Abstract
The study aims to investigate the association between nonalcoholic fatty liver disease (NAFLD) and osteoporosis.
We employed a retrospective cohort study design using the National Health Insurance Research Database in Taiwan. Our study included 2 cohorts: 4318 patients with NAFLD and 17,272 patients without NAFLD for comparison. They were matched by sex and age on the date of enrollment between January 1, 2000 and December 31, 2003. The study population in both groups was observed from the enrollment date until December 31, 2013. The incidence and the risk ratios of subsequent osteoporosis were calculated separately in both cohorts. A Cox proportional hazards model was used to assess the potential confounding variables of NAFLD on the pathogenesis of osteoporosis.
The eligible study participants comprised 4318 patients in the NAFLD and 17,272 in control cohorts. The median follow-up duration was 10.7 and 10.83 years in the NAFLD and control groups, respectively. The risk of new-onset osteoporosis was higher in patients with NAFLD than in the comparison cohort. In addition, the difference of the incidence of new-onset osteoporosis remained significant among the 2 cohorts in the follow-up durations of within 1 year and more than 10 years. Patients with NAFLD were 1.35 times more likely to develop subsequent osteoporosis compared with those without NAFLD (95% confidence interval = 1.20–1.53).
Our finding indicates that NAFLD might increase the risk of developing new-onset osteoporosis. For earlier detection and intervention, screening for osteoporosis in patients with the NAFLD, especially those with lower income and co-morbid with diabetes mellitus and chronic obstructive pulmonary disease, may be recommended.
Keywords: epidemiology, nonalcoholic fatty liver disease, osteoporosis, risk factors
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) refers to the accumulation of fat in the liver that is not caused by excessive alcohol use. In developed countries where the prevalence of obesity is high, NAFLD is a common liver disease; however, NAFLD develops and progresses in an insidious pattern and lacks obvious signs and symptoms for earlier detection.[1] Moreover, laboratory data among the patients with NAFLD may reveal either a normal or mild increase in the serum alanine aminotransferase (ALT) and the aspartate aminotransferase (AST) levels. Some patients are complicated by liver inflammation with varying degrees of hepatic fibrosis and diagnosed with nonalcoholic steatohepatitis. In a published study, in Taiwan, the prevalence of NAFLD was 11.5%.[2] In addition, NAFLD has been proven to be associated with systemic diseases including cardiovascular diseases, diabetes mellitus, and thyroid gland abnormalities.[3]
As for osteoporosis, a skeletal condition caused by systemic low bone mass and microarchitectural damage, resulting in higher probability of fractures. Numerous epidemiologic studies have proven that osteoporosis is prevalent both in western and eastern nations.[4,5] However, similar to the clinical manifestations observed in patients with NAFLD, patients with osteoporosis often show no remarkable clinical signs and symptoms until fractures occur. Therefore, the diagnosis of osteoporosis is easily under-recognized, and the prevalence of the disease is often underestimated.
Osteoporosis is linked to many physical disorder or health-related behaviors such as estrogen deficiency, endocrine disorder, hypertension, and smoking. Interestingly, patients with NAFLD present comorbid clinical profiles similar to osteoporosis. For example, NAFLD is associated with hypertension, dyslipidemia, insulin resistance, and diabetes.[6] Studies have shown that bone mineral density (BMD) probably be affected in some condition and disorder including obesity, depression, nephropathy, and heart failure.[7–10] Duarte et al found that elevated inflammation may play a major role in NAFLD. Patients with NAFLD were considered to have tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) overexpression; impairment in Kupffer cell phagocytosis may also play an important role in NAFLD.[11] Although the exact role of IL-6 in the pathogenesis of NAFLD is still waiting to be determined, elevation of IL-6 levels was also observed in patients with NAFLD.[12–15] An increased production of TNF-α produced by hepatocytes and nonparenchymal cells in patients with NAFLD was noted in several studies.[16–18] Osteopontin, a T-helper 1 cytokine, exacerbates inflammation in several chronic inflammatory diseases including NAFLD.[19] Many risk factors such as systematic inflammation have been identified for abnormal bone turnover and osteoporosis. Chronic inflammation results in the systemic bone loss, one of the mechanisms of osteoporosis. The inflammatory cytokines, especially TNF-α and IL-1, have been implicated in osteoporosis.[20] These inflammatory cytokines may play a crucial role in the development of osteoporosis. Thus, inflammation may be related both to NAFLD and to osteoporosis.
