Supplemental Digital Content is available in the text
Keywords: retroperitoneal fibrosis, rituximab, smoking, treatment
Abstract
Retroperitoneal fibrosis (RPF) refers to a fibro-inflammatory lesion in the retroperitoneum, often anterolateral to the aorta. Most cases are due to IgG4-related disease (IgG4-RD) or are idiopathic. RPF can lead to severe morbidity. Treatment strategies remain poorly-defined. We evaluated the efficacy and safety of rituximab (RTX) for idiopathic or IgG4-related RPF.
We retrospectively reviewed the records of patients who had RPF treated with RTX. Treatment response was determined by assessing changes in both clinical features, including symptoms and laboratory measurements, as well as in the radiographic dimensions of the lesion.
Twenty-six patients with IgG4-related (n = 19) or idiopathic RPF (n = 7) were identified. Patients without histopathological evidence of IgG4-RD on either retroperitoneal biopsies or sampling of extra-retroperitoneal organs were considered to have idiopathic RPF. Of the 26 patients, 19 (73%) received RTX without additional glucocorticoids. All 19 patients who presented with pain reported symptomatic improvement following RTX. Among 25 patients with follow-up imaging, 22 (88%) had radiologic improvement. Among 10 patients with ureteral stents and/or percutaneous nephrostomy tubes, 4 (40%) underwent successful stent or tube removal. Responses to treatment were similar among those treated with RTX monotherapy and those treated with RTX and glucocorticoids. RTX was generally well tolerated, but 3 (12%) patients experienced severe infections.
In this study, RTX for RPF led to resolution of symptoms in all patients and radiographic improvement in the majority. Prospective studies of RTX for RPF are indicated.
1. Introduction
Retroperitoneal fibrosis (RPF) is characterized by fibro-inflammatory tissue in the retroperitoneum, often anterolateral to the aorta and/or its branches (e.g., iliac vessels). The inflammatory process often entraps and compresses surrounding structures including the ureters, leading to hydronephrosis.[1] Patients typically present with abdominal, flank, or back pain, and can also have renal failure secondary to hydronephrosis.[2,3] Untreated RPF can lead to chronic pain, chronic kidney disease, and abdominal aortic aneurysm. The cause of RPF is unknown, but both tobacco and asbestos exposure are risk factors for the condition.[4,5] Although the majority of RPF cases were once considered to be “idiopathic,” IgG4-related disease (IgG4-RD) is now recognized as the underlying cause in many cases.[6,7]
IgG4-RD is hypothesized to be responsible for the a substantial proportion of RPF cases for several reasons.[6] First, RPF is often found in patients with IgG4-RD involving common sites such as the submandibular glands and orbits.[6] Second, many RPF patients have elevated serum IgG4 concentrations.[8] Also third, the histopathological findings in RPF are often indistinguishable from those found in IgG4-RD affecting other organs.[6,9] IgG4-related RPF also has a typical anatomical distribution, involving the peri-aortic region beginning at the infra-renal portion and extending inferiorly to involve the iliac vessels. Nevertheless, confirming the diagnosis of IgG4-related RPF is often challenging. The characteristic histopathologic features are often not observed in specimens obtained through computed tomographically guided needle biopsies because of the small amounts of tissue sampled in this way. Moreover, larger biopsies are not performed routinely unless open procedures are required either to exclude malignancy or to address some advanced complication of RPF, for example, the performance of ureteropexy to free an entrapped ureter. Even when large tissue samples are available, the overwhelming histopathologic picture in RPF is often one of dense fibrosis. Thus, the diagnosis of IgG4-related RPF is often surmised through the combination of data from small biopsies, serological data, radiologic evidence, and findings in extra-retroperitoneal organs.
Glucocorticoids have been the gold standard treatment for idiopathic RPF.[10] However, glucocorticoids are associated with substantial morbidity (e.g., diabetes, weight gain, osteoporosis) and frequent treatment failures in IgG4-RD both during (6–25%) and after glucocorticoid tapering (17%–72%).[10,11] The efficacy of steroid-sparing medications (e.g., mycophenolate mofetil, methotrexate, azathioprine, and tamoxifen) in combination with glucocorticoids for RPF has been evaluated only to a limited degree.[10,12] No clear-cut steroid-sparing regimen has been identified.[13,14] Moreover, it is unclear whether any of the alleged efficacy observed in the combination treatment regimens studied to date is due to the medication combined with glucocorticoids, or simply to effects of glucocorticoids themselves.
