Abstract
Rationale:
Hepatic hemangioma is the third most common pediatric tumor, and it is rare in the neonatal period. This case study presents a rare case of hepatic hemangioma found in a neonate.
Patient concerns:
A girl who was 18 days of age with the emergence of jaundice and an abdominal mass was admitted for physical examination in the local department.
Diagnoses:
An ultrasound showed that the hepatic left lobe was about 9 cm × 7 cm × 7 cm in size. A CT scan indicated a giant hemangioma in the hepatic left lobe. MRI detected a lesion measuring about 92 mm × 71 mm × 68 mm.
Interventions:
The patient was treated with propranolol 3.5 mg PO bid (body weight 3.8 kg) after 1 week of admission for 4 weeks, but the mass did not appear to regress. Surgery was then performed successfully.
Outcomes:
The patient recovered well without recurrence beyond one year.
Lessons:
Imaging strategies and prenatal diagnosis are vital for the diagnosis of infantile hepatic hemangioma. Propranolol is effective in both cutaneous and hepatic multifocal and diffuse hemangioma. Adequate treatment time is necessary to cure the disease. The role of propranolol in massive hepatic hemangioma remains uncertain and needs further investigation.
Keywords: infantile hepatic hemangioma, propranolol, surgical treatment, treatment strategies
1. Introduction
Infantile hepatic hemangioma (IHE) is a vascular liver tumor of infancy (accounting for 1%–5% of all tumors), and it is also the third most common hepatic tumor of children.[1] The histological manifestation of IHE is proliferative endothelial cell lesions; however, most IHEs are clinically silent and eventually resolve spontaneously without incident.[2,3]
Radiological examinations (ultrasound, computed tomography, and magnetic resonance imaging) are commonly used to diagnose the essential features of IHE, but a biopsy should be carefully considered since it may lead to massive bleeding.[2,4] The general natural history shows that small and asymptomatic lesions in children may self-heal without treatment, while larger or symptomatic lesions require active treatment to prevent serious complications, such as congestive heart failure, hypothyroidism, abdominal compartment syndrome, or fulminant hepatic failure that will lead to death.[2] Therapeutic options for IHE include drug treatment (propranolol, corticosteroids, and drugs with strong antiangiogenic effects, such as interferon alpha, cyclophosphamide, vincristine, or actinomycin D), radiotherapy, selective embolization, and surgery (vascular ligation, tumor resection, or liver transplantation).[3] In the present case, a 4-week-old girl presented a large hemangioma on the hepatic left lobe. Although the girl received 4 weeks of propranolol treatment, no expected regression was achieved. We then provided surgical treatment with consent from her parents. The explored strategies for giant hemangioma treatment include the use of propranolol and the timing and choice of embolization or surgery.
2. Case report
An 18-day-old girl was found with jaundice in the local department when admitted for the physical examination for an abdominal mass. The obstetric history of her mother was uncomplicated with one previous healthy boy, and no abdominal mass was found during her prenatal examination. Enhanced CT indicated a hepatic left lobe occupancy, suggesting infantile hepatic hemangioendothelioma. Then, she received blue light treatment that improved her jaundice. Still, since the girl could not receive appropriate treatment in the local hospital, she was admitted to our institution at 28 days after birth.
When the girl was admitted to our institution, the jaundice had disappeared, she was breathing smoothly, had a good appetite, and slept well. After physical examination, her stable heart rate was between 121 and 132 bpm at admission, respiratory rate was 24 bpm, her systolic blood pressure ranged between 85 and 90, and the diastolic was between 44 and 58 mm Hg. No significant yellow pigmentation or vascular papules were found in the skin and sclera, and the liver was palpated approximately 10 cm below the left costal margin, without tenderness.