Several studies suggest NAFLD may be a risk factor for decreased BMD.[7,21–24] In 1 study, NAFLD was significantly associated with a history of osteoporotic fractures in middle-aged and elderly Chinese men.[25] However, in other studies, debate existed regarding the effect of BMD on NAFLD.[26,27]
Based on the debates mentioned above and a few large-scale studies especially in Asia, we hypothesized that NAFLD is associated with osteoporosis. In response to the lack of national data and few longitudinal studies concerning this association, we designed a nationwide population-based study to investigate the possibility of higher risk of developing osteoporosis among patients with NAFLD.
2. Materials and methods
2.1. Data source
The National Health Insurance (NHI) program in Taiwan was instituted in 1995. In this compulsory program, health-related information such as medical data collected from outpatient, inpatient, and emergency is offered to all compatriots, with a coverage rate up to 98%. Prescription, physical and laboratory examinations and diagnostic codes based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) are obtained in the NHI Research Database (NHIRD). The NHIRD was originally managed by the National Health Research Institutes. However, the NHIRD has been transferred to the Health and Welfare Data Science Center, Ministry of Health and Welfare (MOHW) in Taiwan to merge more health-related databases and improve safety issues. In addition, interested researchers could still obtain the data through formal application to the MOHW. The database of the study was the Longitudinal Health Insurance Database 2000 (LHID 2000), which was constructed by systematic and random sampling from the NHIRD and includes data of one million individuals. According to the Taiwanese National Health Research Institutes reports, no significant differences were observed for the distributions of age and sex or the average monthly insurance amount between the data in the LHID 2000 and the original NHIRD.
2.2. Study population
For this retrospective cohort study patients selected from the LHID 2000 are newly diagnosed with NAFLD between January 1, 2000, and December 31, 2003. Patients with NAFLD were defined according to the ICD-9-CM code 571.8 by gastroenterologists. Exclusions included patients diagnosed with osteoporosis (ICD-9-CM code: 733.0, 733.1) between January 1, 1996, and December 31, 1999, and those diagnosed with osteoporosis before the NAFLD diagnosis. Age- and sex-matched patients without NAFLD were selected from the LHID 2000 as the control cohort. All participants were observed until one of the following occurred: diagnosis with osteoporosis (ICD-9-CM code: 733.0, 733.1), withdrawal from the NHI system, death, or study cessation (December 31, 2013). The primary reported clinical outcomes in patients were osteoporosis. Moreover, common comorbidities, including depressive disorder, hypertension, diabetes mellitus, dyslipidemia, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), nephropathy, autoimmune disease, obesity, and congestive heart failure, were compared among the patients in the NAFLD and control cohorts. The study flow is presented in Fig. 1.
Figure 1.
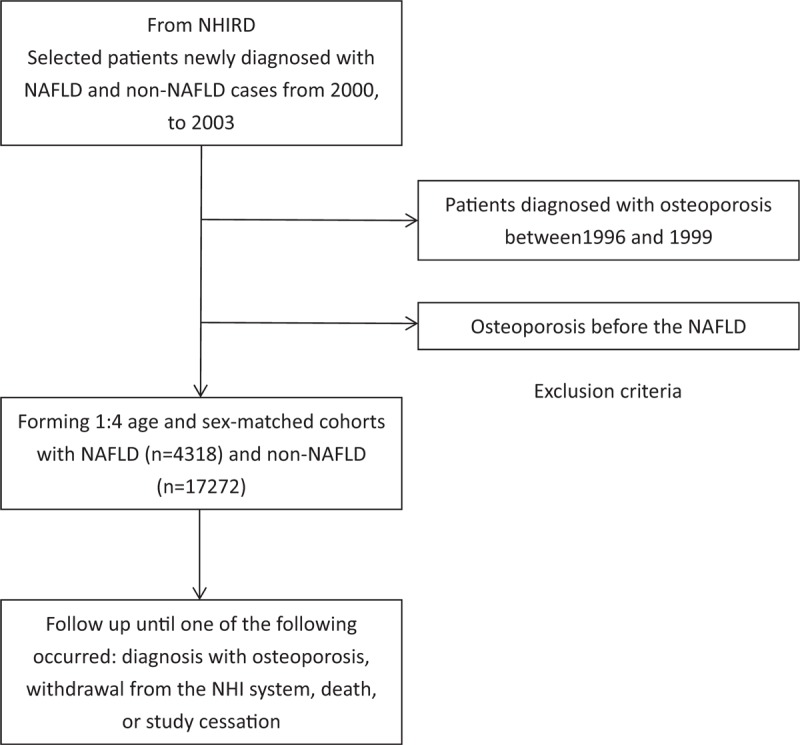
Retrospective cohort study design and study flow chart.
