Abstract
Objective.
While individuals with 22q11.2 deletion syndrome (22q11DS) are at increased risk for a variety of functional impairments and psychiatric disorders, including psychosis, not all individuals with 22q11DS experience negative outcomes. Efforts to further understand which childhood variables best predict adult functional outcomes are needed, especially those that investigate childhood executive functioning abilities.
Methods.
This longitudinal study followed 63 individuals with 22q11DS and 43 control participants over 9 years. Childhood executive functioning ability was assessed using both rater-based and performance-based measures and tested as predictors of young adult outcomes.
Results.
Childhood global executive functioning abilities and parent report of child executive function abilities were the most consistent predictors of young adult outcomes. Study group moderated the relationship between child executive functioning and young adult outcomes for several outcomes such that the relationships were stronger in the 22q11DS sample.
Conclusion.
Rater-based and performance-based measures of childhood executive functioning abilities predicted young adult outcomes in individuals with and without 22q11DS. Executive functioning could be a valuable target for treatment in children with 22q11DS for improving not only childhood functioning but also adult outcomes.
Keywords: 22q11.2 deletion syndrome, developmental disorder, executive functioning, longitudinal, psychosis, adaptive functioning
(22q11DS), also known as velo-cardio-facial syndrome (VCFS), is a genetic disorder caused by the deletion of a segment of chromosome 22 at the q11.2 band (Shapiro, Tassone, Choudhary, & Simon, 2014). 22q11DS affects approximately 1 out of every 1,000–7,000 births (Botto et al., 2003; Grati et al., 2015) and is characterized by immunologic deficiencies, hypocalcemia, congenital cardiac anomalies, palate anomalies and mild facial dysmorphism (Shprintzen, 2000).
Psychotic symptoms are present in nearly 30% of the 22q11DS adult population, and about 22% later develop schizophrenia or schizoaffective disorder (Bassett et al., 2005). In addition to psychosis, attention deficit hyperactivity disorder (ADHD) (15–37% prevalence rates depending upon age) and anxiety disorders (27–35%) are especially prevalent in individuals with 22q11DS (Schneider et al., 2014). Individuals with 22q11DS also have social adjustment problems and lower abilities across multiple domains of cognition (Fung et al., 2015). Intellectual abilities of individuals with 22q11DS are impaired when compared to the typically developing population, with mean full scale IQ’s in the Borderline range of intelligence (Vorstman et al., 2015).
Executive Functioning in 22q11DS
In addition to generalized intellectual deficits, individuals with 22q11DS have executive functioning (EF) impairments (Antshel, Fremont, & Kates, 2008). EF is an umbrella term that encompasses various cognitive domains, including cognitive flexibility, planning, self-regulation, and working memory (Anderson, 2002; Harms, Zayas, Meltzoff, & Carlson, 2014). EF abilities develop in a progressive fashion, emerging in early childhood and developing into adulthood (Bunge & Zelazo, 2006; Maeder et al., 2016). Childhood EF abilities predict adolescent and adult academic and occupational functioning in individuals with and without ADHD (Miller, Nevado-Montenegro, & Hinshaw, 2012).
Inhibition (Shapiro et al., 2014), working memory (Maeder et al., 2016) and cognitive flexibility (Antshel et al., 2010) deficits have been reported in the 22q11DS population. Most of the literature reports lower performance in all EF domains for individuals with 22q11DS when compared to the typically developing population (Antshel et al., 2008). Nonetheless, there are mixed findings about the developmental trajectory of EF in individuals with 22q11DS, and whether EF improves as a function of age and time. Shapiro et al. (2014) reported that working memory develops in the same trajectory as in the typically developing population, just with weaker performance across time compared to age-norms (i.e., standardized scores). Others (Chawner et al., 2017) have similarly reported that EF improves over time yet never reaches the levels of typically developing peers. Conversely, Maeder et al. (2016) found that individuals with 22q11DS do not improve in EF with age and time when compared to age-norms (i.e., standardized scores) in two domains of EF: verbal fluency and auditory/verbal working memory. Antshel and colleagues followed children with 22q11DS into adulthood and reported that after controlling for full scale IQ differences, children with 22q11DS differed significantly from controls in longitudinal trajectories of visual and auditory/verbal working memory yet not in other EF trajectories. In both working memory domains, differences between controls and individuals with 22q11DS increased over time (Antshel, Fremont, Ramanathan, & Kates, 2017).
Executive functions and psychosis.
Cross-sectionally, EF deficits have been reported to have inconsistent associations with psychosis in children with 22q11DS (Lajiness-O’Neill et al., 2006; Niarchou et al., 2014). In adolescence and young adulthood, EF deficits have also been inconsistently associated with psychotic symptoms; some findings show positive associations (Chow, Watson, Young, & Bassett, 2006; van Amelsvoort et al., 2004; Weinberger et al., 2016), yet one study demonstrated no association (Yi et al., 2015). In addition to the inconsistent nature of these findings, cross-sectional studies do not permit investigating the temporal nature of the relationship between EF and psychosis.
Longitudinally, one study reported that childhood EF predicted psychotic symptoms in mid-adolescence (Antshel et al., 2010) yet this study is limited by the short follow-up period of 3 years. In a longer follow-up period of 9 years, in the same cohort, Antshel and colleagues reported that cognitive flexibility trajectories predicted psychotic symptoms in adulthood. Individuals with 22q11DS who developed psychotic symptoms improved less appreciably and continued to demonstrate difficulties with cognitive flexibility relative to individuals with 22q11DS who did not have psychotic symptoms (Antshel et al., 2017). To our knowledge, no other study has considered the association between childhood EF and psychosis using a longitudinal design. Given the consistent associations between childhood EF and adult psychosis noted in the idiopathic psychosis literature (Fusar-Poli et al., 2013), this represents a void in the 22q11DS literature.
Executive functions and psychosocial functional outcomes.
Cross-sectionally, mixed results have been reported about the associations between EF abilities and psychosocial outcomes. For example, in adolescents with 22q11DS, IQ was associated with social competence abilities, while EF was not (Campbell, McCabe, Melville, Strutt, & Schall, 2015). In children with 22q11DS, however, a cross-sectional association between social functioning and EF has been reported (Kiley-Brabeck & Sobin, 2006). Longitudinally, while there have been multiple studies investigating which childhood intellectual variables best predict psychosocial 22q11DS outcomes in adolescence and adulthood (Fiksinski et al., 2017; Gothelf et al., 2013; Hooper et al., 2013; Schneider et al., 2014; Tang et al., 2017), surprisingly none of these studies have investigated EF – all focused on IQ. Given the predictive abilities of childhood EF to later social (Rinsky & Hinshaw, 2011), educational (Sjowall, Bohlin, Rydell, & Thorell, 2017) and occupational (Barkley & Fischer, 2011) functioning outcomes in the non-22q11DS literatures, this also represents a void in the 22q11DS literature.
Rationale for Current Study
While multiple studies have reported EF deficits in 22q11DS across the lifespan, very few studies have investigated the relationship between childhood EF and adult outcomes. The present study aims to fill this research void. While one previous study (Antshel et al., 2017) investigated longitudinal trajectories of EF as predictors for psychosis, the study (a) did not investigate childhood EFs per se as predictors, (b) did not consider parent report of EF as a variable and (c) only focused on psychosis. Thus, we presently know very little about how childhood EF is associated with adult outcomes. In addition to being innovative, this research question also holds potential clinical significance. Findings from the present study may permit a greater understanding of which children with 22q11DS are at greatest risk for negative developmental outcomes, and may facilitate early, targeted intervention and prevention strategies.
