Abstract
Introduction:
The purpose of this study was to report the clinical features, computed tomography (CT) and magnetic resonance imaging (MRI) findings, clinical management, and prognoses of 7 patients with Sertoli–Leydig cell tumors (SLCT) of ovary, and to review the literature of this rare condition.
Methods:
Seven patients with pathologically confirmed ovarian SLCT were included. Their clinical, CT and MRI characteristics (CT images obtained from 6 patients and MR images from 4 patients), clinical management, and prognoses of 7 patients were retrospectively analyzed.
Results:
Patients symptoms included irregular menstruation (n = 3), infertile (n = 1), vaginal bleeding after 7 years of menopause (n = 1), a palpable abdominal mass (n = 1), and abdominal pain (n = 1). Three patients had elevated alpha-fetoprotein (AFP), 1 had elevated cancer antigen 125 (CA125), and 2 had elevated Testosterone (T). The 7 tumors of 7 patients were solid or mixed solid-cystic mass with clear boundaries. The solid components of the tumors showed iso-dense on CT. On MRI, the solid components showed iso- or slightly low signal intensity (SI) on T1-weighted imaging (T1WI), high or slightly high SI on T2WI, and high on diffusion-weighted imaging (DWI) with low apparent diffusion coefficient (ADC) value. On contrast-enhanced CT and MRI, 1 tumor exhibited heterogeneous enhancement consisting of multiple nodules with relatively marked homogeneous enhancement, and other 6 tumors showed moderate or marked and constantly heterogeneous enhancements. All patients were treated with surgical excision. Only 3 had received postoperative chemotherapy. With the exception of 1 patient lost to follow-up, the other 6 patients exhibited tumor-free survival with a median follow-up time of 13.5 months, the longest follow-up time being 24 months.
Conclusion:
The patients of SLCT can present with hormonal magnification and manifest high AFP, CA125, and T levels. SLCT is characterized by a solid or mixed solid-cystic mass on CT/MR scans, and shows marked or moderated heterogeneous and constantly enhancement upon postcontrast study. The clinical characteristics and imaging findings are features and appropriated imaging should be performed whenever an SLCT is suspected.
Keywords: computed tomography (CT), magnetic resonance imaging (MRI), ovary, Sertoli–Leydig cell tumors (SLCT)
1. Introduction
Sertoli–Leydig cell tumors (SLCTs), also known as androblastoma, belong to the sex cord-stromal tumors, which exhibit a testicular pattern of differentiation. SLCTs are rare and account for less than 0.5% of all primary ovarian tumors.[1] It can affect any age group ranging from 2 to 75 years. However, 51% of SLCTs take place in the first 3 decades of life.[2] Clinically, the manifestation of SLCT varies widely, ranging from an asymptomatic clinical profile to extreme virilization. As SLCT is rare, the imaging findings of these tumor are yet to be elucidated. Prognosis of ovarian SLCTs is significantly correlated with degree of tumor grading and staging.[2] Management of SLCT remains challenging owing to the lack of standardized management protocol guidelines. Fertility-sparing surgery is the preferred option in young women. Herein, we provide a retrospective review of the clinical features, computed tomography (CT) and magnetic resonance imaging (MRI) findings, clinical management, and prognoses of 7 cases of SLCT with the aim to improve the knowledge of SLCT of radiologists and oncologists.
2. Patients and methods
2.1. Patients
A total of 7 patients with pathologically proven ovarian SLCT at our hospital from January 2013 and August 2017 were included in our retrospective study. Clinical features, CT and MRI findings, clinical management, pathologic confirmations, and prognoses were recorded. All patients had no any previous pelvic surgery or radiation history. All patients underwent surgical resection for primary tumor and SLCT was pathologically confirmed by our pathologists.
This retrospective study was approved by Service Ethics Committee of Women's Hospital, Zhejiang University School of Medicine (Zhejiang, China) with the following reference number: 20170162. All patients provided written informed consent to participate in this study.
2.2. Multislice CT and MRI examinations
Three patients underwent a contrast-enhanced CT, 1 only had an MRI, and 3 had an MRI and a contrast-enhanced CT.
CT scans were performed using a 16-row scanner (GE Medical Systems, Milwaukee, WI). The main imaging parameters were as follows: 5 mm section thickness reconstructions, 25 cm field of view, 120 kA tube voltage, 300 mA current, and a 512 × 512 matrix. The contrast medium injected was iopromide (ultravist, 300 mg/mL) with a dose of 1.5 mL/kg and an injection rate of 2.5 mL/s who underwent contrast-enhanced CT. None of the patients were allergic to the iodine contrast medium. Contrast-enhanced CT scans were started 50 to 60 seconds after the administration of the contrast agent.