2.3. Statistical analysis
The primary outcome of this study was the incidence of newly diagnosed osteoporosis both in the NAFLD and control group. Independent t test and Chi-squared test were applied to compare the distributions of the demographic characteristics between the 2 groups. To examine potential surveillance bias, subgroups were stratified by duration since the NAFLD diagnosis. Furthermore, a Cox proportional hazard regression model was used to estimate the hazard ratios (HRs) of osteoporosis in the NAFLD and control cohorts. Age, sex, comorbidities relating to NAFLD and osteoporosis, urbanization, and income of the participants were the predictive variables for the analysis. The significance of variables (P < .05) on univariate analysis would be arranged multivariate analysis. We also performed gender subanalysis to estimate the HRs of risk factors for osteoporosis in patients with fatty liver. We calculated follow-up specific risk ratio, since the risk ratio for NAFLD was substantially higher in the first year after osteoporosis diagnosis.
2.4. Ethics
This study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital (VGHKS17-CT12-05). We did not apply written consent for this study because the data were obtained from the LHID 2000, which includes de-identified secondary data. Besides, the Institutional Review Board designed a formal written waiver for the need for consent.
3. Results
The eligible study participants comprised 4318 patients in the NAFLD and 17,272 in control cohorts, respectively. Table 1 shows the demographic and clinical data between the 2 groups. The median age of the patients was 44.94 (interquartile range, 35.60–54.94 and 35.60–54.92) years and the median follow-up duration was 10.7 and 10.83 years in the NAFLD and control groups, respectively. Hypertension, diabetes mellitus, dyslipidemia, and cerebrovascular disease were the 4 most common comorbidities in both groups.
Table 1.
Baseline characteristics of patients with and without fatty liver.
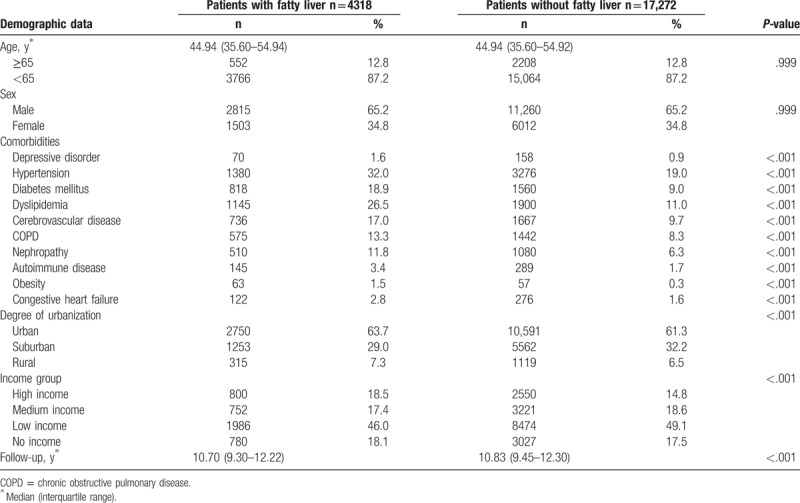
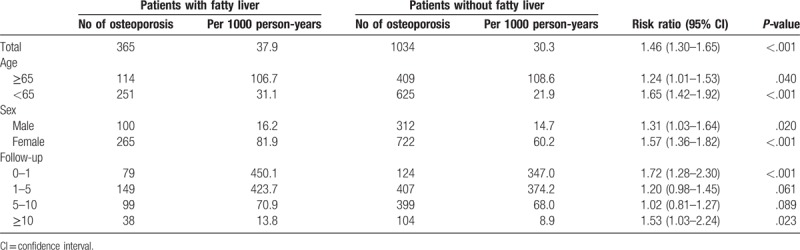
During the follow-up period, the NAFLD group included 365 patients diagnosed with osteoporosis. The incidence was significantly higher in patients in the NAFLD cohort (P < .001) than in those in the control cohort. Moreover, the subanalysis in our study stratified by the duration of follow-up showed that the participants in the NAFLD cohort yielded the highest risk ratio for developing osteoporosis within 1 year of NAFLD diagnosis. Although the risk of developing osteoporosis decreased with time, it revealed statistically significant even more than 10 years after diagnosis (Fig. 2). Compared with patients in the control cohort, patients in the NAFLD cohort tended to have a higher risk of developing osteoporosis (RR = 1.46, 95% confidence interval [CI] = 1.30–1.65) (Table 2).
