Abstract
Rationale
Abnormal mechanosensing of smooth muscle cells (SMCs) resulting from the defective elastin-contractile units has been suggested to drive the formation of thoracic aortic aneurysms (TAAs); however, the precise molecular mechanism has not been elucidated.
Objective
The aim of this study was to identify the crucial mediator(s) involved in abnormal mechanosensing and propagation of biochemical signals during the aneurysm formation and to establish a basis for a novel therapeutic strategy.
Methods and Results
We used a mouse model of postnatal ascending aortic aneurysms (Fbln4SMKO; termed SMKO), in which deletion of Fbln4 leads to disruption of the elastin-contractile units caused by a loss of elastic lamina-SMC connections. In this mouse, upregulation of early growth response-1 (Egr1) and angiotensin converting enzyme leads to activation of angiotensin II signaling. Here we showed that the matricellular protein, thrombospondin-1 (Thbs1), was highly upregulated in SMKO ascending aortas and in human TAAs. Thbs1 was induced by mechanical stretch and Ang II in SMCs, for which Egr1 was required, and reduction of Fbln4 sensitized the cells to these stimuli and led to higher expression of Egr1 and Thbs1. Deletion of Thbs1 in SMKO mice prevented the aneurysm formation in approximately 80% of SMKO; Thbs1−/− (termed DKO) animals and suppressed slingshot-1 and cofilin de-phosphorylation, leading to the formation of normal actin filaments. Furthermore, elastic lamina-SMC connections were restored in DKO aortas and mechanical testing showed that structural and material properties of DKO aortas were markedly improved.
Conclusions
Thbs1 is a critical component of mechanotransduction as well as a modulator of elastic fiber organization. Maladaptive upregulation of Thbs1 results in disruption of elastin-contractile units and dysregulation of actin cytoskeletal remodeling, contributing to the development of ascending aortic aneurysms in vivo. Thbs1 may serve as a potential therapeutic target for treating TAAs.
Subject Terms: Aneurysm, Cell Signaling/Signal Transduction, Genetically Altered and Transgenic Models, Vascular Biology
Keywords: Thoracic aortic aneurysm, mechanotransduction, extracellular matrix, elastic fibers, angiotensin II, aneurism, vascular biology, genetically altered mice, cell signaling
INTRODUCTION
Thoracic aortic aneurysms (TAAs) are life-threatening diseases defined as a permanent abnormal dilatation of the thoracic aorta. It has been shown that TAAs are associated with dysregulation of transforming growth factor beta (TGFβ) signaling 1–3, defects in the extracellular matrix (ECM) 4–7, and mutations in smooth muscle cell (SMC) contractile genes 8, 9. The anatomical and functional unit connecting SMC and elastic fibers in the aortic wall is called elastin-contractile unit and the disruption of this unit was shown to cause TAAs due to abnormal mechanosensing of SMCs 10. The relationship between these defects and aneurysm pathogenesis have begun to be established using various genetic models (reviewed in 11, 12), however, the initiation signal and the signaling pathway necessary for sustaining the aneurysmal expansion in each TAA subtype are not fully understood.
We previously established a mouse model of postnatal TAA by deleting fibulin-4 gene (Fbln4) in vascular SMCs (Fbln4SMKO, termed SMKO). Fibulin-4 is a secreted glycoprotein and is a component of elastic fibers, where it is localized to microfibrils 13. In SMKO aortas, elastic fibers fail to form normal elastic lamina-SMC connections during the early postnatal period, leading to an upregulation of mechanosensitive molecules, such as early growth response 1 (Egr1) and angiotensin converting enzyme (ACE), resulting in a local elevation of Angiotensin II (Ang II) signaling 7, 14. We also showed that an increase in serine/threonine phosphatase slingshot-1 (Ssh1) causes dephosphorylation of cofilin (activated form) and disruption of actin filaments 10. Furthermore, inhibition of Ang II signaling sufficiently prevented the development of ascending aortic aneurysms and improved elastic fiber integrity and restored actin filaments 14. However, the molecule responsible for converting the mechanical stress to biochemical signals that result in aneurysm growth has yet to be determined.
Thrombospondin-1 (Thbs1) is a homotrimeric glycoprotein secreted by various cells, including vascular SMCs and endothelial cells (ECs). Thbs1 negatively regulates cell adhesion, migration, proliferation, cell-cell interaction and angiogenesis, while positively regulates inflammation and activation of latent TGFβ. This broad spectrum of biological functions is attributable to domain structures with distinct binding sites for various ECM molecules and cell surface receptors, such as integrins, CD36 and CD47 15–18. Recent studies have shown that Thbs1 contributes to the development of abdominal aortic aneurysm (AAA) through acceleration of vascular inflammation 19, whereas it was reported that attenuation of Thbs1-directed activation of TGFβ resulted in progression of AAA 20. Thus, the multi-functionality of Thbs1 has complicated the understanding of its role in aortic aneurysms.
Here, we report a dual role for Thbs1 as a mediator of mechanotransduction and critical modulator of arterial wall structure in SMKO mice. We demonstrate that Thbs1 is directly induced by mechanical stretch and Ang II in SMCs and indirectly activates downstream Ssh1-cofilin signaling and leads to actin cytoskeletal remodeling in vivo. Deletion or reduction of Thbs1 restored the disruption of elastic lamina-SMC connections and prevented aneurysm formation, demonstrating a causative role of Thbs1 in aneurysm development in SMKO mice.
METHODS
The authors declare that all supporting data are available within the article and supplementary files.
The authors are responsible for maintaining availability and agree to make materials available to any researchers.
Detailed descriptions are provided in the Online Data Supplement.
RESULTS
Upregulation of Thbs1 in the aneurysmal wall
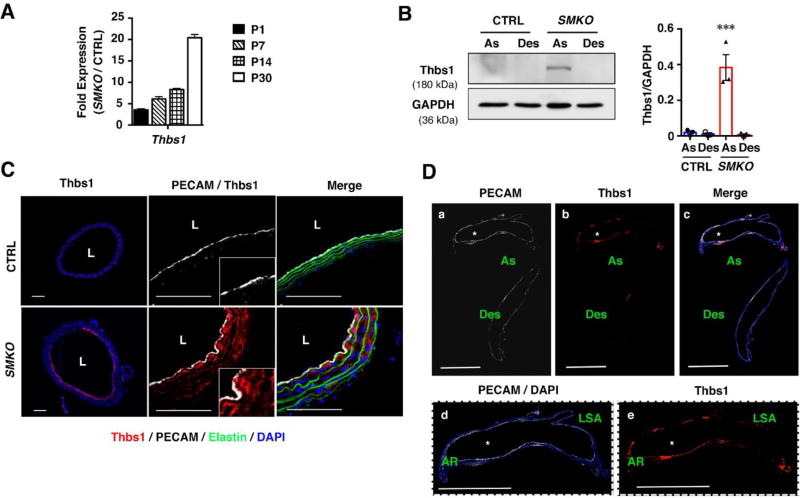
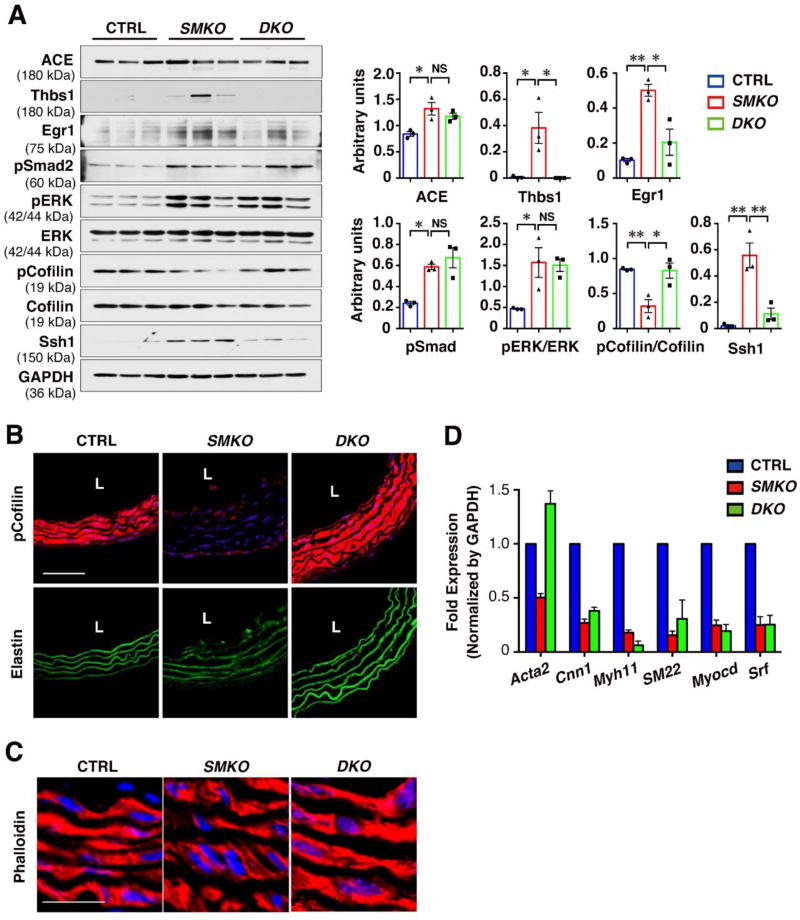
As we previously reported, the aortic wall begins to expand in SMKO aortas at postnatal day (P) 7, and the aneurysm is established by P30 10, 14. We defined this period as a critical therapeutic time window in which aneurysm formation could be prevented by inhibiting Ang II-mediated signaling caused by local upregulation of ACE 14. Transcripts level of Thbs1 in SMKO aortas was already increased at P1, before the aneurysm formation and markedly increased at P30 (Fig. 1A). Western blot analysis comparing the expression of Thbs1 in the ascending (As) and descending (Des) aortas of CTRL and SMKO mice at P30 showed a marked upregulation of Thbs1 specifically in the ascending aortas of SMKO mice (Fig. 1B). Consistent with Western blot data, immunostaining showed strong expression of Thbs1 in the SMKO ascending aortas including the aortic root compared with that of control (CTRL) aortas (Figs. 1C, D). Thbs1 was highly expressed in both endothelial cell (EC) and SMC layers, which was apparent at P9 SMKO aortas (Online Fig. I). Interestingly, although phosphorylated Cofilin (pCofilin) was decreased (=activated) in SMCs of SMKO aortas compared with CTRL, cofilin phosphorylation was maintained in ECs. These data indicated that Thbs1 was highly expressed in the aneurysmal wall prior to and during aneurysmal expansion in SMKO aortas.