Given the efficacy of rituximab (RTX) in the treatment of other manifestations of IgG4-RD and recognition that many idiopathic RPF cases may be IgG4-related RPF, we examined the efficacy of RTX in idiopathic and IgG4-related RPF in a large retrospective cohort.[7,15–18] Most of the patients in this study received no concomitant glucocorticoid treatment.
2. Methods
2.1. Cohort overview
This retrospective study was approved by the Partners Institutional Review Board. All patients provided written informed consent. The Massachusetts General Hospital Center for IgG4-RD maintains a database of all patients referred for evaluation at the center.[16] All patients with IgG4-related or idiopathic RPF treated with RTX between 2010 and 2016 were identified. Data pertaining to demographics, date of diagnosis, symptoms, comorbidities, laboratory values, use of stents or percutaneous nephrostomy tubes, biopsy results, symptomatic response to treatment and adverse events were obtained from the electronic medical records. Acute kidney injury was defined as an abrupt change in renal function, defined as a creatinine rise of 0.3 mg/dL within 48 hours, a 1.5-fold creatinine increase within 7 days or urine volume less than 0.5 mL/kg/hour for 6 hours.[19] Each patient was seen in follow-up between 3 and 6 months following RTX. Aspects of some of these cases have been previously reported, though none in the systematic manner focusing upon the retroperitoneal lesion, as in this series.[16,20]
2.2. Diagnosis
RPF was diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI) following commonly accepted radiographic criteria.[10] Two patients with RPF in atypical anatomic locations had biopsy-proven IgG4-related or idiopathic RPF.[10] A single board-certified radiologist (AS) reviewed all available baseline and follow-up imaging. The diagnosis of IgG4-RD was established by consistent pathology in the appropriate clinical context[21] or according to clinical diagnostic criteria for IgG4-RD.[22] RPF was considered “idiopathic” under the following circumstances: malignancy and infection were clinically or pathologically excluded; secondary causes (e.g., radiation) were not identified; and the patient did not meet clinical diagnostic criteria for IgG4-RD[22] and pathology was not supportive of that diagnosis (e.g., no IgG4+ plasma cells).
2.3. Treatment protocol
All patients received 2 intravenous doses of rituximab (RTX, 1,000 mg) separated by approximately 2 weeks. Patients were pre-medicated with methylprednisolone (100 mg), diphenhydramine (25–50 mg), and acetaminophen (650 mg) prior to each infusion. Additional glucocorticoids, when used, were prescribed at the discretion of the provider.
2.4. Assessing treatment response
Similar to prior studies, a treatment response was defined as improvement in symptoms attributed to RPF, the dimensions of the lesion on CT or MRI, or the resolution of laboratory markers (serum IgG4-concentration, erythrocyte sedimentation rate, or C-reactive protein).[11,12] In patients who required ureteral stents or percutaneous nephrostomy tubes, we also evaluated whether these could be successfully removed post-infusion.[12] Symptom response was based on subjective patient report as documented in clinical documentation from a visit.
For patients with imaging available for review, radiologic dimensions were measured in 3 different axes (anterior–posterior, left lateral, transverse, craniocaudal) by a board-certified radiologist (AS). The maximum transverse measurement in the left paraaortic region was recorded. We assessed the radiologic response among those with imaging 3–6 months after RTX and among those with any follow-up imaging. Although RTX is thought to have its peak impact 3 to 6 months after treatment based on the kinetics of B cell depletion, we chose to evaluate any available follow-up imaging because patients had their subsequent imaging at variable times. A radiologic response was defined as a reduction in lesion size in at least 2 radiographic planes, an approach consistent with other radiologic studies of RPF.[10] Percent radiographic change was measured by averaging the 2 dimensions of greatest change. A decrease in the size of the lesion by ≥ 10% was judged to be an improvement; a reduction in size between 0% and 10% was thought to be stable disease. Similarly, an increase in the size of the lesion by ≥ 10% was considered to be worsening of disease while an increase between 0% and 10% was considered stable disease. For individuals with imaging obtained outside our medical records system but without electronic images available for review, imaging reports were used to assess the presence or absence of a radiologic response. We also assessed inflammatory markers (erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)), and serum IgG4 concentrations prior to and 3–6 months after RTX. We chose a follow-up range of 3 to 6 months because prior studies suggest that this is when RTX has peak efficacy.[23]
2.5. Adverse events
Patient charts were reviewed to determine whether patients had an infusion reaction or developed infection requiring intravenous antibiotics or hospitalization within 6 months of RTX.
