its controlled release on coenzyme A. Colloid. Surface. B 2007, 58, 151–156. [DOI] [PubMed] [Google Scholar]
- (12).Shoichet MS, Polymer scaffolds for biomaterials applications. Macromolecules 2009, 43, 581–591. [Google Scholar]
- (13).Garg T; Goyal AK, Biomaterial-based scaffolds - current status and future directions. Expert Opin. Drug Deliv 2014, 11, 767–789. [DOI] [PubMed] [Google Scholar]
- (14).Toivonen MS; Kurki-Suonio S; Schacher FH; Hietala S; Rojas OJ; Ikkala O, Water-Resistant, transparent hybrid nanopaper by physical cross-linking with chitosan. Biomacromolecules 2015, 16, 1062–1071. [DOI] [PubMed] [Google Scholar]
- (15).Inkinen S; Hakkarainen M; Albertsson AC; Sodergard A, From lactic acid to poly(lactic acid) (PLA): characterization and analysis of PLA and its precursors. Biomacromolecules 2011, 12, 523–532. [DOI] [PubMed] [Google Scholar]
- (16).Sukmana I, Bioactive polymer scaffold for fabrication of vascularized engineering tissue. J. Artif. Organs 2012, 15, 215–224. [DOI] [PubMed] [Google Scholar]
- (17).Guo BL; Finne-Wistrand A; Albertsson AC, Versatile functionalization of polyester hydrogels with electroactive aniline oligomers. J. Polym. Sci. Polym. Chem 2011, 49, 2097–2105. [Google Scholar]
- (18).Hopley EL; Salmasi S; Kalaskar DM; Seifalian AM, Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Bio.Technol. Adv 2014, 32, 1000–1014. [DOI] [PubMed] [Google Scholar]
- (19).Goenka S; Sant V; Sant S, Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [DOI] [PubMed] [Google Scholar]
- (20).Fan ZJ; Wang JQ; Wang ZF; Ran HQ; Li Y; Niu LY; Gong PW; Liu B; Yang SR, One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon 2014, 66, 407–416. [Google Scholar]
- (21).Abarrategi A; Gutierrez MC; Moreno-Vicente C; Hortiguela MJ; Ramos V; Lopez-Lacomba JL; Ferrer ML; del Monte F, Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [DOI] [PubMed] [Google Scholar]
- (22).Harrison BS; Atala A, Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [DOI] [PubMed] [Google Scholar]
- (23).Nair RS; Ameer JM; Alison MR; Anilkumar TV, A gold nanoparticle coated porcine cholecyst-derived bioscaffold for cardiac tissue engineering. Colloid. Surface. B 2017, 157, 130–137. [DOI] [PubMed] [Google Scholar]
- (24).Shevach M; Fleischer S; Shapira A; Dvir T, Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 2014, 14, 5792–5796. [DOI] [PubMed] [Google Scholar]
- (25).Shevach M; Maoz BM; Feiner R; Shapira A; Dvir T, Nanoengineering gold particle composite fibers for cardiac tissue engineering. J. Mater. Chem. B 2013, 1, 5210–5217. [DOI] [PubMed] [Google Scholar]
- (26).Bredas JL; Street GB, Polarons, bipolarons, and solitons in conducting polymers. Accounts Chem. Res 1985, 18, 309–315. [Google Scholar]
- (27).MacDiarmid A; Chiang J; Richter A; Epstein AJ, Polyaniline: a new concept in conducting polymers. Synthetic. Met 1987, 18, 285–290. [Google Scholar]
- (28).MacDiarmid AG, “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Edit 2001, 40, 2581–2590. [DOI] [PubMed] [Google Scholar]
- (29).Ouyang L; Jafari MJ; Cai W; Aguirre LE; Wang C; Ederth T; Inganäs O, The contraction of PEDOT films formed on a macromolecular liquid-like surface. J. Mater. Chem. C 2018, 6, 654–660. [Google Scholar]
- (30).Checkol F; Elfwing A; Greczynski G; Mehretie S; Inganäs O; Admassie S, Highly stable and efficient lignin-PEDOT/PSS composites for removal of toxic metals. Adv. Sustain. Systems 2018, 2, DOI: 10.1002/adsu.201700114. [DOI] [Google Scholar]