Figure 2.
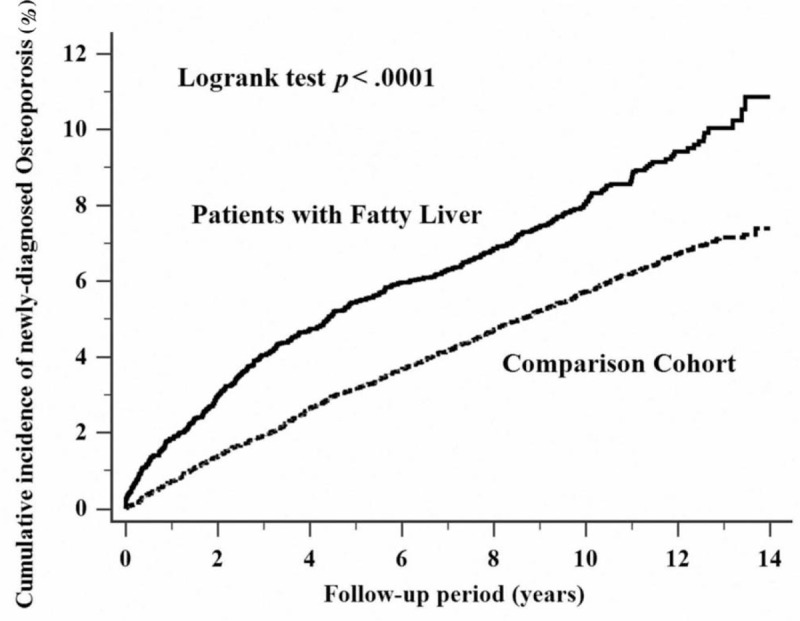
Cumulative incidence comparison of newly diagnosed osteoporosis for patients with (solid line) and without (dashed line) nonalcoholic fatty liver disease.
Table 2.
The incidence of osteoporosis in patients with and without fatty liver.
We applied Cox proportional hazard regression analysis to estimate the HR of newly diagnosed osteoporosis for patients in the NAFLD and control cohorts. We listed the results of the analysis after adjusting for confounding factors and noted the same association between NAFLD and osteoporosis (Table 3).
Table 3.
Analyses of risk factors for osteoporosis in patients with and without fatty liver.
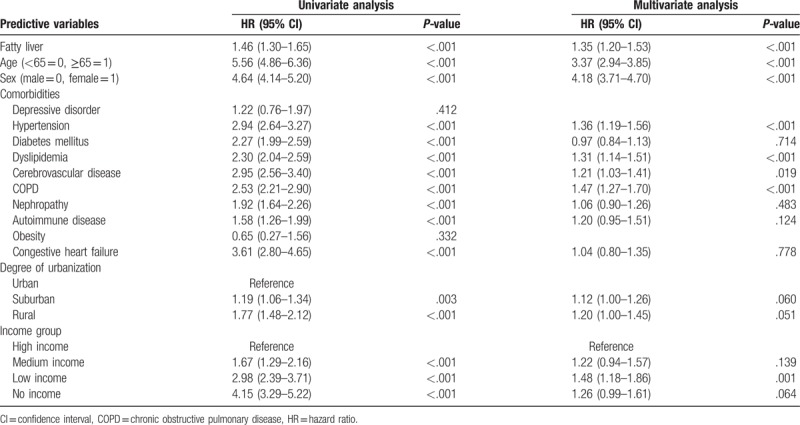
We also performed a Cox proportional hazard regression analysis to detect risk factors for osteoporosis in patients with NAFLD. Predictive variables in comorbidities showed a higher prevalence of diabetes mellitus and COPD among patients in the NAFLD cohort (Table 4). In the gender subanalysis, predictive variables in comorbidities revealed a higher prevalence of COPD among male (Table 5) and dyslipidemia among female (Table 6), respectively.