Figure 1. Thbs1 is highly expressed in aneurysmal wall.
(A) qPCR analysis of Thbs1 from ascending aortas of CTRL (pooled P1, n=12; P7, n=18; P14, n=9; and P30, n=12) and Fbln4SMKO (SMKO, pooled P1, n=12; P7, n=18; P14, n=8; and P30, n=11) mice performed in technical triplicate. (B) Representative Western blots of ascending (As) and descending (Des) aortas of CTRL and SMKO at P30. n=3, Bar are means ± SEM. ***P < 0.001, one-way ANOVA. (C) Cross sections of the ascending aorta from CTRL and SMKO at P30 immunostained with Thbs1 (red), DAPI (blue), PECAM (white). Elastic fibers are green (autofluorescence). n=5. Scale bars are 50 µm. (D) Longitudinal sections of the aorta from SMKO mice (a–c) immunostained with PECAM (white), Thbs1 (red) and merged image with DAPI (blue). As (ascending aorta), Des (descending aorta). Expanded images of ascending aorta (d and e). AR (aortic root), LSA (left subclavian artery). Scale bars are 1 cm. n=5.
Fbln4 deficiency increases the response to Ang II and mechanical stretch, and upregulates Thbs1 in SMCs
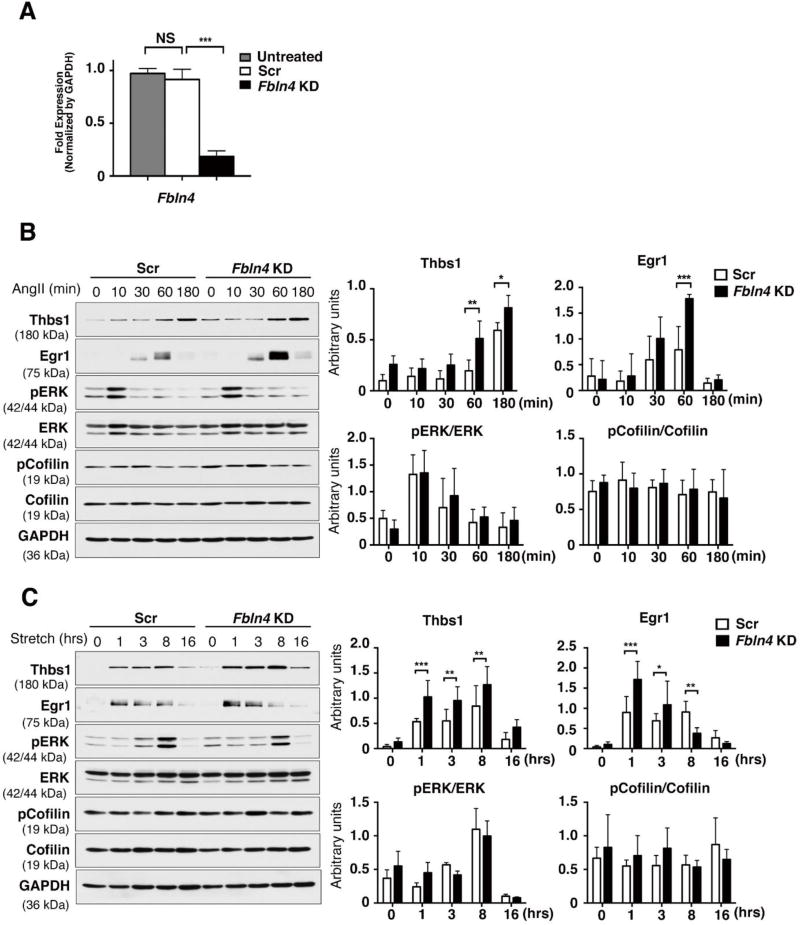
To understand the upstream signals for Thbs1 induction in SMCs, we turned to an in vitro system and performed small interference (si) RNA-mediated knockdown (KD) of Fbln4. Fbln4 or scramble siRNA (Scr; as CTRL) was administered to rat SMCs and the efficiency of KD was confirmed by qPCR (Fig. 2A). We first examined the response of Fbln4 KD cells to Ang II in the absence of serum (Fig. 2B). Ang II induced rapid phosphorylation (p) of ERK in 10 min in both Fbln4 KD and CTRL cells. Consistent with previous reports 21, 22, Egr1 was induced by Ang II in both groups, but the level of Egr1 was significantly higher in Fbln4 KD compared with CTRL. Thbs1 was increased at 60 min in Fbln4 KD cells whereas Thbs1 upregulation was observed at 180 min after Ang II stimulation in CTRL cells. Similar results on the induction of Thbs1 by Ang II were obtained using primary SMCs isolated from P30 SMKO aortas (Online Fig. II), as well as in Fbln4 knockout rat SMCs generated by CRISPR-Cas9 genome editing technology (Online Fig. III). However, neither condition was sufficient to affect the phosphorylation status of cofilin.
Figure 2. Fbln4 deficiency enhances the response to mechanical stress and Ang II in rat SMCs.
(A) qPCR analysis confirming knockdown of Fbln4 in rat SMCs. Total RNA from siRNA-transfected cells were extracted 3 days after transfection. The mRNA levels relative to untreated siRNA are shown. n=4, Bars are means ± SEM. *** P < 0.001, one-way ANOVA. (B) Rat SMCs treated with scramble siRNA (Scr) or Fbln4 siRNA (Fbln4 KD) cultured in serum-free media for 21 hr, then stimulated with Ang II (1 nM) for 10, 30, 60 or 180 min. Cell lysates were analyzed by Western blotting with indicated antibodies. Representative blots and quantification graphs are shown from quadruplicate experiments. Bars are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Two-way ANOVA. (C) Scr- or Fbln4 KD cells were subjected to cyclic stretch (20 % strain, 1.0 Hz) by FlexCell system for 1, 3, 8 or 16 hr. Representative Western blots showing expression of Thbs1, Egr1, pERK, total ERK, pCofilin, total cofilin and GAPDH. Quantification graph are shown from triplicate experiments. Bars are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Two-way ANOVA.