Given the non-22q11DS research findings, we hypothesize that childhood EF will be negatively associated with adult functioning whereas verbal learning and memory (a non-EF ability) will not. More specifically, we hypothesized that childhood EF abilities would be less well developed in children with 22q11DS compared to control participants. Trajectories of EF abilities would be similar between 22q11DS and control participants. Finally, we hypothesized that study group would not have a moderating effect on the relationship between child EF and young adult outcomes.
Method
Participants
This 9-year longitudinal study followed individuals with 22q11DS across four time points, with data collected at baseline, year 3, year 6, and year 9. Children with 22q11DS were initially recruited from a large academic medical center in the northeastern United States during the years 2002–2005. Only those children with a fluorescence in situ hybridization-confirmed deletion of 22q11.2 were included. All families that met inclusion criteria agreed to participate. Specifically, children with 22q11DS, and their age and gender matched siblings were recruited from the 22q11DS Center at SUNY-Upstate Medical University. In addition, age and gender matched community control participants were recruited from local public schools via advertisements. Children in the community control group were excluded if they were not instructed in a general education classroom. In all three groups, children with an identifiable genetic disorder other than 22q11DS or children with an identifiable neurological condition (e.g., traumatic brain injury, pre-term birth) that is known to affect cognitive or psychiatric function were excluded from participation. Given the developmental delays that are associated with 22q11DS, no attempt was made to exclude sibling or community control participants with ADHD and learning disabilities (LD). Children community control group were excluded if they were not instructed in a general education classroom. Due to the lack of significant differences on all independent and dependent variables between our sibling controls and community controls (p’s > .60), the two control groups were combined to form one control group. Our control group consisted of 22 community controls and 21 sibling controls.
For the current project, only participants who had Time 4 (young adult) data and who also had baseline data were included. Our total sample consisted of 63 children with 22q11DS (34 males) and 43 control participants (22 males). No gender differences, X2 (1) = 1.22, p = .543 or differences in age between the two groups existed at both baseline, F (1,104) = 1.98, p = .161: 22q11DS = 12.2 years (SD = 2.3); Controls = 11.8 (SD=2.0), and young adulthood, F (1,104) = 0.63, p = .424: 22q11DS = 21.2 years (SD = 2.2); Controls = 20.9 (SD=2.0). No Hollingshead scale socioeconomic status or racial/ethnicity differences were present between the two groups (p > .05).
In young adulthood, control participants were more likely to live independently (27%) compared to young adults with 22q11DS (10%), X2 (1) = 5.10, p = .024. Likewise, control participants were more likely to be employed (77%) compared to young adults with 22q11DS (46%), X2 (1) = 9.48, p = .002. Within the subsample of those that were employed, control participants worked more hours per week (30.38 hours per week, SD = 9.98) compared to young adults with 22q11DS (14.69 hours per week, SD = 9.51), F (1, 62) = 37.54, p < .001, η2 = .40.
An independent samples t-test found no differences in attrition between our two groups, t (1) = 3.44, p = .222. Furthermore, participants lost to follow-up did not differ from those retained on any relevant Time 1 sociodemographic measures including participant age, gender, and socioeconomic status. In addition, participants lost to follow-up did not differ from those retained on any relevant Time 1 functional or cognitive variables (p > .05). Thus, those participants who completed Time 4 assessments appear representative of the Time 1 sample.
Measures
Young adult intellectual ability measure.
Time 4 Wechsler Adult Intelligence Scale – Third edition.
The Wechsler Adult Intelligence Scale – Third edition (WAIS-III; Wechsler, 1993) is a commonly used psychological test for measuring intellectual abilities. For the current study, only the Full Scale IQ (FSIQ) was used. Please see Supplemental Table 1 for comprehensive table detailing each measure used in the current study.
Young adult parent report dependent variables.
Time 4 Vineland Adaptive Behavior Scales, Second Edition.
The Vineland Adaptive Behavior Scales- 2nd edition (VABS-II; Sparrow, Cicchetti, & Balla, 2005) Parent/Caregiver Rating Form asks parents to rate their adult child’s ability to independently perform behaviors across three domains: Communication, Daily Living Skills, and Socialization. Standard scores (M= 100, SD= 15) are provided for each domain, with higher scores indicating better functioning. For the current study, only the VABS-II Total Composite was used.
Time 4 Behavior Rating Inventory of Executive Functioning - Adult version.
The Behavior Rating Inventory of Executive Functioning – Adult version (BRIEF-A; Roth, Isquith, & Gioia, 2006) is an ecologically standardized measure that assesses a collateral reporter’s views of an adult’s executive functioning in their everyday environment. The BRIEF-A contains 75 items that load onto 9 subdomains. The Global Executive Composite (GEC) Index, which represents the overall EF score, was used in this study. Raw scores are transformed into T scores for results interpretation, with higher scores indicate weaker EF abilities.
Young adult clinician rated dependent variable.
Time 4 Structured Interview for Prodromal Symptoms.
The Structured Interview for Prodromal Symptoms (SIPS; Miller et al., 2003) is a commonly used structured interview that evaluates current psychosis symptoms and clinical risk of psychosis. Previous research indicates that the SIPS has good predictive value of correctly identifying 67% of individuals who later developed psychosis at a 24 month follow up (Miller et al., 2003). In the current study, the full SIPS was administered both to participants and to their parents in young adulthood (Time 4). If a discrepancy existed between raters, the more severe rating was entered by the clinician.
For the current study, only the Positive Symptom domain score was used in analyses. This decision was based upon positive symptoms being more specific to psychosis than negative symptoms (Keshavan, Nasrallah, & Tandon, 2011), especially in a condition such as 22q11DS, in which the majority of individuals have negative symptoms such as social isolation (Schneider et al., 2014). The Positive Symptom domain includes questions related to the presence of positive psychotic symptoms, such as unusual thought content, suspiciousness, ideas of grandiosity or persecution with delusional features, hallucinations, or disorganized speech. Higher scores on the SIPS indicate the presence of more positive symptoms of psychosis. No control participants were reported to have psychotic symptoms. In contrast, 22% of participants with 22q11DS were reported to have psychotic symptoms at Time 4 (p < .001).
Young adult self-report dependent variable measures.
Time 4 ecologically valid functioning measures.
Information regarding the young adult’s living status and occupation was obtained. Living status was dichotomized into independent (living on his/her own) or dependent (living with parents, living in group home, etc.). Employment was dichotomized into employed/non-employed. For those that were employed, a continuous measure of the number of hours per week was obtained.
Time 4 Behavior Rating Inventory of Executive Functioning - Adult version.
The Behavior Rating Inventory of Executive Functioning – Adult version (BRIEF-A; Roth et al., 2006) was used to assess self-report of executive functioning in his/her everyday environment. Please see above for description of the BRIEF-A.
Time 4 Adult Self-Report.
The Adult Self Report (ASR; Achenbach & Rescorla, 2003) scale was used to assess self-perceptions of symptoms in the young adults with 22q11DS. The ASR consists of 123 statements that load onto 8 scales. The ASR has solid psychometric properties (Achenbach & Rescorla, 2003). For the current study, only the ADHD Problems, Internalizing and Externalizing composites were used. This decision was made due to the high prevalence of psychopathology, especially ADHD, in 22q11DS (Schneider et al., 2014), the associations between ADHD and executive dysfunction (Hervey, Epstein, & Curry, 2004) as well as a desire to reduce the likelihood of a Type I error.