Although the examination showed her AFP higher than 1000.0 ng/mL, we did not diagnose her with malignant disease since she was in the neonatal period and the IHE could be affected by the elevated serum AFP. Her ALT was 14.2 U/L and the AST was 40.7 U/ L. Moreover, CTA examination revealed a giant mass in the left lobe of the liver that was rich in blood supply but without obvious arteries, and the left hepatic vein was under compression (Fig. 1). Furthermore, dynamic contrast-enhanced MRI was performed to characterize the lesions in the liver, which included axial T2-weighted images of the liver with and without fat saturation and precontrast axial T1 with fat sat. The lesion was 92 mm × 71 mm × 68 mm, with oval T1-hypointense and T2-hyperintense in the left liver lobe together with a smooth border (Fig. 2). Cardiac Doppler ultrasound showed no obvious abnormalities and normal coagulation function. Her general vital signs were stable at admission. In order to treat her cough and diarrhea, she was treated by spray treatment, oral enteric probiotics, and montmorillonite powder during her hospitalization until she was cured.
Figure 1.
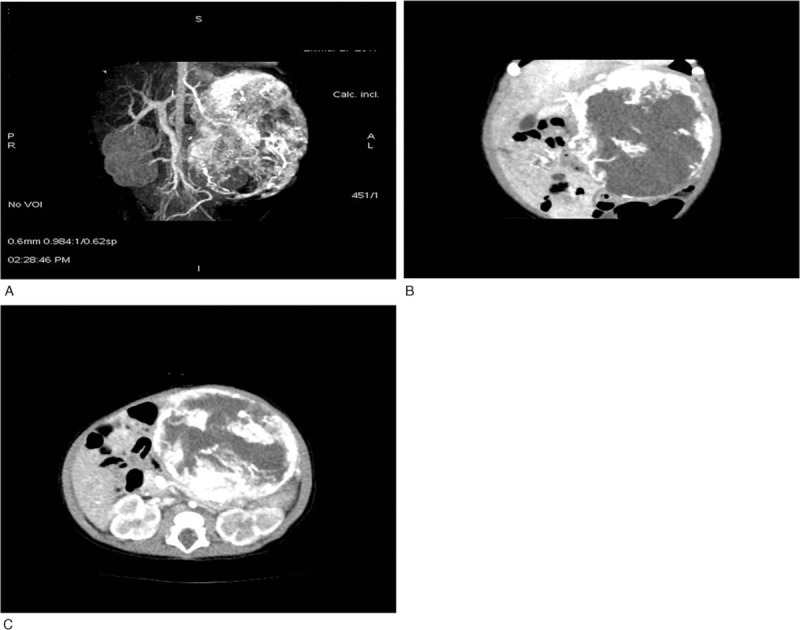
CT scan of the liver prior to the initiation of treatment. (A) CTA volume reconstruction images showing a spherical mass in the left abdomen; the density of the outer layer of the mass is high, and no thick artery is obvious. (B, C) Multiplanar reconstruction. The outer part of the tumor is significantly enhanced, there is large nonenhanced area in the mass, and part of the surrounding tissue and blood vessels are oppressed by the tumor. CT = computed tomography.
Figure 2.
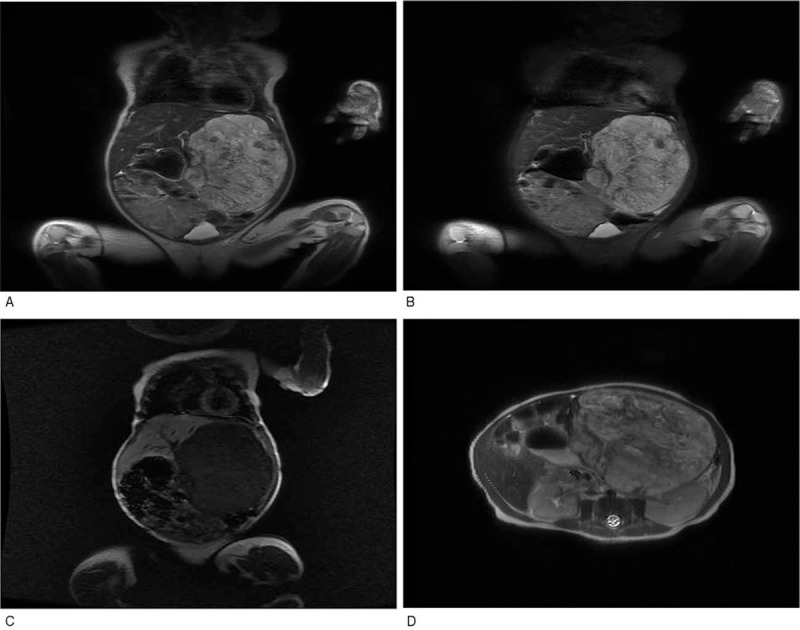
MRI of the patient with IHE. (A, D) (coronal and transverse) T2-weighted image of the liver shows hyperintense lesions relative to the normal liver parenchyma with a smooth border and surrounding oppressed tissue. (B) T2-weighted image with fat saturation of the liver demonstrates multiple hyperintense lesions scattered throughout. (C) Axial T1-weighted image of the liver shows spherical hypointense mass. IHE = infantile hepatic hemangioma.
