Abstract
Background and Purpose:
Whether to resume oral anticoagulation treatment after Intracerebral Hemorrhage (ICH) remains an unresolved question. Previous studies focused primarily on recurrent stroke after ICH. We sought to investigate the association between cardioembolic stroke risk, oral anticoagulation therapy (OAT) resumption, and functional recovery among ICH survivors in the absence of recurrent stroke.
Methods:
we conducted a joint analysis of three observational studies: 1) the multi-center RETRACE study; 2) the Massachusetts General Hospital ICH study (n = 166); 3) the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study (n = 131). We included 941 survivors of ICH in the setting of active OAT for prevention of cardioembolic stroke due to non-valvular atrial fibrillation, and without evidence of ischemic stroke and/or recurrent ICH at one year from the index event. We created univariable and multivariable models to explore associations between cardioembolic stroke risk (based on CHA2DS2-VASc scores) and functional recovery after ICH, defined as achieving modified Rankin scale (mRS) ≤ 3 at one year for participants with mRS > 3 at discharge.
Results:
In multivariable analyses, CHA2DS2-VASc score was associated with decreased likelihood of functional recovery (odds ratio [OR] = 0.83 per 1 point increase, 95% confidence interval [CI] = 0.79 – 0.86) at one year. Anticoagulation resumption was independently associated with a higher likelihood of recovery, regardless of CHA2DS2-VASc score (OR = 1.89, 95% CI = 1.32 – 2.70). We found an interaction between CHA2DS2-VASc score and anticoagulation resumption in terms of association with increased likelihood of functional recovery (interaction p = 0.011).
Conclusion:
Increasing cardioembolic stroke risk is associated with decreased likelihood of functional recovery at 1 year following ICH, but this association was weaker among participants resuming oral anticoagulation therapy. These findings support including recovery metrics in future studies of anticoagulation resumption after ICH.
INTRODUCTION
Intracerebral Hemorrhage (ICH) is the most severe and least treatable form of stroke. ICH accounts for 10–20% of all acute cerebrovascular events and is responsible for about 50% of stroke-related disability and mortality.1, 2 Oral Anticoagulation Treatment (OAT) is associated with increased ICH risk, with about 80% of cases occurring during treatment of underlying atrial fibrillation aimed at preventing cardioembolic stroke.3, 4 Thus, resuming OAT is a major clinical dilemma in ICH care, because the increased risk of recurrent bleeding must be weighed against the beneficial protection from thromboembolic events.5, 6 Previous observational studies have associated OAT resumption with decreased mortality and decreased ischemic stroke risk following OAT-related ICH, as confirmed in a recent meta-analysis of all available data.7–13
OAT resumption was recently found to be associated with improved functional outcome at one year post-ICH, likely reflecting the aforementioned reduction in symptomatic ischemic stroke risk.14 However, atrial fibrillation has also been associated with increased incidence of asymptomatic (i.e. subclinical) ischemic strokes.15, 16 These covert ischemic events may negatively impact outcome after OAT-ICH, even in the absence of clinical symptomatology.17 Prevention of asymptomatic infarcts could therefore represent an additional mechanism linking cardioembolic stroke risk, OAT resumption and post-ICH outcome.
We therefore sought to determine: 1) whether cardioembolic stroke risk (as defined by CHA2DS2-VASc scores) is associated with recovery after OAT-ICH, even in the absence of recurrent stroke; 2) whether OAT resumption alters the association between cardioembolic stroke risk and recovery after OAT-ICH. To test these hypotheses, we collected and analyzed individual-level data from three large studies of ICH: 1) the multi-center RETRACE study of OAT-ICH in Germany; 2) a single-center longitudinal study of ICH conducted in Boston, USA; 3) the US-based multi-center ERICH study.7, 18, 19 This large collaborative effort allowed us to analyze individual level data for consecutive OAT-ICH cases.
METHODS
Data, Materials, and Code Disclosure Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Participating Studies
We present results of data collected by three prospective studies of OAT-related ICH: 1) the multi-center RETRACE (German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage) study, coordinated by the University of Erlangen-Nuremberg (Erlangen, Germany) and conducted from 2006 to 2010 in 19 tertiary care centers across Germany;7 2) the Massachusetts General Hospital (MGH) single-center longitudinal study of ICH conducted in Boston, USA since 1994 and currently ongoing;18 3) the multi-center Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study, coordinated by the University of Cincinnati (Cincinnati, OH, USA) and enrolling ICH cases and controls from 2011 to 2015 at 19 clinical recruitment centers across the USA.19 Because MGH participated as a recruitment center for the ERICH study, ERICH participants enrolled at MGH were only included in the ERICH analyses, to avoid data duplication.