Next, to examine the effects of mechanical stimuli on Thbs1 expression, Scr and Fbln4 KD cells were subjected to cyclic stretch (1Hz, 20% strain) for 16 hours (hr). Fbln4 KD responded to cyclic stretch and upregulated Egr1 and Thbs1 after 1 hr of stimulation, and the amplitude of expression was significantly higher in Fbln4 KD cells compared with CTRL cells (Fig. 2C). Upregulation of pERK peaked at 8 hr in both Fbln4 KD and CTRL cells and the levels were comparable between the two cells, indicating that ERK activation at the late phase was not affected by Fbln4 KD. Since SMCs in SMKO aortas were shown to be less differentiated compared with CTRL SMCs 7 and the responses to Ang II and mechanical stretch can be influenced by the differentiation status of SMCs 23, 24, it is plausible that Fbln4 deficiency exacerbated the response to Ang II and mechanical stretch, leading to the increased expression of Egr1 and Thbs1.
Egr1 is required for upregulation of Thbs1
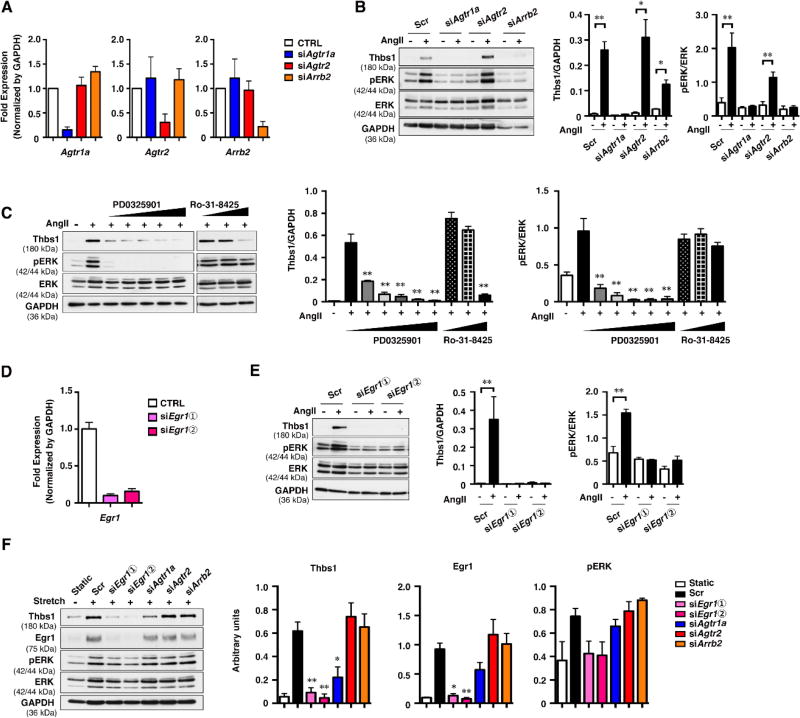
Ang II induces ERK activation by two distinct pathways downstream of Agtr1a, mediated by heterotrimeric guanine nucleotide-binding protein (G protein) and β-arrestin 2 (Arrb2) 25, 26. To examine the involvement of these pathways in Ang II-induced Thbs1 induction, knockdown for Agtr1a, Agtr2 and Arrb2 was performed. The KD efficiency was confirmed by qPCR (Fig. 3A). Cells were cultured in serum- free media overnight and stimulated with Ang II (100 nM) for 3 hr. In Scr-treated CTRL cells, upregulation of Thbs1 and ERK phosphorylation were reproducibly observed (Fig. 3B). Agtr1a KD inhibited ERK phosphorylation and abolished Thbs1 expression, whereas Agtr2 KD showed no effects. Arrb2 KD also showed reduced ERK phosphorylation and suppressed Thbs1 expression.
Figure 3. Upregulation of Thbs1 is mediated by Egr1.
(A and D) qPCR analysis confirming the knockdown of Agtr1a, Agtr2, and Arrb2 (A) and Egr1 (D) in rat SMCs. Total RNA from siRNA-treated cells were extracted 3 days after transfection. The mRNA levels relative to scramble siRNA (Scr) are shown. Bars indicate technical triplicate. (B and E) Scr- or siRNA-treated rat SMCs were cultured in serum-free media for 21 hr and stimulated with or without Ang II (100 nM) for 3 hr. Representative Western blots and quantification graphs are shown. (C) Rat SMCs were cultured in serum-free media for 21 hr, then pretreated with or without MEK inhibitor PD0325901 (1 nM, 10 nM, 100 nM, 1 µM, 10 µM) or PKC inhibitor Ro-31-8425 (100 nM, 1 µM, 10 µM) for 1 hr followed by 3 hr of stimulation with Ang II (100 nM). Representative Western blots and quantification graphs are shown. Bar are means ± SEM, **P < 0.01, two-way ANOVA. (F) Scr- or siRNA-treated rat SMCs were subjected to uniaxial cyclic stretch (20 % strain, 1.0 Hz) in the presence of 20% FBS for 8 hr. Representative Western blots showing Thbs1, Egr1, pERK, total ERK and GAPDH. Quantification graphs are shown from triplicate experiments. Bars are means ± SEM. *P < 0.05, **P < 0.01. One-way ANOVA.
Since it has been reported that G protein-mediated ERK phosphorylation requires the activation of protein kinase C (PKC) 25, 26, we tested the effect of a PKC inhibitor Ro-31-8425 on Ang II-induced Thbs1 expression (Fig. 3C). Ro-31-8425 suppressed Thbs1 expression in a dose dependent manner, however, it had no effects on ERK phosphorylation. In contrast, pretreatment with PD0325901, a MEK inhibitor, completely blocked pERK and suppressed Thbs1 expression. These data suggested that there were pERK-dependent and -independent pathways downstream of G-proteins that regulate expression of Thbs1. As it has been reported that Ang II-induced ERK activation is mediated by Egr1 27 and Egr1 is a known activator of Thbs1 28, we tested whether Ang II-induced Thbs1 expression was dependent on Egr1 in rat SMCs. Egr1 was knocked-down by 2 sets of siRNA (Fig. 3D) and AngII was administered to induce Thbs1 expression. Egr1 KD abolished phosphorylation of ERK as well as Ang II-induced Thbs1 expression for both sets of siRNA (Fig. 3E). These results indicated that Egr1 was an essential mediator of Thbs1 downstream of Ang II-Agtr1a.
We also examined the regulation of Thbs1 by mechanical stretch in SMCs. We used Agtr1a, Agrt2, Arrbs2, and Egr1 KD cells and subjected them to cyclic stretch (1 Hz, 20% strain) for 8 hr. As Fig. 3F shows, Thbs1 was induced by cyclic stretch in Scr-treated cells with upregulation of Egr1 and pERK. Agtr1a KD modestly suppressed the increase in Thbs1 expression with stretch, without affecting Egr1. Agtr2 KD or Arrb2 KD did not change the effect of stretch on Thbs1 expression or Egr1 expression. In contrast, Egr1 KD completely suppressed Thbs1 expression and pERK upregulation in response to stretch, indicating that Egr1 is an essential regulator of Thbs1 expression downstream of mechanical stress.