The girl was diagnosed with hepatic hemangioma after multidisciplinary diagnosis. She was treated with propranolol (3.5 mg PO bid/body weight 3.8 kg) after 1 week of admission, and she developed no signs of bradycardia, asthma, hypotension, or other adverse effects. Four weeks after starting propranolol, the left lobe of the liver was still 80 mm × 98 mm × 65 mm, and the mass was considered not to have regressed, perhaps caused by a thick artery supply. After evaluation and consent from her parents, she had surgery. A dark brown hemangioma was found located in the left lobe of the liver with a clear border and medium texture, measuring about 9 cm × 7 cm × 7 cm. The mass partially adhered to the omentum, and the ligamentum teres hepatitis was ligated. The portal vein, left hepatic vein, and the completely resected hemangioma were blocked after separating the left triangle ligament. The diameter of the left external branch of the portal vein was about 1.0 cm, while no significant difference of the left lateral branches was found between the left hepatic artery and vein during the ligation.
The girl recovered well after given hemostasis, prevention against infection, and other liver-protecting therapies, and her hepatic angioendothelioma was confirmed by pathological diagnosis (Fig. 3). Ten days after surgery, enhanced CT showed no obvious residual hemangioma (Fig. 4). Therefore, the patient was discharged from the hospital 2 weeks after the removal, and no recurrence happened beyond one year.
Figure 3.
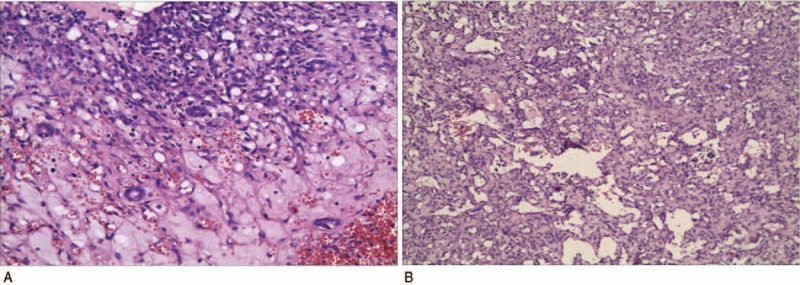
Pathological results of the patient with IHE. (A) Small bile ducts are observed in the tumor. (B) The tumor tissue is composed of different sizes of vascular lacunar-like structure, and the vasculature is surrounded with loose fiber interstitial filling. IHE = infantile hepatic hemangioma.
Figure 4.