Participant Enrollment and Baseline Data Acquisition
Eligible participants were: 1) diagnosed with primary ICH (confirmed on CT scan); 2) age 18 years or older at time of ICH; 3) without history of prior ICH (before the index event at enrollment); 4) without history of functional impairment and/or dependence before ICH; 5) on active oral anticoagulation therapy (OAT) for prevention of cardioembolic stroke due to previously diagnosed non-valvular atrial fibrillation at time of ICH; 6) alive and eligible for potential OAT resumption (i.e. with care not focused on comfort measures only) at time of discharge from acute care; 7) not diagnosed with recurrent ICH or ischemic stroke at one year follow-up.7, 14, 18, 19 Informed consent for participation in each individual study (including data sharing) was obtained from all participants or their legally designated surrogates. For the purpose of these analyses, OAT resumption was defined as active use of Vitamin K Antagonists (VKA) agents, according to previously published criteria.14 Data on demographics, medical history, pre-ICH medication exposures and laboratory results were obtained via structured interview of patients and/or informants at time of enrollment.7, 14, 18, 19 We supplemented information obtained in-person via review of available medical records at each individual institution. Medical records were also reviewed to determine functional status at discharge (defined as modified Rankin Scale [mRS]). Available information was used to compute values for the CHA2DS2-VASc score.7, 18, 19 Of note, ICH was not considered a “stroke” for the purpose of calculating CHA2DS2-VASc scores.14 Institutional Review Boards or Ethical Committees reviewed and approved all study protocols.
Longitudinal Follow-up
Follow-up information was obtained via phone interviews, aimed at identifying: 1) OAT resumption status; 2) mortality; 3) modified Rankin Scale (mRS) captured using the Simplified mRS Questionnaire;20 4) recurrent stroke events, including both recurrent ICH and ischemic stroke.7, 14, 18, 19 All follow-up phone interviews and outcome adjudications were conducted by the coordinating center for each of the participating studies. OAT resumption was defined in an identical manner as for eligibility criteria listed above. Interviews were conducted at 3 months, 6 months and one year from index ICH, based on established protocols.17–19 Patient-derived follow-up information was corroborated by review of pertinent medical records.
Statistical Methods
Overall Analysis Framework
Given the heterogeneity in design and recruitment procedures across participating studies, we used a meta-analysis framework to compile results from individual datasets.14, 21 This approach allows for formal exploration of effect heterogeneity using validated statistical tools.22 We initially conducted separate univariable and multivariable analyses (see below) for the RETRACE, MGH-ICH and ERICH studies. Results from multivariable analyses from each individual study were then meta-analyzed using a conservative inverse variance-based random effects method (DerSimonian-Laird).21 Cochran Q test was used to estimate heterogeneity, followed by calculation of I2.22 Effect size heterogeneity was considered significant for heterogeneity p values < 0.10 or I2 > 0.20.
Variables’ Definition and Handling
We defined recovery in functional status based on difference in mRS scores at one year post-ICH vs. discharge from acute care. We employed two different definitions of post-ICH recovery: 1) functional recovery, i.e. transition to mRS ≤ 3 at one year after ICH among those with mRS > 3 at discharge; 2) mRS score change during follow-up, with positive values indicating increasing mRS over time, and negative numbers indicating decreasing mRS (i.e. recovery). We also conducted the following secondary analyses: 1) including participants experiencing recurrent ICH (n = 18); 2) repeating all analyses after exclusion of individuals experiencing death during follow-up, since some deaths may have been caused by non-diagnosed strokes.
Univariable and Multivariable Models
Categorical variables were compared using Fisher exact test (2-tailed) and continuous variables using the Mann-Whitney rank-sum or unpaired t test. Multivariable analyses utilized logistic regression or ordinal logistic regression. We initially included in multivariable modeling all factors associated with outcomes of interest in univariable analyses with p<0.20. All variables included in the CHA2DS2-VASc score (e.g. age) were only entered as part of the score itself, to avoid likely multicollinearity issues. We subsequently used backward elimination procedures to arrive at a minimal model including only variables associated with functional recovery or with OAT resumption status at p<0.05. We initially conducted interaction analyses between CHA2DS2-VASc score and OAT resumption using interaction terms in multivariable analyses. We subsequently repeated multivariable analyses within strata defined by CHA2DS2-VASc scores (0 to 2, 3 to 4, 5 to 6, and 7 to 9) to approximately subdivide participants in equal-sized groups. We conducted model diagnostics including: 1) testing the proportional odds assumption (Brant test); 2) evaluation of multi-collinearity via VIF calculation (pre-specified removal of variables with VIF≥3, none qualified); 3) visual inspection of residual vs. fitted value plots; 4) goodness-of-fit evaluation via the Hosmer-Lemshow test. For all analyses we considered p values < 0.05 (2-tailed) statistically significant, after adjustment for multiple testing using the False Discovery Rate (FDR) method.23 All analyses were performed with R v3.4.4 (the R Project for Statistical Computing), including the car, generalhoslem, brant and meta packages.