- (31).Ravichandran R; Sundarrajan S; Venugopal JR; Mukherjee S; Ramakrishna S, Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Guimard NK; Gomez N; Schmidt CE, Conducting polymers in biomedical engineering. Prog. Polym. Sci 2007, 32, 876–921. [Google Scholar]
- (33).Hardy JG; Lee JY; Schmidt CE, Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Bio.Tech 2013, 24, 847–854. [DOI] [PubMed] [Google Scholar]
- (34).Thompson BC; Richardson RT; Moulton SE; Evans AJ; O’Leary S; Clark GM; Wallace GG, Conducting polymers, dual neurotrophins and pulsed electrical stimulation - dramatic effects on neurite outgrowth. J. Control. Release 2010, 141, 161–167. [DOI] [PubMed] [Google Scholar]
- (35).Harris AR; Wallace GG, Organic electrodes and communications with excitable cells. Adv. Funct. Mater 2017, 1700587. [Google Scholar]
- (36).Mawad D; Stewart E; Officer DL; Romeo T; Wagner P; Wagner K; Wallace GG, A single component conducting polymer hydrogel as a scaffold for tissue engineering. Adv. Funct. Mater 2012, 22, 2692–2699. [Google Scholar]
- (37).Kaur G; Adhikari R; Cass P; Bown M; Gunatillake P, Electrically conductive polymers and composites for biomedical applications. Rsc Adv. 2015, 5, 37553–37567. [Google Scholar]
- (38).Schmidt CE; Shastri VR; Vacanti JP; Langer R, Stimulation of neurite outgrowth using an electrically conducting polymer. P. Natl. A. Sci 1997, 94, 8948–8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Qazi TH; Rai R; Boccaccini AR, Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials 2014, 35, 9068–9086. [DOI] [PubMed] [Google Scholar]
- (40).Li MY; Guo Y; Wei Y; MacDiarmid AG; Lelkes PI, Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [DOI] [PubMed] [Google Scholar]
- (41).Zhao F; Shi Y; Pan LJ; Yu GH, Multifunctional nanostructured conductive polymer gels: Synthesis, properties, and applications. Accounts Chem. Res 2017, 50, 1734–1743. [DOI] [PubMed] [Google Scholar]
- (42).Balint R; Cassidy NJ; Cartmell SH, Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [DOI] [PubMed] [Google Scholar]
- (43).Liu S; Wang J; Zhang D; Zhang P; Ou J; Liu B; Yang S, Investigation on cell biocompatible behaviors of polyaniline film fabricated via electroless surface polymerization. Appl. Surf. Sci 2010, 256, 3427–3431. [Google Scholar]
- (44).Zhang Z; Roy R; Dugré FJ; Tessier D; Dao LH, In vitro biocompatibility study of electrically conductive polypyrrole-coated polyester fabrics. J. Biomed. Mater. Res. A 2001, 57, 63–71. [DOI] [PubMed] [Google Scholar]
- (45).Humpolicek P; Kasparkova V; Sáha P; Stejskal J, Biocompatibility of polyaniline. Synthetic. Met 2012, 162, 722–727. [Google Scholar]
- (46).Ramanaviciene A; Kausaite A; Tautkus S; Ramanavicius A, Biocompatibility of polypyrrole particles: an in-vivo study in mice. J. Pharm. Pharmacol 2007, 59, 311–315. [DOI] [PubMed] [Google Scholar]
- (47).Liu L; Li P; Zhou G; Wang M; Jia X; Liu M; Niu X; Song W; Liu H; Fan Y, Increased proliferation and differentiation of pre-osteoblasts MC3T3-E1 cells on nanostructured polypyrrole membrane under combined electrical and mechanical stimulation. J. Biomed. Nanotechnol 2013, 9, 1532–1539. [DOI] [PubMed] [Google Scholar]
- (48).Min Y; Liu Y; Poojari Y; Wu JC; Iii BEH; Rosol TJ; Epstein AJ, Self-doped polyaniline-based interdigitated electrodes for electrical stimulation of osteoblast cell lines. Synthetic. Met 2014, 198, 308–313. [Google Scholar]
- (49).Green RA; Lovell NH; Wallace GG; Poole-Warren LA, Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [DOI] [PubMed] [Google Scholar]
- (50).Wang L; Wu YB; Hu TL; Guo BL; Ma PX, Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [DOI] [PubMed] [Google Scholar]