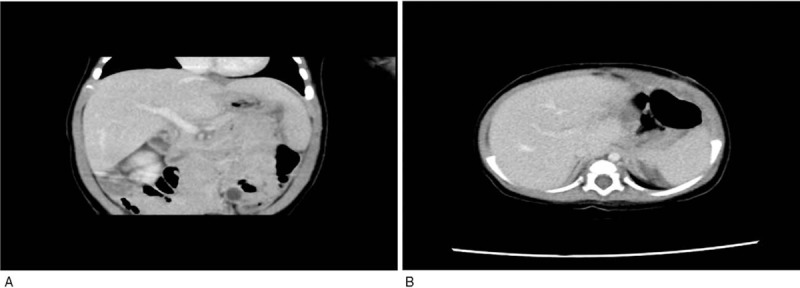
(A, B) Enhanced CT images were reviewed 15 days after the operation, and no recurrence of IHE was observed in the liver. CT = computed tomography, IHE = infantile hepatic hemangioma.
3. Discussion
IHE is the most common benign liver tumor of infants (12%–20% of all infants) and is classified into 3 types, namely, focal, multiple, and diffuse.[3] The liver is the most common extracutaneous site of infantile hemangioma (IH) involvement. The average age of IHEs starts at 47 days (range 1–365 days) and they are mainly diagnosed in the first quarter after birth.[5] Many hepatic hemangiomas are asymptomatic and remain undetected, while others may lead to anemia, consumptive coagulopathy, vomiting, abdominal compartment syndrome, and congestive heart failure. Congestive heart failure might be caused by high volume arteriovenous or portovenous shunting, and the mortality rate of infants during the 1968 to 1995 period was up to 18% caused by hepatic hemangiomas.[6] Hepatic hemangiomas, also referred to as hepatic hemangioendotheliomas, follow the same natural history and biological and morphologic features as cutaneous IH, some of which can coexist in the same patient.[3]
The effect of prenatal examination on detecting fetal abdominal masses is obvious. Most abdominal tumors will be found by ultrasound during pregnancy. Unfortunately, the patient in the current case showed no abnormalities during any prenatal examination, which may have been due to the fact that the prenatal examination was carried out by an unskilled physician.
Imaging is vital to diagnose these lesions. Ultrasound shows a hypoechoic lesion full of blood flow signals.[7] CT scanning reveals the lesion in a low-density area when the contrast agent is injected into the vena, and the area is first enhanced at the edge and then enters into the center. After a short while, the focus area has the same density as the liver. The time for using MRI is longer than for CT or ultrasound, but it can accurately demonstrate a lesion because of the high spatial resolution. To avoid the risk of prolonged sedation, we keep the infant awake (up to 4 hours before the exam), then apply a chloral hydrate enema. This is more acceptable to parents than anesthesia. MRI scans include axial T1 with fat saturation, contrast-enhanced dynamic T1-weighted images after contrast injection, and axial T2-weighted images of the liver with and without fat saturation. The lesions are hypointense relative to the liver on T1-weighted sequences and hyperintense on T2-weighted sequences. Some large lesions can show central necrosis and hemorrhage.[7] We can confirm the diagnosis by CT scan or MRI generally. If the child's symptoms and imaging performances are not typical, further examination, such as biopsy under ultrasound, laparoscopic exploration, or even technetium-99 m-labeled red blood cell scans should be considered.[8] All of these methods are invasive and some hepatic hemangiomas can lead to life-threatening complications, such as high-output heart failure and hypothyroidism. IHE tissue has high D3 iodothyronine deiodinase, and D3 iodothyronine deiodinase can convert T4 and T3 to inactive metabolites. When the IHE replaces most of the liver tissue, consumptive hypothyroidism can occur.[8,9]
The use of corticosteroids for the treatment of cutaneous IH has been the standard treatment until recently. Leaute-Labreze et al[10] found that using propranolol was highly effective for cutaneous IH in 2008. Propranolol is a nonselective B-blocker. The mechanism of propranolol acting on IH is still being investigated. Vasoconstriction, endothelial cell apoptosis, and decreased angiogenesis have been proposed to explain how propranolol affects IH.[11] Many practitioners have adopted propranolol as the first line therapy, and it has been proven effective in treating diffuse hepatic infantile hemangiomas in the last few years. Recent studies have shown that propranolol can successfully treat multifocal and diffuse intrahepatic hemangiomas in combination with other medical therapies, such as systemic corticosteroids and vincristine.[12,13] The effect of propranolol on IH seems to be related to the diameter of the hemangioma.[12] Most studies evaluating propranolol for IHE have involved small patient numbers with dosages of 2 to 3.5 mg/kg/day divided bid to tid. The time of response onset varies from 4.3 weeks to 8.7 weeks.[14] Propranolol can also be used in infants with steroids and vincristine resistance.[13]
The side effects of using propranolol are rare in both cutaneous and hepatic IH. Common adverse events include hypotension, bradycardia, sleep disturbance, and acrocyanosis. Most side effects occur within 1 to 3 hours after oral administration and are transient.[15] Serious adverse events are rare, and include reports of symptomatic hypotension, bradycardia, bronchospasm, hypoglycemia, and even hypoglycemic seizures. Avoiding prolonged fasting is suggested and it is recommended not to be used during episodes of acute respiratory disease and bronchial spasm.[16] The use of propranolol to treat symptomatic hepatic IH requires further study to determine the optimal dosage. How to use propranolol as a monotherapy or in conjunction with steroids and vincristine requires further study and clinical trials.