RESULTS
Study Participants
After application of inclusion and exclusion criteria, we analyzed data for 941 OAT-ICH survivors (Figure 1). Participants’ characteristics are presented in Table 1. OAT resumption rate was lower in the ERICH study (20%) compared to the RETRACE (29%) and MGH-ICH (33%) studies (p < 0.05 for both comparisons). All other medical history characteristics (including CHA2DS2-VASc score) did not differ among studies (all p > 0.20). A total of 262/941 (29%) OAT-ICH survivors resumed oral anticoagulation during follow-up. Median time to OAT resumption was 32 days (Inter-Quartile Range [IQR] 24 – 65). In univariable analyses ICH location, antiplatelet use during follow-up and CHA2DS2-VASc score were the only patient characteristics associated with OAT resumption status (Table 2). Detailed information on patient characteristics by OAT resumption status and study are presented in Supplemental Table I. Multivariable analyses confirmed that higher CHA2DS2-VASc scores were associated with OAT resumption (OR 1.74, 95% CI 1.16 – 2.61, p=0.008). We found no association between OAT resumption status and mRS at discharge from acute ICH hospitalization (OR 0.86, 95% CI 0.51 – 1.46, p=0.58).
Figure 1. Participant Enrollment and Eligibility / Exclusion Criteria.
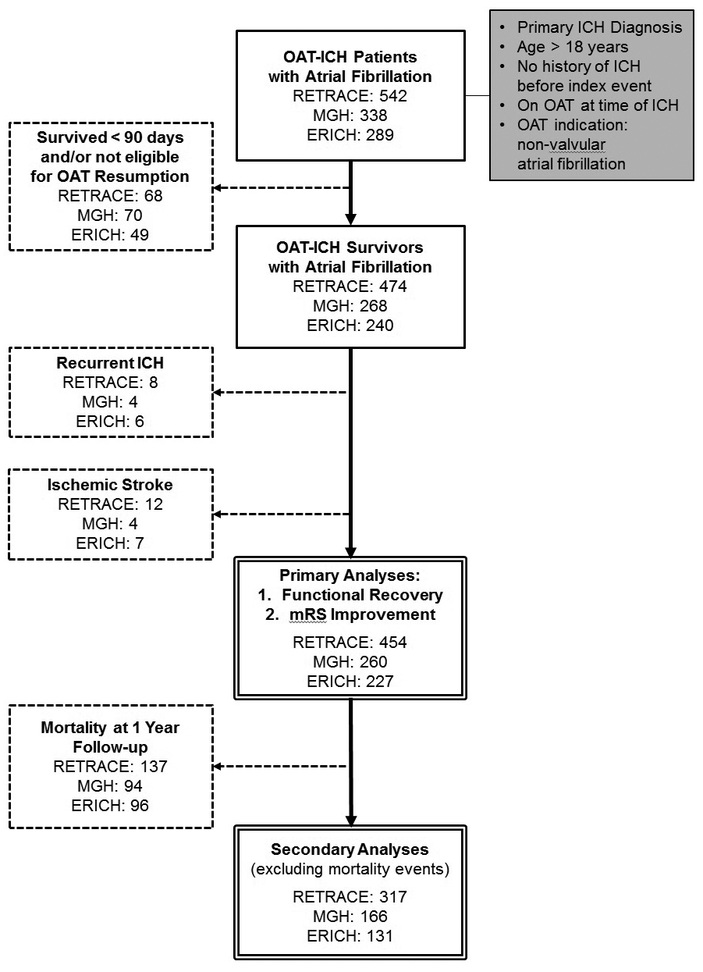
Flow-chart summarizing sequential application of eligibility/exclusion criteria for all planned analyses. Solid border boxes in the center report number of patients fulfilling eligibility criteria at each stage and for each participating study. The double-border boxes at the bottom indicates participants selected for analyses mentioned in the Results section. Initial criteria for eligibility are listed in the grey background box on the right-hand side. Dashed lines connect to dashed boxes listing criteria for exclusion from analysis and number of participants excluded as a result in each participating study.</P/>
Abbreviations: ICH = Intracerebral Hemorrhage; Abbreviations: mRS = modified Rankin Scale, OAT = Oral Anticoagulation Treatment
Table 1.