2.6. Statistical analysis
Categorical variables are reported using frequency and percentage. Continuous variables are reported using median and interquartile range (IQR) because of the non-normal distribution of these variables. All statistical tests were performed using SAS 9.3 (SAS Institute, Cary, NC).
3. Results
3.1. Baseline features
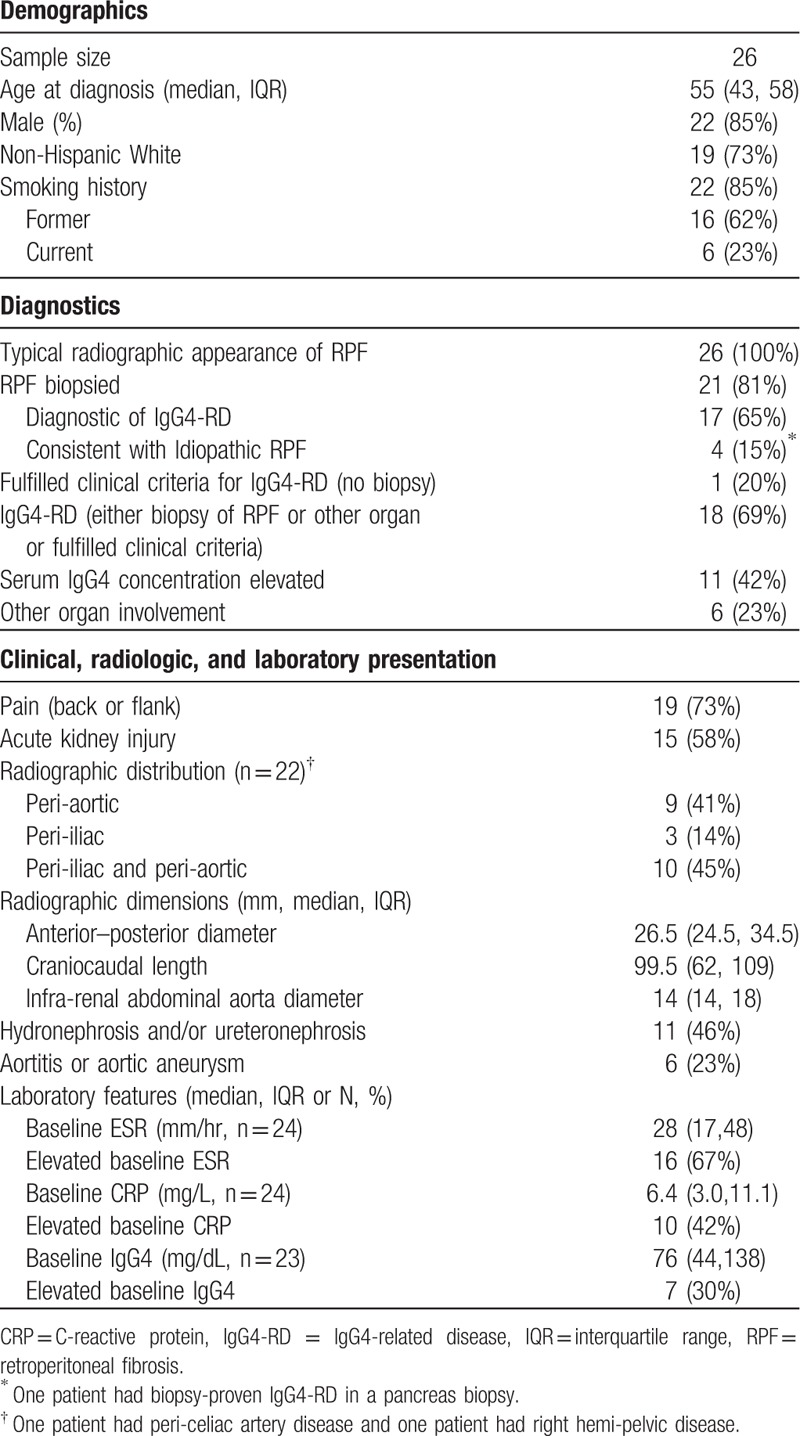
Twenty-six patients with idiopathic or IgG4-related RPF were treated with RTX (Table 1). The median age at diagnosis was 55 years (IQR 43, 58). The median time from diagnosis or symptom onset to RTX was 12.5 months (IQR 3, 21). The majority of patients were male (85%), non-Hispanic white (73%), and former or current smokers (85%). Six (23%) patients had clinicopathologic evidence of IgG4-RD in another organ.
