Abstract
Introduction
Calpains represent a family of neutral, calcium-dependent proteases, which modify the function of their target proteins by partial truncation. These proteases have been implicated in numerous cell functions, including cell division, proliferation, migration, and death. In the CNS, where calpain-1 and calpain-2 are the main calpain isoforms, their activation has been linked to synaptic plasticity as well as to neurodegeneration. This review will focus on the role of calpain2 in acute neuronal injury and discuss the possibility of developing selective calpain-2 inhibitors for therapeutic purposes.
Areas covered
This review covers the literature showing how calpain-2 is implicated in neuronal death in a number of pathological conditions. The possibility of developing new selective calpain-2 inhibitors for treating these conditions is discussed.
Expert opinion
As evidence accumulates that calpain-2 activation participates in acute neuronal injury, there is interest in developing therapeutic approaches using selective calpain-2 inhibitors. Recent data indicate the potential use of such inhibitors in various pathologies associated with acute neuronal death. The possibility of extending the use of such inhibitors to more chronic forms of neurodegeneration is discussed.
Keywords: Calpain-2, neuronal death, inhibitors, traumatic brain injury, acute glaucoma
1. Introduction
While calcium-activated neutral proteases (CANP) were discovered in 1964 by Guroff [1], the terms calpain and calpastatin, its endogenous inhibitor, were introduced in the 1980s [2]. Since then, many studies have been directed at understanding the physiological as well as the pathological function(s) of this family of proteases in the brain and other organs. We initially proposed in 1984 that calpain played a critical role in synaptic plasticity and learning and memory [3], and this hypothesis was recently confirmed by studies performed in calpain-1 knock-out (KO) mice [4, 5]. Since the original hypothesis was proposed, studies related to the functions of calpain in the brain have mostly focused on the potential critical roles of calpain in neuronal death and neurodegeneration [6, 7, 8, 9, 10, 11, 12, 13]. While there is strong evidence that calpain plays a role in neurodegeneration, the major issue plaguing the literature is that there are only a handful of studies addressing the question of which calpain isoform(s) is (are) involved and of the signaling pathways leading to neurodegeneration. Since the identification of calcium-dependent neutral proteases by Guroff [1], a plethora of calpain isoforms have been identified and we now know that calpains constitute a family of enzymes with at least 15 members [14, 15]. Several studies have addressed the specific roles each of these proteases play in human diseases [16]. The muscle specific calpain-3, as defects in the gene encoding calpain-3 lead to a particular type of dystrophy, limb-girdle muscular dystrophy 2A (LGMD-2A) [17, 18]. However, calpain-3 has a number of unique features, which set it apart from the more typical calpain isoforms [19]. There is also good evidence for a link between calpain-10 and diabetes mellitus, based on genetic studies [20]. More recently, calpain-14 has been linked to eosinophilic esophagitis, due to its abundance in the upper gastro-intestinal tract [21]. In the brain, the major calpain isoforms are calpain-1, aka μ-calpain, calpain-2, aka m-calpain, and calpain-5. Mutations of calpain-5 have recently been associated with autoimmune uveitis and photoreceptor degeneration [22]. We previously reviewed the roles of calpain in synaptic plasticity and this topic will not be addressed here [4]. The present review will focus on the role calpain-2 is playing in acute neuronal death and on the mechanisms linking calpain-2 activation to neuronal death. It will also review the evidence indicating that calpain-2 is a good target to develop selective inhibitors, which could be developed for the treatment of various neurological disorders associated with acute neuronal death. Finally, we will discuss the possibility that these inhibitors could also be useful for the treatment of chronic neurodegenerative disorders.
2. Calpain properties
Of the 15 isoforms, calpain-1 and −2 are ubiquitously expressed, predominantly in mammalian brain and have been the most extensively studied. Calpain-1 and −2 are generally soluble and are present in both neurons and glia. For activity, calpain-1 or calpain-2 require their association with a small regulatory subunit (calpain-S1, formerly known as calpain-4) to form functional heterodimeric proteins. The large catalytic subunit for calpain-1 or −2 contains four major domains. Domain I is the N-terminal anchor helix region of the large subunit, which can undergo autolysis following calpain activation by Ca2+ [23]. Domain II comprises two protease core domains (PC1 and PC2), which fuse to form the active cysteine catalytic region upon Ca2+ binding onto each core domain [24]. Domain III is involved in binding Ca2+ and phospholipids [25]. Domain IV exhibits a penta-EF-hand calcium-binding domain and contributes to the heterodimer formation [26].
The intracellular cytosolic calcium concentration is generally estimated to be 50−300 nM [27], much lower than its extracellular concentration, approximately 2 mM. In response to an extracellular signal, calcium can be either released from intracellular stores, such as the endoplasmic reticulum (ER) and mitochondria, or can cross plasma membranes through ionotropic receptors and voltage-gated Ca2+ channels. As a result, intracellular calcium concentration is estimated to rise to tens of μM at most, which would be high enough to activate calpain-1, but, at least in principle, not high enough to activate calpain-2. As calpain-2 activation has been shown to require close to mM calcium concentration, alternative in vivo activation mechanisms for calpain-2 have been suggested. The finding that calpain-2 could be activated by extracellular signal-regulated kinase (ERK)-mediated direct phosphorylation at its serine 50 without increased intracellular Ca2+ concentration [28, 29] provided evidence for the existence of such mechanisms. We showed that both EGF and BDNF could activate calpain-2 by ERK-mediated phosphorylation in dendritic spines of hippocampal neurons [30].
The availability of crystal structures for rat calpain-1, calpain-2 and calpain-9 has provided a wealth of information regarding the mechanisms of calpain activation, the mechanism of inhibition by the endogenous inhibitor calpastatin, and more generally, the potential structural requirements for designing calpain inhibitors [31, 32, 33, 34, 35]. Nevertheless, it has been extremely difficult to design selective inhibitors for the various calpain isoforms, thereby limiting the understanding of their respective functions [19]. The availability of calpain-1 KO mice generated by the laboratory of Dr. Chishti provided an invaluable tool to better understand the functions of this particular calpain isoform, and we previously reviewed some of the data generated using these KO mice [12]. Unfortunately, calpain-2 knock-out mice are embryonically lethal, thereby limiting the types of studies that can be performed with these mutants. Conditional knock-out of the small regulatory subunit, calpain-S1 or calpain-4, has been successfully performed but these mice lacked both calpain-1 and calpain-2 activity, thereby limiting the interpretation of the data generated with these mutant mice. Nevertheless, it was reported that these mice are impaired in synaptic plasticity, but are also resistant to injury produced by excitotoxicity and mitochondrial toxicity [36]. To our knowledge there are no data available regarding knock-out mice for the other calpain isoforms.
3. Calpain-2 and acute neuronal injury
3.1. Mechanisms linking calpain-2 to neuronal injury
As mentioned above, there is an extensive literature linking calpain activation with neurodegeneration. However, very few studies have explored the specific contributions of calpain-1 and calpain-2 in neurodegeneration. Using primary neuronal cultures, we showed that calpain-2, but not calpain-1 activation was responsible for NMDA-induced excitotoxicity through the activation of STEP [37]. A similar study indicated that down-regulation of calpain-2 but not calpain-1 increased neuronal survival following NMDA treatment of cultured hippocampal neurons [38]. Calpains have a large number of potential target proteins, belonging to many classes, including membrane receptors and ion channels, cytoskeletal proteins, protein kinases and phosphatases, transcription factors, as well as regulatory proteins [10]. In general, calpain-mediated truncation does not lead to the elimination of the target protein, but it alters its function for a duration related to the half-life of the protein. Consequently, calpain activation can modify a very large number of cellular functions for significant periods of time. It has been difficult to determine under various experimental conditions which of the calpain target(s) is (are) responsible for the alterations in cell functions triggered by calpain activation. Figure 1 illustrates various cellular functions modified by calpain activation, and when known, by calpain-2 activation, which have been associated with neuronal injury.