Time 4 Social Adjustment Scale - Self-Report.
The Social Adjustment Scale – Self-Report (SAS-SR; Weissman, 1999) is a 54-item self-report scale that measures social adjustment. The measure is intended for individual’s ages 17 years and older and identifies six social role areas: work, social and leisure activities, relationships with extended family, role as a spouse or partner, parental role, and role within the family unit. An area is not assessed if the respondent indicates that the questions are not relevant to them (i.e., if the respondent does not have children or is not married). Each item is rated on a five-point Likert scale (1=always, 5=never). Scores are transformed into T-scores (M= 50, SD= 10) based on a normative sample, with higher scores indicating more social impairment. For the current study, only the SAS-SR Total Composite was used.
Time 4 Trait Emotional Intelligence Questionnaire-Short Form.
The Trait Emotional Intelligence Questionnaire – Short Form (TEIQue-SF; Cooper & Petrides, 2010) is a 30-item questionnaire based on the full, 153 –item TEIQue (Petrides, 2009), which is designed to measure 15 facets of emotional intelligence, including traits of adaptability, assertiveness, emotion expression, emotion perception, emotion regulation, emotion management, impulse control, relationships, self-esteem, self-motivation, social awareness, stress management, trait empathy, trait happiness and trait optimism. Two items from each of these facets of emotional intelligence were included in the Short Form, based on their correlation with total facet scores from the full form. A single, global score for Emotional Intelligence is derived and was used in the current study. The Short Form has solid psychometric properties (Cooper & Petrides, 2010).
Childhood Executive Functioning Predictors
Wechsler Intelligence Scale for Children- 3rd edition.
The Wechsler Intelligence Scale for Children-3rd edition (WISC-III; Wechsler, 1991) Digit Span was used to assess working memory.
Gordon Diagnostic System.
The Gordon Diagnostic System (GDS; Gordon, 1983) is a performance based continuous performance test (CPT) that measures sustained attention and response inhibition. For the present study, only the standardized omission and commission errors score were used in the analyses.
Tower of London.
The Tower of London (TOL; Shallice, 1982) has demonstrated good construct validity as a measure of planning (Culbertson & Zillmer, 1998). The total number of moves was used in analyses.
Wisconsin Card Sorting Test.
The Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Talley, Kay, & Curtiss, 1993) is a task that measures cognitive flexibility. Standard scores for perseverative errors and non-perseverative errors was used in our analyses. The WCST was manually administered.
Stroop Color-Word Test.
The Stroop Color-Word Test (Golden, 1978) measures cognitive flexibility, selective attention and response inhibition. The interference T-score was used in the present study.
Visual Span Test.
The Visual Span Test (VSPAN; Davis, 1998) is a computer-based test that assesses visual working memory abilities. The forward and backward span standardized z-scores were used in the current study.
Behavior Rating Inventory of Executive Functioning.
The Behavior Rating Inventory of Executive Functioning (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000) is an ecologically standardized measure that assesses a parents’ views of a child’s executive functioning in their everyday environment. For the current study, only the two composites, the Behavioral Regulation Index (BRI) and the Metacognitive Index (MI) were used.
Childhood Non-Executive Functioning Predictor
To assess the specificity of these findings to EF, a learning/memory measure was used as a control variable.
California Verbal Learning Test-Children’s version
The California Verbal Learning Test-Children’s Version (CVLT-C; Delis, Kramer, Kaplan, & Ober, 1994) measures auditory/verbal learning and working memory. Standardized scores for List A Trial 1 (recall after hearing the list once), List A Trial 5 (recall after hearing the list five times), List B (interference), Short Delay Recall, Long Delay Recall and Perseverations/Intrusions were used in analyses.
Procedures
Informed consent and assent was attained from parents and participants and all data included in this manuscript was obtained in compliance with regulations of SUNY-Upstate Medical University. At both time periods, a doctoral-level examiner administered all psychological tests to participants in a quiet room. Parents completed all parent-report rating scales in a separate room.
Statistical Analyses
All analyses were conducted in SPSS Statistics Version 23, with statistical significance set to p < .05. When appropriate, effect size is reported using eta squared (η2) or Cohen’s d. First, T-tests were used to examine group mean differences between children with 22q11DS and control children on baseline EF abilities and adaptive behavior. Repeated measures ANOVA was then used to examine parent reported EF trajectories in children with 22q11DS and control children from baseline to young adulthood.
Due to the multivariate and intercorrelated nature of the EF data collected from participants at Time 1, principal components analysis (PCA) was used as a data reduction technique to identify the key components of EF. Twelve different EF variables, including both rater-based and performance-based measures, were included in the principal components analyses, as listed in Table 2. In addition, CVLT-C variables were included in the PCA to test the specificity of our findings to EF. PCA on these 18 items was conducted using Varimax rotation and Kaiser normalization. All components with Eigen values greater than 1 were considered to be meaningful factors. A loading cutoff of 0.4 was used to determine which EF measures loaded onto each identified factor. PCA was conducted across the total sample.
Table 2.
Rotated Component Matrix from Principal Components Analysis of Time 1 Executive Functioning Measures
| Rotated Component Matrix | ||||||
|---|---|---|---|---|---|---|
| Component |
||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| CVLT – C List A Trial 1 | 0.573 | |||||
| CVLT – C List A Trial 5 | 0.851 | |||||
| CVLT – C Short Delay Recall | 0.849 | |||||
| CVLT – C Long Delay Recall | 0.847 | |||||
| CVLT – C Perseverations | 0.533 | |||||
| CVLT – C Intrusions | −0.417 | −0.508 | ||||
| WCST – Perseverative Errors | 0.741 | |||||
| WCST – Non-Perseverative Errors | 0.700 | |||||
| WISC – III Digit Span Backward | 0.560 | |||||
| Tower of London - Total | −0.418 | −.479 | −0.453 | |||
| GDS - Total Correct | 0.814 | |||||
| GDS - Commissions | 0.839 | |||||
| VSPAN Forward | 0.830 | |||||
| VSPAN Backward | 0.721 | |||||
| BRIEF - Parent Report - Behavioral Regulation Index | 0.877 | |||||
| BRIEF - Parent Report - Metacognition Index | 0.833 | |||||
| Stroop Interference Score | 0.647 | |||||
| WISC – III Digit Span Forward | 0.677 | |||||
Note: BRIEF = Behavioral Rating Inventory of Executive Functioning; WISC-III = Wechsler Intelligence Scale for Children- 3rd edition; WCST = Wisconsin Card Sorting Test; Stroop = Stroop Color-Word Test; CVLT-C = California Verbal Learning Test-Children’s version. VSPAN = Visual Span. GDS = Gordon Diagnostic System Continuous Performance Test.
Before proceeding with analyses examining childhood EF as a predictor of young adult outcomes in individuals with 22q11DS, all young adult outcome variables of interest were examined for normality. For normally distributed outcome variables, stepwise linear regression was utilized with the first step including the relevant baseline (childhood) dependent variable as a covariate, and the second step including the EF factors identified through the PCA.
For non-normally distributed outcome variables, negative binomial regression was used. Similar to the linear regression analyses, the first step in the negative binomial regressions included the relevant baseline (childhood) dependent variable. Models that were trending toward significance were re-examined using only factors identified as being significant predictors of previously examined outcomes, to increase statistical power. For each outcome variable that was significantly predicted by childhood EF among individuals with 22q11DS, models were reexamined, adding baseline IQ as a first step, in order to determine if EF uniquely predicts these outcomes, or if IQ might be driving these relationships.