Characteristics of Participants by Enrollment Study
| Variable | RETRACE | MGH | ERICH |
|---|---|---|---|
| No. of Individuals | 454 | 260 | 227 |
| Demographics | |||
| Age* | 73.6 (7.8) | 72.1 (9.3) | 71.2 (11.3) |
| Sex (female) | 178 (39) | 103 (40) | 74 (32) |
| Medical History | |||
| Hypertension | 386 (85) | 205 (79) | 182 (80) |
| Diabetes Mellitus | 145 (32) | 58 (21) | 67 (29) |
| Hyperlipidemia | 156 (34) | 73 (28) | 113 (50) |
| Prior Stroke | 134 (30) | 55 (21) | 59 (26) |
| CHA2DS2-VASc+ | 5 (4 – 6) | 5 (4 – 6) | 5 (4 – 6) |
| ICH Characteristics | |||
| ICH Location | |||
| - Lobar | 191 (42) | 118 (45) | 83 (37) |
| - Non-lobar | 263 (58) | 142 (55) | 144 (63) |
| ICH Volume (cc)+ | 10.5 (4.5 – 27.2) | 12.1 (3.3 – 25.6) | 10.3 (2.8 – 23.0) |
| Discharge mRS+ | 4 (3 – 5) | 4 (3 – 5) | 4 (3 – 5) |
| Follow-up Medication Exposures | |||
| OAT Resumption | 131 (29) | 86 (33) | 45 (20) |
| Antiplatelet Agents | 218 (48) | 109 (42) | 71 (31) |
values displayed as mean (standard deviation)
values displayed as median (inter-quartile range)
All values displayed as count (%) unless otherwise specified.
Abbreviations: ICH = Intracerebral Hemorrhage; mRS = modified Rankin Scale; OAT = Oral Anticoagulation Treatment
Table 2.
Characteristics of Participants by Anticoagulation Resumption Status.
| Variable | OAT Resumption | No OAT Resumption | p |
|---|---|---|---|
| No. of Individuals | 262 | 679 | - |
| Age (mean, SD)* | 71.5 (8.9) | 72.6 (9.4) | 0.17 |
| Sex (female) | 97 (37) | 258 (38) | 0.85 |
| CHA2DS2-VASc score + | 5 (5 – 6) | 4 (4 – 6) | 0.012 |
| ICH Location | 0.003 | ||
| - Lobar | 81 (31) | 311 (46) | |
| - Non-lobar | 181 (69) | 368 (54) | |
| ICH Volume (median, IQR)+ | 10.8 (3.4 – 24.4) | 11.9 (4.3 – 26.4) | 0.081 |
| Discharge mRS (median, IQR)+ | 3 (3 – 4) | 3 (3 – 4) | 0.25 |
| Antiplatelet Agents | 20 (8) | 378 (56) | < 0.001 |
values displayed as mean (standard deviation)
values displayed as median (inter-quartile range)
All values displayed as count (%) unless otherwise specified.
Abbreviations: ICH = Intracerebral Hemorrhage; mRS = modified Rankin Scale; OAT = Oral Anticoagulation Treatment
Cardioembolic Risk and Functional Recovery after OAT-ICH
We first sought to examine the association between cardioembolic stroke risk and functional recovery one year following OAT-ICH in univariable analyses. We found that increasing cardioembolic stroke risk (i.e. higher CHA2DS2-VASc score) was associated with decreased likelihood of functional recovery at one year (Fisher’s exact p<0.001 for all combined data) and with change in mRS score over time (p<0.001 for all combined data). Among other variables listed in Table 1, only discharge mRS was also associated with likelihood of functional recovery and mRS change over time (both p < 0.001 for all combined data).
Multivariable analyses (Table 3) confirmed that CHA2DS2-VASc score was associated with both functional recovery and mRS score change at one year after ICH. Upon restricting our analyses to participants who did not experience death during follow-up (n = 614, Figure 1) we found that CHA2DS2-VASc score was still associated with both functional recovery (OR 0.85, 95% CI 0.77 – 0.93, p<0.001) and mRS score change (OR 0.88, 95% CI 0.83 – 0.93, p<0.001) at one year after ICH. Re-introduction of individuals experiencing recurrent ICH, also resulted in CHA2DS2-VASc scores being associated with functional recovery (OR 0.85, 95% CI 0.77 – 0.92, p<0.001) and mRS score change (OR 0.88, 95% CI 0.82 – 0.93, p<0.001). All multivariable analyses showed no evidence of between-studies heterogeneity in effect size (I2 = 0% for all analyses, and all heterogeneity p-values > 0.20).
Table 3.