Deletion of Thbs1 attenuates aneurysm formation in SMKO aortas
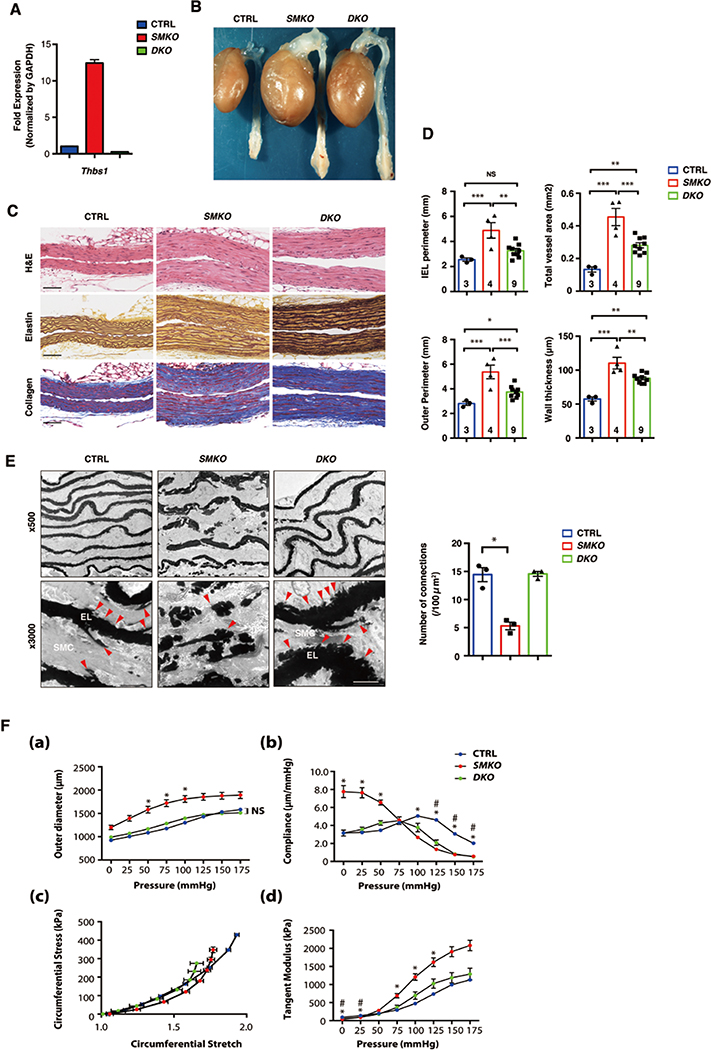
To test whether Thbs1 contributed to aneurysm formation in SMKO mice, we generated SMKO mice on a Thbs1-null background (SMKO;Thbs1−/−; termed DKO). qPCR confirmed the absence of Thbs1 mRNA in DKO aortas (Fig. 4A). The aneurysms were examined at 3 months of age and compared with respective CTRL littermates. Aneurysms were mostly prevented in DKO mice (30/38, 78.9%) and some of SMKO;Thbs1+/− mice (12/25, 48%) also showed amelioration of the aneurysm (Fig. 4B and Table 1). Morphology of SMCs and collagen deposition in rescued DKO aortas were comparable to that of CTRL aortas and DKO aortas contained intact elastic fibers that were visualized by Hart’s staining (Fig. 4C). The internal elastic lamina (IEL) perimeter and outer perimeter were smaller in DKO compared to SMKO aortas, however, the aortic wall was thicker in DKO aortas compared to CTRL (Fig. 4D). We hypothesized that the deletion of Thbs1 enhanced elastic fiber maturation and prevented aneurysm formation. To test this hypothesis, we performed in vitro elastogenesis assays using neonatal human dermal fibroblasts (HDFs) 29 and examined the function of Thbs1 in elastic fiber assembly by siRNA (Online Fig. IVA). Scr siRNA-treated cells (CTRL) did not affect elastic fiber formation whereas FBLN4 siRNA suppressed the deposition of elastin onto the fibrillin-1-positive microfibrils (Online Fig. IVB). THBS1 siRNA-treated cells were indistinguishable from Scr-treated cells and the combination of THBS1 and FBLN4 siRNA did not improve elastic fiber assembly. These results showed that Thbs1 was not directly involved in elastic fiber assembly in vitro.
Figure 4. Deletion of Thbs1 attenuates aneurysm formation in SMKO aortas.
(A) qPCR analysis of Thbs1 on aortas harvested from P30 CTRL (pooled n=12), SMKO (pooled n=11) and DKO (pooled n=3) mice in technical triplicate. (B) Gross photos of CTRL, SMKO and SMKO crossed with Thbs1 null mice (SMKO; Thbs1−/−, DKO) at 3 months of age. (C) Histological images of cross sections of the ascending aorta from P30 CTRL, SMKO and DKO stained with hematoxylin and eosin (H&E), Hart’s (elastin) and Masson trichrome (collagen). Scale bars, 20 µm. (D) Morphometric analysis showing IEL perimeter, outer perimeter, total vessel area and wall thickness. Bars are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA. Number of animals are indicated in each bar. (E) Electron microscopy (EM) images from P30 CTRL (n=3), SMKO (n=3) and DKO (n=3) ascending aortas. Elastic lamina (EL) -SMC connections seen in CTRL (red arrowheads) were significantly decreased in SMKO and restored in DKO. Scale bars, 2 µm. Quantification of the connection between EL and SMC by Kruskal-Wallis test with Dunn’s multiple comparisons is shown. (F) Mechanical testing using CTRL (n=7), SMKO (n=7) and DKO (n=3) aortas at P30. (a) Aortic pressure-outer diameter curves, (b) Aortic pressure-compliance curves, (c) circumferential stress-stretch plots, and (d) tangent modulus. Bars are means ± SEM. Symbols indicate significant difference (P < 0.05) between CTRL and SMKO (*), and CTRL and DKO (#). One-way ANOVA with Tukey’s post-hoc test.
Table 1.
Summary of aneurysm phenotype in SMKO mice with Thbs1 deficiency.
| GENOTYPE | PHENOTYPE | ||
|---|---|---|---|
| Aneurysm | Dilatation or normal | Total number (rescue ratio) | |
| SMKO; Thbs1−/− (DKO) | 8 | 30 | 38 (78.9 %) |
| SMKO; Thbs1+/− | 13 | 12 | 25 (48.0 %) |
| SMKO; Thbs1+/+ (SMKO) | 33 | 0 | 33 (0 %) |
To gain more insights into the ultrastructure of DKO aortas, we performed electron microscopy, compared with CTRL and SMKO aortas. Consistent with the previous findings, SMKO aorta showed severe disruption of elastic fibers and a loss of connections between the elastic laminae and SMCs; connections that were evident in CTRL aortas (arrows in CTRL, Fig. 4E). Surprisingly, the elastic laminae in rescued DKO aortas were thicker and much more organized compared to SMKO aortas and contained numerous short elastin extensions extending toward the SMCs (arrowheads in DKO, Fig. 4E). These findings suggested that the deletion of Thbs1 promoted the organization of elastic fibers in vivo. To examine the impact of improved elastic fiber organization on biomechanics of the aorta, we performed pressure-diameter experiments for DKO aortas and compared with CTRL and SMKO aortas 30, 31. The outer diameter of DKO aortas exhibited pressure-dependent dilatation and significant differences were not found at any pressures compared to CTRL (Fig. 4Fa). Compliance, the inverse of stiffness, was higher in SMKO aortas between 0–50 mm Hg and became significantly lower above physiological pressures (>100 mmHg, Fig. 4Fb). DKO aortas showed lower compliance than SMKO aortas between 0–50 mmHg similar to CTRL, however, it was significantly decreased compared with CTRL between 125–170 mmHg and behaved similar to SMKO aortas (Fig. 4Fb). We then examined the material properties of DKO aortas by calculating tangent modulus using stress-stretch relationship (Fig. 4Fc, d). Tangent modulus of DKO aortas was significantly lower than SMKO aortas but did not differ from that of CTRL at any pressures, indicating the restoration of the material properties in DKO aortas. Taken together, prevention of aneurysm development was supported by normalization of material properties and improved quality of elastic fibers.
Deletion of Thbs1 suppresses abnormal mechanotransduction in the aneurysmal wall
To determine which signaling pathways were affected in DKO aortas, we performed Western blot analyses to compare the signaling molecules dysregulated in SMKO, including ACE, Thbs1, Egr1, pSmad2, pERK, pCofilin and Ssh1. While ACE and pERK remained high in DKO aortas, Ssh1, and Egr1 were significantly decreased and pCofilin was increased in DKO aortas (Fig. 5A). Since Thbs1 is known to activate latent TGFβ, the rescue might have been achieved through inhibition of TGFβ. However, pSmad2 levels were unchanged in DKO aortas (Fig. 5A), indicating that the rescue was independent of canonical TGFβ signaling and that activation of latent TGFβ by Thbs1 was not extensive in SMKO animals. Immunostaining results confirmed that pCofilin levels were restored to CTRL levels in DKO aortas (Fig. 5B). Accordingly, actin filaments in DKO aortas were continuous compared with those of SMKO aortas (Fig. 5C). Interestingly, qPCR analysis revealed that expressions of SMC contractile genes were unchanged compared to SMKO aorta except upregulation of Acta2 (Fig. 5D). Since Acta2 is the major actin isotype in SMCs, Acta2 upregulation may have been contributed to the increased actin filaments.
Figure 5. Thbs1 is upstream of Ssh1-cofilin pathway leading to aneurysm formation.