- (51).Wang L; Wu YB; Guo BL; Ma PX, Nanofiber yarn/hydrogel core-shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. Acs Nano 2015, 9, 9167–9179. [DOI] [PubMed] [Google Scholar]
- (52).Spearman BS; Hodge AJ; Porter JL; Hardy JG; Davis ZD; Xu T; Zhang X; Schmidt CE; Hamilton MC; Lipke EA, Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells. Acta Biomater. 2015, 28, 109–120. [DOI] [PubMed] [Google Scholar]
- (53).Xu H; Holzwarth JM; Yan Y; Xu P; Zheng H; Yin Y; Li S; Ma PX, Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Chattopadhyay DK; Raju K, Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci 2007, 32, 352–418. [Google Scholar]
- (55).Broda CR; Lee JY; Sirivisoot S; Schmidt CE; Harrison BS, A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering. J. Biomed. Mater. Res. A 2011, 98, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Simitzi C; Ranella A; Stratakis E, Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 2017, 51, 21–52. [DOI] [PubMed] [Google Scholar]
- (57).Boyan BD; Lohmann CH; Dean DD; Sylvia VL; Cochran DL; Schwartz Z, Mechanisms involved in osteoblast response to implant surface morphology. Annu. Rev. Mater. Res 2001, 31, 357–371. [Google Scholar]
- (58).Guo BL; Finne-Wistrand A; Albertsson AC, Electroactive hydrophilic polylactide surface by covalent modification with tetraaniline. Macromolecules 2012, 45, 652–659. [Google Scholar]
- (59).Hardy JG; Khaing ZZ; Xin S; Tien LW; Ghezzi CE; Mouser DJ; Sukhavasi RC; Preda RC; Gil ES; Kaplan DL, Into the groove: instructive silk-polypyrrole films with topographical guidance cues direct DRG neurite outgrowth. J. Biomat. Sci-Polym. E 2015, 26, 1327–1342. [DOI] [PubMed] [Google Scholar]
- (60).Guo BL; Glavas L; Albertsson AC, Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci 2013, 38, 1263–1286. [Google Scholar]
- (61).Zelikin AN; Lynn DM; Farhadi J; Martin I; Shastri V; Langer R, Erodible conducting polymers for potential biomedical applications. Angew. Chem. Int. Edit 2002, 41, 141–144. [DOI] [PubMed] [Google Scholar]
- (62).Guimard NK; Sessler JL; Schmidt CE, Toward a biocompatible and biodegradable copolymer incorporating electroactive oligothiophene units. Macromolecules 2008, 42, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Cui HT; Liu YD; Deng MX; Pang X; Zhang PBA; Wang XH; Chen XS; Wei Y, Synthesis of biodegradable and electroactive tetraaniline grafted poly(ester amide) copolymers for bone tissue engineering. Biomacromolecules 2012, 13, 2881–2889. [DOI] [PubMed] [Google Scholar]
- (64).Cui H; Cui L; Zhang P; Huang Y; Wei Y; Chen X, In situ electroactive and antioxidant supramolecular hydrogel based on cyclodextrin/copolymer inclusion for tissue engineering repair. Macromol. Biosci 2014, 14, 440–450. [DOI] [PubMed] [Google Scholar]
- (65).Cui H; Wang Y; Cui L; Zhang P; Wang X; Wei Y; Chen X, In vitro studies on regulation of osteogenic activities by electrical stimulus on biodegradable electroactive polyelectrolyte multilayers. Biomacromolecules 2014, 15, 3146–3157. [DOI] [PubMed] [Google Scholar]
- (66).Liu Y; Hu J; Zhuang X; Zhang P; Wei Y; Wang X; Chen X, Synthesis and characterization of novel biodegradable and electroactive hydrogel based on aniline oligomer and gelatin. Macromol. Biosci 2012, 12, 241–250. [DOI] [PubMed] [Google Scholar]
- (67).Guo BL; Finne-Wistrand A; Albertsson AC, Facile synthesis of degradable and electrically conductive polysaccharide hydrogels. Biomacromolecules 2011, 12, 2601–2609. [DOI] [PubMed] [Google Scholar]