Multivariable Analyses of Recovery after Oral Anticoagulation ICH
| Variable | Functional Recovery* | |||||||
|---|---|---|---|---|---|---|---|---|
| RETRACE | MGH | ERICH | All Studies | |||||
| OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | |
|
CHA2DS2-VASc Score (for 1 point increase) |
0.85 (0.73 – 0.99) |
0.036 | 0.78 (0.59 – 1.02) |
0.075 | 0.84 (0.68 – 1.04) |
0.11 | 0.83 (0.79 – 0.86) |
<0.001 |
| OAT Resumption | 1.88 (1.05 – 3.37) |
0.035 | 2.10 (1.04 – 4.25) |
0.041 | 1.77 (0.98 – 3.19) |
0.061 | 1.89 (1.32 – 2.70) |
<0.001 |
| Variable | mRS Score Change** | |||||||
| RETRACE | MGH | ERICH | All Studies | |||||
| OR (95% CI) | p |
OR (95% CI) |
p |
OR (95% CI) |
p |
OR (95% CI) |
p | |
|
CHA2DS2-VASc Score (for 1 point increase) |
0.90 (0.83 – 0.98) |
0.012 | 0.82 (0.70 – 0.96) |
0.013 | 0.85 (0.74 – 0.98) |
0.025 | 0.88 (0. 82 – 0.93) |
<0.001 |
| OAT Resumption | 1.66 (1.10 – 2.51) |
0.017 | 1.77 (1.13 – 2.77) |
0.014 | 1.58 (1.06 – 2.36) |
0.027 | 1.66 (1.30 – 2.12) |
<0.001 |
All analyses adjusted for discharge modified Rankin scale, ICH location, and exposure to antiplatelet agents.
defined as achieving mRS ≤3 if mRS > 3 at discharge
difference in mRS score between one-year follow-up and discharge, effect size calculated for one-point decrease in mRS
Abbreviations: 95% CI = 95% Confidence Interval, mRS = modified Rankin scale, OAT = Oral Anticoagulation Treatment, OR = Odds Ratio.
OAT Resumption and Functional Recovery after OAT-ICH
In univariable analyses, OAT resumption was associated with functional recovery at one year (Fisher’s exact p < 0.001 for all combined data) and with mRS score change over time (p < 0.001 for all combined data). Multivariable analyses confirmed that OAT resumption was independently associated with higher likelihood of functional recovery and mRS decrease at one year after ICH, regardless of CHA2DS2-VASc score (Table 3). We present the combined effects of CHA2DS2-VASc score and OAT resumption on functional recovery after ICH in Figure 2. Upon restricting our analyses to individuals who did not experience mortality during follow-up we found that OAT resumption was still associated with increased likelihood of functional recovery (OR 1.85, 95% CI 1.20 – 2.85, p = 0.006) and mRS decrease over time (OR 1.66, 95% CI 1.30 – 2.12, p < 0.001). OAT resumption was still associated with likelihood of functional recovery (OR 1.89, 95% CI 1.32 – 2.71, p = 0.001) and mRS decrease over time (OR 1.64, 95% CI 1.32 – 2.04, p < 0.001) after re-introduction of individuals who experienced recurrent ICH during follow-up.
Figure 2. CHA2DS2-VASc Score, Oral Anticoagulation Resumption and Functional Recovery after Intracerebral Hemorrhage.
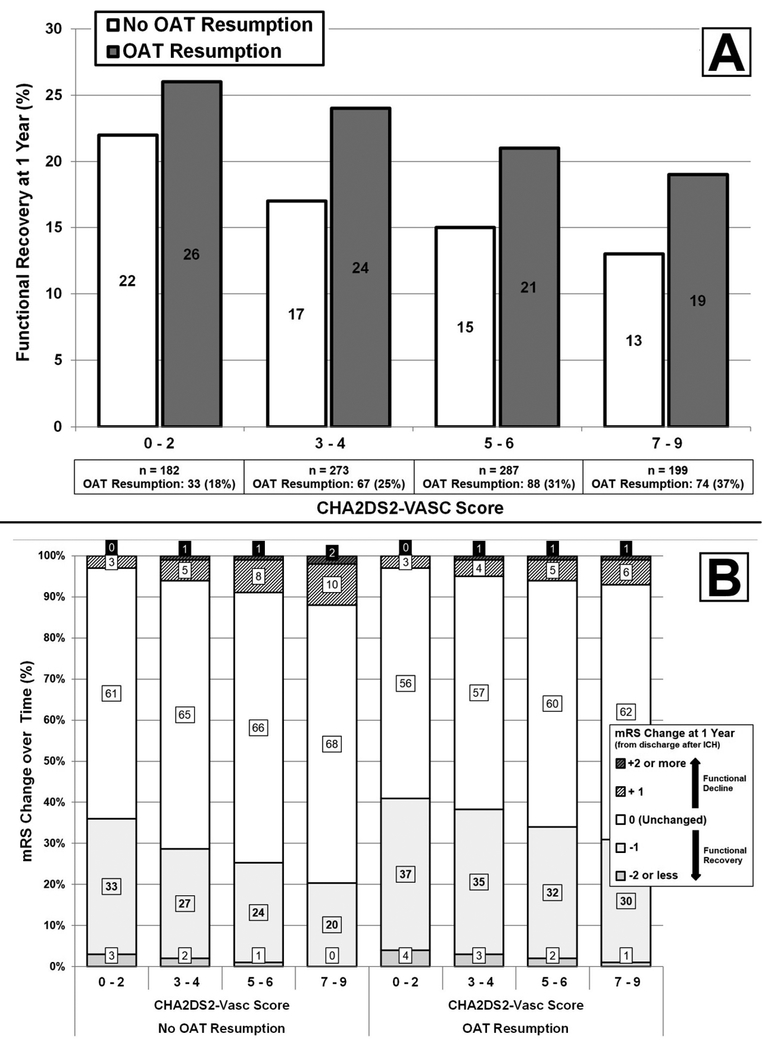
A. Bar graph summarizing the relationship between CHA2DS2-VASc Score / OAT Resumption and functional recovery, defined as achieving mRS < 3 for ICH survivors with mRS ≥ 3 at discharge. B. Bar graph summarizing the relationship between CHA2DS2-VASc Score / OAT Resumption and change in mRS between discharge after acute ICH and follow-up at one year.