Table 4.
Analyses of risk factors for osteoporosis in patients with fatty liver.
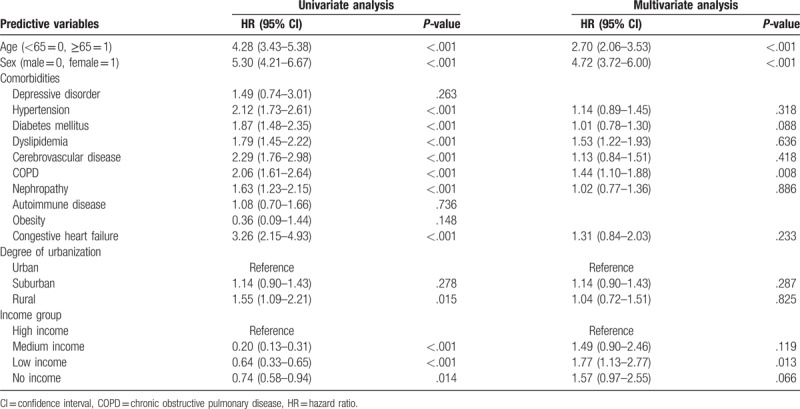
Table 5.
Analyses of risk factors for osteoporosis in patients with fatty liver (male).
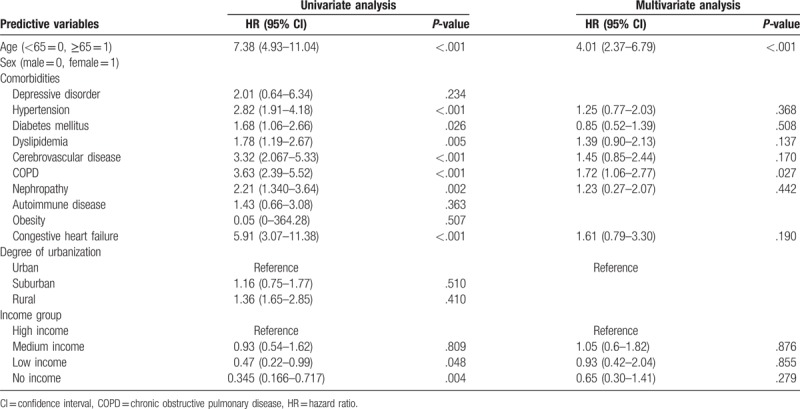
Table 6.
Analyses of risk factors for osteoporosis in patients with fatty liver (female).
4. Discussion
The present study, based on a large-scale data set, indicated that NAFLD was significantly associated with an increased risk of subsequent osteoporosis. Furthermore, our analysis revealed that patients with NAFLD are prevalently comorbid with hypertension, dyslipidemia, COPD, and low income were significantly than in the patients without NAFLD.
Numerous studies tried to identify the association between NAFLD and osteoporosis.[7,21,22,25] However, few large-scale cohort study could be found in the literature; therefore, we conducted a study to observe the incidence and subsequent risk of osteoporosis among patients with NAFLD. Our nationwide study benefitted from a large cohort study, and the selection process of our study design was unbiased.