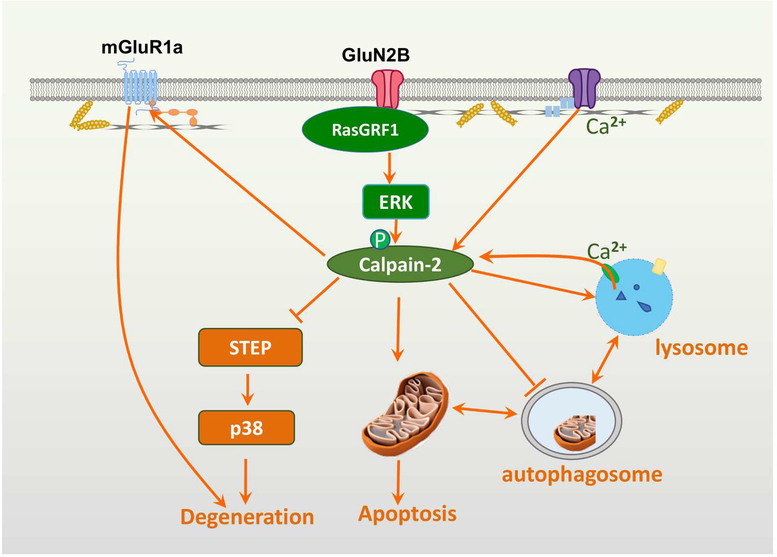
Figure 1: Schematic representation of the various pathways regulated by calpain-2 and leading to neuronal death.
Various pathways leading to neuronal death are represented in this figure. Calpain-2 activation is shown downstream of NR2B and its associated RasGRF1, which leads to ERK activation and calpain-2 phosphorylation/activation. Several targets of calpain-2 are also represented, including the STEP/p38 pathway, which has long been shown to contribute to neuronal death. Calpain has often been shown to trigger apoptosis through the degradation/inactivation of several pro-survival proteins and the degradation/activation of pro-death proteins. Several studies have also linked calpain activation to the regulation of autophagy, which is generally considered to be a pro-survival mechanism, and a recent report clearly showed that calpain-2 activation inhibits autophagy. Similarly, a calpain-cathepsin hypothesis for Alzheimer’s disease has been proposed, suggesting that calpain activation could elicit the release of lysosomal proteases in the cell cytosol, thus contributing to neuronal damage. Importantly, apoptotic pathways, autophagy and lysosomes are interacting with each other to provide a balance between cell survival and cell death. We previously reported that calpain, by truncating the C-terminal domain of mGluR1a eliminates the pro-survival effect of this receptor stimulation, while maintaining its pro-degenerating component, related to increase intracellular calcium release [69].
We discussed elsewhere the notion that calpain-2 is associated with a multi-protein complex, which includes extrasynaptic NMDARs, and how activation of extrasynaptic NMDARs could result in calpain-2 activation [12, 37]. Briefly, NR2B subunits are enriched in extrasynaptic NMDARs [39], and their activation is critical for excitotoxicity [40]. Furthermore, NR2B directly binds RasGRF1, which provides a link between NMDAR activation and ERK activation [41]. As mentioned above, ERK activation directly phosphorylates and activates calpain-2 [30]; thus, this pathway is likely responsible for the prolonged activation of calpain-2 following stimulation of extrasynaptic NMDA receptors. Numerous studies have shown that calpain cleaves striatal-enriched tyrosine phosphatase (STEP), generating inactive fragments, resulting in activation of p38 and downstream cell death signaling pathways [42, 43].
Under many conditions, whether a cell survives or dies depends on the relative effectiveness of the pro-survival process of autophagy or of the pro-death process of apoptosis [44]. A recent study demonstrated that calpain-2 inhibition or knock-down enhanced autophagy and reduced cell death after ischemia/reperfusion in liver [45]. Furthermore, the authors showed that calpain-2, by cleaving Atg3 and Atg7, suppressed autophagy and enhanced liver sensitivity to ischemia/reperfusion injury. Atg5 is also regulated by calpain cleavage [46, 47]. Non- selective calpain inhibitors promoted mTOR-independent autophagy and rescued Huntington’s disease phenotypes in zebrafish [48]. However, the specific roles of calpain-1 and calpain-2 in these processes are not clear. Other studies have also indicated that calpain activation can switch cellular programs from autophagy to apoptosis [49, 50, 51]. On the other hand, calpain activation has been repeatedly shown to be involved in stimulating apoptosis pathways through multiple mechanisms [52]. Calpains cleave several members of the Bcl-2 family of proteins, including Bax, Bid, and Bcl-xL, leading to cytochrome c release [53, 54, 55] and caspase-3 activation. Caspase-3 can further activate calpain by compromising the membrane permeability to Ca2+ and by degrading calpastatin [56]. Calpain also converts pro-caspase-7 to caspase-7 [57]. More recently, mitochondrial calpain-2 in rat heart was found to activate the mitochondrial permeability transition pore (mPTP) through truncation of ND6 on complex I, which could further contribute to apoptosis [58]. Thus, an increasing number of studies indicates that calpain2 activation prevents autophagy, while it stimulates apoptosis. The specific roles of calpain-1 in autophagy remain to be determined. Considering the multiple cross-talks between these 2 cellular processes, it is highly likely that calpain-2 activation represents a critical step towards cell death.
Over the last 20 years, Dr. Yamashima has developed the calpain-cathepsin hypothesis to account for several features of neuronal death in Alzheimer’s disease (AD) [59, 60, 61, 62]. A main feature of this hypothesis is the truncation of carbonylated Hsp70.1 by calpain, leading to the destabilization of lysosomal membranes and the release of cathepsins in neuronal cytoplasm. Incorporated in this hypothesis is the concept that oxidative stress, which has often been associated with AD [63], could stimulate the formation of carbonylated Hsp70.1, and calpain activation through disruption of mitochondrial function. Reactive oxygen species (ROS), which accumulate as a result of mitochondrial dysfunction, have been shown to activate calpain and in particular, calpain-2 in several types of cells and under a variety of experimental conditions [64, 65, 66]. There is therefore a link between calpain-2 activation, lysosomal dysfunction and potentially neuronal death in AD. However, it is worth noting that calpain activation in mouse models of Alzheimer’s disease may be an artifact of APP overexpression [67]. Thus, the mouse model should be chosen cautiously when studying the roles of calpain in AD.
As autophagy is now recognized to be a key regulator of cell function and in particular in neuronal health and disease [68], it is clear that the interactions between calpain-2, autophagy, lysosomes and apoptosis represent a key component of the pathways leading from calpain-2 activation to neuronal death. It is important to note though that we still do not have much information regarding the specific roles of calpain-1 and calpain-2 in these processes, and that further work is needed to get this information. Our own work has clearly demonstrated a selective role for calpain-2 in acute neuronal death, as discussed below.
We previously identified another mechanism linking calpain activation to neuronal death through the truncation of mGluR1α, which is related to NMDA receptor stimulation-induced calpain activation [69]. Under normal conditions, mGluR1α receptors are coupled to PI3K-Akt signaling and their activation is neuroprotective. Although mGluR1α activation leads to calcium release from internal stores, the extent of calcium release does not produce significant toxic effects. Following NMDA receptor stimulation or onset of ischemia, calpain is activated leading to mGluR1α truncation. As a result, the neuroprotective effect of the mGluR1α-PI3KAkt signaling cascade is disrupted. In addition, mGluR1α-dependent calcium release from intracellular stores further contributes to calcium overload due to calcium influx through NMDA receptors and thus enhances neurotoxicity. Together, NMDA receptor activation followed by calpain-mediated truncation of mGluR1α constitutes a positive feedback loop for excitotoxicity. Table I summarizes the various calpain targets, which have been associated with these different mechanisms of neuronal death. It is interesting to note that calpain-2, by regulating such a variety of pathways leading to neuronal death, plays a central role in linking numerous extracellular stimuli both acute and potentially chronic to neuronal death.