(A) Western blots showing downregulation of Ssh1 expression and upregulation of pCofilin levels in P30 DKO aortas. Quantification of Western blots are shown in right graphs. Bars are means ± SEM. *P < 0.05, **P < 0.01, one-way ANOVA. (B and C) Cross sections of the ascending aorta from P30 CTRL, SMKO and DKO immunostained with pCofilin (red in B), Phalloidin (red in C) and DAPI (blue). Elastic fibers are green (autofluorescence). n=3. Scale bars are 50 µm in (B), 100 µm in (C). (D) qPCR analysis of SMC-specific genes from ascending aortas of CTRL (pooled n=12), SMKO (pooled n=11) and DKO (pooled n=3) mice performed in technical triplicate.
Next, to test the possibility that Thbs1 directly inhibited phosphorylation of cofilin in SMCs in the absence of Fbln4, we used Fbln4KO rat SMCs (described in Online Fig. III) and primary SMCs isolated from SMKO aortas (described in Online Fig. II) and treated with recombinant Thbs1 (rThbs1) for 6 hr followed by Western blot analysis. The phosphorylation status of cofilin was not affected by addition of rThbs1 in Fbln4KO rat SMCs (Online Fig. VA) or primary SMKO SMCs (Online Fig. VB). It has been reported that Thbs1 inhibits pCofilin in macrophage 32, which cell type is frequently observed in experimental aortic aneurysms 33. Therefore, we evaluated the presence of macrophages in the aneurysmal wall of CTRL, SMKO and DKO mice (Online Fig. VI). qPCR analysis showed an increase in Cd68 and Mcp1 transcripts in SMKO but not in DKO aortas (Online Fig. VIA). However, immunostaining for CD68 in SMKO or DKO aortas showed no accumulation of CD68-positive macrophages (Online Fig. VIB), consistent with our previous findings 7.
It is of note that Thbs1 was highly expressed in ECs of SMKO aortas from P9 (Online Fig. I); however, phosphorylation of cofilin was unaffected. To examine if Thbs1 in ECs contributed to the formation of aneurysms via acting on their receptors, we generated double knockout mice for SMKO and Cd36 (SMKO; Cd36−/−) as well as SMKO and Cd47 (SMKO;Cd47−/−), both of which are known Thbs1 receptors expressed in ECs 34, 35. Both double knockout mice were evaluated for the presence of aneurysms by gross observations at P30 (Online Fig. VIIA). A large aneurysm was observed in the ascending aortas of SMKO;Cd36−/− and SMKO; Cd47−/− animals with severe tortuous descending aortas. Histological observations revealed thick aortic wall and disrupted elastic fibers in SMKO as well as SMKO;Cd36−/− and SMKO; Cd47−/− animals (Online Fig. VIIB). Morphometric analysis showed that IEL perimeter, outer perimeter, total vessel area, and wall thickness were largely comparable among SMKO, SMKO;Cd36−/− and SMKO; Cd47−/− mice (Online Fig. VIIC). These data indicate that Thbs1 does not directly alter phosphorylation of cofilin in SMCs and CD36 nor CD47 is involved in the aneurysm formation in SMKO mice.
Thrombospondin-1 is increased in human TAAs
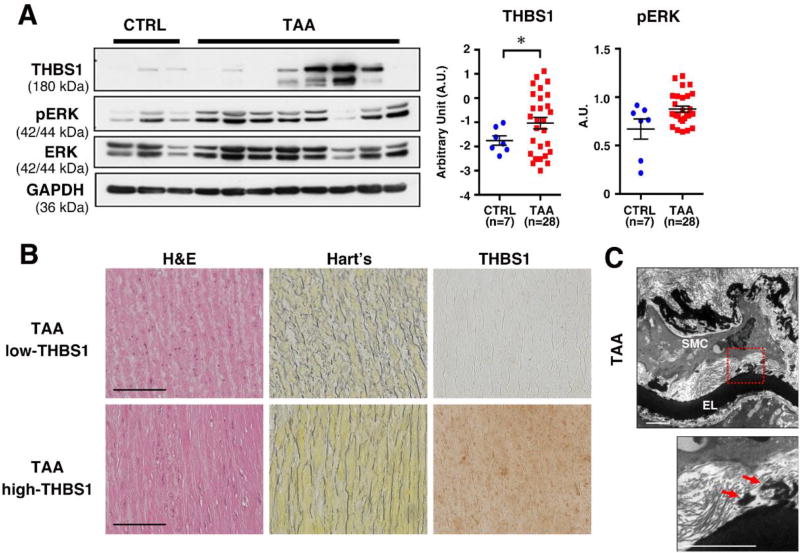
Finally, to evaluate if the THBS1-mediated pathway was involved in the pathogenesis of TAA in humans, we examined the expression of THBS1 and pERK levels in TAA samples from non-syndromic sporadic TAA patients that underwent surgery. Aortic wall punch biopsies obtained from patients undergoing coronary artery bypass surgery (CABG) were designated as non-aneurysmal CTRL. There were no differences in sex, age, metabolic rate, blood pressure and cardiac functions between CTRL and TAA (Table 2). Western blot analysis revealed a significant increase in THBS1 levels in aneurysmal tissues compared to CTLR (Fig. 6A). In contrast, pERK levels were comparable between CTRL and TAA samples. Histological analysis of TAA with high THBS1 (n=6) and low THBS1 (n=4) both showed elastic fiber fragmentation and the degree of fragmentation seems comparable between the two groups (Fig. 6B). THBS1 levels did not correlate with clinical features (Online Fig. VIII). Electron microscopy images from TAA with high THBS1 exhibited the same loss of elastic lamina-SMC connections that was observed in SMKO aortas (Fig. 6C). Taken together, the data demonstrated that THBS1 elevation was associated with non-syndromic sporadic TAA in humans.
Table 2.
Characterization of CTRL and TAA patients.
| CTRL (17 Patients) |
TAA (28 Patients) |
P-VALUE (t-TEST) |
|
|---|---|---|---|
| Male gender | 76.5 % | 60.7 % | - |
| Median of age (mini – max; years-old) | 69 (50 – 81) | 66 (47 – 79) | 0.357 |
| Body mass index (BMI; kg/m2) | 23.40 ± 3.72 | 22.65 ± 3.35 | 0.487 |
| Heart rate (HR; bpm) | 75.76 ± 11.38 | 72.29 ± 12.16 | 0.346 |
| Systolic blood pressure (SBP; mmHg) | 127.82 ± 17.58 | 131.46 ± 19.36 | 0.530 |
| Aortic diameter (mm) | 37.0 ± 4.88 | 53.28 ± 7.50 | 0.001* |
| Ejection fraction (EF; %) | 52.88 ± 16.67 | 61.21 ± 10.05 | 0.075 |
| Internal ventricular septum (IVS; mm) | 9.87 ± 1.87 | 10.82 ± 1.88 | 0.117 |
| left ventricular posterior wall (LVPW; mm)) | 10.10± 1.48 | 10.46 ± 1.72 | 0.490 |
P < 0.05, unpaired t-test.
Figure 6. THBS1 expression is significantly increased in human TAA.
(A) Representative Western blots showing the comparison of THBS1, GAPDH and pERK levels in aortic tissues of control (n=7, pooled 2–3 tissues per group) and TAA samples (n=28). Log-converted value of THBS1/GAPDH was compared using unpaired t-test with Welch correction. pERK/GAPDH value was compared by unpaired t-test. * P < 0.05. (B) Representative histological images of cross sections of the ascending aorta from human TAA tissues from high THBS1 group (n=6) and low THBS1 group (n=4) stained with hematoxylin and eosin (H&E), Hart’s (elastin) and anti-THBS1. Bars are 50 µm. (C) EM images of the ascending aortas from human TAA tissues. The lower panel is an enlargement of the area shown in red box. Arrows indicate loss of connections between elastic lamina (EL) and SMCs. n=1. Scale bar, 2 µm.
DISCUSSION
In this study, we reported the upregulation of Thbs1 in TAAs of mice and humans and showed that genetic deletion of Thbs1 prevented aneurysms in SMKO mice. In SMCs, Thbs1 expression was markedly induced by mechanical stretch and Ang II, and Egr1 was required for the induction of Thbs1. Deletion of Fbln4 in vitro enhanced the responses to these stimuli and led to the increased expression of Egr1 and Thbs1, further contributing to the transduction of mechanical stress into biochemical signals in SMCs. Aortas from DKO mice showed restoration of elastic lamina-SMC connections with numerous short elastin extensions and suppression of Ssh1-cofilin signaling, revealing the unrecognized role of Thbs1 as a modulator of arterial wall integrity.