As a final step, for each outcome variable that was significantly predicted by childhood EF among individuals with 22q11DS, models were re-examined including control participants. Only the EF factors that were significant predictors for individuals with 22q11DS were included in these models, and study group and interaction terms between study group and each EF factor were added to determine if study group has a moderating effect on the relationship between EF and young adult outcomes.
Results
Executive Functioning in 22q and Control Children
Childhood variables.
Consistent with our hypothesis, children with 22q11DS performed worse on all childhood EF measures compared to control children (all p’s ≤ .01), except for Stroop interference, GDS total, and digit span forward scores. Effect sizes ranged from medium to large (Cohens d = 0.5 – 1.9). Children with 22q11DS also had significantly more impaired functioning in each domain of adaptive behavior measured by the Vineland at Time 1 (all p’s < .001). Effect sizes were all large (Cohens d = 1.2 – 1.6). See Table 1 for a summary of means and standard deviations for participants with and without 22q11DS on baseline measures.
Table 1.
Baseline Cognitive Functioning and Adaptive Functioning
| Time 1 Measure | 22q11DS N = 63 Mean(SD) |
Control N = 43 Mean(SD) |
Effect Size Cohen’s d |
|---|---|---|---|
| CVLT-C - List A Trial 1 *** | −0.94 (1.02) | −0.20 (0.84) | 0.79 |
| CVLT-C - List A Trial 5 *** | −1.09 (1.31) | −0.12 (1.02) | 0.83 |
| CVLT-C - Short Delay Recall *** | −1.07 (1.22) | −0.08 (0.94) | 0.91 |
| CVLT-C - Long Delay Recall *** | −1.09 (1.19) | −0.14 (0.94) | 0.89 |
| CVLT-C - Perseverations | −0.30 (0.84) | −0.19 (0.75) | 0.14 |
| CVLT-C - Intrusions | 0.05 (.83) | −0.26 (1.15) | 0.31 |
| BRIEF- Parent Report- Behavioral Regulation Index *** | 62.61 (13.32) | 51.08 (9.69) | 0.99 |
| BRIEF- Parent Report- Metacognition Index *** | 66.65 (10.58) | 53.45 (10.79) | 1.24 |
| Stroop Interference Score | 46.02 (8.85) | 47.65 (7.96) | 0.19 |
| Tower of London- Total *** | 141.98 (48.22) | 117.15 (23.41) | 0.66 |
| VSPAN Forward *** | −0.86 (0.82) | −0.19 (0.82) | 0.82 |
| VSPAN Backward *** | −1.35 (1.03) | −0.40 (1.01) | 0.93 |
| GDS - Total Correct | −0.94 (2.11) | −0.35 (2.19) | 0.27 |
| GDS - Commissions ** | −3.00 (4.28) | −1.08 (3.01) | 0.52 |
| WCST - Perseverative Errors *** | 68.69 (13.17) | 93.48 (12.93) | 1.90 |
| WCST - Non Perseverative Errors ** | 80.35 (14.16) | 87.48 (14.18) | 0.50 |
| WISC-III - Full Digit Span Forward | 0.06 (0.83) | 0.21 (0.77) | 0.19 |
| WISC-III - Full Digit Span Backward *** | −0.85 (0.64) | −0.29 (0.64) | 0.88 |
| WISC-III - Full Scale IQ *** | 71.94 (12.49) | 100.27 (12.81) | 2.24 |
| Vineland- Communication *** | 70.34 (18.60) | 91.44 (16.69) | 1.19 |
| Vineland- Daily Living *** | 66.50 (19.77) | 93.61 (14.63) | 1.56 |
| Vineland- Socialization *** | 76.43 (19.02) | 98.54 (15.20) | 1.28 |
| Vineland- Composite *** | 66.80 (16.30) | 92.98 (15.80) | 1.63 |
Note: BRIEF = Behavioral Rating Inventory of Executive Functioning; WISC-III = Wechsler Intelligence Scale for Children- 3rd edition; WCST = Wisconsin Card Sorting Test; Stroop = Stroop Color-Word Test; CVLT-C = California Verbal Learning Test-Children’s version. VSPAN = Visual Span. GDS = Gordon Diagnostic System Continuous Performance Test.
p < .05;
p < .01;
p < .001.
Parent reported EF developmental trajectory.
A 2×2 repeated measures ANOVA examining the effects of time (from T1 to T4) and diagnostic status (22q vs. control) on the BRIEF-A GEC revealed main effects of both time (F(1, 50) = 18.87, p < .001, η2 = .27) and diagnosis (F(1, 50) = 48.91, p < .001, η2 = .49), such that parent-reported EF problems declined slightly from time 1 to time 4 in both groups, and EF was more impaired in 22q11DS than controls at both times. Consistent with our hypothesis, there was no significant interaction between group and time (F(1, 50) = .16, p = .69), suggesting that participants with 22q11DS begin with, and maintain significantly greater parent- reported impairments in EF compared to control participants.
Principal Components Analysis
Principal Components Analysis utilizing Varimax rotation and Kaiser normalization was conducted on the entire sample with all Time 1 EF and verbal learning measures and resulted in a six factor solution. Factor 1 explained 31% of the variance (Eigen value = 5.64), consisted of all CVLT-C measures (trial 1, trial 5, short delay performance, long delay performance, intrusions, and perseverations) and was determined to represent an auditory verbal learning and memory factor. Higher scores on this factor indicate better verbal learning and memory. Factor 2 explained 10% of the variance (Eigen value = 1.76), consisted of WISC-III digit span backward, WCST perseverative and non-perseverative errors, and TOL measures and was determined to represent a global EF factor. Higher scores on this factor indicate better global EF. Factor 3 explained 9% of the variance (Eigen value = 1.64), consisted of GDS commissions, GDS total correct, CVLT-C intrusions, and TOL measures and was determined to represent a response inhibition factor. Higher scores on this factor indicate better response inhibition. Factor 4 explained 7% of the variance (Eigen value = 1.20), consisted of VSPAN forward, VSPAN backward, and TOL and was determined to represent a visual working memory factor. Higher scores on this factor indicate better visual working memory. Factor 5 explained 6% of the variance (Eigen value = 1.10), consisted of BRIEF metacognition and behavioral regulation indices and was determined to represent a parent report of child EF factor. Lower scores on this factor indicate better parent-reported EF. Factor 6 explained 6% of the variance (Eigen value = 1.05), consisted of Stroop Interference and WISC-III digit span forward, and was determined to represent a self-monitoring factor. Higher scores on this factor indicate better self-monitoring. See Table 2 for the rotated component matrix. For interpretation of the following results, it is important to note that for factor 5, lower scores are better, whereas for all other PCA factors, higher scores are better.
Childhood EF as a Predictor of Young Adult Outcomes
Using the 6 factors identified through the principal components analysis, childhood EF was examined as a predictor of young adult outcomes.
Predicting young adult psychopathology.
Negative binomial regression revealed that for individuals with 22q11DS, childhood EF significantly predicted SIPS clinician rated positive symptoms of psychosis in young adulthood, with factors 5 (parent report of childhood EF; B = 2.02, p < .05) and 6 (self-monitoring; B = −3.41, p < .01) reaching significance. Across all factors, and consistent with our hypothesis, worse childhood EF skills were predictive of greater SIPS symptoms of psychosis in young adulthood. Factors 5 and 6 remained significant when baseline IQ was added as a covariate in the model, suggesting that childhood EF is a unique predictor of young adult psychotic symptoms.