Table I:
Selected targets of calpain-2 in various signaling pathways leading to neuronal death.
| Signaling pathway | Substrate | Consequence of cleavage | references |
|---|---|---|---|
| Glutamate receptormediated signaling | GluA1 mGluR1a NR2A NR2B PSD95 p35/p39 STEP |
Stimulates internalization Inhibits neuroprotection and enhances neurodegeneration Decreased excitability and Uncoupling from pro-survival pathways Aberrant activation of Cdk5 Cleavage activates p38 |
[69, 138, 139, 140, 141, 142, 143] |
| Apoptosis | Bax Bcl-xL Bid Caspase-3 Pro-Caspase-7 ND6 p53 |
Leads to cytochrome C release from mitochondria, and decreases pro-survival pathway Unknown Activates caspase-7 Stimulates mPTP Stimulates apoptosis |
[53, 54, 57, 144, 145] |
| Autophagy | Atg3 Atg5 Atg7 PTEN |
Inhibits autophagy Regulates autophagy through mTORC1 |
[45, 47] [104, 146] |
| Lysosome | carbonylated Hsp70.1 | [62] |
3.2. Role of calpain-2 in acute glaucoma
In order to further analyze the role of calpain-2 in acute neurodegeneration in vivo, we used a model of acute glaucoma in mice consisting in a brief period of increased intraocular pressure (IOP) [70]. Calpain activation had been previously involved in retinal cell death induced by NMDAR activation [71, 72], although the contribution of calpain-1 and calpain-2 had not been analyzed. The results indicated that, while calpain-1 was briefly activated following increased IOP, calpain-2 activation was delayed and prolonged. Likewise, injection of a relatively selective calpain-2 inhibitor (C2I), Z-Leu-Abu-CONH-CH2-C6H3 (3, 5-(OMe)2) [37, 73, 74] (see Fig. 2D for the structure), prevented RGC death and prevented loss of vision when injected 2 h after increased IOP. This inhibitor has a Ki of 25 nM against purified calpain-2 versus a Ki of 1.3 μM against calpain-1, indicating that it has a 50-fold selectivity for calpain-2 over calpain-1 [37]. In addition, the extent of RGC death was larger in calpain-1 KO than in WT mice [70]. Several mechanisms have previously been involved in RGC death in acute glaucoma [75, 76, 77], including calpain activation [78]. Our results clearly indicated that calpain-1 and calpain-2 play opposite functions in increased IOP-induced retinal damage, with calpain-1 being neuroprotective, since retinal damage was exacerbated in calpain-1 KO mice, as compared to WT mice. Calpain-1 activation leads to the stimulation of a survival pathway previously identified in a different system [37], consisting in calpain-1 mediated cleavage of PHLPP1 leading to activation of the pro-survival Akt pathway [37]. In contrast, calpain-2 is neurodegenerative, as evidenced by the significant protection against retinal damage provided by a selective calpain-2 inhibitor [70]. Several features could account for the differential roles of calpain-1 and calpain-2 in acute retinal damage. Firs, these two calpain isoforms exhibit a different time-course of activation; calpain-1 is rapidly and briefly activated following increased IOP, while calpain-2 activation is delayed and prolonged. Calpain-1 activation is likely due to the rapid and transient stimulation of synaptic NMDA receptors, composed NR2A subunits, as we previously reported [37, 73]. On the other hand, delayed calpain-2 activation is likely due to the stimulation of extrasynaptic NMDA receptors, composed of NR2B subunits, as a result of glutamate spill-over or inhibition of glutamate transport known to take place following ischemia [79, 80]. As discussed above, calpain-2 triggers the degradation and inhibition of the phosphatase STEP, which activates STEP substrate p38, resulting in neuronal death [37, 42]. This interpretation is consistent with the differential roles of NR2A- and NR2B-containing NMDA receptors in NMDA-induced neurotoxicity in retina [81]. Autophagy has also been shown to be activated following increased IOP in the retina [82, 83], although in this case, it appears that autophagy activation could be related to neuronal death and not neuroprotection. On the other hand, a recent study suggests that compromised autophagic activation could be involved in retinal damage [84]. Whether this represents a protective or a degenerative mechanism is not completely clear, nor is it clear if it takes place in neurons or in glial cells. Apoptosis has also been proposed to participate in RGC death in various models of retinal damage [85].
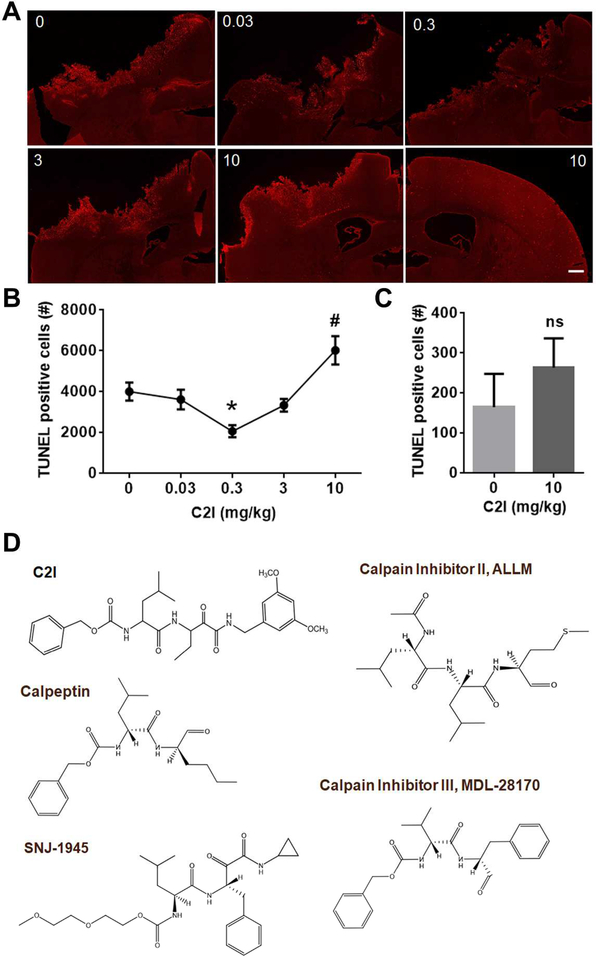
Figure 2: Effects of various doses of a selective calpain-2 inhibitor on cell death following TBI.
(A) TUNEL staining in the ipsilateral or contralateral side of the brain 24 h after control cortical impact in adult WT mice. 0, 0.03, 0.3, 3 or 10 mg/kg of a selective calpain-2 inhibitor Z-LeuAbu-CONH-CH2-C6H3 (3, 5-(OMe)2) was injected intraperitoneally 1 h after TBI. The picture at the bottom right is the staining in contralateral side. All other pictures are staining in ipsilateral side. Scale bar, 500 μm.
(B) Quantification of number of cells labeled with TUNEL staining following TBI. Total numbers of TUNEL-positive cells in 6 coronal sections (Bregma 0.50, −0.58, −1.58, −1.94, −2.30, −2.70 mm) of each brain were counted to provide the total numbers of TUNEL-positive cells for each animal. TUNEL-positive cells in both ipsilateral and contralateral sides of each section were counted. Data are means ± SEM. N = 3 – 5 (animals). * p < 0.05, 0 vs 0.3. # p < 0.05, 10 vs any other groups.
(C) Quantification of TUNEL staining in brain sections collected 23 h after intraperitoneal injection of a selective calpain-2 inhibitor (0 or 10 mg/kg) to naive WT mice. TUNEL-positive cells in both ipsilateral and contralateral sides of each section were counted. Data are means ± SEM. N = 3 (animals). Ns, no significant difference, two-tailed t-test.