Radiotherapy was used to treat infants with IHE decades ago, but evidence showed very limited efficacy and the therapy was harmful to normal adjacent liver tissue as found by long-term follow-up.[6] Due to the negative response, this therapy has since been eliminated.
Congenital focal hepatic hemangiomas show a less predictable response to propranolol therapy. This may be associated with arteriovenous shunts. In our case, we used propranolol for one month and reviewed the size of the lesion by ultrasound, but no obvious changes were detected. This may have been due to the short period of using propranolol, and some arteriovenous shunting may have occurred. To avoid life-threatening complications, such as high-output heart failure and hypothyroidism, we decided on surgery after consultation with the parents. Embolization could have been considered in our case, but there are few surgeons skilled enough to carry out embolization in small infants.
It is generally believed that IHE, which is defined as having a diameter larger 5 cm, is an indication for embolization.[17] However, the technique is usually a complex procedure, requiring significant technical skills and knowledge specific to these lesions. Embolization should be considered in a setting of IHE when the symptoms include rupture, the presence of high-output cardiac failure, a large shunt, and failed medical therapy using propranolol.[17] The wrong choice of the embolic agent may result in pulmonary embolization.[18] Persistent patent foramen ovale can also lead to systemic migration of embolic material within coronary, cerebral, or other vital arteries, causing obstruction.
4. Conclusion
Appropriate imaging strategies and prenatal diagnosis are vital for the diagnosis of IHE. Propranolol has become the first-line treatment for both cutaneous and hepatic IH because of the rapid onset resulting in clinical improvement and few serious side effects. Propranolol is also considered to be effective in multifocal and diffuse hemangiomas. Adequate treatment time is necessary to cure the disease. Embolization should be considered carefully in IHEs. If the above treatments are ineffective, surgery is the only choice. More randomized studies with multicenter participation should be performed in the next few years. Lack of sufficient data can lead to inadequate and inaccurate treatment. More knowledge of IHEs and their optimal treatment is needed.
Acknowledgment
We thank Drs Haowei Zhao, Chao Sun, Qi Wang, Zhicheng Zhou, Wenyi Lu, Ruze Tang, and Xiaobo Liu (Children's Hospital of Soochow University).
Author contributions
Conceptualization: Jianlei Chen.
Data curation: Peng Cai.
Formal analysis: Jie Zhu.
Methodology: Bin Wu.
Project administration: Zhicheng Gu.
Resources: Shungen Huang.
Writing – original draft: Zhenwei Zhu.
Writing – review & editing: Jian Wang.
Footnotes
Abbreviations: CT = computed tomography, MRI = magnetic resonance imaging.
ZZ, PC, ZG, SH, and JW contributed equally to this study.
Consent for publication: Informed written consent was obtained from the patient for publication of this case report and accompanying images.