Stepwise linear regression revealed no significant effects of childhood EF on ASR young adult self-reported internalizing problems among individuals with 22q11DS. However, there were significant effects of childhood EF on ASR young adult self-reported externalizing problems. Factors 2 (global EF; B = −7.42, p < .05) and 5 (parent report of childhood EF; B = 12.61, p < .001) significantly predicted ASR self-reported externalizing problems in young adults with 22q11DS. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that EF is a unique predictor of externalizing problems. Factor 2 was no longer significant, however this is likely due to the fact that factor 2 includes digit span, which is a subcomponent of IQ. Inconsistent with our hypothesis, when control participants were included in the model predicting externalizing problems, and study group was examined as a potential moderator with factors 2 and 5, there was a significant study group x factor 2 interaction (B = −8.30, p < .01). This suggests that the relationship between global EF and young adult externalizing problems is different for individuals with and without 22q11DS. More specifically, worse childhood global EF is associated with greater young adult externalizing problems for individuals with 22q11DS, but not for control participants. Please see Supplemental Tables 2 – 7 for regression analyses including IQ as a covariate.
There was a trend toward significance when predicting self-reported ASR young adult ADHD problems from childhood EF among individuals with 22q11DS (F = 2.233, p = .078). When only factors 2, 5, and 6 were included in the model, the model became significant (F = 3.75, p < .05), with factors 2 (global EF; B = −4.55, p < .01) and 5 (parent report of childhood EF; B = 6.45, p < .01) significantly predicting ASR young adult ADHD problems. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that EF is a unique predictor of ADHD problems. Factor 2 was no longer significant, however this is likely due to the fact that factor 2 includes digit span, which is a subcomponent of IQ. Also inconsistent with our hypothesis, when control participants were included in the model predicting ADHD problems, and study group was examined as a potential moderator with factors 2 and 5, there was a significant study group x factor 2 interaction (B = −8.79, p < .01). This suggests that the relationship between global EF and young adult ADHD problems is different for individuals with and without 22q11DS. More specifically, worse childhood global EF is associated with greater young adult ADHD problems for individuals with 22q11DS, but not for individuals without 22q11DS.
Predicting young adult psychosocial functioning.
For young adult social, emotional, and intellectual functioning outcomes, stepwise linear regression revealed that childhood EF significantly predicted VABS-II parent-reported young adult adaptive behavior among individuals with 22q11DS, even after accounting for childhood adaptive behavior. More specifically, Factor 5 (parent report of childhood EF) was a significant predictor of VABS-II parent-reported young adult adaptive behavior (B = 7.79, p < .01). When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that parent report of childhood EF is a unique predictor of young adult adaptive behavior. When control participants were included in the model predicting adaptive functioning, and study group was examined as a potential moderator with factor 5, there was not a significant interaction (p > .05). Consistent with our hypothesis, this suggests that childhood EF predicts adaptive functioning similarly for individuals with and without 22q11DS.
Stepwise linear regression revealed a trend toward significance when predicting SAS-SR young adult self-reported social adjustment among individuals with 22q11DS (F = 1.884, p = .132). When only factors 2, 5, and 6 were included in the model, the model was significant (F = 2.884, p < .05), with factor 5 (parent report of child EF; B = 9.40, p < .01) significantly predicting social adjustment, after covarying for childhood adaptive behavior. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that EF is a unique predictor of SAS-SR young adult self-reported social adjustment. When control participants were included in the model predicting social adjustment, and study group was examined as a moderator with factor 5, there was not a significant interaction (p > .05). Consistent with our hypothesis, this suggests that childhood EF predicts young adult social adjustment similarly for individuals with and without 22q11DS.
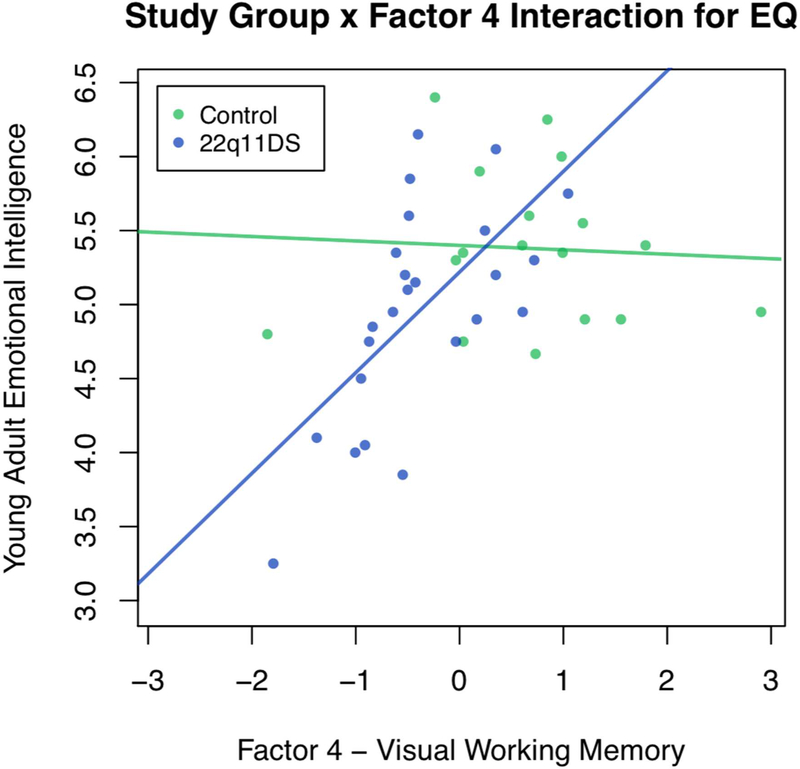
Childhood EF also significantly predicted TEIQue-SF young adult emotional intelligence abilities among individuals with 22q11DS, even when accounting for childhood adaptive behavior. More specifically, factors 4 (visual working memory; B = .574, p < .01) and 5 (parent report of child EF; B = −.399, p < .05) were significant predictors of TEIQue-SF emotional intelligence. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that parent report of childhood EF is a unique predictor of emotional intelligence. Factor 4 was no longer significant, possibly because working memory is a subcomponent of IQ. When control participants were included in the model predicting emotional intelligence, and study group was examined as a moderator with factors 4 and 5, there was a significant study group x factor 4 interaction (B = .512, p < .05). Inconsistent with our hypothesis, this suggests that the relationship between visual working memory and young adult emotional intelligence is different for individuals with and without 22q11DS. More specifically, worse childhood visual working memory is associated with lower TEIQue-SF emotional intelligence for individuals with 22q11DS, but not for individuals without 22q11DS. See Figure 1 for a visual depiction of this interaction.
Figure 1.
Study Group as a Moderator of the Relationship between Factor 4 – Visual Working Memory and Young Adult Emotional Intelligence
Note. EQ = Emotional Intelligence.
Finally, with regard to employment and independent living status, no childhood EF variables significantly predicted young adult status. Likewise, for those that were employed in young adulthood, no childhood EF variables predicted number of hours worked per week. See Table 3 for a summary of the significant regression analyses predicting young adult outcomes for individuals with 22q11DS.
Table 3.