(D) Structure of Z-Leu-Abu-CONH-CH2-C6H3 (3, 5-(OMe)2) and of other calpain inhibitors. See complete list of calpain inhibitors and their structure here: http://calpain.net/reagents/inhibitors.html
3.3. Role of calpain-2 in a mouse model of traumatic brain injury (TBI)
Calpain activation has long been shown to be involved in the pathology of TBI [86, 87, 88]. Various spectrin breakdown products (SBDPs) generated by calpain activation have been extensively used as biomarkers for TBI in the CSF or in blood [89, 90, 91, 92, 93, 94, 95]. However, none of these studies have addressed the respective roles of calpain-1 and calpain-2 in the associated neuronal damage. This was due in part to the lack of isoform selective calpain inhibitors, as well as the lack of markers for calpain-1 and calpain-2 activation. These limitations could account for several conflicting results. In particular, the calpain inhibitor AK295 and ALLM was reported to protect the cytoskeletal structure of injured neurons and to attenuate motor and cognitive deficits after TBI [96, 97]. However, these results were not confirmed in more recent studies using different calpain inhibitors. Overexpression of the endogenous calpain inhibitor, calpastatin, could reduce calpain activation [98], but did not prevent neuronal death [99]. Two other calpain inhibitors, SNJ-1945 and MDL-28170, which were shown to cross the blood brain barrier, did not exhibit significant efficacy in a model of control cortical impact [100, 101]. We were able to determine the time-course of activation of calpain-1 and calpain-2 following TBI by using several tools applied to both WT and calpain-1 KO mice. Changes in SBDP levels represent both calpain-1 and calpain-2 activation, as spectrin can be cleaved by both calpain-1 and calpain-2 [102]. However, when the analysis is done in calpain-1 KO mice, SBDP represents calpain-2 activation only, and the difference between results obtained with both types of mice, could be attributed to calpain-1 activation [70, 103]. We previously showed that PTEN was selectively cleaved by calpain-2 but not calpain-1 [104] and analysis of the changes in PTEN under various experimental conditions provides a marker for calpain-2 activation, with the prediction that there should be no difference in these changes between WT and calpain-1 KO mice. Using these tools, we found that calpain-1 is also rapidly and transiently activated in the cortex surrounding the impact site after TBI. Activation peaked at 6 h after TBI but was no longer present by 24 h after TBI. On the other hand, calpain-2 activation was delayed, starting between 4 and 8 h after TBI, but was very prolonged and was still present 3 days after TBI [103]. Moreover, using TUNEL as well as fluoro-Jade staining to identify dying cells, we were able to show that the extent of calpain-2 activation was linearly related to the extent of cell death, indicating that calpain-2 activation is a critical step in the cascade of events triggered by TBI and leading to cell death. Systemic administration of C2I, 1 or 4 h after TBI, significantly reduced calpain-2 activation and the number of degenerating cells in the cortex surrounding the impact site, further demonstrating the neurodegenerative role of calpain-2. In addition, while calpain-2 activation following TBI was not different in WT and calpain-1 KO mice, the extent of cell death was significantly greater in calpain-1 KO mice, further emphasizing the neuroprotective role of calpain-1 activation [103]. Our results are therefore not only in complete agreement with previous studies showing that non-selective calpain inhibitors could inhibit overall calpain activation (without distinguishing which calpain isoform was targeted) following TBI, but they also account for the observation that they failed to provide neuroprotection. Indeed, it would be difficult to predict the effects of non-selective calpain inhibitors, as they will critically depend on the time of injection as well as on the relative effects on calpain-1 and calpain-2. In particular, we performed a dose-response with C2I administered 1 h after TBI and determined the extent of cell death 24 h later (Fig. 2). As reported, a low dose of C2I provided protection, but increasing doses failed to produce protection and the highest dose exacerbated neuronal death in both ipsilateral and contralateral sides of the lesion. This dose-response curve is very similar to what we previously observed with C2I in fear conditioning, with low doses facilitating learning and high doses inhibiting learning [105]. We interpreted these results as evidence that the low doses of C2I inhibit calpain-2, while higher doses inhibit both calpain-1 and calpain-2 These results would suggest that inhibiting both calpain-2 and calpain-1 results in more neuronal death than in control.
The role of autophagy in neuronal injury following TBI is also highly controversial. While several studies support the notion that autophagy is impaired after TBI, thus limiting its neuroprotective function [106, 107, 108], other studies indicate that autophagy activation contributes to neuronal damage [109, 110, 111]. More studies are needed to better characterize the contributions of the various pathways described in Figure 1 to neuronal death following TBI.
4. Calpain inhibitors and neurodegenerative diseases
Our results strongly support the idea of using selective calpain-2 inhibitors in acute models of neuronal death, including TBI, stroke and possibly spinal cord injury, and other forms of ischemic neuronal injuries. Neurodegeneration usually refers to more chronic forms of neuronal damage and is a hallmark of numerous human disorders. Numerous reviews have discussed the role of calpain in neurodegeneration in general [11, 112], and in stroke [113, 114] and in traumatic brain injury (TBI) [86, 115]. Likewise, numerous studies have attempted to use calpain inhibitors to reduce neurodegeneration in both stroke and TBI [114, 116, 117, 118, 119, 120, 121, 122, 123]. Moreover, many reviews have discussed the potential use of calpain inhibitors in more chronic neurodegenerative diseases [112, 124, 125, 126, 127]. As discussed above, very few studies have addressed the respective roles of calpain-1 and calpain-2 in chronic neurodegenerative diseases. Based on our results, we would predict that non-selective calpain inhibitors would probably not represent a viable therapeutic approach, as by inhibiting calpain-1, they would block the normal neuroprotective function and roles in synaptic plasticity of calpain1, which is maintained in adult brain. In addition to acute neuronal injury, calpain-2 has also been involved in the pathology of chronic neurodegenerative diseases. In particular, calpain-2 but not calpain-1 activation is concentrated in neurofibrillary tangles and induces degradation of nicotinic acetylcholine receptor α4 and causes cholinergic impairments in AD [128, 129, 130]. We recently reported that TBI-induced calpain-2 activation triggered rapid oligomerization of tau in the brain [131]. We found that following TBI, calpain-2 cleaved and inhibited a tyrosine phosphatase named PTPN13, aka FAP-1. PTPN13 inhibition caused the activation of the tyrosine kinase c-Abl and enhanced tyrosine phosphorylation of tau, which lead to tau oligomerization. Tau oligomers play a critical role in the initiation and spreading of tau pathology leading to AD [132, 133, 134, 135]. Thus, we discovered a novel link between TBI, calpain-2 and increased risk of AD.
Calpain-2 has also been implicated in Wolfram syndrome, a genetic disorder associated with diabetes and neurodegeneration [136]. This disease is due to the loss of function of two genes, Wolfram Syndrome 1 (WFS1) and Wolfram Syndrome 2 (WFS2), which encode transmembrane proteins of the ER. WFS2 is a negative regulator of calpain-2, which is elevated in iPS cells from human Wolfram syndrome patients. Calpain-2 might also been involved in the pathogenesis of spino-cerebellar ataxia type 3 (SCA3) [137]. SCA3 is a polyglutamine disease in which mutated ataxin is more susceptible to calpain-2-mediated truncation. It has been suggested that calpain-2-mediated ataxin fragments trigger the formation of aggregates and neurodegeneration. It is thus tempting to suggest that a similar process takes place in many forms of neurodegenerative diseases associated with the accumulation of protein aggregates. If this were to be the case, a selective calpain-2 inhibitor could become a potential therapeutic approach for many neurodegenerative disorders.
5. Conclusions
Calpain has been proposed to play a critical role in neurodegeneration for a long time. Its prolonged activation by calcium, as well as the numerous proteins related to cell death cascades calpain could regulate made it a potential target for the development of neuroprotective strategies. However, despite many attempts by several pharmaceutical and biotech companies to develop calpain inhibitors, none have made it to clinical trials. The existence of a large number of calpain isoforms, with many of them without clear functions, coupled with the lack of selective inhibitors could account for the difficulties in understanding the roles of these calpain isoforms in physiological as well as pathological conditions. The situation started to change when we discovered that calpain-1 and calpain-2 played opposite functions in both synaptic plasticity and neuronal protection/neuronal death [12, 37, 73]. As we discussed elsewhere, these opposite functions of calpain-1 and calpain-2 are due, at least in part, to their interactions with different signaling pathways due to their associations with different PDZ binding partners. Calpain-1 has an atypical PDZ binding site in its C-terminal domain, while calpain-2 exhibits a typical PDZ binding site in its C-terminal domain. While calpain-1 is neuroprotective due to the activation of pro-survival pathways when activated, calpain-2 is neurodegenerative and the review summarized several neurodegenerative pathways regulated by calpain-2 activation. These findings account for many of the failure to clearly demonstrate neuroprotective effects of nonselective calpain inhibitors, as well as the discrepancies reported in the literature regarding the effects of various calpain inhibitors. Our results further emphasize the need to use selective calpain-2 inhibitors to obtain clear neuroprotective effects, at least in two models of acute neuronal injury, acute glaucoma and TBI [70, 103]. Considering that our studies also indicated that calpain-2 activation was delayed and prolonged, they provide support for the idea of developing such selective calpain-2 inhibitors for the post-treatment of a variety of conditions associated with acute neuronal death. It is important to note that a blood biomarker related to brain calpain activation has been identified, an N-terminal fragment of spectrin generated by calpain-mediated truncation (SNTF), and its blood levels during the hours following brain injury have previously been shown to correlate with the severity of neurologic outcomes in TBI [94].Application of this blood biomarker could greatly facilitate the clinical development of a selective calpain-2 inhibitor, although it remains to be determined whether it is generated by calpain-1 or calpain-2 activation.