Novel role of Thbs1 in mediating mechanical stress in the aneurysmal wall
Thbs1 is maintained at low levels in the postnatal vessels and elevated in various vascular diseases, including pulmonary arterial hypertension 36, aortic aneurysms 37, atherosclerosis 38, cerebral cavernous malformations 39, and ischemia reperfusion injury 40. The role of Thbs1 in these conditions seem to be highly context dependent but is largely associated with activation of TGFβ and/or inflammatory cytokine production. For example, in AAAs, Thbs1 promotes macrophage recruitment and contributes to adventitial inflammation during aneurysm formation 19, whereas blockade of Thbs1-directed TGFβ activation or Thbs1 deficiency promotes inflammation and accelerates AAA 20, 38. Since central pathogenesis of TAA is different from that of AAA and a reduced contribution of inflammation has been observed 41, we speculated that the mechanistic role of Thbs1 in TAA might be distinct from that of AAA. Our current study shows that pathologic Thbs1 contributes to abnormal mechanosensing of SMCs and is essential for development of TAAs in SMKO mice.
The pathway(s) involved in Thbs1 upregulation has been investigated in various cell types and Ang II is generally known as an upstream regulator of Thbs1 42, 43. TGFβ by forming a positive feedback loop, also induces Thbs1 expression in a Smad2-dependent manner 44 and this pathway can be amplified by Ang II, as TGFβ is a transcriptional target of Ang II 45. In addition, recent studies have shown that physical factors such as hypoxia and disturbed flow induce Thbs1 36, 46. In SMKO aortas, Thbs1 is highly upregulated in ECs and inner portions of SMCs in the ascending aortas, supporting the relationship between physical factors and Thbs1 expression. Thbs1 is more robustly upregulated in Fbln4 KD SMCs than CTRL in response to mechanical stretch, indicating that SMKO SMCs may be exquisitely sensitive to changes in mechanical stimuli, such as increased pulse pressure 14, decreased compliance, and increased elastic modulus observed in SMKO aorta. The unique and complex mechanical environment of the ascending aorta 47 in combination with altered SMC mechanosensing may contribute to localized Thbs1 signaling. Whereas Ang II quickly activates ERK via Agtr1a and upregulates Thbs1 in isolated SMCs, mechanical stretch induces Thbs1 at 8 hr with delayed ERK activation. Intriguingly, in both cases, Egr1 is essential for Thbs1 induction. Taken together, our results suggest that Thbs1 is a mechanosensitive matricellular protein upregulated by Egr1 that activates ERK, which in turn induces Egr1 and produces Ang II via upregulation of ACE, generating a feed forward loop for aneurysm expansion in the ascending aorta.
Deletion of Thbs1 prevents aneurysm formation with restored elastic lamina-SMC connections in SMKO aorta
Thbs1 is a matriellular protein known to mediate cell-matrix interactions with a limited role in tissue morphogenesis. Based on our results using DKO mice, the deletion of Thbs1 is sufficient to prevent aneurysm formation in SMKO mice. Surprisingly, the DKO aorta showed better quality of elastic fibers with restored connections between elastic lamina and SMCs and actin cytoskeletal abnormality is corrected. This suggests that Thbs1 is a negative regulator of elastic fiber organization. While we failed to make a direct link between Thbs1 and inhibition of elastic fiber assembly in vitro, there are possible explanations for the potential contribution of Thbs1 in elastic fiber formation. First, electron-microscopy analysis showed numerous elastic extensions between elastic laminae and SMCs, indicating the restoration of the elastin-contractile units, thus the vessel wall was stabilized in DKO mice. The absence of antiadhesive activity of Thbs1 may contribute to the increased formation of elastic lamina-SMC connections in DKO mice. This is the first demonstration that a loss of Thbs1 improves the elastic fiber organization in the structurally compromised aortas. Second, it has previously been shown that inhibition of versican, which is a large extracellular matrix proteoglycan, promotes tropoelastin synthesis and elastic fiber formation in human leiomyosarcoma SMCs 48. Since versican forms a functional complex with Thbs1 and is co-localized on microfibrils 49, deletion of Thbs1 may have disrupted the inhibitory complex and facilitate elastic fiber formation in DKO aortas.
Thbs1 is hardly expressed in CTRL aortas whereas it is highly expressed in ECs and SMCs of SMKO aortas. Our knockout study using Cd36−/− and Cd47−/− mice did not lead to the rescue of aneurysms in SMKO, indicating that the function of Thbs1 is not mediated via these receptors. However, we failed to determine whether the function of Thbs1 was cell autonomous in SMCs or EC-derived Thbs1 acted on neighboring SMCs. A recent study showed that deletion of an AT1 receptor in ECs attenuates the development of ascending aneurysms in Marfan model mice, where pathological changes are usually seen in SMCs 50. Therefore, it is possible in SMKO mice that an AT1 receptor drives thrombospondin-1 production in ECs, which is delivered and acts on SMC in a non-cell autonomous manner. It will be informative to determine the cell type responsible for production and execution of the deleterious effect of Thbs1 during aneurysm formation.
As we have previously shown, the activity of cofilin, which is regulated by its phosphatase Ssh1, causes abnormal actin cytoskeletal remodeling in SMKO aortas 10. Although the expression of ACE and pERK remained unchanged, Ssh1 levels are decreased and phosphorylation of cofilin is increased in DKO aortas. As a result, actin filaments are continuous compared with those of SMKO aortas. It is likely that restoration of elastic fibers in the absence of Thbs1 indirectly suppresses the Ssh1-cofilin pathway in DKO aortas. Alternatively, since Thbs1 has been shown to bind the actin cytoskeleton via its cell surface receptors 51 and/or to increase phosphorylation of FAK in SMCs 52, the absence of Thbs1 may have altered the actin filaments via the alteration of focal adhesions in SMCs.
Application to human aortic aneurysms
Imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound are currently used for routine diagnosis and monitoring of aneurysms. However, these imaging tools cannot be used to accurately determine the pre-clinical status and predict the timing for dissection or rupture of aneurysms (reviewed in 53). It has been reported that aortic size does not always provide an accurate prediction for Type A or Type B aortic dissection 54–56. Therefore, there is a pressing need for the discovery of blood-borne biomarkers that enable us to assess the dissection or rupture risk in a timely manner. Recent analysis of differentially expressed proteins in aortic aneurysms in human patients showed that Thbs1 is increased in TAAs 37, 57. Although the association of Thbs1 and aortic aneurysms is not fully established in humans, it was suggested that a high serum concentration of Thbs1 was associated with slower growth of AAA in humans 58. Intra-arterial thrombus secretome analysis showed a negative association between Thbs1 and AAA 59. Our mouse study demonstrates that Thbs1 is directly involved in TAA formation and an increase in Thbs1 transcripts is already evident before developing a distinct aneurysm. Although our model fails to address the relevance of Thbs1 in aortic dissection, temporal analysis reveals that it could potentially predict the pre-clinical status of the aneurysm. Second application is to develop anti-THBS1 therapy for prevention/control of aneurysm growth. Ang II receptor blockers have been suggested as a new drug to treat aneurysm growth in patients with Marfan syndrome 45, however, large clinical trials comparing the effects between losartan and β-blocker failed to establish the advantage of losartan over atenolol 60. Directly targeting Thbs1 and inhibiting maladaptive upregulation of Thbs1 may be effective in controlling the aneurysm growth, especially those with a high level of THBS1 at the early clinical stage of aneurysm. Further analysis with human serum Thbs1 levels and its association with imaging data will provide a basis for Thbs1-targeted therapy as a potential treatment for TAA.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Defective extracellular matrix (ECM) is one of the mechanisms for thoracic aortic aneurysm (TAA) formation.
Alterations in cytoskeletal remodeling in smooth muscle cells (SMCs) is observed in TAA.
Loss of fibulin-4 (Fbln4) causes TAA with disruption of elastin-SMC connections and abnormal mechanosensing of SMCs.
The initial signal that triggers abnormal mechanosensing leading to TAA is unknown.
What New Information Does This Article Contribute?
Thrombospondin-1 (Thbs1) is induced by mechanical stretch and angiotensin II in SMCs, and Early growth response 1 (Egr1) is required for this response.
Thbs1 is highly upregulated in the aneurysm lesions in Fbln4 mutant mice and TAA patients.
Deletion of Thbs1 in Fbln4 mutant mice restores elastin-SMC connections and organization of elastic fibers, preventing TAA.
Thbs1 may be a potential therapeutic target for TAA.