Statistically Significant Young Adult Outcomes from Childhood Executive Functioning in Individuals with 22q11DS
| Young Adult Outcomes | Factor 1 B | Factor 2 B | Factor 3 B | Factor 4 B | Factor 5 B | Factor 6 B |
|---|---|---|---|---|---|---|
| SIPS - Positive Symptoms of Psychosis | .057 | .334 | 1.39 | .632 | 2.02* | −3.41** |
| ASR - Externalizing Problems | −3.30 | −7.42* | −1.16 | .510 | 12.61*** | −.622 |
| ASR - ADHD Problems | −4.56** | 6.45** | −.984 | |||
| VABS-II Adaptive Functioning | 1.67 | 2.93 | −1.41 | 1.58 | −7.79** | −1.58 |
| SAS-SR Social Adjustment | −2.21 | 9.40** | 1.20 | |||
| TEIQue-SF Emotional Intelligence | .016 | .264 | .175 | .574** | −.399* | −.040 |
Note. SIPS = Structured Interview for Prodromal Symptoms; ASR = Adult Self-Report scale. VABS-II = Vineland Adaptive Behavior Scales- 2nd edition; SAS-SR = Social Adjustment Scale - Self-report; TEIQue-SF = Trait Emotional Intelligence Questionnaire - Short Form.
p < .05
p < .01
p < .001.
Discussion
Consistent with previous literature, children with 22q11DS displayed significantly impaired executive functioning compared to control children (Shapiro et al., 2014). A more innovative finding, however, is that childhood EF was predictive of several key outcome variables in young adults with 22q11DS, including parent report of adaptive behavior, positive symptoms of psychosis, emotional intelligence, and self-reported social adjustment and externalizing problems. While previous literature suggests that 22q11DS is associated with the functional impairments and psychopathology examined in the present study (Maeder et al., 2016), our study is the first to examine whether childhood EF predicts young adult functioning in individuals with 22q11DS. The findings of the present study suggest that EF may be a worthwhile target for intervention among children with 22q11DS, in order to reduce the likelihood that these children will go on to develop negative outcomes in young adulthood. While some authors (Cutler-Landsman, 2012) have previously recommended that parents and educators of children with 22q11DS target EF development due to concurrent difficulties, our data are the first to indicate that childhood EF abilities also have clear distal effects well into adulthood.
Across the six factors identified in this study, only EF factors were significant predictors. Parent report of child EF was the most consistent predictor of young adult outcomes and was a stronger predictor than the psychological tests of executive functioning. This may be explained by the fact that parents are reporting on their observations of their child’s EF in real-life situations, whereas neuropsychological tests of EF examine children’s EF in a more controlled environment, which may have less ecological validity. This is consistent with findings from (Barkley & Fischer, 2011), who reported that EF ratings are better predictors of adult outcomes than EF tests, as these tests may not be capturing the same complexity of EF required in real-world situations.
Our findings suggest that EF is a unique predictor of young adult outcomes for individuals with 22q11DS. The auditory verbal learning factor (factor 1) did not significantly predict any of the outcomes examined, while four out of five EF factors were significant predictors of at least one outcome. Additionally, parent-reported EF continued to be a significant predictor even after IQ was included as a covariate in each model, suggesting the predictive power of EF cannot be explained simply by differences in IQ. In light of the controversy inherent in the neuropsychology field about covarying for IQ in neurodevelopmental disorder populations (Dennis et al., 2009), these data provide clear guidance to clinicians: Childhood EF abilities are a relevant factor to consider, over and above IQ.
Study group moderated the relationship between childhood EF and young adult outcomes for some outcomes but not for others. Study group did not moderate the relationship between childhood EF and young adult adaptive behavior or social adjustment. This suggests that EF predicts adaptive behavior and social adjustment similarly for individuals with and without 22q11DS. However, study group moderated the relationship between childhood EF and emotional intelligence, externalizing problems, and ADHD problems. For each of these outcomes, lower childhood EF scores were associated with poorer young adult outcomes for individuals with 22q11DS, but not for individuals without 22q11DS. Of note, there were no interactions of study group with factor 5 (parent-report of childhood EF), suggesting that parent-reported EF predicts outcomes similarly across individuals with and without 22q11DS.
Our finding that childhood deficits in executive functions predict positive prodromal symptoms in adulthood are generally consistent with several longitudinal studies that have reported deficits in cognitive functioning in children who eventually develop idiopathic schizophrenia (Cannon et al., 2000; Dickson, Laurens, Cullen, & Hodgins, 2012; Fuller et al., 2002; Seidman, Buka, Goldstein, & Tsuang, 2006; Woodberry, Giuliano, & Seidman, 2008). Although many of these studies relied on global IQ scores rather than individual neuropsychological tests, specific developmental delays in the cognitive skills involved in working memory have been observed (Reichenberg, 2010). Although we are not aware of longitudinal studies that specifically predict emotional intelligence in adulthood, deficits in emotional intelligence have been observed in both individuals with 22q11DS and idiopathic schizophrenia, attesting to the importance of identifying early predictors of deficits in this skill set (Ho et al., 2012; Kee et al., 2009).
Given the predictive utility of EF, implementing receiver operating characteristic (ROC) curves to detect cut-off scores for low EF performance and risk for poor outcomes could improve detection of high risk children. Future analyses could also integrate the results of these analyses with other variables that may best predict resilience / “real world” adaptive functioning in young adults with 22q11DS.
Limitations
The present study was not without limitations. The smaller number of participants with 22q11DS and control participants who had Time 4 data likely limited our power for detecting smaller effects of childhood EF on young adult outcomes. In spite of our limited sample size, however, several significant effects of EF on developmental outcomes were detected, and the subsample of participants examined in this study were not demographically different from the total sample, suggesting that these results would likely generalize. A second limitation is the number of analyses conducted and the potential for type I error. We chose not to apply a correction for multiple testing because of our small sample size and the potential increase in type II error. A study with low power also has a lower positive predictive value. Thus, these results should be considered preliminary until other groups can independently replicate these findings. A third limitation is that we were unable to examine teacher reports of children’s EF. Thus, we were unable to include information about children’s EF in the school setting, which may differ from their EF observed in other settings. Future research should also consider examining teacher report to address the limitations of common method variance, as in the present study, parent report is used for both predictor and outcome variables. Having both teacher and parent report would provide information about convergent validity, which would indicate greater reliability of findings.
Additionally, the present study did not consider the potential impact of treatment. It is possible that participants with 22q11DS received treatment at some point during the 9-year time period in which this study was conducted. Treatment may have impacted young adult outcomes for children with 22q11DS. Likewise, the majority of young adults with 22q11DS (and controls) were living at home in young adulthood yet our analyses did not consider how supportive those home environments may be. Thus, we do not know the extent to which support systems may moderate our findings. Similarly, due to insufficient statistical power, we did not consider that psychiatric symptoms may moderate our results. Thus, we do not know the extent to which our findings may be less driven by 22q11DS and more impacted by psychopathology. Our neuropsychological test battery did not include a performance validity test (PVT). Thus, the issue of suboptimal participant effort may have impacted our results. Finally, for the analyses, standardized scores were used for all variables except for the Tower of London. Thus, our Tower of London results may be differentially impacted by the PCA analyses and should be viewed with caution.
Conclusions/Implications
Childhood EF is an important predictor of young adult outcomes among individuals with and without 22q11DS. This has important implications for clinical practice, as it suggests that EF could be a valuable target for treatment in children with 22q11DS. This study provides support for assessing the efficacy of preventative cognitive remediation (CR) interventions for children with 22q11DS, such as the computer-based CR intervention investigated by Mariano and colleagues (Mariano, Tang, Kurtz, & Kates, 2015, 2016). That is, Mariano and colleagues administered a longitudinal, computer-based cognitive remediation program (Bracy et al., 1999) to 21 adolescents with 22q11DS, and observed that participants exhibited significant improvements in working memory, shifting attention, and cognitive flexibility. These improvements were maintained over a six-month period that followed the intervention program.