6. Expert Opinion
In the 80s and 90s, several pharmaceutical and biotech companies initiated drug discovery and development programs focusing on calpain. However, all the efforts to bring a calpain inhibitor to the clinic failed. Today, a handful of companies continue the search for calpain inhibitors and their possible therapeutic indications. As more basic information on the properties and functions of the various calpain isoforms has emerged, it is clear now that several calpain isoforms could be interesting targets for drug development. The argument developed in this review supports the notion that calpain-2 is an attractive candidate for the treatment of acute neuronal death associated with several neurological disorders. However, we also discussed the large number of downstream effectors leading to neuronal death, which could potentially complicate the clinical development of such inhibitors. More studies are needed to determine whether calpain-2 could also be an attractive candidate for more chronic forms of neurodegeneration, such as Alzheimer’s, Huntington’s and Parkinson’s disease. As mentioned above, the availability of a blood biomarker related to brain calpain activation could facilitate the clinical development of selective calpain-2 inhibitors, inasmuch as prevention of the appearance of SNTF in the blood could be used as an outcome of clinical trials.
Article Highlights.
Calpain-2 is neurodegenerative and several downstream signaling pathways lead to neuronal death.
Calpain-2 activation is delayed and prolonged in two models of acute neuronal injury, acute glaucoma and TBI.
In TBI, the extent of cell death is directly related to calpain-2 activation.
A selective calpain-2 inhibitor provides a highly significant degree of neuroprotection when administered after the injury in both acute glaucoma and TBI.
A selective calpain-2 inhibitor could be a potential candidate for the treatment of severalneurological disorders associated with acute neuronal death.
Acknowledgments
Funding
This work was supported by grants P01NS045260–01 from NINDS (PI: Dr. C.M. Gall), and grant R21NS057128 from NINDS to M. Baudry, and grant R15MH101703 from NIMH to X. Bi. X. Bi is also supported by funds from the Daljit and Elaine Sarkaria Chair.
Footnotes
Declaration of Interest
Y. Wang, X. Bi and M. Baudry are co-founders of NeurAegis, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1. •.Guroff G A neutral, calcium-activated proteinase from the soluble fraction of rat brain.The Journal of Biological Chemistry. 1964;239(1):149–155. Original report of the existence of calcium-dependent protease. [PubMed] [Google Scholar]
- 2.Murachi T, Tanaka K, Hatanaka M, et al. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Advances in Enzyme Regulation. 1981;19:407–424. [DOI] [PubMed] [Google Scholar]
- 3. •.Lynch G, Baudry M. The biochemistry of memory- A new and specific hypothesis. Science. 1984;224(4653):1057–1063. This manuscript was the first manuscript developing the hypothesis that calpain plays acritical role in synaptic plasticity and learning and memory. [DOI] [PubMed] [Google Scholar]
- 4.Baudry M, Chou MM, Bi X. Targeting calpain in synaptic plasticity. Expert Opinion on Therapeutic Targets. 2013;17(5):579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu G, Liu Y, Wang Y, et al. Different patterns of electrical activity lead to long-term potentiation by activating different intracellular pathways. Journal of Neuroscience. 2015;35(2):621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. •.Nixon RA, Saito KI, Grynspan F, et al. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann N Y Acad Sci. 1994. December 15;747:77–91. PubMed PMID: Interesting review reporting the role of calpain in aging and AD. [DOI] [PubMed] [Google Scholar]
- 7.Vanderklish PW, Bahr BA. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. International Journal of Experimental Pathology. 2000;81(5):323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. •.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiological Reviews. 2003;83(3):731–801. Extensive review of the calpain system. [DOI] [PubMed] [Google Scholar]
- 9.Camins A, Verdaguer E, Folch J, et al. Involvement of calpain activation in neurodegenerative processes. CNS Drug Reviews. 2006;12(2):135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. •.Bevers MB, Neumar RW. Mechanistic role of calpains in postischemic neurodegeneration. Journal of Cerebral Blood Flow & Metabolism. 2008;28(4):655–673., Very interesting review relating calpains to mecahnisms of cell death. [DOI] [PubMed] [Google Scholar]
- 11.Vosler P, Brennan C, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Molecular Neurobiology. 2008;38(1):78–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. •.Baudry M, Bi X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016. February 10. doi: 10.1016/j.tins.2016.01.007.PubMed PMID: , Our recent review discussing the opposite functions of calpain-1 and calpain-2 in plasticity and neurodegeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Liu MC, Wang KK. Calpain in the CNS: from synaptic function to neurotoxicity. Sci Signal. 2008;1(14):re1. doi: 10.1126/stke.114re1. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Sorimachi H, Ono Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovascular Research. 2012;96(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macqueen DJ, Wilcox AH. Characterization of the definitive classical calpain family of vertebrates using phylogenetic, evolutionary and expression analyses. Open Biology. 2014;4(4):130219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Wang KK. The calpain family and human diseases. Trends in Molecular Medicine. 2001;7(8):355–362. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann JS, Bushby KM. Advances in the molecular genetics of the limb-girdle type of autosomal recessive progressive muscular dystrophy. Current Opinion in Neurology. 1996;9(5):389. [DOI] [PubMed] [Google Scholar]
- 18.Gallardo E, de Luna N, Diaz-Manera J, et al. Comparison of dysferlin expression in human skeletal muscle with that in monocytes for the diagnosis of dysferlin myopathy. PloS One. 2011;6(12):e29061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ••.Ono Y, Saido TC, Sorimachi H. Calpain research for drug discovery: challenges and potential. Nature Reviews Drug Discovery. 2016, Excellent review on the potential of calpain inhibitors for various disorders. [DOI] [PubMed] [Google Scholar]
- 20.Harris F, Biswas S, Singh J, et al. Calpains and their multiple roles in diabetes mellitus. Annals of the New York Academy of Sciences. 2006;1084(1):452–480. [DOI] [PubMed] [Google Scholar]
- 21.Litosh VA, Rochman M, Rymer JK, et al. Calpain-14 and its association with eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahajan VB, Skeie JM, Bassuk AG, et al. Calpain-5 mutations cause autoimmune uveitis, retinal neovascularization, and photoreceptor degeneration. PLoS Genetics. 2012;8(10):e1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa K, Masumoto H, Sorimachi H, et al. Dissociation of m-calpain subunits occurs after autolysis of the N-terminus of the catalytic subunit, and is not required for activation. Journal of Biochemistry. 2001. November;130(5):605–11. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 24.Moldoveanu T, Hosfield CM, Lim D, et al. A Ca 2+ switch aligns the active site of calpain. Cell. 2002;108(5):649–660. [DOI] [PubMed] [Google Scholar]
- 25.Reverter D, Sorimachi H, Bode W. The structure of calcium-free human m-calpain: implications for calcium activation and function. Trends in Cardiovascular Medicine. 2001;11(6):222–229. [DOI] [PubMed] [Google Scholar]