- (68).Guo BL; Finne-Wistrand A; Albertsson AC, Degradable and electroactive hydrogels with tunable electrical conductivity and swelling behavior. Chem. Mater 2011, 23, 1254–1262. [Google Scholar]
- (69).Gharibi R; Yeganeh H; Rezapour-Lactoee A; Hassan ZM, Stimulation of wound healing by electroactive, antibacterial, and antioxidant polyurethane/siloxane dressing membranes: in vitro and in vivo evaluations. ACS Appl. Mater. Inter 2015, 7, 24296–24311. [DOI] [PubMed] [Google Scholar]
- (70).Chen J; Dong RN; Ge J; Guo BL; Ma PX, Biocompatible, biodegradable, and electroactive polyurethane-urea elastomers with tunable hydrophilicity for skeletal muscle tissue engineering. ACS Appl. Mater. Inter 2015, 7, 28273–28285. [DOI] [PubMed] [Google Scholar]
- (71).Chen J; Guo B; Eyster TW; Ma PX, Super stretchable electroactive elastomer formation driven by aniline trimer self-assembly. Chem. Mater 2015, 27, 5668–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Deng Z; Guo Y; Zhao X; Li L; Dong R; Guo B; Ma PX, Stretchable degradable and electroactive shape memory copolymers with tunable recovery temperature enhance myogenic differentiation. Acta Biomater. 2016, 46, 234–244. [DOI] [PubMed] [Google Scholar]
- (73).Zhao Q; Qi HJ; Xie T, Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci 2015, 49, 79–120. [Google Scholar]
- (74).Zhao Q; Zou W; Luo Y; Xie T, Shape memory polymer network with thermally distinct elasticity and plasticity. Sci. Adv 2016, 2, e1501297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Zhao X; Dong R; Guo B; Ma PX, Dopamine-incorporated dual bioactive electroactive shape memory polyurethane elastomers with physiological shape recovery temperature, high stretchability, and enhanced C2C12 myogenic differentiation. ACS Appl. Mater. Inter 2017, 9, 29595–29611. [DOI] [PubMed] [Google Scholar]
- (76).Ma PX, Biomimetic materials for tissue engineering. Adv.Drug Deliver. Rev 2008, 60, 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Holzwarth JM; Ma PX, Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Arioz I; Erol O; Bakan G; Dikecoglu FB; Topal AE; Urel M; Dana A; Tekinay AB; Guler MO, Biocompatible electroactive tetra(aniline)-conjugated peptide nanofibers for neural differentiation. ACS Appl Mater Interfaces 2018, 10, 308–317. [DOI] [PubMed] [Google Scholar]
- (79).Jiang T; Carbone EJ; Lo KWH; Laurencin CT, Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci 2015, 46, 1–24. [Google Scholar]
- (80).Sun B; Long YZ; Zhang HD; Li MM; Duvail JL; Jiang XY; Yin HL, Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci 2014, 39, 862–890. [Google Scholar]
- (81).Zhang J; Qiu K; Sun B; Fang J; Zhang K; Hany E-H; Al-Deyab SS; Mo X, The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J. Mater. Chem. B 2014, 2, 7945–7954. [DOI] [PubMed] [Google Scholar]
- (82).Ostrovidov S; Ebrahimi M; Bae H; Nguyen HK; Salehi S; Kim SB; Kumatani A; Matsue T; Shi X; Nakajima K, Gelatin–polyaniline composite nanofibers enhanced excitation–contraction coupling system maturation in myotubes. ACS Appl. Mater. Inter 2017, 9, 42444–42458. [DOI] [PubMed] [Google Scholar]
- (83).Xie J; MacEwan MR; Willerth SM; Li X; Moran DW; Sakiyama-Elbert SE; Xia Y, Conductive core–sheath nanofibers and their potential application in neural tissue engineering. Adv. Funct. Mater 2009, 19, 2312–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Shafei S; Foroughi J; Stevens L; Wong CS; Zabihi O; Naebe M, Electroactive nanostructured scaffold produced by controlled deposition of PPy on electrospun PCL fibres. Res. Chem. Intermediat 2017, 43, 1235–1251. [Google Scholar]