Table 1.
Baseline demographics and features.
3.2. IgG4-RD vs idiopathic RPF
Retroperitoneal biopsies were obtained in 21 (81%) patients. In 17 (81%) of these patients, pathology features consistent with IgG4-RD were present.[6] One patient with nonspecific RPF biopsy features had biopsy-proven IgG4-RD in the pancreas and was therefore classified as having IgG4-related RPF. In the remaining 3 patients who were biopsied, malignancy and infection were excluded but specific features of IgG4-RD were absent. These 3 patients were considered to have idiopathic RPF. Of the 5 patients without biopsies, one met clinical diagnostic criteria[22] for IgG4-RD. The other 4 had a lesion with a radiologic distribution typical of IgG4-related RPF but were considered idiopathic RPF because of a lack of other specific features and the absence of clinical suspicion of infection or malignancy. In summary, 19 (73%) patients had IgG4-related RPF and 7 (27%) patients had idiopathic RPF.
3.3. Clinical presentation
Back or flank pain were the most common symptoms attributable to RPF (n = 19, 73%). Fifteen (58%) patients had acute kidney injury attributed to ureteral compression from RPF at the time of diagnosis. The majority (12, 63%) of patients with IgG4-related RPF had no other organ involvement by IgG4-RD. Baseline ESR and CRP concentrations were available for 24 patients and baseline IgG4 concentration measurements were available for 23. The ESR, CRP, and serum IgG4 concentrations were elevated in 16 (67%), 10 (42%), and 7 (30%) patients, respectively, among those in whom measurements were available.
3.4. Radiologic features
Twenty-six (100%) patients had typical radiographic features of RPF. Twenty-three (88%) patients had a classic radiologic distribution of IgG4-related RPF (i.e., peri-aortic, extending anterolaterally). Three (12%) had only peri-iliac fibrosis and 9 (35%) had only peri-aortic fibrosis. Eight (31%) had both peri-iliac and peri-aortic fibrosis. Three (12%) had peri-iliac, peri-aortic and peri-caval fibrosis. The remaining 3 patients did not have “classic” IgG4-related RPF distributions. One had peri-renal fibrosis, one had disease around the celiac axis, and one had disease inferior to the third portion of the duodenum. All 3 patients with nonclassic distributions met criteria for IgG4-RD. Two (peri-renal and peri-duodenal) had biopsies consistent with IgG4-RD and the third (peri-celiac axis) met clinical criteria for IgG4-RD. Six patients (23%) had radiographic evidence of aortitis (e.g., vessel wall thickening or enhancement) or aneurysmal dilation of the abdominal aorta.
3.5. Treatment approach
Table 2 includes details of the treatments used. Ten (39%) patients had previously failed glucocorticoids. Among cases with information about prior glucocorticoid dosages, the glucocorticoid courses consisted of at least 20 mg of prednisone (range 20–80 mg/day), with slow tapers over at least one month (Supplemental Table 1). In 9 of these 10 patients, the disease recurred either during tapering or following discontinuation of glucocorticoids. In one patient, poorly controlled diabetes unrelated to IgG4-RD (e.g., no overt pancreatic disease) but induced by glucocortocoids prohibited the continued use of that treatment.