The disruption of elastin-SMC connections plays a critical role in aneurysm formation, not only by damaging the structural integrity of the aortic wall but also by altering mechanotransduction and cytoskeletal remodeling of SMCs. By using SMC-specific knockout for Fbln4 (termed SMKO), we show that disruption of elastin-SMC connections causes maladaptive upregulation of Thbs1 in the ascending aorta prior to the formation of aneurysms. Thbs1 expression is induced by mechanical stretch and angiotensin II in SMCs, which is mediated by Egr1 and enhanced by a loss of fibulin-4. Although Thbs1 has a minimal role in the development of the aorta, the deletion of Thbs1 in Fbln4 SMKO mice significantly suppresses aneurysm formation. This is due to restoration of elastin-SMC connections and improvement of material property of the aorta. We also show that Thbs1 is upregulated in TAA patients. Further analysis of blood and tissue concentrations of Thbs1 associated with each type of TAA are necessary for evaluation of the potential application of Thbs1 blocking antibody/inhibitors for treatment of TAA.
Acknowledgments
We thank G. Urquhart, K. Matsumura, M. Masuda, J.V. Alves and S. Sato for technical assistance, N.A. Abumrad for providing Cd36-null mice, P.A. Oldenborg for providing Cd47-null mice, Y. Jinzenji and L. J. Ringuette for assistance with electron microscopy, and we acknowledge technical support from the Facility for Electron Microscopy Research (FEMR) at McGill University and the Histology Core Laboratory at UT Southwestern Medical Center. We appreciate R. Ann Word for critical reading of the manuscript.
SOURCES OF FUNDING
This work was supported in part by grants from the MEXT KAKENHI (Grant Number JP 17H04289), The Naito Foundation, Astellas Foundation for Research on Metabolic Disorders to HY and Grant-in Aid for Young Scientists (B) (Grant Number 15K20898), Japan Heart Foundation Research grant, The Inamori Foundation, Japan Foundation for Applied Enzymology and Takeda Science Foundation to YY, National Institutes of Health (NIH HL105314 and HL115560) to JEW, and the National Sciences and Engineering Council of Canada (NSERC RGPIN 355710-13) to ECD. CAL was supported in part by the MEXT Inter-University Exchange Project.
Nonstandard Abbreviations and Acronyms
- TAA
Thoracic aortic aneurysm
- AAA
Abdominal aortic aneurysm
- SMKO
Smooth muscle cell-specific knockout
- DKO
SMKO;Thbs1 knockout (−/−)
- P
Postnatal day
- p
Phosphorylated
- SMC
Smooth muscle cell
- EC
Endothelial cell
- ECM
Extra cellular matrix
- Ang II
Angiotensin II
- TGFβ
Transforming growth factor β
- Thbs1
Thrombospondin-1
- Egr1
Early growth response 1
- ACE
Angiotensin converting enzyme
- PKC
Protein kinase C
- ERK
Extracellular signal-regulated kinase
- MEK
Mitogen-activated protein kinase kinase
- Ssh1
Slingshot-1
- G protein
Heterotrimeric guanine nucleotide-binding protein
- IEL
Internal elastic lamina
- CABG
Coronary artery bypass surgery
- MMP
Matrix metalloproteinase
- CD36, CD47
Cluster of differentiation #
- DAPI
4’, 6-diamidino-2-phenylindole
- Acta2
α-smooth muscle actin
- AR
Aortic root
- LSA
Left subclavian artery
- PECAM
Platelet and endothelial cell adhesion molecule
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
Footnotes
DISCLOSURES
None.
References
- 1.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of tgf-beta activation contributes to pathogenesis in marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 2.Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, Ihara M, Kinoshita A, Yoshiura K, Junien C, Kajii T, Jondeau G, Ohta T, Kishino T, Furukawa Y, Nakamura Y, Niikawa N, Boileau C, Matsumoto N. Heterozygous tgfbr2 mutations in marfan syndrome. Nat Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in tgfbr1 or tgfbr2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 4.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 5.Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A:2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: A potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 9.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha-actin (acta2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 10.Yamashiro Y, Papke CL, Kim J, Ringuette LJ, Zhang QJ, Liu ZP, Mirzaei H, Wagenseil JE, Davis EC, Yanagisawa H. Abnormal mechanosensing and cofilin activation promote the progression of ascending aortic aneurysms in mice. Sci Signal. 2015;8:ra105. doi: 10.1126/scisignal.aab3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milewicz DM, Prakash SK, Ramirez F. Therapeutics targeting drivers of thoracic aortic aneurysms and acute aortic dissections: Insights from predisposing genes and mouse models. Annual review of medicine. 2017;68:51–67. doi: 10.1146/annurev-med-100415-022956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: A novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78:1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Yamashiro Y, Papke CL, Ikeda Y, Lin Y, Patel M, Inagami T, Le VP, Wagenseil JE, Yanagisawa H. Angiotensin-converting enzyme-induced activation of local angiotensin signaling is required for ascending aortic aneurysms in fibulin-4-deficient mice. Sci Transl Med. 2013;5:183ra158, 181–111. doi: 10.1126/scitranslmed.3005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto-Pantoja DR, Kaur S, Roberts DD. Cd47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015;50:212–230. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of tgf-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 18.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Morgan S, Ren J, Wang Q, Annis DS, Mosher DF, Zhang J, Sorenson CM, Sheibani N, Liu B. Thrombospondin-1 (tsp1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res. 2015;117:129–141. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishna SM, Seto SW, Jose RJ, Biros E, Moran CS, Wang Y, Clancy P, Golledge J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin ii-infused apolipoprotein-e-deficient mouse. Arterioscler Thromb Vasc Biol. 2015;35:389–398. doi: 10.1161/ATVBAHA.114.304732. [DOI] [PubMed] [Google Scholar]
- 21.Day FL, Rafty LA, Chesterman CN, Khachigian LM. Angiotensin ii (atii)-inducible platelet-derived growth factor a-chain gene expression is p42/44 extracellular signal-regulated kinase-1/2 and egr-1-dependent and mediated via the atii type 1 but not type 2 receptor. Induction by atii antagonized by nitric oxide. J Biol Chem. 1999;274:23726–23733. doi: 10.1074/jbc.274.34.23726. [DOI] [PubMed] [Google Scholar]
- 22.Simo-Cheyou ER, Tan JJ, Grygorczyk R, Srivastava AK. Stim-1 and orai-1 channel mediate angiotensin-ii-induced expression of egr-1 in vascular smooth muscle cells. J Cell Physiol. 2017;232:3496–3509. doi: 10.1002/jcp.25810. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Guerrero E, Midgley VC, Khachigian LM. Angiotensin ii induction of pdgf-c expression is mediated by at1 receptor-dependent egr-1 transactivation. Nucleic Acids Res. 2008;36:1941–1951. doi: 10.1093/nar/gkm923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanjare M, Agarwal N, Gerecht S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. American journal of physiology Cell physiology. 2015;309:C271–281. doi: 10.1152/ajpcell.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei H, Ahn S, Barnes WG, Lefkowitz RJ. Stable interaction between beta-arrestin 2 and angiotensin type 1a receptor is required for beta-arrestin 2-mediated activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 2004;279:48255–48261. doi: 10.1074/jbc.M406205200. [DOI] [PubMed] [Google Scholar]
- 26.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and g protein-mediated erk activation by the angiotensin ii receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 27.Guillemot L, Levy A, Raymondjean M, Rothhut B. Angiotensin ii-induced transcriptional activation of the cyclin d1 gene is mediated by egr-1 in cho-at(1a) cells. J Biol Chem. 2001;276:39394–39403. doi: 10.1074/jbc.M103862200. [DOI] [PubMed] [Google Scholar]
- 28.Zhao HY, Ooyama A, Yamamoto M, Ikeda R, Haraguchi M, Tabata S, Furukawa T, Che XF, Zhang S, Oka T, Fukushima M, Nakagawa M, Ono M, Kuwano M, Akiyama S. Molecular basis for the induction of an angiogenesis inhibitor, thrombospondin-1, by 5-fluorouracil. Cancer Res. 2008;68:7035–7041. doi: 10.1158/0008-5472.CAN-07-6496. [DOI] [PubMed] [Google Scholar]