- (85).Ku SH; Lee SH; Park CB, Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials 2012, 33, 6098–6104. [DOI] [PubMed] [Google Scholar]
- (86).Chen M-C; Sun Y-C; Chen Y-H, Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [DOI] [PubMed] [Google Scholar]
- (87).Baniasadi H; SA AR; Mashayekhan S, Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol 2015, 74, 360–366. [DOI] [PubMed] [Google Scholar]
- (88).Chen J; Ge J; Guo B; Gao K; Ma PX, Nanofibrous polylactide composite scaffolds with electroactivity and sustained release capacity for tissue engineering. J. Mater. Chem. B 2016, 4, 2477–2485. [DOI] [PubMed] [Google Scholar]
- (89).Ma X; Ge J; Li Y; Guo B; Ma PX, Nanofibrous electroactive scaffolds from a chitosan-grafted-aniline tetramer by electrospinning for tissue engineering. Rsc Adv. 2014, 4, 13652–13661. [Google Scholar]
- (90).Guex A; Spicer C; Armgarth A; Gelmi A; Humphrey E; Terracciano C; Harding S; Stevens M, Electrospun aniline-tetramer-co-polycaprolactone fibers for conductive, biodegradable scaffolds. MRS Cummun, 2017, 7, 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Liu YD; Cui HT; Zhuang XL; Wei Y; Chen XS, Electrospinning of aniline pentamer-graft-gelatin/PLLA nanofibers for bone tissue engineering. Acta Biomater. 2014, 10, 5074–5080. [DOI] [PubMed] [Google Scholar]
- (92).Lau HK; Kiick KL, Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules 2015, 16, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Li GF; Wu J; Wang B; Yan SF; Zhang KX; Ding JX; Yin JB, Self-healing supramolecular self-assembled hydrogels based on poly(L-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [DOI] [PubMed] [Google Scholar]
- (94).Ryu JH; Lee Y; Kong WH; Kim TG; Park TG; Lee H, Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [DOI] [PubMed] [Google Scholar]
- (95).Guo BL; Gao Q-Y, Preparation and properties of a pH/temperature-responsive carboxymethyl chitosan/poly (N-isopropylacrylamide) semi-IPN hydrogel for oral delivery of drugs. Carbohyd. Res 2007, 342, 2416–2422. [DOI] [PubMed] [Google Scholar]
- (96).Guiseppi-Elie A, Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 2010, 31, 2701–2716. [DOI] [PubMed] [Google Scholar]
- (97).Zhao WF; Glavas L; Odelius K; Edlund U; Albertsson AC, Facile and green approach towards electrically conductive hemicellulose hydrogels with tunable conductivity and swelling behavior. Chem. Mater 2014, 26, 4265–4273. [Google Scholar]
- (98).Zhao WF; Glavas L; Odelius K; Edlund U; Albertsson AC, A robust pathway to electrically conductive hemicellulose hydrogels with high and controllable swelling behavior. Polymer 2014, 55, 2967–2976. [Google Scholar]
- (99).Zhao WF; Nugroho RWN; Odelius K; Edlund U; Zhao CS; Albertsson AC, In situ cross-linking of stimuli-responsive hemicellulose microgels during spray drying. ACS Appl. Mater. Inter 2015, 7, 4202–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Zhao J; Zhao X; Guo BL; Ma PX, Multifunctional interpenetrating plymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules 2014, 15, 3246–3252. [DOI] [PubMed] [Google Scholar]
- (101).Zhao J; Guo BL; Ma PX, Injectable alginate microsphere/PLGA-PEG-PLGA composite hydrogels for sustained drug release. Rsc Adv. 2014, 4, 17736–17742. [Google Scholar]
- (102).Zhang L; Wang L; Guo BL; Ma PX, Cytocompatible injectable carboxymethyl chitosan/N-isopropylacrylamide hydrogels for localized drug delivery. Carbohyd. Polym 2014, 103, 110–118. [DOI] [PubMed] [Google Scholar]