Table 2.
Treatment approach.

RTX was used as monotherapy in the majority of patients (n = 19, 73%) and in combination with glucocorticoids in 7 patients (27%). Among patients who received concurrent prednisone and rituximab, the dose range was 10–40 mg, with a median dose of 20 mg (Supplemental Table 1). Ten patients (39%) required ureteral stents or percutaneous nephrostomy tubes to relieve hydronephrosis caused by ureteral compression.
3.6. Treatment response
Table 3 describes the response to treatment in this cohort. Of the 19 patients with baseline symptoms (e.g., pain), all (100%) had improvement. Of the 25 patients with follow-up imaging, 14 had repeat imaging 3 to 6 months after RTX (median 109 days, IQR 86–125 days).,. Of these, 12 patients had images available for review. Eight had a decrease of ≥ 10% (range: 10%–56%) in the size of the lesion (Figs. 1 and 2), 3 had a decrease between 3% and 9%, and one had an increase of 16%. Among these patients, the average reduction in the size of the lesion was 16% (±19%). Two patients with outside imaging 3 to 6 months after RTX had significant improvement in the size of the lesion, although measurements of the lesion before and after treatment were unavailable. Thus, among those with follow-up imaging 3 to 6 months after RTX, 13 (93%) had either improvement or stability in the size of the lesion. Twenty-one (84%) of the 25 patients with imaging at any time after the first RTX infusion had radiologic improvement; 3 patients had stability in their disease and one, as above, had worsening. Overall, the mean reduction in size in 2-dimensions was 23% (±21%).
Table 3.
Treatment response.

Figure 1.
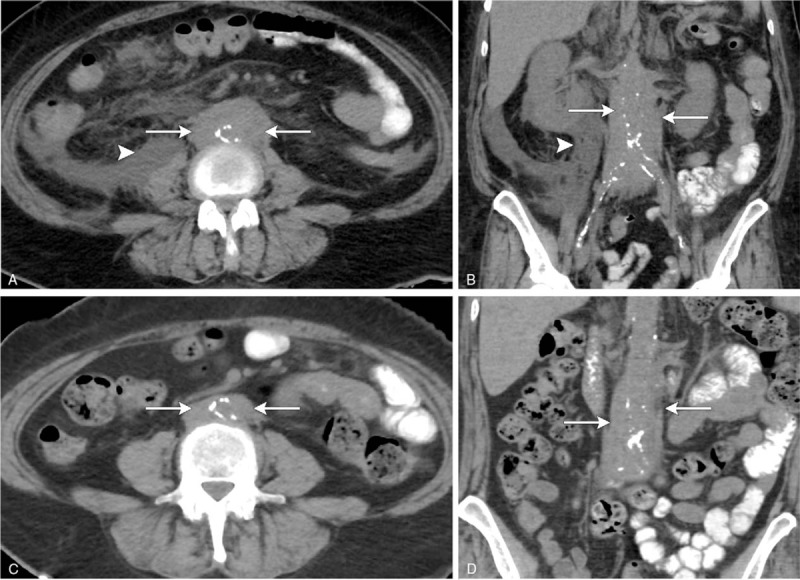
(A–D) A 56-year-old woman with retroperitoneal fibrosis and bilateral hydronephrosis presenting to the emergency room with right flank pain. Axial (A) and coronal (B) nonenhanced CT scans the abdomen demonstrates circumferential soft tissue surrounding the infrarenal abdominal aorta, extending to the level of the common iliac arteries (white arrows). There is retroperitoneal fluid present in the right perirenal fascia, likely secondary to a ruptured renal calyx (white arrows). Axial (C) and coronal (D) nonenhanced CT scans the abdomen following treatment in the same patient shows significant decrease in periaortic soft tissue (white arrows) and resolution of retroperitoneal fluid following nephrostomy drainage. CT = computed tomography.
Figure 2.