Abbreviations: mRS = modified Rankin Scale, OAT = Oral Anticoagulation Treatment
In order to clarify the relationship among OAT resumption, CHA2DS2-VASc score and functional recovery, we performed interaction analyses (Supplemental Table II). CHA2DS2-VASc score and OAT resumption showed an interaction in associating with likelihood of functional recovery (interaction p = 0.011) and mRS decrease (p = 0.012) at one year after ICH. This resulted in participants with higher CHA2DS2-VASc score demonstrating larger effect sizes for the association between OAT resumption and functional recovery. This observation was confirmed by conducting analyses of association between OAT resumption and functional recovery after stratification by CHA2DS2-VASc score (Table 4).
Table 4.
Oral Anticoagulation Resumption, CHA2DS2-VASc Score and Recovery after Intracerebral Hemorrhage
| CHA2DS2-VASc Score | OAT Association with Functional Recovery* | OAT Association with mRS Score Change** | ||||
|---|---|---|---|---|---|---|
| OR | OR 95% CI |
p | OR | OR 95% CI |
p | |
| 0 – 2 | 0.69 | 0.36 – 1.32 | 0.27 | 0.70 | 0.35 – 1.40 | 0.32 |
| 3 – 4 | 1.33 | 0.99 – 1.79 | 0.061 | 1.11 | 1.00 – 1.24 | 0.061 |
| 5 – 6 | 1.98 | 1.14 – 3.44 | 0.016 | 1.49 | 1.07 – 2.07 | 0.018 |
| 7 – 9 | 2.55 | 1.16 – 5.59 | 0.021 | 1.95 | 1.19 – 3.18 | 0.008 |
All analyses adjusted for discharge modified Rankin scale, ICH location, and exposure to antiplatelet agents.
defined as achieving mRS ≤3 if mRS > 3 at discharge
difference in mRS score between one-year follow-up and discharge, effect size calculated for one-point decrease in mRS
Abbreviations: 95% CI = 95% Confidence Interval, mRS = modified Rankin Scale, OAT = Oral Anticoagulation Treatment, OR = Odds Ratio, SE = Standard Error
DISCUSSION
We present evidence from combined analyses of three large observational studies, identifying associations between higher cardioembolic stroke risk and lower likelihood of functional recovery at one year after ICH, in the absence of recurrent ICH or ischemic stroke. We also demonstrated that OAT resumption lowered the effect size of associations between cardioembolic stroke risk and functional recovery at one year post-ICH. These findings indicate that cardioembolic stroke risk may play a negative role in recovery after ICH, thus adding an additional target endpoint for future studies of OAT resumption after primary ICH.
Cardioembolic stroke risk and oral anticoagulation resumption are expected to influence recovery after OAT-ICH by affecting ischemic stroke rate, with associated additional disability.14 In order to focus on potential associations with functional recovery, we limited our analysis to individuals who did not experience recurrent ICH or subsequent ischemic stroke. It is possible that less severe embolic infarcts not causing clinically-evident deficits may have resulted in enough CNS damage to hinder post-ICH recovery. Atrial fibrillation is associated with a hypercoagulable state, and up to 30% of patients with atrial fibrillation demonstrated evidence of micro embolism via transcranial Doppler ultrasonography.24–26 These observations point to the most likely mechanism linking atrial fibrillation with covert infarcts, i.e. occult cardioembolism.15 Of note, previous studies on ischemic stroke associated with atrial fibrillation also uncovered associations between embolic stroke risk and outcome.17 Due to the lack of surveillance imaging, we are unfortunately unable to further test this hypothesis in our study. Atrial fibrillation has also been associated with cognitive impairment (especially in terms of memory performance), even among stroke-free patients.15 Previous studies clarified that ICH survivors are at high risk for dementia;27, 28 atrial fibrillation may further increase risk for cognitive impairment, in itself a major obstacle to rehabilitation and recovery after stroke.29 A number of additional biological observations can also be invoked to account for our findings, including decreased cerebral perfusion (as a result of reduced cardiac output) and pro-inflammatory state due to atrial fibrillation.30 Finally, increasing CHA2DS2-VASc scores may represent an increasing burden of co-morbid conditions negatively affecting post-ICH recovery. However, we identified an interaction between cardioembolic stroke risk and OAT resumption, suggesting that embolic risk (influenced by OAT resumption) is likely to be central to the association between CHA2DS2-VASc scores and functional outcome.