Proinflammatory cytokines (TNF-related-activation-induced cytokine) and IL-6 are related to osteoclast bone resorption.[20] Osteopontin acts both in osteoblastic and in osteoclastic functions by inhibiting bone mineral growth.[28] High serum osteopontin levels suggested a significant risk factor for menopausal osteoporosis.[29] These studies support that inflammation has a major impact on bone metabolism leading to osteoporosis. Vitamin D deficiency may result in osteoporosis. Roth et al[30] found that vitamin D deficiency exacerbates NAFLD and increases hepatic inflammation in rat pathways. In 1 case–control study patients with NAFLD tended to have a decrease in serum 25(OH)D levels.[31] A significant relationship between vitamin D deficiency and NAFLD was found in Korea.[32] Zhai et al[33] suggest that vitamin D levels have an association with NAFLD, especially in East China. Vitamin D deficiency leads to osteomalacia, thin or brittle bones, which increases risks for osteoporosis.[34] The association between vitamin D levels in NAFLD and patients with osteoporosis needs more investigation. NAFLD might be a risk factor for subsequent osteoporosis because of increased insulin resistance and leptin levels. Pirgon et al[7] reported that NAFLD cohorts had a lower BMD than the non-NAFLD cohorts through the mechanism of increased insulin resistance. Activation of inflammatory pathways both in NAFLD and insulin resistance is discussed in a study[35]; another study suggested elderly patients with type 2 diabetes mellitus are prone to develop osteoporosis.[36] The serum osteocalcin, which expresses mainly by osteoblast, increases insulin secretion. However, osteocalcin was decreased in patients with NAFLD.[37] In summary, the results of these previous studies implies that patients with NAFLD with insulin resistance may have associated risk for osteoporosis.
In our study, the results of multivariate analysis among the patients with NAFLD revealed that COPD could be seen as potential risk factors for the subsequent development of osteoporosis. As for the association with COPD and osteoporosis, studies have shown that COPD is associated with osteoporosis.[38–40] The possible mechanisms may be related to the release of proinflammatory factors (e.g., IL-1, IL-6, CRP, and TNF-α) caused by chronic inflammation and imbalance between proteases and their inhibitors in patients with COPD.[40] Additionally, frequent glucocorticoid use and vitamin D deficiency are also possible risks of osteoporosis development in patients with COPD.[41] Therefore, we observed that patients with NAFLD comorbid with COPD have a higher risk of developing osteoporosis.
Further analyses considering genders showed that, among the male patients with NAFLD, COPD was a significant risk factor for the subsequent development of osteoporosis. Jaramillo et al[42] found that male smokers with COPD have a significant risk of low BMD and vertebral fractures than female smokers as our finding.
Dyslipidemia could be seen as potential risk factors for the subsequent development of osteoporosis among the female patients with NAFLD. Under the NHI program in Taiwan, almost all patients diagnosed with dyslipidemia would be treated. Statin treatment was widely used. A meta-analysis showed that statin treatment may be associated with an increased BMD at the total hip and the lumbar spine and may have a greater effect on male patients than on female patients.[43] Statins may play a role in gender differences.
Several limitations to our study have been identified. Although NHIRD included a large amount of data, it does not include personal information on patients, such as smoking, lack of sun exposure and calcium supplements, body mass index, and family history of osteoporosis, which are contributing factors to the development of osteoporosis. Therefore, we could not survey these potential confounding variables. Second, a diagnostic delay of osteoporosis may be present in our study. Thus, the outcome of our study might not back up the hypothesis that NAFLD leads to osteoporosis.
Conclusion about the association between NAFLD and osteoporosis is positive in our study. NAFLD is prevalent in developed countries. Further long-term and well-designed prospective studies are needed to address the association between NAFLD and osteoporosis. For earlier detection, screening for osteoporosis in patients with NAFLD might be recommended and should be considered in future public health policy planning.
Acknowledgment
The authors thank the Research Center of Medical informatics at Kaohsiung Veterans General Hospital for technical assistance. The study is based on data from the NHIRD provided by the Bureau of National Health Insurance (BNHI) in Taiwan and managed by National Health Research Institutes (NHRI). The authors express their gratitude to the government organization BNHI and the nonprofit foundation NHRI.
Author Contributions
Hon-Jhe Chen was responsible for study design, data collection, data analysis and manuscript revision. Hao-Yu Yang was responsible for manuscript editing and revision. Kuang-Chieh Hsueh, Min-Wei Huang, Cheng-Che Shen, Ru-Yi Chen, Hsien-Chung Yu, Tzu-Lin Wang supervised the study and taught the technique.
Conceptualization: Ru-Yi Chen.