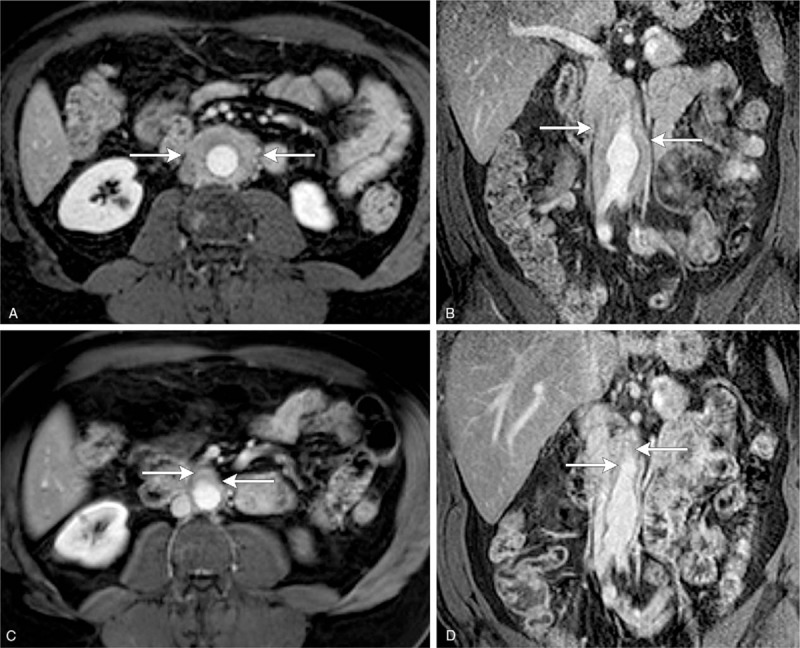
(A–D) A 56-year-old man with retroperitoneal fibrosis involving the infrarenal aorta extending into the common iliac arteries. Axial (A) and coronal T1-weighted gadolinium-enhanced MRI images of the upper abdomen demonstrate circumferential enhancing soft tissue surrounding the abdominal aorta (white arrows). Post-treatment axial (C) and coronal (D) T1-weighted gadolinium enhanced MRI images show significant decrease in periaortic soft tissue following rituximab treatment (white arrows).
Of the 10 patients with stent(s) or percutaneous nephrostomy tube(s), 4 (40%) had the stent(s) or tube(s) removed. All 4 patients in whom the stent(s) or tube(s) were removed successfully were treated with rituximab monotherapy. All patients who became stent- or nephrostomy tube-free had their stent(s) or tube(s) for <1 year prior to RTX. In contrast, 4 of the 6 patients whose stents or percutaneous nephrostomy tubes were not removed had disease duration for >1 year prior to RTX.
Of those with an elevated ESR at baseline (n = 16), 4 (25%) had normalization of their ESR 3 to 6 months after RTX, 3 (19%) remained elevated, and 9 (56%) did not have follow-up levels drawn within 3 to 6 months of RTX. Of those with an elevated CRP at baseline (n = 10), 2 (20%) had a normalization of their CRP 3 to 6 months after RTX, 4 (40%) remained high and 4 (40%) did not have follow-up levels drawn within 3 to 6 months of RTX. Of those with an elevated serum IgG4 concentration at baseline (n = 7), 1 (13%) had a normalization of their serum IgG4 concentration 3 to 6 months after RTX, 4 (50%) remained elevated and 3 (38%) did not have follow-up levels drawn within 3 to 6 months of RTX.
3.7. RTX monotherapy treatment response
Among those who received RTX without concomitant glucocorticoids (n = 19, n = 15 with any follow-up radiology, n = 12 with imaging available for review), all (n = 19, 100%) had symptomatic improvement and eleven (92%) had radiologic improvement (3 had >25% size reduction). Although the numbers of patients with baseline and follow-up measurements of ESR, CRP, and serum IgG4 concentrations were too small for meaningful analysis, similar trends to those seen in the entire RTX-treated cohort were observed.