- 26.Hosfield CM, Elce JS, Davies PL, et al. Crystal structure of calpain reveals the structural basis for Ca2+‐dependent protease activity and a novel mode of enzyme activation. The EMBO Journal. 1999;18(24):6880–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maravall M, Mainen Z, Sabatini B, et al. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophysical Journal. 2000;78(5):2655–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glading A, Bodnar RJ, Reynolds IJ, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004. March;24(6):2499–512. PubMed PMID: ; PubMed Central PMCID: PMCPMC355832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao H, Chou J, Baty CJ, et al. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptormediated activation. Molecular and Cellular Biology. 2006;26(14):5481–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. •.Zadran S, Jourdi H, Rostamiani K, et al. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinasedependent phosphorylation. The Journal of Neuroscience. 2010;30(3):1086–1095., First report that brain calpain-2 could be activated by ERK-mediated phosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biology. 2007;8(6):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis TL, Walker JR, Finerty PJ, et al. The crystal structures of human calpains 1 and 9 imply diverse mechanisms of action and auto-inhibition. Journal of Molecular Biology. 2007;366(1):216–229. [DOI] [PubMed] [Google Scholar]
- 33.Qian J, Cuerrier D, Davies PL, et al. Cocrystal Structures of Primed Side-Extending αKetoamide Inhibitors Reveal Novel Calpain-Inhibitor Aromatic Interactions. Journal of Medicinal Chemistry. 2008;51(17):5264–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature. 2008;456(7220):409–412. [DOI] [PubMed] [Google Scholar]
- 35.Campbell RL, Davies PL. Structure–function relationships in calpains. Biochemical Journal. 2012;447(3):335–351. [DOI] [PubMed] [Google Scholar]
- 36.Amini M, Ma C-l, Farazifard R, et al. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. Journal of Neuroscience. 2013;33(13):5773–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. •.Wang Y, Briz V, Chishti A, et al. Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci. 2013. November 27;33(48):18880–92. doi: 10.1523/JNEUROSCI.3293-13.2013. PubMed PMID: ; PubMed Central PMCID: PMCPMC3841454., First report that calpain-1 and calpain-2 play opposite functions in neuroprotection/neuronal death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevers MB, Lawrence E, Maronski M, et al. Knockdown of m‐calpain increases survival of primary hippocampal neurons following NMDA excitotoxicity. Journal of Neurochemistry. 2009;108(5):1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papouin T, Oliet SH. Organization, control and function of extrasynaptic NMDA receptors. Phil Trans R Soc B. 2014;369(1654):20130601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chazot PL. The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Current Medicinal Chemistry. 2004;11(3):389–396. [DOI] [PubMed] [Google Scholar]
- 41.Krapivinsky G, Krapivinsky L, Manasian Y, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40(4):775–784. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Kurup P, Zhang Y, et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009. July 22;29(29):9330–43. doi: 10.1523/JNEUROSCI.2212-09.2009. PubMed PMID: ; PubMed Central PMCID: PMCPMC2737362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladding CM, Sepers MD, Xu J, et al. Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington’s disease mouse model. Human Molecular Genetics. 2012;21(17):3739–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Messer JS. The cellular autophagy/apoptosis checkpoint during inflammation. Cellular and Molecular Life Sciences. 2016:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. •.Zhao Q, Guo Z, Deng W, et al. Calpain 2-mediated autophagy defect increases susceptibility of fatty livers to ischemia–reperfusion injury. Cell Death & Disease. 2016;7(4):e2186 Interesting report illustrating the role of calpain-2 in the regulation of autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia H-g, Zhang L, Chen G, et al. Control of basal autophagy by calpain1 mediated cleavage of ATG5. Autophagy. 2010;6(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nature Cell Biology. 2006;8(10):1124. [DOI] [PubMed] [Google Scholar]
- 48.Williams A, Sarkar S, Cuddon P, et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nature Chemical Biology. 2008;4(5):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordy C, He Y- W. The crosstalk between autophagy and apoptosis: where does this lead? Protein & Cell. 2012;3(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukhopadhyay S, Panda PK, Sinha N, et al. Autophagy and apoptosis: where do they meet? Apoptosis. 2014;19(4):555–566. [DOI] [PubMed] [Google Scholar]
- 51.Corazzari M, Fimia GM, Piacentini M. Dismantling the autophagic arsenal when it is time to die: concerted AMBRA1 degradation by caspases and calpains. Autophagy. 2012;8(8):1255–1257. [DOI] [PubMed] [Google Scholar]
- 52.Harwood SM, Yaqoob MM, Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Annals of Clinical Biochemistry. 2005;42(6):415–431. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. The Journal of Cell Biology. 2000;150(4):887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi WS, Lee EH, Chung CW, et al. Cleavage of Bax is mediated by caspase‐dependent or‐independent calpain activation in dopaminergic neuronal cells: Protective role of Bcl‐2. Journal of Neurochemistry. 2001;77(6):1531–1541. [DOI] [PubMed] [Google Scholar]
- 55.Takano J, Tomioka M, Tsubuki S, et al. Calpain Mediates Excitotoxic DNA Fragmentation via Mitochondrial Pathways in Adult Brains: evidence from calpastatin mutant mice. Journal of Biological Chemistry. 2005;280(16):16175–16184. [DOI] [PubMed] [Google Scholar]
- 56.Wang KK. Calpain and caspase: can you tell the difference? Trends in Neurosciences. 2000;23(1):20–26. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz‐Vela A, de Buitrago GG, Martínez‐A C. Implication of calpain in caspase activation during B cell clonal deletion. The EMBO Journal. 1999;18(18):4988–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shintani-Ishida K, Yoshida K-i. Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia–reperfusion. International Journal of Cardiology. 2015;197:26–32. [DOI] [PubMed] [Google Scholar]
- 59.Yamashima T Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Progress in Neurobiology. 2000;62(3):273–295. [DOI] [PubMed] [Google Scholar]
- 60.Yamashima T Ca 2+-dependent proteases in ischemic neuronal death: a conserved ‘calpain–cathepsin cascade’from nematodes to primates. Cell Calcium. 2004;36(3):285–293. [DOI] [PubMed] [Google Scholar]
- 61.Yamashima T Reconsider Alzheimer’s disease by the ‘calpain–cathepsin hypothesis’—a perspective review. Progress in Neurobiology. 2013;105:1–23. [DOI] [PubMed] [Google Scholar]
- 62.Can Yamashima T. ‘calpain-cathepsin hypothesis’ explain Alzheimer neuronal death? Ageing Research Reviews. 2016;32:169–179. [DOI] [PubMed] [Google Scholar]
- 63.Clausen A, Xu X, Bi X, et al. Effects of the superoxide dismutase/catalase mimetic EUK207 in a mouse model of Alzheimer’s disease: protection against and interruption of progression of amyloid and tau pathology and cognitive decline. Journal of Alzheimer’s Disease. 2012;30(1):183–208. [DOI] [PubMed] [Google Scholar]
- 64.Páramo B, Montiel T, Hernández-Espinosa DR, et al. Calpain activation induced by glucose deprivation is mediated by oxidative stress and contributes to neuronal damage. The International Journal of Biochemistry & Cell Biology. 2013;45(11):2596–2604. [DOI] [PubMed] [Google Scholar]
- 65.Yokoyama Y, Maruyama K, Yamamoto K, et al. The role of calpain in an in vivo model of oxidative stress-induced retinal ganglion cell damage. Biochemical and Biophysical Research Communications. 2014;451(4):510–515. [DOI] [PubMed] [Google Scholar]
- 66. •.Chang H, Sheng JJ, Zhang L, et al. ROS‐Induced Nuclear Translocation of Calpain‐2 Facilitates Cardiomyocyte Apoptosis in Tail‐Suspended Rats. Journal of Cellular Biochemistry. 2015;116(10):2258–2269., Interesting paper illustrating the role of calpain-2 in apoptosis. [DOI] [PubMed] [Google Scholar]
- 67.Saito T, Matsuba Y, Yamazaki N, et al. Calpain activation in Alzheimer’s model mice is an artifact of APP and presenilin overexpression. Journal of Neuroscience. 2016;36(38):9933–9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menzies FM, Fleming A, Caricasole A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93(5):1015–1034. [DOI] [PubMed] [Google Scholar]
- 69.Xu W, Wong TP, Chery N, et al. Calpain-mediated mGluR1α truncation: a key step in excitotoxicity. Neuron. 2007;53(3):399–412. [DOI] [PubMed] [Google Scholar]