- 29.Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, Sakai LY, Koli K, Naitoh M, von Melchner H, Suzuki S, Rifkin DB, Nakamura T. Latent tgf-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc Natl Acad Sci U S A. 2013;110:2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301:H221–229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le VP, Yamashiro Y, Yanagisawa H, Wagenseil JE. Measuring, reversing, and modeling the mechanical changes due to the absence of fibulin-4 in mouse arteries. Biomech Model Mechanobiol. 2014;13:1081–1095. doi: 10.1007/s10237-014-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csanyi G, Feck DM, Ghoshal P, Singla B, Lin H, Nagarajan S, Meijles DN, Al Ghouleh I, Cantu-Medellin N, Kelley EE, Mateuszuk L, Isenberg JS, Watkins S, Pagano PJ. Cd47 and nox1 mediate dynamic fluid-phase macropinocytosis of native ldl. Antioxid Redox Signal. 2017;26:886–901. doi: 10.1089/ars.2016.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Yoshimura K, Aoki H, Tsutsui H, Noda T, Sagara J, Taniguchi S, Takahashi M. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin ii-induced aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:127–136. doi: 10.1161/ATVBAHA.114.303763. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and cd47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, Irwin D, Li M, McKeon BA, Riddle S, Dale Brown R, Morgan LE, Evans CM, Hernandez-Saavedra D, Bandeira A, Maloney JP, Bull TM, Janssen WJ, Stenmark KR, Tuder RM, Graham BB. Tgf-beta activation by bone marrow-derived thrombospondin-1 causes schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler K, Borges LF, Ho-Tin-Noe B, Jondeau G, Michel JB, Vranckx R. Angiogenesis and remodelling in human thoracic aortic aneurysms. Cardiovascular research. 2014;104:147–159. doi: 10.1093/cvr/cvu196. [DOI] [PubMed] [Google Scholar]
- 38.Moura R, Tjwa M, Vandervoort P, Van Kerckhoven S, Holvoet P, Hoylaerts MF. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in apoe−/− mice. Circ Res. 2008;103:1181–1189. doi: 10.1161/CIRCRESAHA.108.185645. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Ramirez MA, Fonseca G, Zeineddine HA, Girard R, Moore T, Pham A, Cao Y, Shenkar R, de Kreuk BJ, Lagarrigue F, Lawler J, Glass CK, Awad IA, Ginsberg MH. Thrombospondin1 (tsp1) replacement prevents cerebral cavernous malformations. J Exp Med. 2017;214:3331–3346. doi: 10.1084/jem.20171178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol. 2005;207:358–366. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 41.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: Focus on smooth muscle cell contractile dysfunction. Annual review of genomics and human genetics. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 42.Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. Angiotensin ii induces thrombospondin-1 production in human mesangial cells via p38 mapk and jnk: A mechanism for activation of latent tgf-beta1. Am J Physiol Renal Physiol. 2004;286:F278–287. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- 43.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin ii sustains brain inflammation in mice via tgf-beta. J Clin Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakagawa T, Li JH, Garcia G, Mu W, Piek E, Bottinger EP, Chen Y, Zhu HJ, Kang DH, Schreiner GF, Lan HY, Johnson RJ. Tgf-beta induces proangiogenic and antiangiogenic factors via parallel but distinct smad pathways. Kidney Int. 2004;66:605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 45.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim CW, Pokutta-Paskaleva A, Kumar S, Timmins LH, Morris AD, Kang DW, Dalal S, Chadid T, Kuo KM, Raykin J, Li H, Yanagisawa H, Gleason RL, Jr, Jo H, Brewster LP. Disturbed flow promotes arterial stiffening through thrombospondin-1. Circulation. 2017;136:1217–1232. doi: 10.1161/CIRCULATIONAHA.116.026361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagenseil JE. Bio-chemo-mechanics of thoracic aortic aneurysms. Curr Opin Biomed Eng. 2018;5:50–57. doi: 10.1016/j.cobme.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keire PA, Bressler SL, Mulvihill ER, Starcher BC, Kang I, Wight TN. Inhibition of versican expression by sirna facilitates tropoelastin synthesis and elastic fiber formation by human sk-lms-1 leiomyosarcoma smooth muscle cells in vitro and in vivo. Matrix Biol. 2016;50:67–81. doi: 10.1016/j.matbio.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuznetsova SA, Issa P, Perruccio EM, Zeng B, Sipes JM, Ward Y, Seyfried NT, Fielder HL, Day AJ, Wight TN, Roberts DD. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J Cell Sci. 2006;119:4499–4509. doi: 10.1242/jcs.03171. [DOI] [PubMed] [Google Scholar]
- 50.Galatioto J, Caescu CI, Hansen J, Cook JR, Miramontes I, Iyengar R, Ramirez F. Cell type-specific contributions of the angiotensin ii type 1a receptor to aorta homeostasis and aneurysmal disease-brief report. Arterioscler Thromb Vasc Biol. 2018;38:588–591. doi: 10.1161/ATVBAHA.117.310609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saumet A, de Jesus N, Legrand C, Dubernard V. Association of thrombospondin-1 with the actin cytoskeleton of human thrombin-activated platelets through an alphaiibbeta3- or cd36-independent mechanism. Biochem J. 2002;363:473–482. doi: 10.1042/0264-6021:3630473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gahtan V, Wang XJ, Ikeda M, Willis AI, Tuszynski GP, Sumpio BE. Thrombospondin-1 induces activation of focal adhesion kinase in vascular smooth muscle cells. J Vasc Surg. 1999;29:1031–1036. doi: 10.1016/s0741-5214(99)70244-2. [DOI] [PubMed] [Google Scholar]
- 53.Balmforth D, Harky A, Adams B, Yap J, Shipolini A, Roberts N, Uppal R, Bashir M. Is there a role for biomarkers in thoracic aortic aneurysm disease? Gen Thorac Cardiovasc Surg. 2017 doi: 10.1007/s11748-017-0855-0. [DOI] [PubMed] [Google Scholar]
- 54.Trimarchi S, Jonker FH, Hutchison S, Isselbacher EM, Pape LA, Patel HJ, Froehlich JB, Muhs BE, Rampoldi V, Grassi V, Evangelista A, Meinhardt G, Beckman J, Myrmel T, Pyeritz RE, Hirsch AT, Sundt TM, 3rd, Nienaber CA, Eagle KA. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type b aortic dissection. J Thorac Cardiovasc Surg. 2011;142:e101–107. doi: 10.1016/j.jtcvs.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 55.Kim EK, Choi SH, Sung K, Kim WS, Choe YH, Oh JK, Kim DK. Aortic diameter predicts acute type a aortic dissection in patients with marfan syndrome but not in patients without marfan syndrome. J Thorac Cardiovasc Surg. 2014;147:1505–1510. doi: 10.1016/j.jtcvs.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 56.Mansour AM, Peterss S, Zafar MA, Rizzo JA, Fang H, Charilaou P, Ziganshin BA, Darr UM, Elefteriades JA. Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology. 2018;139:139–146. doi: 10.1159/000481930. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto K, Satoh K, Maniwa T, Tanaka T, Okunishi H, Oda T. Proteomic comparison between abdominal and thoracic aortic aneurysms. Int J Mol Med. 2014;33:1035–1047. doi: 10.3892/ijmm.2014.1627. [DOI] [PubMed] [Google Scholar]
- 58.Krishna SM, Seto SW, Jose R, Li J, Moxon J, Clancy P, Crossman DJ, Norman P, Emeto TI, Golledge J. High serum thrombospondin-1 concentration is associated with slower abdominal aortic aneurysm growth and deficiency of thrombospondin-1 promotes angiotensin ii induced aortic aneurysm in mice. Clin Sci (Lond) 2017;131:1261–1281. doi: 10.1042/CS20160970. [DOI] [PubMed] [Google Scholar]
- 59.Moxon JV, Padula MP, Clancy P, Emeto TI, Herbert BR, Norman PE, Golledge J. Proteomic analysis of intra-arterial thrombus secretions reveals a negative association of clusterin and thrombospondin-1 with abdominal aortic aneurysm. Atherosclerosis. 2011;219:432–439. doi: 10.1016/j.atherosclerosis.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, Benson DW, Braverman AC, Chen S, De Backer J, Gelb BD, Grossfeld PD, Klein GL, Lai WW, Liou A, Loeys BL, Markham LW, Olson AK, Paridon SM, Pemberton VL, Pierpont ME, Pyeritz RE, Radojewski E, Roman MJ, Sharkey AM, Stylianou MP, Wechsler SB, Young LT, Mahony L Pediatric Heart Network I. Atenolol versus losartan in children and young adults with marfan's syndrome. The New England journal of medicine. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.