3.8. Adverse events
Three patients (12%) had possible infusion reactions, all of which were managed conservatively and none of which interfered with the completion of treatment. Three patients (12%) had 4 severe infections requiring intravenous antibiotics or hospitalization within 6 months of RTX. One patient, who was also on glucocorticoids at the time, developed a cutaneous herpes zoster infection 3 months after RTX; this resolved with antiviral therapy. A second patient, with end-stage renal disease on dialysis and insulin-dependent diabetes, was hospitalized and successfully treated for multifocal pneumonia and Clostridium difficile colitis 2 months after RTX. Of note, this patient's end-stage renal disease developed following the diagnosis of RPF and was attributable to AKI in the setting of infection and hemorrhage. The third patient required intravenous antibiotics on 2 occasions for complicated urinary tract infections; the first episode occurred while the patient was also on glucocorticoids.
4. Discussion
The findings of this retrospective study of patients with idiopathic or IgG4-related RPF suggest that peripheral B cell depletion with RTX is a well tolerated and effective intervention. In contrast to prior studies evaluating the efficacy of other potential glucocorticoid-sparing agents in RPF,[10,13,14] RTX was used as monotherapy in the majority of patients in this study. These results have implications for the management of RPF, a disease in which glucocorticoid-sparing approaches are particularly appealing given that this older patient population is at increased risk for glucocorticoid toxicities (i.e., osteoporosis, diabetes).
In this cohort, the median decrease in the size of the lesion following rituximab was 23% which is somewhat less than the 50% median reduction observed in a randomized controlled trial comparing prednisone to tamoxifen for remission maintenance following a month of high dose steroids, conducted by Vaglio et al.[10] However, important differences exist between that study and ours. Nearly 40% of our cohort had relapsed during or following a glucocorticoid taper, suggesting that our cohort may have had more severe disease. In addition, all patients in the trial by Vaglio et al, as well as in studies evaluating the efficacy of mycophenolate mofetil and methotrexate, were treated with high dose glucocorticoids.[10,13,14] Moreover, because our study was retrospective, the timing of repeat imaging was nonuniform whereas all patients had protocolized reassessments in the prospective trials.[10,13,14] Despite these differences, all patients in our study had symptomatic responses to treatment, as in studies of glucocorticoids with tamoxifen[10] and mycophenolate mofetil.[13] The majority of patients in our study also had radiologic improvement. Future prospective studies might compare RTX and glucocorticoids in a head-to-head comparison.
There was significant variability in the extent of radiologic improvement and the ability to remove stents/tubes following RTX. This did not appear to be related to glucocorticoid use, since those with the greatest reduction in radiologic dimensions and those in whom stents or tubes could be removed were treated with RTX monotherapy. We found that disease duration prior to RTX was shorter (generally less than one year) among patients whose stents or tubes were removed, suggesting that disease duration may impact response to treatment. Another factor affecting the variability in the response to therapy may have been the variable timing of follow-up imaging. Future prospective trials with standardized follow-up imaging and proscribed attempts at stent or tube removal are necessary.
There were several striking features of our cohort. First, we confirmed prior observations that RPF patients are often current or former smokers. In our cohort, 85% of patients had tobacco exposure, a figure strikingly similar to that reported by Goldoni et al[24] (84%). Future studies might investigate how tobacco exposure could contribute to the development of RPF and whether smokers and nonsmokers respond differently to treatment. Second, in contrast to prior studies, the CRP and serum IgG4 concentrations were normal in most patients at baseline. This may reflect the fact that 50% of our cohort had prior exposure to glucocorticoids. However, this observation highlights that alternative methods of assessing treatment response, such as imaging or novel biomarkers, are required in this patient population. Of note, similar observations have been made in general IgG4-RD cohorts in which approximately 30% of patients have a normal serum IgG4 concentration and the majority have a normal CRP.[7,8] The percentage of IgG4-related RPF patients who elevated acute phase reactants or elevated serum IgG4 concentrations appears to be substantially lower than the percentage of patients with IgG4-RD and other organ involvement.
We included both idiopathic and IgG4-related RPF subsets in this study because the distinction between these conditions remains poorly defined in both the literature and clinical practice.[6,10] The diagnosis of IgG4-RD can be difficult to establish when RPF is the sole disease manifestation for several reasons. The peri-aortic location of the lesion often makes it less amenable to minimally invasive biopsy approaches. Moreover, when biopsy samples are obtained, they are often small and it is difficult to identify specific features of IgG4-RD (e.g., storiform fibrosis, obliterative phlebitis) in the small and often highly fibrotic tissue.[8] The low number of idiopathic RPF cases limited a comparison of treatment response between idiopathic and IgG4-related RPF.