MR examinations were performed using on a 1.5-T scanner (Signa HDxt; GE Healthcare, Milwaukee, WI) with a phased-array abdominal coil. The patients laid in a supine position and breathed freely during acquisition. The sequences were obtained as follows: axial spin echo (SE) T1-weighted imaging (T1WI) [time of repetition (TR)/time of echo (TE), 340 ms/10 ms]; axial fast SE T2-weighted imaging (T2WI) with and without fat saturation (TR/TE, 8000 ms/83 ms and 4000 ms/98 ms, respectively); and sagittal and coronal fast SE T2WI (TR/TE, 8000 ms/83 ms). Diffusion-weighted imaging (DWI) was obtained in axial planes at b values of 0, 800 s/mm2 (TR/TE 4600 ms/72 ms). The triple-phase dynamic MR-enhanced scans were performed in the axial, sagittal, and coronal planes immediately after the intravenous administration of Gadopentetate dimeglumine (Magnevistb; Bayer Schering, Guangzhou, China) at a dose of 0.2 mmol/kg of body weight and a rate of 2 to 3 mL/s. The scanning parameters were as follows: 5 mm slice thickness, 1.2 mm gap, 256 to 320 × 256 to 320 matrix, 250 to 296 mm × 250to 340 mm field of view and four excitations. The scanning range was from the inferior pubic symphysis to the renal hilum and extended beyond the dome of the tumor in cases with huge masses.
2.3. Image analysis
The imaging characteristic of the tumors were retrospectively evaluated by 2 trained radiologists in consensus. We analyzed the nature of the primary lesion: the tumor location, size, contour, density, signal intensity, degree of enhancement, calcification, and lymph node. Size indicates the largest tumor diameter observed on axial scans. Lymph node metastasis was considered positive if either of the following was shown: solitary lymph node larger than 10 mm in minimal diameter or clustered lymph nodes larger than 6 mm in minimal diameter. Apparent diffusion coefficient (ADC) value as measured on ADC maps, a circular region of interest (ROI) of at least 1 cm2 was placed at targeted areas with the possibly lowest ADC values in the solid components of the tumor, by referring to conventional MRI and avoiding areas such as hemorrhage, necrosis, and major vascular structures. At least 3 measurements were obtained and averaged.
2.4. Pathological examination
The histology of the primary tumor was reviewed by an expert pathologist. The pathological tumor stage (tumor-node-metastasis stage) was assessed according to the criteria established by the 7th edition of the American Joint Committee (AJCC) on Cancer staging manual.[3] The histological technique consisted of routine hematoxylin and eosin (H&E) staining and immunohistochemical evaluation. Immunohistochemical analysis included AFP, Calretinin, and Inhibin, CD99, CD30, SMA, CK, Vimentin.
3. Results
3.1. Clinical features.
From January 2013 and August 2017, a total of 7 patients with histologically proven ovarian SLCT were retrospectively analyzed. The clinical features of 7 patients were reviewed and the findings summarized in Table 1. The age of the patients was from 16 to 63 years with the mean age of 34 years. There were 2 patients over 50 years of age.
Table 1.
Clinical and surgical details of the 7 cases.
Four patients showed androgenic-excess manifestations, of whom 3 presented with irregular menstruation, 1 was infertile, no virilization, voice deepening, male pattern baldness, and so on. One patient showed estrogenic-excess manifestations, who presented with irregular vaginal bleeding after 7 years of menopause. The other 2 patients had no endocrine symptoms, who presented with a palpable abdominal mass or abdominal pain.
Serum level of CA199, CEA, and β-HCG (human chorionic gonadotropin) were all within normal ranges. Three patients had elevated AFP (8.6, 919.8, and 1881 ng/mL; normal, < 7 ng/mL), 1 had elevated CA125 (37.1 μ/mL; normal range, <35 μ/mL, and 2 had elevated T (4.7 and 12.8 nmol/L; normal range,1.3∼2.8 nmol/L). After the operation, AFP, CA125, and T level came back to normal.
3.2. Image analysis
All 7 patients had a single tumor representing ovarian SLCT. Tumors were located in the left ovary in 4 patients, and the right ovary in the remaining 3 patients. The diameter of the tumors ranged from 2.3 to 27 cm (mean diameter, 10.3 cm) and 6 tumors were oval and 1 was lobulated. All the tumors were well-defined. CT and MRI characteristics of all the tumors are summarized in Table 2, and representative patients are illustrated in Figs. 1 to 4.
Table 2.
Summary of CT/MRI findings.
Figure 1.