Data curation: Kuang-Chieh Hsueh.
Formal analysis: Cheng-Che Shen.
Investigation: Hon-Jhe Chen.
Methodology: Hsien-Chung Yu.
Project administration: Hao-Yu Yang.
Resources: Tzu-Lin Wang.
Software: Tzu-Lin Wang.
Supervision: Kuang-Chieh Hsueh, Cheng-Che Shen, Ru-Yi Chen, Hsien-Chung Yu, Tzu-Lin Wang.
Validation: Hon-Jhe Chen, Hao-Yu Yang.
Visualization: Hon-Jhe Chen.
Writing – original draft: Hon-Jhe Chen.
Writing – review & editing: Hao-Yu Yang.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMD = bone mineral density, BNHI = Bureau of National Health Insurance, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, HR = hazard ratios, ICD-9-CM = International Classification of Diseases Ninth Revision Clinical Modification, IL = interleukin, LHID 2000 = Longitudinal Health Insurance Database 2000, MOHW = Ministry of Health and Welfare, NAFLD = nonalcoholic fatty liver disease, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, RR = risk ratio, TNF = tumor necrosis factor.
H-JC and H-YY contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- [2].Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of Taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol 2006;40:745–52. [DOI] [PubMed] [Google Scholar]
- [3].Kumar P, Saini M, Kumar D, et al. Estimation of endogenous levels of osteopontin, total antioxidant capacity and malondialdehyde in seminal plasma: application for fertility assessment in buffalo (Bubalus bubalis) bulls. Reprod Domest Anim 2017;52:221–6. [DOI] [PubMed] [Google Scholar]
- [4].Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health 2016;16:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cavalli L, Guazzini A, Cianferotti L, et al. Prevalence of osteoporosis in the Italian population and main risk factors: results of BoneTour Campaign. BMC Musculoskelet Disord 2016;17:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–23. [DOI] [PubMed] [Google Scholar]
- [7].Pirgon O, Bilgin H, Tolu I, et al. Correlation of insulin sensitivity with bone mineral status in obese adolescents with nonalcoholic fatty liver disease. Clin Endocrinol 2011;75:189–95. [DOI] [PubMed] [Google Scholar]
- [8].Whooley MA, Kip KE, Cauley JA, et al. Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999;159:484–90. [DOI] [PubMed] [Google Scholar]
- [9].Jamal SA. Bone mass measurements in men and women with chronic kidney disease. Curr Opin Nephrol Hypertens 2010;19:343–8. [DOI] [PubMed] [Google Scholar]
- [10].Gerber Y, Melton LJ, 3rd, Weston SA, et al. Osteoporotic fractures and heart failure in the community. Am J Med 2011;124:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Duarte N, Coelho IC, Patarrao RS, et al. How inflammation impinges on NAFLD: a role for Kupffer cells. BioMed Res Intern 2015;2015:984578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, et al. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:18070–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Polyzos SA, Kountouras J, Polymerou V, et al. Vaspin, resistin, retinol-binding protein-4, interleukin-1, and interleukin-6 in patients with nonalcoholic fatty liver disease. Ann Hepatol 2016;15:705–14. [DOI] [PubMed] [Google Scholar]
- [14].Kumar R, Prakash S, Chhabra S, et al. Association of proinflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian J Med Res 2012;136:229–36. [PMC free article] [PubMed] [Google Scholar]
- [15].Genc H, Dogru T, Kara M, et al. Association of plasma visfatin with hepatic and systemic inflammation in nonalcoholic fatty liver disease. Ann Hepatol 2013;12:548–55. [PubMed] [Google Scholar]
- [16].Fan JG, Li F, Cai XB, et al. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol 2007;22:1086–91. [DOI] [PubMed] [Google Scholar]
- [17].Orlic L, Mikolasevic I, Bagic Z, et al. Chronic kidney disease and nonalcoholic fatty liver disease-is there a link? Gastroenterol Res Pract 2014;2014:847539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity 2008;16:1394–9. [DOI] [PubMed] [Google Scholar]
- [19].Morimoto J, Kon S, Matsui Y, et al. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr Drug Targets 2010;11:494–505. [DOI] [PubMed] [Google Scholar]