Findings of the present study suggest improving children’s EF may reduce the likelihood of a variety of negative outcomes in later development. Additionally, parent report of childhood EF was a consistent predictor of young adult outcomes. This finding has important implications for neuropsychologists, as it suggests the information collected through parent-report measures of EF may be more valuable for identifying children at risk for negative developmental outcomes than information collected through behavioral paradigm measures of executive functioning.
Supplementary Material
Supplemental Table 1
Measures Used Across Time Points
Supplemental Table 2
Negative Binomial Regression Predicting Young Adult SIPS - Positive Symptoms of Psychosis from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 3
Regression Predicting Young Adult ASR Externalizing Problems from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 4
Regression Predicting Young Adult ASR ADHD Problems from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 5
Regression Predicting Young Adult VABS-II Adaptive Functioning from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 6
Regression Predicting Young Adult SAS-SR Social Adjustment from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 7
Regression Predicting Young Adult TEIQue-SF Emotional Intelligence from Childhood Executive Functioning in Individuals with 22q11DS
Acknowledgements
The authors have no conflicts of interest to report. This project was funded by the National Institutes of Health grant number R01MH064824 to Wendy R. Kates, Ph.D. The authors are additionally grateful to the families that participated in the project.
References
- Achenbach TM, & Rescorla LA (2003). Manual for the ASEBA adult forms and profiles. Burlington, VT: University of Vermont. [Google Scholar]
- Anderson P (2002). Assessment and development of executive function (EF) during childhood. Child Neuropsychology, 8, 71–82. doi: 10.1076/chin.8.2.71.8724 [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, & Kates WR (2008). The neurocognitive phenotype in velocardio-facial syndrome: a developmental perspective. Developmental Disability Research Review, 14(1), 43–51. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Ramanathan S, & Kates WR (2017). Predicting Cognition and Psychosis in Young Adults With 22q11.2 Deletion Syndrome. Schizophrenia Bulletin, 43(4), 833–842. doi: 10.1093/schbul/sbw135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, & Kates WR (2010). Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry, 49(4), 333–344. [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, & Fischer M (2011). Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: self-reported executive function (EF) deficits versus EF tests. Developmental Neuropsychology, 36(2), 137–161. doi: 10.1080/87565641.2010.549877 [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Husted J, Weksberg R, Caluseriu O, Webb GD, & Gatzoulis MA (2005). Clinical features of 78 adults with 22q11 deletion syndrome. American journal of medical genetics. Part A, 138(4), 307–313. doi: 10.1002/ajmg.a.30984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Campbell RM (2003). A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics, 112(1 Pt 1), 101–107. [DOI] [PubMed] [Google Scholar]
- Bracy OD, Oakes AL, Cooper RS, Watkins D, Watkins M, Brown DE, & Jewell C (1999). The effects of cognitive rehabilitation therapy techniques for enhancing the cognitive/intellectual functioning of seventh- and eighth-grade children. Journal of Cognitive Technology, 4, 19–27. [Google Scholar]
- Bunge SA, & Zelazo PD (2006). A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science, 15(3), 118–121. doi: 10.1111/j.0963-7214.2006.00419.x [DOI] [Google Scholar]
- Campbell LE, McCabe KL, Melville JL, Strutt PA, & Schall U (2015). Social cognition dysfunction in adolescents with 22q11.2 deletion syndrome (velo-cardio-facial syndrome): relationship with executive functioning and social competence/functioning. Journal of Intellectual Disability Research, 59, 845–859. doi: 10.1111/jir.12183 [DOI] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, & Hadley T (2000). Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophrenia Bulletin, 26(2), 379–393. [DOI] [PubMed] [Google Scholar]
- Chawner S, Doherty JL, Moss H, Niarchou M, Walters JTR, Owen MJ, & van den Bree MBM (2017). Childhood cognitive development in 22q11.2 deletion syndrome: case-control study. British Journal of Psychiatry, 211(4), 223–230. doi: 10.1192/bjp.bp.116.195651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Watson M, Young DA, & Bassett AS (2006). Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophenia Research, 87(1–3), 270–278. doi: 10.1016/j.schres.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, & Petrides KV (2010). A psychometric analysis of the trait emotional intelligence questionnaire-short form (TEIQue-SF) using item response theory. Journal of Personality Assessment, 92, 449–457. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, & Zillmer EA (1998). The tower of london(DX): a standardized approach to assessing executive functioning in children. Archives of Clinical Neuropsychology, 13(3), 285–301. [PubMed] [Google Scholar]
- Cutler-Landsman D (2012). Educating children with velo-cardio-facial syndrome (also known as 22q11.2 deletion syndrome and DiGeorge syndrome) San Diego, CA: Plural Publishing. [Google Scholar]
- Delis D, Kramer JH, Kaplan E, & Ober BA (1994). California Verbal Learning Test - Children’s version. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why intelligence quotient is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15(3), 331–343. doi: 10.1017/S1355617709090481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson H, Laurens KR, Cullen AE, & Hodgins S (2012). Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychological Medicine, 42(4), 743–755. doi: 10.1017/S0033291711001693 [DOI] [PubMed] [Google Scholar]
- Fiksinski AM, Breetvelt EJ, Duijff SN, Bassett AS, Kahn RS, & Vorstman JAS (2017). Autism Spectrum and psychosis risk in the 22q11.2 deletion syndrome. Findings from a prospective longitudinal study. Schizophrenia Research, 188, 59–62. doi: 10.1016/j.schres.2017.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, & Andreasen NC (2002). Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. American Journal of Psychiatry, 159(7), 1183–1189. [DOI] [PubMed] [Google Scholar]
- Fung WAL, Butcher NJ, Costain G, Andrade DM, Boot E, Chow EWC, Bassett AS (2015). Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genetics in medicine : official journal of the American College of Medical Genetics, 17(8), 599–609. doi: 10.1038/gim.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, Yung A (2013). The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry, 70(1), 107–120. doi: 10.1001/jamapsychiatry.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G, Isquith P, Guy S, & Kenworthy L (2000). Behavior Rating Inventory of Executive Functioning (BRIEF). Lutz, FL: PAR, Inc. [Google Scholar]
- Golden JC (1978). Stroop Color and Word Test. Chicago: Stoelting Company. [Google Scholar]
- Gordon M (1983). The Gordon Diagnostic System. DeWitt, NY: Gordon Systems. [Google Scholar]
- Gothelf D, Schneider M, Green T, Debbane M, Frisch A, Glaser B, Eliez S (2013). Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. Journal of the American Academy of Child and Adolescent Psychiatry, 52(11), 1192–1203 e1193. doi: 10.1016/j.jaac.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Grati FR, Molina Gomes D, Ferreira JC, Dupont C, Alesi V, Gouas L, Vialard F (2015). Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenatal Diagnosis. doi: 10.1002/pd.4613 [DOI] [PubMed] [Google Scholar]
- Harms MB, Zayas V, Meltzoff AN, & Carlson SM (2014). Stability of executive function and predictions to adaptive behavior from middle childhood to pre-adolescence. Frontiers in Psychology, 5(331). doi: 10.3389/fpsyg.2014.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, & Curtiss G (1993). Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Hervey AS, Epstein JN, & Curry JF (2004). Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology, 18(3), 485–503. [DOI] [PubMed] [Google Scholar]