- (103).Deng Z; Guo Y; Zhao X; Ma PX; Guo BL, Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater 2018, 30, 1729–1742. [Google Scholar]
- (104).Qu J; Zhao X; Ma PX; Guo B, Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 10.1016/j.actbio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- (105).Mawad D; Artzy-Schnirman A; Tonkin J; Ramos J; Inal S; Mahat MM; Darwish N; Zwi-Dantsis L; Malliaras GG; Gooding JJ, Electroconductive hydrogel based on functional poly (ethylenedioxy thiophene). Chem. Mater 2016, 28, 6080–6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Ding H; Zhong M; Kim YJ; Pholpabu P; Balasubramanian A; Hui CM; He H; Yang H; Matyjaszewski K; Bettinger CJ, Biologically derived soft conducting hydrogels using heparin-doped polymer networks. Acs Nano 2014, 8, 4348–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Zhao X; Guo B; Ma PX, Single component thermo-gelling electroactive hydrogels from poly (caprolactone)–poly (ethylene glycol)–poly (caprolactone)-graft-aniline tetramer amphiphilic copolymers. J. Mater. Chem. B 2015, 3, 8459–8468. [DOI] [PubMed] [Google Scholar]
- (108).Wu Y; Guo B; Ma PX, Injectable electroactive hydrogels formed via host–guest interactions. ACS Macro Lett. 2014, 3, 1145–1150. [DOI] [PubMed] [Google Scholar]
- (109).Qu J; Zhao X; Ma PX; Guo BL, pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [DOI] [PubMed] [Google Scholar]
- (110).Li LC; Ge J; Ma PX; Guo BL, Injectable conducting interpenetrating polymer network hydrogels from gelatin-graft-polyaniline and oxidized dextran with enhanced mechanical properties. Rsc Adv. 2015, 5, 92490–92498. [Google Scholar]
- (111).Li L; Ge J; Guo B; Ma PX, In situ forming biodegradable electroactive hydrogels. Polym. Chem 2014, 5, 2880–2890. [Google Scholar]
- (112).Dong R; Zhao X; Guo B; Ma PX, Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl. Mater. Inter 2016, 8, 17138–17150. [DOI] [PubMed] [Google Scholar]
- (113).Cui H; Shao J; Wang Y; Zhang P; Chen X; Wei Y, PLA-PEG-PLA and its electroactive tetraaniline copolymer as multi-interactive injectable hydrogels for tissue engineering. Biomacromolecules 2013, 14, 1904–1912. [DOI] [PubMed] [Google Scholar]
- (114).Jin E; Zhang Z; Lian H; Chen X; Xiao C; Zhuang X; Chen X, Injectable electroactive hydrogels based on Pluronic® F127 and tetraaniline copolymer. Eur. Polym. J 2017, 88, 67–74. [Google Scholar]
- (115).Kashi M; Baghbani F; Moztarzadeh F; Mobasheri H; Kowsari E, Green synthesis of degradable conductive thermosensitive oligopyrrole/chitosan hydrogel intended for cartilage tissue engineering. Int. J. Biol. Macromol 2018, 107, 1567–1575. [DOI] [PubMed] [Google Scholar]
- (116).Atoufi Z; Zarrintaj P; Motlagh GH; Amiri A; Bagher Z; Kamrava SK, A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: synthesis, characterization, drug release and cell culture study. J. Biomat. Sci-Polym. E 2017, 28, 1617–1638. [DOI] [PubMed] [Google Scholar]
- (117).Zhao X; Wu H; Guo B; Dong R; Qiu Y; Ma PX, Anti-bacterial antioxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [DOI] [PubMed] [Google Scholar]
- (118).Shahini A; Yazdimamaghani M; Walker KJ; Eastman MA; Hatami-Marbini H; Smith BJ; Ricci JL; Madihally SV; Vashaee D; Tayebi L, 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed 2014, 9, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Zhang M; Guo B, Electroactive 3D scaffolds based on silk fibroin and water-borne polyaniline for skeletal muscle tissue engineering. Macromol. Biosci 2017, 17, 1700147. [DOI] [PubMed] [Google Scholar]