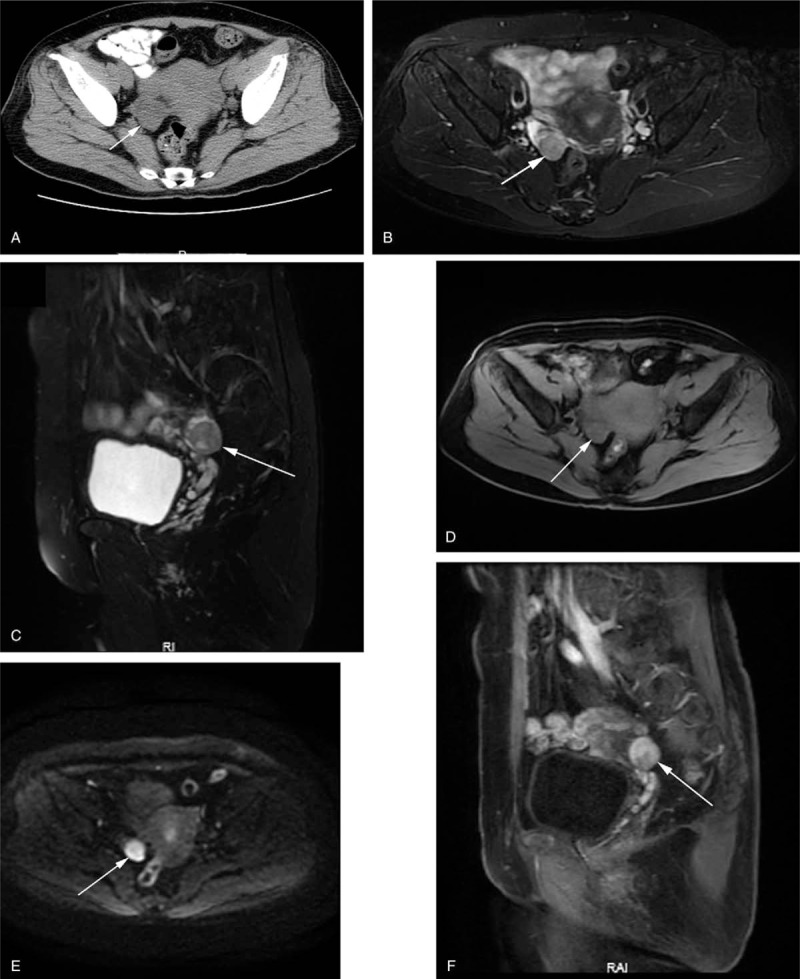
SLCT in a 63-year-old woman with vaginal bleeding after 7 years of menopause. (A) Axial CT showed an isodense solid nodule (arrow) in right ovary. Unenhanced MRI showed the lesion had slightly high SI on fat saturation T2WI (B, C), and iso-SI on fat saturation T1WI (D). (E) Axial DWI (b-factor: 800 mm/s2) demonstrated high SI compared with that of the peripheral muscles. (F) Sagittal contrast-enhanced T1WI with fat saturation showed that the lesion was heterogeneous marked enhancement.
Figure 4.
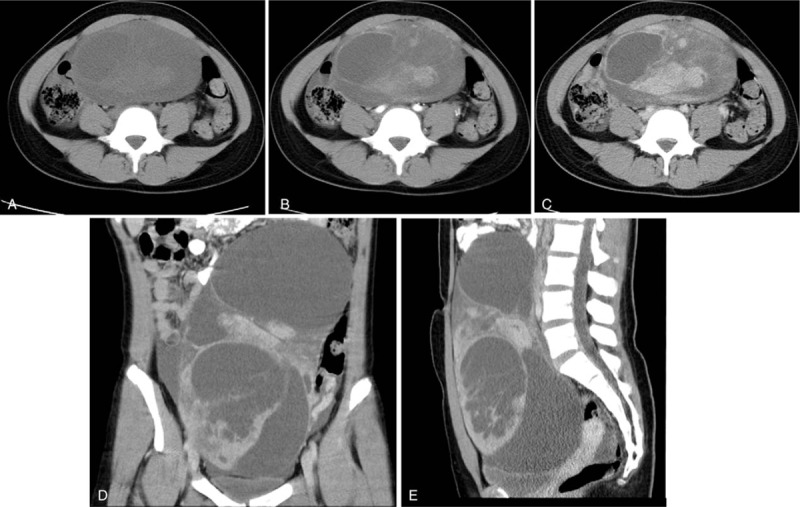
SLCT in a 16-year-old woman with abdominal pain. (A) Axial CT shows a huge multilocular cystic mass with solid area and mural nodules. (B–E) Contrast-enhanced CT showing heterogeneous marked and constant enhancement.
Four patients were solid tumors, with the diameters smaller than 5 cm (Figs. 1, 2). The tumors of 3 patients who had CT scans, showed isodense, and no calcification was observed. Other 3 tumors, which had MRI scans, showed iso-SI on T1WI, slightly high SI on T2WI, and high SI on DWI, with the average ADC value was 1.19 × 10−3 mm2/s (ranged from 1.14 to 1.23 × 10−3 mm2/s). On contrast-enhanced CT and MRI, 1 tumor exhibited heterogeneous marked enhancement consisting of multiple nodules with relatively marked homogeneous enhancement compared with the surroundings (Fig. 2). Other 3 tumors showed heterogeneous marked and constantly enhancement.