- [20].Lacativa PG, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol 2010;54:123–32. [DOI] [PubMed] [Google Scholar]
- [21].Targher G, Lonardo A, Rossini M. Nonalcoholic fatty liver disease and decreased bone mineral density: is there a link? J Endocrinol Invest 2015;38:817–25. [DOI] [PubMed] [Google Scholar]
- [22].Moon SS, Lee YS, Kim SW. Association of nonalcoholic fatty liver disease with low bone mass in postmenopausal women. Endocrine 2012;42:423–9. [DOI] [PubMed] [Google Scholar]
- [23].Eshraghian A. Bone metabolism in nonalcoholic fatty liver disease: vitamin D status and bone mineral density. Minerva Endocrinol 2017;4:164–72. [DOI] [PubMed] [Google Scholar]
- [24].Bang KB, Cho YK. Comorbidities and metabolic derangement of NAFLD. J Lifestyle Med 2015;5:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li M, Xu Y, Xu M, et al. Association between nonalcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle-aged and elderly Chinese. J Clin Endocrinol Metab 2012;97:2033–8. [DOI] [PubMed] [Google Scholar]
- [26].Upala S, Jaruvongvanich V, Wijarnpreecha K, et al. Nonalcoholic fatty liver disease and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab 2016;35:685–93. [DOI] [PubMed] [Google Scholar]
- [27].Lee SH, Yun JM, Kim SH, et al. Association between bone mineral density and nonalcoholic fatty liver disease in Korean adults. J Endocrinol Invest 2016;39:1329–36. [DOI] [PubMed] [Google Scholar]
- [28].Ishijima M, Rittling SR, Yamashita T, et al. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J Exp Med 2001;193:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology 2011;53:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology 2012;55:1103–11. [DOI] [PubMed] [Google Scholar]
- [31].Targher G, Bertolini L, Scala L, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2007;17:517–24. [DOI] [PubMed] [Google Scholar]
- [32].Park D, Kwon H, Oh SW, et al. Is vitamin D an independent risk factor of nonalcoholic fatty liver disease?: A cross-sectional study of the healthy population. J Korean Med Sci 2017;32:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhai HL, Wang NJ, Han B, et al. Low vitamin D levels and nonalcoholic fatty liver disease, evidence for their independent association in men in East China: a cross-sectional study (Survey on prevalence in East China for metabolic diseases and risk factors (SPECT-China). Br J Nutr 2016;115:1352–9. [DOI] [PubMed] [Google Scholar]
- [34].Zhen D, Liu L, Guan C, et al. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: its relationship to osteoporosis and lifestyle factors. Bone 2015;71:1–6. [DOI] [PubMed] [Google Scholar]
- [35].Toonen EJ, Mirea A-M, Tack CJ, et al. Activation of proteinase 3 contributes to nonalcoholic fatty liver disease and insulin resistance. Mol Med 2016;22: doi: 10.2119/molmed.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xia J, Zhong Y, Huang G, et al. The relationship between insulin resistance and osteoporosis in elderly male type 2 diabetes mellitus and diabetic nephropathy. Ann Endocrinol (Paris) 2012;73:546–51. [DOI] [PubMed] [Google Scholar]
- [37].Sinn DH, Gwak GY, Rhee SY, et al. Association between serum osteocalcin levels and nonalcoholic fatty liver disease in women. Digestion 2015;91:150–7. [DOI] [PubMed] [Google Scholar]
- [38].Liu SF, Kuo HC, Liu GH, et al. Inhaled corticosteroids can reduce osteoporosis in female patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Abbasi M, Zohal M, Atapour B, et al. Prevalence of osteoporosis and its risk factors in men with COPD in Qazvin. Int J Chronic Dis 2016;2016:4038530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis 2016;11:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Silva DR, Coelho AC, Dumke A, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care 2011;56:961–8. [DOI] [PubMed] [Google Scholar]
- [42].Jaramillo JD, Wilson C, Stinson DS, et al. Reduced bone density and vertebral fractures in smokers. Men and COPD patients at increased risk. Ann Am Thorac Soc 2015;12:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].An T, Hao J, Sun S, et al. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int 2017;28:47–57. [DOI] [PubMed] [Google Scholar]