Our study has several limitations. First and foremost, the observational nature of the participating studies implies the potential for confounding bias (especially severity and indication bias). Lack of randomization in OAT resumption may have led to preferential assignment to anticoagulation of patients at higher likelihood to experience functional recovery to begin with. However, our findings suggest that individuals with higher CHA2DS2-VASc scores were more likely to resume OAT. As these individuals were also shown to be less likely to achieve functional recovery, one would expect OAT resumption to also be associated with worse post-ICH outcome. On the contrary, individuals with higher CHA2DS2-VASc scores (especially those restarted on OAT) may also have more healthcare resources devoted to their recovery by dint of their high-risk status and comorbidity burden, thus leading to residual confounding influencing our analyses. Ultimately, only a randomized clinical trial of OAT resumption after ICH (ideally including serial MRI imaging of silent infarcts) would provide definitive validation of our findings. Additionally, we opted to exclude patients with atrial fibrillation who experienced ICH without OAT exposure, because they are very unlikely to start OAT after ICH (usually due to low cardioembolic stroke risk), and therefore would add limited power to test our main hypotheses. Our study also displays several notable strengths. Thanks to a large collaborative effort, we gathered individual-level data for a large number of OAT-ICH survivors, with in-depth capture of baseline and follow-up outcome information. Several recent studies explored the risk and benefits of OAT resumption following primary ICH.7–11 However, none explored the association with functional outcome, and whether oral anticoagulation modifies this association. We also gathered OAT-ICH cases from a variety of clinical settings (tertiary care academic centers, community-based academic hospitals, and community institutions), thus greatly enhancing the generalizability of our findings. Finally, we demonstrated consistent findings across all three included studies.
Overall, our findings strongly support including recovery metrics in the design of randomized clinical trials of anticoagulation resumption after OAT-ICH. Additionally, future studies aimed at assessing the potential role of covert cardioembolic events (e.g. via surveillance neuroimaging) are warranted to better elucidate the pathophysiology of recovery after OAT-ICH. Finally, future studies should explore the association between cardioembolic risk, OAT resumption and long-term outcomes in multiple functional domains, including motor and cognitive recovery.
SUMMARY
In conclusion, we present evidence from a large collaborative effort identifying novel associations between cardioembolic stroke risk and functional recovery after OAT-related ICH. We also found evidence that these associations are weaker among individuals resuming oral anticoagulation, especially those with the highest cardioembolic stroke risk. Randomized clinical trials are needed to more accurately guide optimal decision making on resuming OAT for these patients and their healthcare providers. Ideally, these future studies will benefit from including detailed recovery metrics among post-ICH outcomes of interest.
Supplementary Material
Acknowledgments
Author Disclosures:
Ms. Murphy, Dr. Kuramatsu, Ms. Leasure, Dr. Kamel, Ms. Kourkoulis, Ms. Schwab, Dr. Elm, Dr. Tran, Dr. Greenberg, Dr. Viswanathan, Dr. Schwab, Dr. Shi, Dr. Kittner, Dr. Testai, Dr. Woo, Dr. Langefeld, Dr. James, Dr. Koch, Dr. Huttner and Dr. Sheth report no disclosures. Dr. Falcone is supported by Yale Pepper Scholar Award (P30AG021342) and the Neurocritical Care Society Research Fellowship. Dr. Sansing is supported by R01NS097728 and R01NS095993. Dr. Gurol is supported by K23NS083711. Dr. Anderson is supported by R01NS103924, K23NS086873, AHA 18SFRN34250007, and has received compensation for consultation services from ApoPharma, Inc. Dr. Rosand is supported by R01NSO36695, UM1HG008895, R01NS093870, R24NS092983, and has received compensation for consultation services from Pfizer Inc. and Boehringer Ingelheim. Dr. Biffi is supported by K23NS100816.