Figure 2.
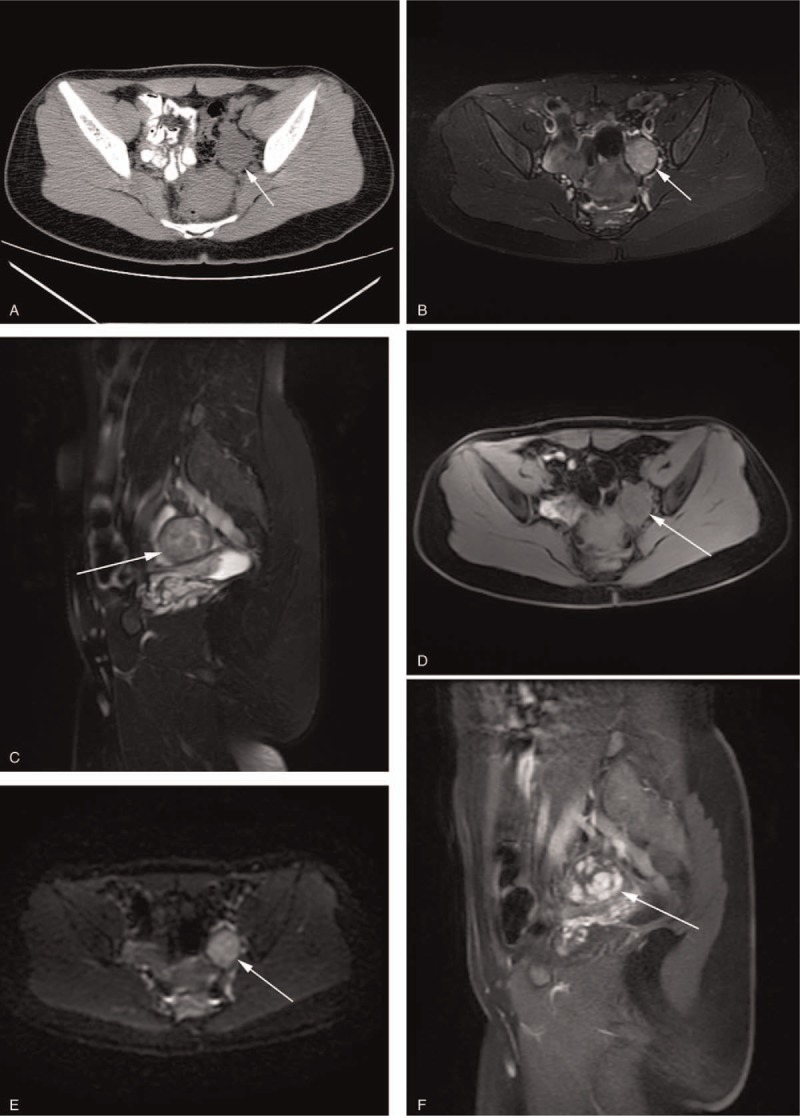
SLCT in a 28-year-old woman with irregular menstruation. (A) Axial CT showed an isodense solid nodule (arrow) in left ovary. Unenhanced MRI showed the lesion had slightly high SI on fat saturation T2WI (B, C), and iso-SI on fat saturation T1WI (D). (E) Axial DWI (b-factor: 800 mm/s2) demonstrated high SI compared with that of the peripheral muscles. (F) Sagittal contrast-enhanced T1WI with fat saturation showed heterogeneous marked enhancement consisting of multiple nodules with relatively homogeneous marked enhancement.
One tumor with a diameter of 7.5 cm manifested as a solid mass with multiple small cysts in the central tumor region (Fig. 3). The solid components were isodense, accompanied by hemorrhagic patchy hyperdense in the tumor. On MRI, the solid components of the tumor showed slightly low SI on T1WI, high SI on T2WI and DWI, with the average ADC value being 1.24 × 10−3 mm2/s, along with hemorrhagic patchy low SI on T2WI inside the tumor. On contrast-enhanced CT and MRI, the tumor had heterogeneous marked and constantly enhancement
Figure 3.