- (120).Chen J; Yu M; Guo B; Ma PX; Yin Z, Conductive nanofibrous composite scaffolds based on In-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid. Interf. Sci 2018, 514, 517–527. [DOI] [PubMed] [Google Scholar]
- (121).Li LC; Yu M; Ma PX; Guo BL, Electroactive degradable copolymers enhancing osteogenic differentiation from bone marrow derived mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 471–481. [DOI] [PubMed] [Google Scholar]
- (122).Guex AG; Puetzer JL; Armgarth A; Littmann E; Stavrinidou E; Giannelis EP; Malliaras GG; Stevens MM, Highly porous scaffolds of PEDOT: PSS for bone tissue engineering. Acta Biomater. 2017, 62, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Xie M; Wang L; Ge J; Guo B; Ma PX, Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl. Mater. Inter 2015, 7, 6772–6781. [DOI] [PubMed] [Google Scholar]
- (124).Meng S; Zhang Z; Rouabhia M, Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J. Bone. Miner. Metab 2011, 29, 535–544. [DOI] [PubMed] [Google Scholar]
- (125).Levenberg S; Rouwkema J; Macdonald M; Garfein ES; Kohane DS; Darland DC; Marini R; Van Blitterswijk CA; Mulligan RC; D’Amore PA, Engineering vascularized skeletal muscle tissue. Nat. Biotechnol 2005, 23, 879. [DOI] [PubMed] [Google Scholar]
- (126).Koning M; Harmsen MC; van Luyn MJ; Werker P, Current opportunities and challenges in skeletal muscle tissue engineering. J. Tissue Eng. Regen. M 2009, 3, 407–415. [DOI] [PubMed] [Google Scholar]
- (127).Mohamadali M; Irani S; Soleimani M; Hosseinzadeh S, PANi/PAN copolymer as scaffolds for the muscle cell-like differentiation of mesenchymal stem cells. Polym. Advan. Technol 2017, 28, 1078–1087. [Google Scholar]
- (128).Jun I; Jeong S; Shin H, The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009, 30, 2038–2047. [DOI] [PubMed] [Google Scholar]
- (129).Dong R; Zhao X; Guo B; Ma PX, Biocompatible elastic conductive films significantly enhanced myogenic differentiation of myoblast for skeletal muscle regeneration. Biomacromolecules 2017, 18, 2808–2819. [DOI] [PubMed] [Google Scholar]
- (130).Wu YB; Wang L; Guo BL; Shao YP; Ma PX, Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [DOI] [PubMed] [Google Scholar]
- (131).Zhang Z; Rouabhia M; Wang Z; Roberge C; Shi G; Roche P; Li J; Dao LH, Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artif. Organs 2007, 31, 13–22. [DOI] [PubMed] [Google Scholar]
- (132).Shi Z; Gao H; Feng J; Ding B; Cao X; Kuga S; Wang Y; Zhang L; Cai J, In situ synthesis of robust conductive cellulose/polypyrrole composite aerogels and their potential application in nerve regeneration. Angew. Chem. Int. Edit 2014, 53, 5380–5384. [DOI] [PubMed] [Google Scholar]
- (133).Sun B; Wu T; Wang J; Li D; Wang J; Gao Q; Bhutto MA; El-Hamshary H; Al-Deyab SS; Mo X, Polypyrrole-coated poly (L-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous membranes promoting neural cell proliferation and differentiation with electrical stimulation. J. Mater. Chem. B 2016, 4, 6670–6679. [DOI] [PubMed] [Google Scholar]
- (134).Wu Y; Wang L; Hu T; Ma PX; Guo B, Conductive micropatterned polyurethane films as tissue engineering scaffolds for Schwann cells and PC12 cells. J. Colloid. Interf. Sci 2018, 518, 252–262. [DOI] [PubMed] [Google Scholar]
- (135).Wang S; Sun C; Guan S; Li W; Xu J; Ge D; Zhuang M; Liu T; Ma X, Chitosan/gelatin porous scaffolds assembled with conductive poly (3, 4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B 2017, 5, 4774–4788. [DOI] [PubMed] [Google Scholar]
- (136).Wu Y; Feng S; Zan X; Lin Y; Wang Q, Aligned electroactive TMV nanofibers as enabling scaffold for neural tissue engineering. Biomacromolecules 2015, 16, 3466–3472. [DOI] [PubMed] [Google Scholar]