- Ho JS, Radoeva PD, Jalbrzikowski M, Chow C, Hopkins J, Tran WC, Bearden CE (2012). Deficits in mental state attributions in individuals with 22q11.2 deletion syndrome (velo-cardio-facial syndrome). Autism Research, 5(6), 407–418. doi: 10.1002/aur.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Curtiss K, Schoch K, Keshavan MS, Allen A, & Shashi V (2013). A longitudinal examination of the psychoeducational, neurocognitive, and psychiatric functioning in children with 22q11.2 deletion syndrome. Research in Developmental Disabilities, 34(5), 1758–1769. doi: 10.1016/j.ridd.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee KS, Horan WP, Salovey P, Kern RS, Sergi MJ, Fiske AP, Green MF (2009). Emotional intelligence in schizophrenia. Schizophrenia Research, 107(1), 61–68. doi: 10.1016/j.schres.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Nasrallah HA, & Tandon R (2011). Schizophrenia, “Just the Facts” 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophrenia Research, 127(1–3), 3–13. doi: 10.1016/j.schres.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley-Brabeck K, & Sobin C (2006). Social skills and executive function deficits in children with the 22q11 Deletion Syndrome. Applied Neuropsychology, 13(4), 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajiness-O’Neill R, Beaulieu I, Asamoah A, Titus JB, Bawle E, Ahmad S, Pollack R (2006). The neuropsychological phenotype of velocardiofacial syndrome (VCFS): relationship to psychopathology. Archives of Clinical Neuropsychology, 21(2), 175–184. [DOI] [PubMed] [Google Scholar]
- Maeder J, Schneider M, Bostelmann M, Debbané M, Glaser B, Menghetti S, Eliez S. (2016). Developmental trajectories of executive functions in 22q11.2 deletion syndrome. Journal of Neurodevelopmental Disorders, 8(1), 10. doi: 10.1186/s11689-016-9141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano MA, Tang K, Kurtz M, & Kates WR (2015). Cognitive remediation for adolescents with 22q11 deletion syndrome (22q11DS): a preliminary study examining effectiveness, feasibility, and fidelity of a hybrid strategy, remote and computer-based intervention. Schizophrenia Research, 166(1–3), 283–289. doi: 10.1016/j.schres.2015.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano MA, Tang K, Kurtz M, & Kates WR (2016). Examining the durability of a hybrid, remote and computer-based cognitive remediation intervention for adolescents with 22q11.2 deletion syndrome. Early Intervention Psychiatry. doi: 10.1111/eip.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Nevado-Montenegro AJ, & Hinshaw SP (2012). Childhood executive function continues to predict outcomes in young adult females with and without childhood-diagnosed ADHD. Journal of Abnormal Child Psychology, 40(5), 657–668. doi: 10.1007/s10802-011-9599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, Woods SW (2003). Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin, 29(4), 703–715. [DOI] [PubMed] [Google Scholar]
- Niarchou M, Zammit S, van Goozen SH, Thapar A, Tierling HM, Owen MJ, & van den Bree MB (2014). Psychopathology and cognition in children with 22q11.2 deletion syndrome. British Journal of Psychiatry, 204(1), 46–54. doi: 10.1192/bjp.bp.113.132324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides KV (2009). Technical manual for the Trait Emotional Intelligence Questionnaires (TEIQue). London: London Psychometric Laboratory. [Google Scholar]
- Reichenberg A (2010). The assessment of neuropsychological functioning in schizophrenia. Dialogues in Clinical Neuroscience, 12(3), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinsky JR, & Hinshaw SP (2011). Linkages between childhood executive functioning and adolescent social functioning and psychopathology in girls with ADHD. Child Neuropsychology, 17(4), 368–390. doi: 10.1080/09297049.2010.544649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, & Gioia G (2006). Behavior Rating Inventory of Executive Function®–Adult Version (BRIEF-A). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree M, Eliez S (2014). Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. American Journal of Psychiatry, 171(6), 627–639. doi: 10.1176/appi.ajp.2013.13070864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, & Tsuang MT (2006). Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology, 28(2), 225–242. doi: 10.1080/13803390500360471 [DOI] [PubMed] [Google Scholar]
- Shapiro HM, Tassone F, Choudhary NS, & Simon TJ (2014). The development of cognitive control in children with chromosome 22q11.2 deletion syndrome. Frontiers in Psychology, 5, 566. doi: 10.3389/fpsyg.2014.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ (2000). Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Mental Retardation and Developmental Disability Research Review, 6(2), 142–147. doi: [DOI] [PubMed] [Google Scholar]
- Sjowall D, Bohlin G, Rydell AM, & Thorell LB (2017). Neuropsychological deficits in preschool as predictors of ADHD symptoms and academic achievement in late adolescence. Child Neuropsychology, 23(1), 111–128. doi: 10.1080/09297049.2015.1063595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, & Balla D (2005). Vineland Adaptive Behavior Scales, Second Edition (Vineland-II). San Antonio, TX: Pearson Education. [Google Scholar]
- Tang SX, Moore TM, Calkins ME, Yi JJ, McDonald-McGinn DM, Zackai EH, Gur RE (2017). Emergent, remitted and persistent psychosis-spectrum symptoms in 22q11.2 deletion syndrome. Translational Psychiatry, 7(7), e1180. doi: 10.1038/tp.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, & Murphy D (2004). Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophrenia Research, 70(2–3), 223–232. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, Bassett AS (2015). Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2014.2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1991). Wechsler Intelligence Scale for Children - Third edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (1993). Wechsler Adult Intelligence Scale - 3rd edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weinberger R, Yi J, Calkins M, Guri Y, McDonald-McGinn DM, Emanuel BS, Gothelf D (2016). Neurocognitive profile in psychotic versus nonpsychotic individuals with 22q11.2 deletion syndrome. European Neuropsychopharmacology, 26(10), 1610–1618. doi: 10.1016/j.euroneuro.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Weissman MM (1999). Social Adjustment Scale- Self-report (SAS-SR) User’s Manual. North Tonawanda, NY: Multi-Health Systems, Inc. [Google Scholar]
- Woodberry KA, Giuliano AJ, & Seidman LJ (2008). Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry, 165(5), 579–587. doi: 10.1176/appi.ajp.2008.07081242 [DOI] [PubMed] [Google Scholar]
- Yi JJ, Calkins ME, Tang SX, Kohler CG, McDonald-McGinn DM, Zackai EH, Gur RE (2015). Impact of psychiatric comorbidity and cognitive deficit on function in 22q11.2 deletion syndrome. Journal of Clinical Psychiatry, 76(10), e1262–1270. doi: 10.4088/JCP.14m09197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1
Measures Used Across Time Points
Supplemental Table 2
Negative Binomial Regression Predicting Young Adult SIPS - Positive Symptoms of Psychosis from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 3
Regression Predicting Young Adult ASR Externalizing Problems from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 4
Regression Predicting Young Adult ASR ADHD Problems from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 5
Regression Predicting Young Adult VABS-II Adaptive Functioning from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 6
Regression Predicting Young Adult SAS-SR Social Adjustment from Childhood Executive Functioning in Individuals with 22q11DS
Supplemental Table 7
Regression Predicting Young Adult TEIQue-SF Emotional Intelligence from Childhood Executive Functioning in Individuals with 22q11DS