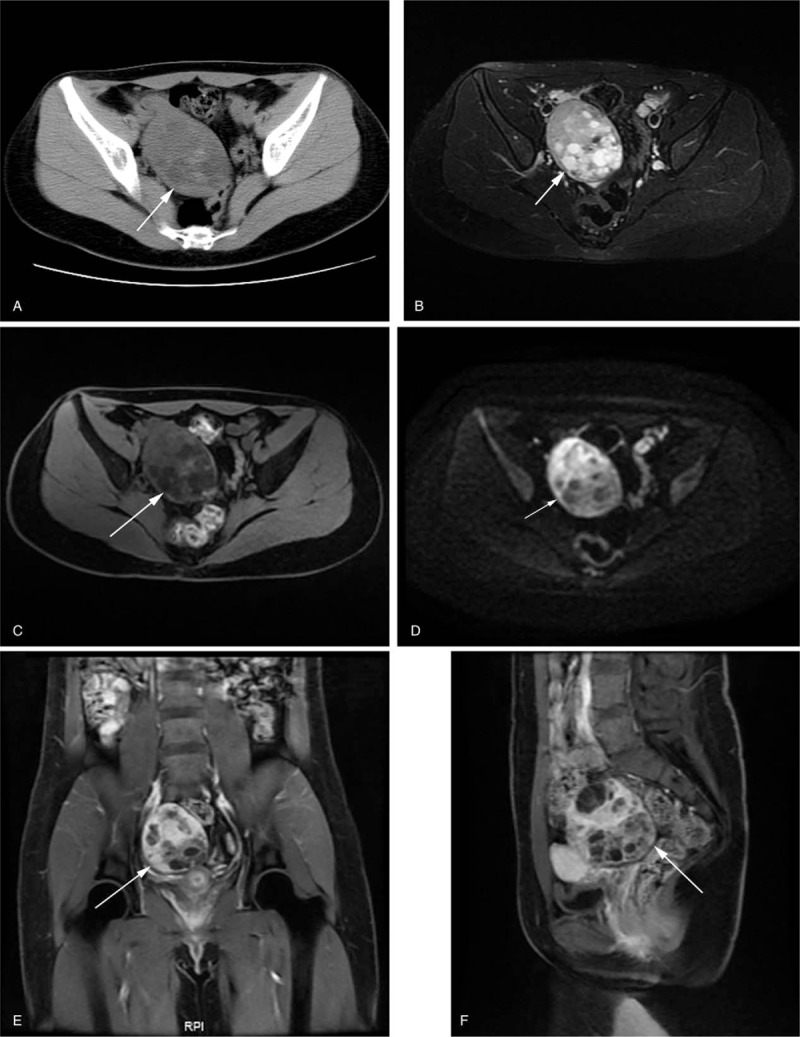
SLCT in a 21-year-old woman with irregular menstruation. (A) Axial CT showed a solid mass with multiple small cysts in the central tumor region. The solid component was isodense, accompanied by hemorrhagic patchy hyperdense in the tumor. On MRI, the solid component of the tumor showed high SI on fat saturation T2WI (B), slightly low SI on fat saturation T1WI (C), and high SI on DWI (D) along with hemorrhagic patchy low SI on fat saturation T2WI inside the tumor. (E) Coronal and (F) sagittal contrast-enhanced T1WI with fat saturation showed heterogeneous marked and constant enhancement.
Two tumors with the diameter of 24.5 and 27 cm appeared as huge multilocular cystic masses with irregularly thickened septa and mural nodules, which extended from the pelvic cavity to the upper abdomen (Fig. 4). There was no obvious calcification in the masses. The solid components were isodense on nonenhanced CT, and heterogeneous marked and constantly enhancement on contrast-enhanced CT. The density of the cystic components was the same as those of the urine. No MRI scans was performed in the 2 tumors.
3.3. Management and follow-up
Surgery was the primary treatment for all patients. According to the international federation of obstetrics and gynecology (FIGO) staging system [n], 4 patients were classified as IA, 3 as IC. Five patients (2 as IA, 3 as IC) underwent unilateral salpingo-oophorectomy along with omentectomy, appendectomy, and pelvic lymphadenectomy in order to preserve fertility, of whom 3 exhibited poorly differentiated and 2 exhibiting moderately differentiated. Another 2 patients (2 as IA) exhibiting poorly differentiated underwent total hysterectomy with bilateral salpingo-oophorectomy, peritoneal biopsies, omentectomy, and pelvic and paraaortic lymph node dissection. There was no evidence of regional lymph node enlargement and metastatic in all patients at diagnosis. Only case 1, case 2, and case 4 had received 4 cycles of postoperative chemotherapy with BEP (bleomycin, etoposide, cisplatin); other 4 patients gave up chemotherapy due to some certain reasons. Case 6 has lost to follow-up, and the other 6 cases exhibited tumor-free survival with a median follow-up time of 13.5 months, the longest follow-up time being 24 months.