Funding Sources:
The authors’ work on this study was supported by funding from the US National Institute of Health (K23NS100816, U01NS069763, R01NS093870, P50NS051343 and R01AG26484) and by the Johannes and Frieda Marohn Foundation. The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: Systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2014;85:660–667 [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet neurology. 2010;9:167–176 [DOI] [PubMed] [Google Scholar]
- 3.da Silva IR, Provencio JJ. Intracerebral hemorrhage in patients receiving oral anticoagulation therapy. J Intensive Care Med. 2015;30:63–78 [DOI] [PubMed] [Google Scholar]
- 4.Cervera A, Amaro S, Chamorro A. Oral anticoagulant-associated intracerebral hemorrhage. Journal of neurology. 2012;259:212–224 [DOI] [PubMed] [Google Scholar]
- 5.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke; a journal of cerebral circulation. 2003;34:1710–1716 [DOI] [PubMed] [Google Scholar]
- 6.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: The decision to anticoagulate patients with atrial fibrillation. Circulation. Cardiovascular quality and outcomes. 2011;4:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836 [DOI] [PubMed] [Google Scholar]
- 8.Poli D, Antonucci E, Dentali F, Erba N, Testa S, Tiraferri E, et al. Recurrence of ich after resumption of anticoagulation with vk antagonists: Chirone study. Neurology. 2014;82:1020–1026 [DOI] [PubMed] [Google Scholar]
- 9.Claassen DO, Kazemi N, Zubkov AY, Wijdicks EFM, Rabinstein AA. Restarting anticoagulation therapy after warfarin-associated intracerebral hemorrhage. Archives of neurology. 2008;65:1313–1318 [DOI] [PubMed] [Google Scholar]
- 10.Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: A nationwide cohort study. Circulation. 2015;132:517–525 [DOI] [PubMed] [Google Scholar]
- 11.Chao TF, Liu CJ, Liao JN, Wang KL, Lin YJ, Chang SL, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133:1540–1547 [DOI] [PubMed] [Google Scholar]
- 12.Murthy SB, Gupta A, Merkler AE, Navi BB, Mandava P, Iadecola C, et al. Restarting anticoagulant therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke; a journal of cerebral circulation. 2017;48:1594–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korompoki E, Filippidis FT, Nielsen PB, Del Giudice A, Lip GYH, Kuramatsu JB, et al. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology. 2017;89:687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biffi A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Annals of neurology. 2017;82:755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, et al. Association between atrial fibrillation and silent cerebral infarctions: A systematic review and meta-analysis. Annals of internal medicine. 2014;161:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell MJ, Eikelboom JW, Yusuf S, Diener HC, Hart RG, Smith EE, et al. Effect of apixaban on brain infarction and microbleeds: Averroes-mri assessment study. American heart journal. 2016;178:145–150 [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Yamada T, Torii T, Furuta K, Matsumoto S, Yoshimura T, et al. Pre-admission chads2, cha2ds2-vasc, and r2chads2 scores on severity and functional outcome in acute ischemic stroke with atrial fibrillation. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2015;24:1629–1635 [DOI] [PubMed] [Google Scholar]
- 18.Biffi A, Anderson C, Battey TW, Ayres A, Greenberg SM, Viswanathan A, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;9:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo D, Rosand J, Kidwell C, McCauley JL, Osborne J, Brown MW, et al. The ethnic/racial variations of intracerebral hemorrhage (erich) study protocol. Stroke; a journal of cerebral circulation. 2013;44:e120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified rankin scale questionnaire: Reproducibility over the telephone and validation with quality of life. Stroke; a journal of cerebral circulation. 2011;42:2276–2279 [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 22.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957 [DOI] [PubMed] [Google Scholar]
- 23.Hsueh HM, Chen JJ, Kodell RL. Comparison of methods for estimating the number of true null hypotheses in multiplicity testing. J Biopharm Stat. 2003;13:675–689 [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Nozawa T, Okumura K, Jong-Dae L, Shimizu A, Yano K. Prothrombotic activity is increased in patients with nonvalvular atrial fibrillation and risk factors for embolism. Chest. 2004;126:687–692 [DOI] [PubMed] [Google Scholar]
- 25.Barber M, Tait RC, Scott J, Rumley A, Lowe GD, Stott DJ. Dementia in subjects with atrial fibrillation: Hemostatic function and the role of anticoagulation. J Thromb Haemost. 2004;2:1873–1878 [DOI] [PubMed] [Google Scholar]
- 26.Kumral E, Balkir K, Uzuner N, Evyapan D, Nalbantgil S. Microembolic signal detection in patients with symptomatic and asymptomatic lone atrial fibrillation. Cerebrovasc Dis. 2001;12:192–196 [DOI] [PubMed] [Google Scholar]
- 27.Biffi A, Bailey D, Anderson CD, Ayres AM, Gurol EM, Greenberg SM, et al. Risk factors associated with early vs. Delayed dementia after intracerebral hemorrhage. JAMA Neurol. 2016;73:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedictus MR, Hochart A, Rossi C, Boulouis G, Henon H, van der Flier WM, et al. Prognostic factors for cognitive decline after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2015;46:2773–2778 [DOI] [PubMed] [Google Scholar]
- 29.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: Still an incomplete picture. International journal of stroke : official journal of the International Stroke Society. 2013;8:38–45 [DOI] [PubMed] [Google Scholar]
- 30.Hui DS, Morley JE, Mikolajczak PC, Lee R. Atrial fibrillation: A major risk factor for cognitive decline. American heart journal. 2015;169:448–456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