- 70. •.Wang Y, Lopez D, Davey PG, et al. Calpain-1 and calpain-2 play opposite roles in retinal ganglion cell degeneration induced by retinal ischemia/reperfusion injury. Neurobiol Dis. 2016. September;93:121–8. doi: 10.1016/j.nbd.2016.05.007. PubMed PMID: , Manuscript reporting the opposite roles of calpain-1 and calpain-2 in a model of acute glaucoma in mice. [DOI] [PubMed] [Google Scholar]
- 71.Chiu K, Lam TT, Ying Li WW, et al. Calpain and N-methyl-d-aspartate (NMDA)induced excitotoxicity in rat retinas. Brain Res. 2005. June 7;1046(1–2):207–15. doi: 10.1016/j.brainres.2005.04.016. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 72.Shimazawa M, Suemori S, Inokuchi Y, et al. A novel calpain inhibitor, ((1S)-1-((((1S)-1Benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-me thylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester (SNJ-1945), reduces murine retinal cell death in vitro and in vivo. J Pharmacol Exp Ther. 2010. February;332(2):380–7. doi: 10.1124/jpet.109.156612. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Zhu G, Briz V, et al. A molecular brake controls the magnitude of long-term potentiation. Nat Commun. 2014;5:3051. doi: 10.1038/ncomms4051. PubMed PMID: ; PubMed Central PMCID: PMCPMC3895372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z, Ortega-Vilain A-C, Patil GS, et al. Novel peptidyl α-keto amide inhibitors of calpains and other cysteine proteases. Journal of Medicinal Chemistry. 1996;39(20):4089–4098. [DOI] [PubMed] [Google Scholar]
- 75.Almasieh M, Wilson AM, Morquette B, et al. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012. March;31(2):152–81. doi: 10.1016/j.preteyeres.2011.11.002. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Cioffi GA, Cull G, et al. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002. April;43(4):1088–94. PubMed PMID: . [PubMed] [Google Scholar]
- 77.Chi W, Li F, Chen H, et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc Natl Acad Sci U S A. 2014. July 29;111(30):11181–6. doi: 10.1073/pnas.1402819111. PubMed PMID: ; PubMed Central PMCID: PMCPMC4121847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russo R, Berliocchi L, Adornetto A, et al. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2:e144. doi: 10.1038/cddis.2011.29. PubMed PMID: ; PubMed Central PMCID: PMCPMC3122060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014. April 16;82(2):279–93. doi: 10.1016/j.neuron.2014.03.030. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 80.Brassai A, Suvanjeiev R-G, Bán E-G, et al. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Research Bulletin. 2015;112:1–6. [DOI] [PubMed] [Google Scholar]
- 81.Bai N, Aida T, Yanagisawa M, et al. NMDA receptor subunits have different roles in NMDA-induced neurotoxicity in the retina. Molecular Brain. 2013;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piras A, Gianetto D, Conte D, et al. Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure. PloS One. 2011;6(7):e22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei T, Kang Q, Ma B, et al. Activation of autophagy and paraptosis in retinal ganglion cells after retinal ischemia and reperfusion injury in rats. Experimental and Therapeutic Medicine. 2015;9(2):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liton PB. The autophagic lysosomal system in outflow pathway physiology and pathophysiology. Experimental Eye Research. 2016;144:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Almasieh M, Wilson AM, Morquette B, et al. The molecular basis of retinal ganglion cell death in glaucoma. Progress in Retinal and Eye Research. 2012;31(2):152–181. [DOI] [PubMed] [Google Scholar]
- 86. ••.Liu S, Yin F, Zhang J, et al. The role of calpains in traumatic brain injury. Brain Injury. 2014;28(2):133–137., Interesting paper discussing the role of calpains in TBI. [DOI] [PubMed] [Google Scholar]
- 87.Kampfl A, Posmantur R, Zhao X, et al. Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy: a review and update. Journal of Neurotrauma. 1997;14(3):121–134. [DOI] [PubMed] [Google Scholar]
- 88.Wang KK, Larner SF, Robinson G, et al. Neuroprotection targets after traumatic brain injury. Current Opinion in Neurology. 2006;19(6):514–519. [DOI] [PubMed] [Google Scholar]
- 89.Pike BR, Zhao X, Newcomb JK, et al. Regional calpain and caspase‐3 proteolysis of α‐spectrin after traumatic brain injury. Neuroreport. 1998;9(11):2437–2442. [DOI] [PubMed] [Google Scholar]
- 90.Pike BR, Flint J, Dutta S, et al. Accumulation of non‐erythroid αII‐spectrin and calpain‐cleaved αII‐spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. Journal of Neurochemistry. 2001;78(6):1297–1306. [DOI] [PubMed] [Google Scholar]
- 91.Pineda JA, Lewis SB, Valadka AB, et al. Clinical significance of α II-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. Journal of Neurotrauma. 2007;24(2):354–366. [DOI] [PubMed] [Google Scholar]
- 92.Brophy GM, Pineda JA, Papa L, et al. αII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. Journal of Neurotrauma. 2009;26(4):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. •.Mondello S, Robicsek SA, Gabrielli A, et al. αII-spectrin breakdown products (SBDPs):diagnosis and outcome in severe traumatic brain injury patients. Journal of Neurotrauma. 2010;27(7):1203–1213., Important paper showing that the levels of SBDPs predict long-term outcomes after TBI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. •.Siman R, Giovannone N, Hanten G, et al. Evidence That the Blood Biomarker SNTF Predicts Brain Imaging Changes and Persistent Cognitive Dysfunction in Mild TBI Patients. Front Neurol. 2013;4:190. doi: 10.3389/fneur.2013.00190. PubMed PMID: ; PubMed Central PMCID: PMCPMC3831148., Important paper showing that the levels of SNTF predict long-term outcomes after TBI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siman R SNTF is a blood biomarker for the diagnosis and prognosis of sports-related concussion. Google Patents; 2014. [Google Scholar]
- 96.Posmantur R, Kampfl A, Siman R, et al. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77(3):875–888. [DOI] [PubMed] [Google Scholar]
- 97.Saatman KE, Murai H, Bartus RT, et al. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Natl Acad Sci U S A. 1996. April 16;93(8):3428–33. PubMed PMID: ; PubMed Central PMCID: PMCPMC39625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan X-X, Jeromin A. Spectrin breakdown products (SBDPs) as potential biomarkers for neurodegenerative diseases. Current Translational Geriatrics and Experimental Gerontology Reports. 2012;1(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoch KM, Reyn CR, Bian J, et al. Brain injury‐induced proteolysis is reduced in a novel calpastatin‐overexpressing transgenic mouse. Journal of Neurochemistry. 2013;125(6):909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thompson SN, Carrico KM, Mustafa AG, et al. A pharmacological analysis of the neuroprotective efficacy of the brain- and cell-permeable calpain inhibitor MDL-28170 in the mouse controlled cortical impact traumatic brain injury model. J Neurotrauma. 2010. December;27(12):2233–43. doi: 10.1089/neu.2010.1474. PubMed PMID: ; PubMed Central PMCID: PMCPMC2996835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bains M, Cebak JE, Gilmer LK, et al. Pharmacological analysis of the cortical neuronal cytoskeletal protective efficacy of the calpain inhibitor SNJ-1945 in a mouse traumatic brain injury model. J Neurochem. 2013. April;125(1):125–32. doi: 10.1111/jnc.12118. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 102.Seubert P, Baudry M, Dudek S, et al. Calmodulin stimulates the degradation of brain spectrin by calpain. Synapse. 1987;1(1):20–24. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Liu Y, Lopez D, et al. Protection against TBI-induced neuronal death with post-treatment with a selective calpain-2 inhibitor in mice. Journal of Neurotrauma. 2017. (ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. •.Briz V, Hsu Y-T, Li Y, et al. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. The Journal of Neuroscience. 2013;33(10):4317–4328., First report that PTEN is selectively cleaved by calpain-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Wang Y, Zhu G, et al. A calpain-2 selective inhibitor enhances learning & memory by prolonging ERK activation. Neuropharmacology. 2016. February 18;105:471–477. doi: 10.1016/j.neuropharm.2016.02.022. PubMed PMID: 26907807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarkar C, Zhao Z, Aungst S, et al. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10(12):2208–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lipinski MM, Wu J, Faden AI, et al. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxidants & Redox Signaling. 2015;23(6):565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun L, Zhao M, Wang Y, et al. Neuroprotective effects of miR-27a against traumatic brain injury via suppressing FoxO3a-mediated neuronal autophagy. Biochemical and Biophysical Research Communications. 2017;482(4):1141–1147. [DOI] [PubMed] [Google Scholar]