- (137).Ghasemi-Mobarakeh L; Prabhakaran MP; Morshed M; Nasr-Esfahani MH; Ramakrishna S, Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [DOI] [PubMed] [Google Scholar]
- (138).Lee JY; Bashur CA; Goldstein AS; Schmidt CE, Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Yow S-Z; Lim TH; Yim EK; Lim CT; Leong KW, A 3D electroactive polypyrrole-collagen fibrous scaffold for tissue engineering. Polymers-Basel 2011, 3, 527–544. [Google Scholar]
- (140).Pires F; Ferreira Q; Rodrigues CA; Morgado J; Ferreira FC, Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. BBA-Gen. Subjects 2015, 1850, 1158–1168. [DOI] [PubMed] [Google Scholar]
- (141).Li D-F; Wang W; Wang H-J; Jia X-S; Wang J-Y, Polyaniline films with nanostructure used as neural probe coating surfaces. Appl. Surf. Sci 2008, 255, 581–584. [Google Scholar]
- (142).Di L; Wang L-P; Lu Y-N; He L; Lin Z-X; Wu K-J; Ren Q-S; Wang J-Y, Protein adsorption and peroxidation of rat retinas under stimulation of a neural probe coated with polyaniline. Acta Biomater. 2011, 7, 3738–3745. [DOI] [PubMed] [Google Scholar]
- (143).Guex AA; Vachicouras N; Hight AE; Brown MC; Lee DJ; Lacour SP, Conducting polymer electrodes for auditory brainstem implants. J. Mater. Chem. B 2015, 3, 5021–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Wu YB; Wang L; Guo BL; Ma PX, Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. Acs Nano 2017, 11, 5646–5659. [DOI] [PubMed] [Google Scholar]
- (145).Hsiao CW; Bai MY; Chang Y; Chung MF; Lee TY; Wu CT; Maiti B; Liao ZX; Li RK; Sung HW, Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials 2013, 34, 1063–72. [DOI] [PubMed] [Google Scholar]
- (146).Wang W; Tan B; Chen J; Bao R; Zhang X; Liang S; Shang Y; Liang W; Cui Y; Fan G, An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018, 160, 69–81. [DOI] [PubMed] [Google Scholar]
- (147).Jahromi MAM; Zangabad PS; Basri SMM; Zangabad KS; Ghamarypour A; Aref AR; Karimi M; Hamblin MR, Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv.Drug Deliver. Rev 2018, 123, 33–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).GhavamiNejad A; Park CH; Kim CS, In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application. Biomacromolecules 2016, 17, 1213–1223. [DOI] [PubMed] [Google Scholar]
- (149).Tandon B; Magaz A; Balint R; Blaker JJ; Cartmell SH, Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug. Deliv. Rev 2017, 10.1016/j.addr.2017.12.012. [DOI] [PubMed] [Google Scholar]
- (150).Guo BL; Sun Y; Finne-Wistrand A; Mustafa K; Albertsson AC, Electroactive porous tubular scaffolds with degradability and non-cytotoxicity for neural tissue regeneration. Acta Biomater. 2012, 8, 144–153. [DOI] [PubMed] [Google Scholar]
- (151).Zare EN; Lakouraj MM; Mohseni M, Biodegradable polypyrrole/dextrin conductive nanocomposite: synthesis, characterization, antioxidant and antibacterial activity. Synthetic. Met 2014, 187, 9–16. [Google Scholar]
- (152).Zhao X; Li P; Guo BL; Ma PX, Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [DOI] [PubMed] [Google Scholar]
- (153).Kim KS; Yeum JH; Ajayan PM; Mollah ML; Aijaz MO; Dar MA; Al-Ahmari A; Karim MR, Conducting and biopolymer based electrospun nanofiber membranes for wound healing applications. Curr. Nanosci 2016, 12, 220–227. [Google Scholar]
- (154).Moutsatsou P; Coopman K; Georgiadou S, Biocompatibility assessment of conducting PANI/chitosan nanofibers for wound healing applications. Polymers-Basel 2017, 9, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]