3.4. Pathological analysis
All 7 tumors displayed similar microscopic characteristic, including Sertoli cells and Leydig cells, among other possible components (Fig. 5). Sertoli cells were arranged in various patterns, forming hollow tubules, poorly developed tubules, and cords in an edematous stroma. Leydig cells had the eosinophilic cytoplasm, and were seen isolated or in small aggregated, admixed in between Sertoli cell aggregates. Of 5 cases with poorly differentiated SLCT, 3 had heterologous elements and 1 had a retiform pattern. Heterologous elements were confined to the AFP-producing epithelium component in 1 case and gastrointestinal mucinous epithelium component in 2. The immunohistochemical results included positive calretinin, inhibin, AFP, and CD99, which was observed in 5, 5, 1, and 4 cases, respectively. Negativity for SMA, CD30, and EMA was observed in 3, 3, and 3 cases.
Figure 5.
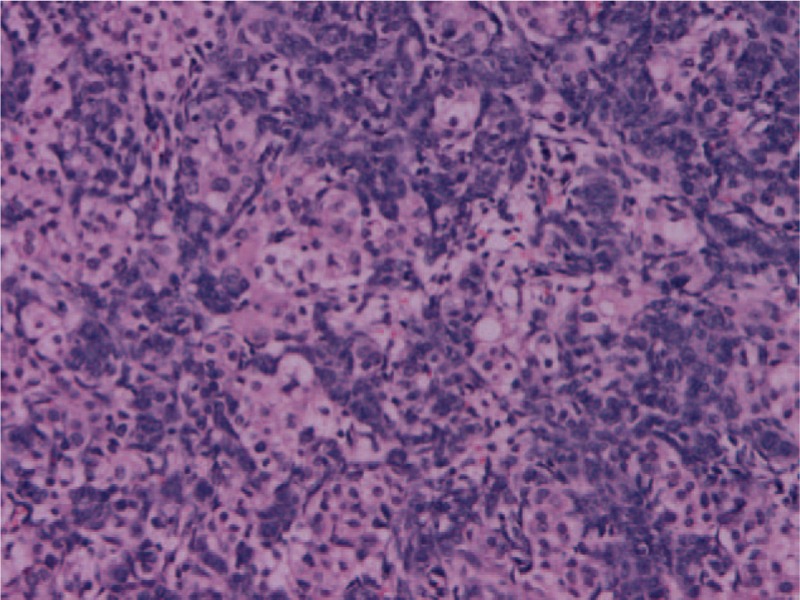
The most common histological features of Sertoli–Leydig cell tumors of the ovary is Sertoli cells are arranged in hollow tubules, cords, sheets, and aggregates, and with adjacent Leydig cells showing eosinophilic cytoplasm (H&E, 200 × ).
4. Discussion
SLCTs are exceedingly unusual neoplasm that belongs to a group of sex cord stromal tumors of ovary. Patients with SLCT present most commonly in second and third decades of life.[1,2] Less than 10% of SLCTs happened prior menarche or postmenopausal.[4,5] The vast majority of SLCTs are confined to a unilateral ovary, especially the right side as shown in our study (4/7, 57.1%). Bilateral SLCTs are exceptionally rare accounting for approximately 1.5% to 2.0% of all SLCT cases,[6,7] and none in our cases.
About one-third of SLCTs are associated with androgen-excess manifestations, including virilization: oligomenorrhea, amenorrhea, breast atrophy, hirsutism, acne, deepening of the voice, clitoris hypertrophy, or balding. T and other androgens may be increased. Occasionally, SLCTs have estrogen-excess manifestations, such as menorrhagia/metrorrhagia, postmenopausal bleeding, or diagnostic curettage, which typically reveal an irregular proliferative endometrium, hyperplasia, or endometrial carcinoma.[7,8] About half of SLCTs have no endocrine manifestation, and patients presenting symptoms may be related to abdominal mass effect such as a feeling of heaviness, abdominal discomfort or pain, ascites, or tumor rupture.[9] In our study, 4 patients showed androgen-excess manifestations, including 1 patient showed infertility, the other 3 had irregular menstruation, and 2 patients had elevated T, which is corresponded to the previous report.[7] One patient presented with estrogen-excess manifestations, who had irregular vaginal bleeding after 7 years of menopause. The remaining 2 patients had no endocrine symptoms.
AFP is a major plasma glycoprotein produced by the liver, yolk sac cells, and fetal gastrointestinal tract. On rare occasions, AFP can also be produced by Sertoli cells, Leydig cells, and heterologous components, such as hepatocytes and/or gastrointestinal epithelium.[10,11] Despite few reports of ovarian SLCT associated with raised levels of AFP, they are the most common AFP-producing nongerm cell tumor of the ovary. In our study, 3 patients had elevated serum level of AFP, and the patients were younger than 30 years, which is similar to the studies.[11] Serum AFP fell to undetectable levels postoperatively.