This work was supported by the Suzhou Science Foundation (Grant No. KJXW2015014), Science Foundation of Suzhou Science and Technology Bureau (Grant No. SYS201634, SYS201758), and the Natural Science Foundation of Jiangsu Higher Education Institutions (Grant No. 17KJD320002).
The authors have no conflicts of interest to disclose.
References
- [1].Rawal N, Yazigi N. Pediatric liver transplantation. Pediatr Clin North Am 2017;64:677–84. [DOI] [PubMed] [Google Scholar]
- [2].van der Meijs BB, Merks JH, de Haan TR, et al. Neonatal hepatic haemangioendothelioma: treatment options and dilemmas. Pediatr Radiol 2009;39:277–81. [DOI] [PubMed] [Google Scholar]
- [3].Christison-Lagay ER, Burrows PE, Alomari A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg 2007;42:62–7. discussion 67-68. [DOI] [PubMed] [Google Scholar]
- [4].Cabrita SV, Goncalves S, Rodrigues H, et al. Antenatal diagnosis of congenital hepatic hemangioma: a case report. Cases J 2009;2:6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Konrad D, Ellis G, Perlman K. Spontaneous regression of severe acquired infantile hypothyroidism associated with multiple liver hemangiomas. Pediatrics 2003;112(6 pt 1):1424–6. [DOI] [PubMed] [Google Scholar]
- [6].Boon LM, Burrows PE, Paltiel HJ, et al. Hepatic vascular anomalies in infancy: a twenty-seven-year experience. J Pediatr 1996;129:346–54. [DOI] [PubMed] [Google Scholar]
- [7].Bosemani T, Puttgen KB, Huisman TA, et al. Multifocal infantile hepatic hemangiomas—imaging strategy and response to treatment after propranolol and steroids including review of the literature. Eur J Pediatr 2012;171:1023–8. [DOI] [PubMed] [Google Scholar]
- [8].Stanley P, Gates GF, Eto RT, et al. Hepatic cavernous hemangiomas and hemangioendotheliomas in infancy. AJR Am J Roentgenol 1977;129:317–21. [DOI] [PubMed] [Google Scholar]
- [9].Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000;343:185–9. [DOI] [PubMed] [Google Scholar]
- [10].Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51. [DOI] [PubMed] [Google Scholar]
- [11].Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol 2010;163:269–74. [DOI] [PubMed] [Google Scholar]
- [12].Vergine G, Marsciani A, Pedini A, et al. Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatic hemangioma. Horm Res Paediatr 2012;78:256–60. [DOI] [PubMed] [Google Scholar]
- [13].Sarialioglu F, Erbay A, Demir S. Response of infantile hepatic hemangioma to propranolol resistant to high-dose methylprednisolone and interferon-alpha therapy. Pediatr Blood Cancer 2010;55:1433–4. [DOI] [PubMed] [Google Scholar]
- [14].Hermans DJ, van Beynum IM, Schultze Kool LJ, et al. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: a study of 20 cases with matched historical controls. J Am Acad Dermatol 2011;64:833–8. [DOI] [PubMed] [Google Scholar]
- [15].Broeks IJ, Hermans DJ, Dassel AC, et al. Propranolol treatment in life-threatening airway hemangiomas: a case series and review of literature. Int J Pediatr Otorhinolaryngol 2013;77:1791–800. [DOI] [PubMed] [Google Scholar]
- [16].de Graaf M, Breur JM, Raphael MF, et al. Adverse effects of propranolol when used in the treatment of hemangiomas: a case series of 28 infants. J Am Acad Dermatol 2011;65:320–7. [DOI] [PubMed] [Google Scholar]
- [17].Menapace D, Mitkov M, Towbin R, et al. The changing face of complicated infantile hemangioma treatment. Pediatr Radiol 2016;46:1494–506. [DOI] [PubMed] [Google Scholar]
- [18].Lord DJ, Chennapragada SM. Embolization in neonates and infants. Tech Vasc Interv Radiol 2011;14:32–41. [DOI] [PubMed] [Google Scholar]