- 109.Clark RS, Bayir H, Chu CT, et al. Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy. 2008;4(1):88–90. [DOI] [PubMed] [Google Scholar]
- 110.Luo C-L, Li B-X, Li Q-Q, et al. Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience. 2011;184:54–63. [DOI] [PubMed] [Google Scholar]
- 111.Wang D, Zhang J, Jiang W, et al. The role of NLRP3-CASP1 in inflammasome-mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal-dependent impairment of learning and memory ability. Autophagy. 2017;13(5):914–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yildiz-Unal A, Korulu S, Karabay A. Neuroprotective strategies against calpain-mediated neurodegeneration. Neuropsychiatric Disease and Treatment. 2015;11:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koumura A, Nonaka Y, Hyakkoku K, et al. A novel calpain inhibitor,((1S)-1 ((((1S)-1benzyl-3-cyclopropylamino-2, 3-di-oxopropyl) amino) carbonyl)-3-methylbutyl) carbamic acid 5-methoxy-3-oxapentyl ester, protects neuronal cells from cerebral ischemia-induced damage in mice. Neuroscience. 2008;157(2):309–318. [DOI] [PubMed] [Google Scholar]
- 114.Anagli J, Han Y, Stewart L, et al. A novel calpastatin-based inhibitor improves postischemic neurological recovery. Biochemical and Biophysical Research Communications. 2009;385(1):94–99. [DOI] [PubMed] [Google Scholar]
- 115.Kobeissy FH, Liu MC, Yang Z, et al. Degradation of βII-Spectrin Protein by Calpain-2 and Caspase-3 Under Neurotoxic and Traumatic Brain Injury Conditions. Molecular Neurobiology. 2015;52(1):696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cagmat EB, Guingab-Cagmat JD, Vakulenko AV, et al. Potential Use of Calpain Inhibitors as Brain Injury Therapy. 2015. [PubMed] [Google Scholar]
- 117.Siklos M, BenAissa M, Thatcher GR. Cysteine proteases as therapeutic targets: does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharmaceutica Sinica B. 2015;5(6):506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hong SC, Goto Y, Lanzino G, et al. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke. 1994. March;25(3):663–9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 119.Bartus RT, Hayward NJ, Elliott PJ, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994. November;25(11):2265–70. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 120.Bartus RT, Baker KL, Heiser AD, et al. Postischemic administration of AK275, a calpain inhibitor, provides substantial protection against focal ischemic brain damage. J Cereb Blood Flow Metab. 1994. July;14(4):537–44. doi: 10.1038/jcbfm.1994.67. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 121.Li PA, Howlett W, He QP, et al. Postischemic treatment with calpain inhibitor MDL 28170 ameliorates brain damage in a gerbil model of global ischemia. Neurosci Lett. 1998. May 8;247(1):17–20. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 122.Markgraf CG, Velayo NL, Johnson MP, et al. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke. 1998. January;29(1):152–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 123.Tsubokawa T, Solaroglu I, Yatsushige H, et al. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006. July;37(7):1888–94. doi: 10.1161/01.STR.0000227259.15506.24. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 124.Stracher A Calpain inhibitors as therapeutic agents in nerve and muscle degeneration. Annals of the New York Academy of Sciences. 1999;884(1):52–59. [DOI] [PubMed] [Google Scholar]
- 125. •.Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Current Drug Targets-CNS & Neurological Disorders. 2003;2(3):173–189., Interesting review on the role of calpains in neurodegeneration. [DOI] [PubMed] [Google Scholar]
- 126.Camins A, Verdaguer E, Folch J, et al. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19(8):453–460. [DOI] [PubMed] [Google Scholar]
- 127.Samantaray S, Ray SK, Banik NL. Calpain as a potential therapeutic target in Parkinson’s disease. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug TargetsCNS & Neurological Disorders). 2008;7(3):305–312. [DOI] [PubMed] [Google Scholar]
- 128.Adamec E, Mohan P, Vonsattel JP, et al. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathologica. 2002;104(1):92–104. [DOI] [PubMed] [Google Scholar]
- 129. •.Grynspan F, Griffin W, Cataldo A, et al. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer’s disease. Brain Research. 1997;763(2):145–158., Important paper showing that calpain-2 is associated with tangles in AD. [DOI] [PubMed] [Google Scholar]
- 130.Yin Y, Wang Y, Gao D, et al. Accumulation of human full-length tau induces degradation of nicotinic acetylcholine receptor α4 via activating calpain-2. Scientific Reports. 2016;6:27283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. •.Wang Y, Hall RA, Lee M, et al. The tyrosine phosphatase PTPN13/FAP-1 links calpain-2, TBI and tau tyrosine phosphorylation. Scientific Reports. 2017;7, This paper reports the existence of a new link between calpain-2 and tau phosphorylation/aggregation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berger Z, Roder H, Hanna A, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. Journal of Neuroscience. 2007;27(14):3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.A Lasagna-Reeves C, L Castillo-Carranza D, R Jackson G, et al. Tau oligomers as potential targets for immunotherapy for Alzheimer’s disease and tauopathies. Current Alzheimer Research. 2011;8(6):659–665. [DOI] [PubMed] [Google Scholar]
- 134.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Scientific Reports. 2012;2:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu S, Kanekura K, Hara T, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proceedings of the National Academy of Sciences. 2014;111(49):E5292–E5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hübener J, Weber JJ, Richter C, et al. Calpain-mediated ataxin-3 cleavage in the molecular pathogenesis of spinocerebellar ataxia type 3 (SCA3). Human Molecular Genetics. 2013;22(3):508–518. [DOI] [PubMed] [Google Scholar]
- 138.Lu X, Rong Y, Baudry M. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neuroscience letters. 2000;286(2):149–153. [DOI] [PubMed] [Google Scholar]
- 139.Yuen EY, Ren Y, Yan Z. Postsynaptic density-95 (PSD-95) and calcineurin control the sensitivity of N-methyl-D-aspartate receptors to calpain cleavage in cortical neurons. Molecular Pharmacology. 2008;74(2):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gascón S, Sobrado M, Roda JM, et al. Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Molecular Psychiatry. 2008;13(1):99–114. [DOI] [PubMed] [Google Scholar]
- 141.Su SC, Tsai L-H. Cyclin-dependent kinases in brain development and disease. Annual Review of Cell and Developmental Biology. 2011;27:465–491. [DOI] [PubMed] [Google Scholar]
- 142.Meyer DA, Torres-Altoro MI, Tan Z, et al. Ischemic stroke injury is mediated by aberrant Cdk5. Journal of Neuroscience. 2014;34(24):8259–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress in Neurobiology. 2014;115:157–188. [DOI] [PubMed] [Google Scholar]
- 144.Chen M, He H, Zhan S, et al. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. Journal of Biological Chemistry. 2001;276(33):30724–30728. [DOI] [PubMed] [Google Scholar]
- 145.Atencio IA, Ramachandra M, Shabram P, et al. Calpain inhibitor 1 activates p53dependent apoptosis in tumor cell lines. Cell Growth and Differentiation. 2000;11(5):247–253. [PubMed] [Google Scholar]
- 146.Jung CH, Ro S-H, Cao J, et al. mTOR regulation of autophagy. FEBS Letters. 2010;584(7):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]