The radiological findings of SLCT are not well known, as few studies have described the imaging feature of the disease. Edge and Compton [4] reported that SLCTs may be purely solid but are often partly solid and partly cystic or rarely cystic, and most of them have solid and cystic components, with an average diameter ranging from 3 to 11 cm (range 1.5–30 cm). Pure cystic SLCTs are extremely unusual.[9] On CT, SLCT often show solitary, soft tissue density adnexal masses with heterogeneous avid contrast enhancement by the solid components of the tumors. Calcification is uncommon.[4,11–13] On MRI, the solid components of SLCT appear various signal on T2WI, depending on the amount of fibrous stroma.[13–16] Cystic portions usually display high SI on T2WI and low signal on T1WI, but sometimes noticeable high SI on T1-weighted images and low SI on T2-weighted images because of hemorrhage.[17] In our study, the tumors showed solid or solid-cystic mixed masses, and the diameter ranged from 2.3 to 27 cm (mean diameter, 10.3 cm), which were consistent with the reports.[4] The solid components of the tumors displayed isodense on CT, iso- or slightly low signal SI on T1WI, high or slightly high SI on T2WI, and had marked or moderated heterogeneous and constant enhancement on the contrast study, which is similar to the general gynecologic tumor. The imaging characteristics of the cystic components mostly corresponded to those previous reports.[17] In addition, almost the solid components of the tumors in our study demonstrated high signal on DWI with low ADC value. It is believed that this is related to the distribution of substantial tumor cells resulting in restricted movement of water molecules.
Histologically, SLCT are composed of variable combinations of Sertoli cells and Leydig cells, among other possible components. They are generally divided into the following histologic subtypes: well-differentiated, moderately differentiated, poorly differentiated, and with heterologous elements and retiform and mixed.[5,18,19] Heterologous elements occur in approximately 20% of SLCT and can divided into 2 types: endodermal elements, with the commonest being gastrointestinal epithelium; and mesenchymal elements, such as cartilage or skeletal muscle. In our study, 3 tumors had heterologous elements, including AFP-producing epithelium component in 1 and gastrointestinal mucinous epithelium component in 2. One tumor had a retiform pattern.
The prognosis of SLCT is significantly correlated with tumor stage, differentiation, and postoperative chemotherapy. Surgery is the main method to deal with SLCT. Postoperative chemotherapy, radiotherapy, or a combination of both may also be considered in the patients with above-mentioned poor prognostic factors.[4,5] In a previously published study, the overall 5-year survival rate for moderately differentiated and poorly differentiated SLCT is 80%.[20,21] Therefore, early identification, appropriate initial surgery, and selective use of chemotherapy and radiotherapy offer the best prognosis. In the present cases, all patients were treated with related operation according to the actual situation. Postoperatively, only 3 patients received adjuvant systemic chemotherapy, and the other 4 patients gave up chemotherapy due to some certain reasons. So far, the exception of 1 patient had lost to follow-up, other 6 patients were under complete remission at the last follow-up, and long-term follow-up was highly advised. Long-term follow-up is highly advised in all the patients.
Like in every study, our study also has some limitations. First, patient selection bias existed because of the retrospective nature. Second, our sample size was relatively small. A larger study would be required to definitively establish the characteristic features of ovarian SLCT. Third, follow-up time is short. Long-term follow-up is highly advised.
5. Conclusion
Most SLCTs not only presented in the first 3 decades of life in young patients but were also seen in postmenopausal women. The patients of SLCT can present with hormonal magnification and manifest high AFP, CA125, and T levels. SLCT is usually solid or mixed solid-cystic mass with clear boundaries. The main characteristics are the solid components displaying isodense on CT, iso- or slightly low SI on T1WI, high or slightly high SI on T2WI, high SI on DWI with low ADC value, and marked or moderated heterogeneous and constant enhancement upon postcontrast study. We hope our observation will help Radiologists and oncologists to be aware of the possibility of SLCT in an appropriate clinical setting, especially in young woman with a probably malignant unilateral ovarian mass with high AFP and/ or T levels.
Author contributions
Investigation: Qiong Xu.
Methodology: Qiong Xu.
Resources: Qiong Xu, Yu Zou, Xiao Fei Zhang.
Writing – original draft: Qiong Xu.
Supervision: Yu Zou, Xiao Fei Zhang.
Writing – review & editing: Yu Zou.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, AFP = alpha-fetoprotein, CA125 = cancer antigen 125, CA199 = cancer antigen 199, CEA = carcinoembryonic antigen, CT = computed tomography, DWI = diffusion-weighted imaging, HCG = human chorionic gonadotropin, MRI = magnetic resonance imaging, ROI = region of interest, SI = signal intensity, SLCT = Sertoli–Leydig cell tumors, T = Testosterone, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
Methodology: retrospective, observational, performed at 1 institution.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. No study subjects or cohorts have been previously reported.
The authors have nothing to disclose and report no conflicts of interest.
References
- [1].Gui T, Cao D, Shen K, et al. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol 2012;127:384–9. [DOI] [PubMed] [Google Scholar]
- [2].Akman L, Ertas IE, Gokcu M, et al. Ovarian Sertoli-Leydig cell tumors: a multicenter long-term clinicopathological analysis of 27 patients. J Cancer Res Ther 2016;12:290–4. [DOI] [PubMed] [Google Scholar]
- [3].Kim HS, Song YS. International Federation of Gynecology and Obstetrics (FIGO) staging system revised: what should be considered critically for gynecologic cancer? J Gynecol Oncol 2009;20:135–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [5].Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol 1985;9:543–69. [DOI] [PubMed] [Google Scholar]
- [6].Zamurovic M, Soldo V, Cutura N. Bilateral poorly differentiated Sertoli-Leydig ovarian tumor associated with dysgerminoma: case report. Eur J Gynaecol Oncol 2013;34:575–6. [PubMed] [Google Scholar]
- [7].Marcelino M, Nobre E, Conceição J, et al. A rare case of hyperandrogenism: bilateral Leydig cell tumor of the ovary. Acta Med Port 2010;23:113–8. [PubMed] [Google Scholar]
- [8].Demidov VN, Lipatenkova J, Vikhareva O, et al. Imaging of gynecological disease (2): clinical and ultrasound characteristics of Sertoli cell tumors, Sertoli-Leydig cell tumors and Leydig cell tumors. Ultrasound Obstet Gynecol 2008;31:85–91. [DOI] [PubMed] [Google Scholar]
- [9].Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992-1999. Gynecol Oncol 2005;97:519–23. [DOI] [PubMed] [Google Scholar]
- [10].Ching B, Klink A, Wang L. Pathologic quiz case: a 22-year-old woman with a large right adnexal mass. Poorly differentiated Sertoli-Leydig cell tumor of the right ovary with retiform differentiation and heterologous elements (mucinous components). Arch Pathol Lab Med 2004;128:e93–5. [DOI] [PubMed] [Google Scholar]
- [11].Guo M, Lim JC, Wojcik EM. Pelvic washing cytology of ovarian Sertoli-Leydig-cell tumor with retiform pattern: a case report. Diagn Cytopathol 2003;29:28–30. [DOI] [PubMed] [Google Scholar]
- [12].Nwogu LC, Showalter JA, Roy S, et al. Retiform Sertoli-Leydig cell tumor in a 38-year-old woman: a case report, retrospective review, and review of current literature. Case Rep Pathol 2017;2017:3421832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].El-Bahrawy M. Alpha-fetoprotein-producing non-germ cell tumours of the female genital tract. Eur J Cancer 2010;46:1317–22. [DOI] [PubMed] [Google Scholar]
- [14].Horta M, Cunha TM, Marques RC, et al. Ovarian Sertoli-Leydig cell tumor with heterologous elements of gastrointestinal type associated with elevated serum alpha-fetoprotein level: an unusual case and literature review. J Radiol Case Rep 2014;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mooney EE, Nogales FF, Tavassoli FA. Hepatocytic differentiation in retiform Sertoli-Leydig cell tumors: distinguishing a heterologous element from Leydig cells. Hum Pathol 1999;30:611–7. [DOI] [PubMed] [Google Scholar]
- [16].Gagnon S, Têtu B, Silva EG, et al. Frequency of alpha-fetoprotein production by Sertoli-Leydig cell tumors of the ovary: an immunohistochemical study of eight cases. Mod Pathol 1989;2:63–7. [PubMed] [Google Scholar]
- [17].Azuma A, Koyama T, Mikami Y, et al. A case of Sertoli-Leydig cell tumour of the ovary with a multilocular cystic appearance on CT and MR imaging. Pediatr Radiol 2008;38:898–901. [DOI] [PubMed] [Google Scholar]
- [18].Kozan P, Chalasani S, Handelsman DJ, et al. A Leydig cell tumor of the ovary resulting in extreme hyperandrogenism, erythrocytosis, and recurrent pulmonary embolism. J Clin Endocrinol Metab 2014;99:12–7. [DOI] [PubMed] [Google Scholar]
- [19].Outwater EK, Wagner BJ, Mannion C, et al. Sex cord-stromal and steroid cell tumors of the ovary. Radiographics 1998;18:1523–46. [DOI] [PubMed] [Google Scholar]
- [20].Foti PV, Attinà G, Spadola S, et al. MR imaging of ovarian masses: classification and differential diagnosis. Insights Imaging 2016;7:21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Litta P, Saccardi C, Conte L, et al. Sertoli-Leydig cell tumors: current status of surgical management: literature review and proposal of treatment. Gynecol Endocrinol 2013;29:412–7. [DOI] [PubMed] [Google Scholar]