Our study has many strengths. First, the majority of patients were treated with RTX monotherapy and received no additional glucocorticoids except a standard dose of methylprednisolone to minimize the risk of infusion reactions. Second, this is the largest number of patients with RPF treated with RTX in the literature. Two our knowledge, only 2 RTX-treated cases have been reported previously.[25] Third, a single radiologist, blinded to the symptomatic response, reviewed all available radiology to measure the radiographic response to treatment. Despite these strengths, there are also limitations. This was a retrospective study and, as such, the collection of data was not uniform which may impact the interpretation of the radiographic response to treatment and changes in certain laboratory measures following treatment. The retrospective nature of the study may also underestimate the frequency with which patients with ureteral stents or nephrostomy tubes can discontinue these devices, since there was not a protocolized schedule for attempts at device removal.
In conclusion, we found that RTX was effective in the vast majority of patients with RPF in terms of symptom improvement and diminution in the size of the RPF lesions. RTX was administered without glucocorticoids in the majority of patients and was well-tolerated. A prospective study comparing a B cell depletion strategy to glucocorticoids is an important next step.
Author contributions
Conceptualization: Zachary S Wallace, Amita Sharma, John H Stone.
Data curation: Rachel Wallwork, Zachary S Wallace, John H Stone.
Formal analysis: Rachel Wallwork, Zachary S Wallace, Amita Sharma, John H Stone.
Investigation: Rachel Wallwork, Zachary S Wallace, Cory A Perugino, Amita Sharma, John H Stone.
Methodology: Zachary S Wallace, Amita Sharma, John H Stone.
Project administration: Zachary S Wallace, Amita Sharma, John H Stone.
Resources: John H Stone.
Software: Zachary S Wallace.
Supervision: Zachary S Wallace, John H Stone.
Validation: Zachary S Wallace, Cory A Perugino, Amita Sharma, John H Stone.
Visualization: Amita Sharma.
Writing – original draft: Rachel Wallwork, Zachary S Wallace.
Writing – review & editing: Rachel Wallwork, Zachary S Wallace, Cory A Perugino, Amita Sharma, John H Stone.
Supplementary Material
Footnotes
Abbreviations: CRP = C-reactive protein, CT = computed tomography, ESR = erythrocyte sedimentation rate, IgG4-RD = IgG4-related disease, IQR = interquartile range, MRI = magnetic resonance imaging, RPF = retroperitoneal fibrosis, RTX = rituximab.
RW and ZSW are the co-first authors.
Funding: Dr ZSW received funding in the form of a Scientist Development Award from the Rheumatology Research Foundation, Fund for Medical Discovery from the Executive Committee on Research at Massachusetts General Hospital, as well as an NIH Loan Repayment Award. Dr Stone has previously received funding from Genentech for the study of rituximab in ANCA-associated vasculitis and IgG4-related disease.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet 2006;367:241–51. [DOI] [PubMed] [Google Scholar]
- [2].Scheel PJ, Jr, Feeley N. Retroperitoneal fibrosis: the clinical, laboratory, and radiographic presentation. Medicine 2009;88:202–7. [DOI] [PubMed] [Google Scholar]
- [3].Yachoui R, Sehgal R, Carmichael B. Idiopathic retroperitoneal fibrosis: clinicopathologic features and outcome analysis. Clin Rheumatol 2016;35:401–7. [DOI] [PubMed] [Google Scholar]
- [4].Vaglio A, Maritati F. Idiopathic retroperitoneal fibrosis. J Am Soc Nephrol 2016;ASN-2015101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Uibu T, Oksa P, Auvinen A, et al. Asbestos exposure as a risk factor for retroperitoneal fibrosis. Lancet 2004;363:1422–6. [DOI] [PubMed] [Google Scholar]
- [6].Khosroshahi A, Carruthers MN, Stone JH, et al. Rethinking Ormond's disease: “idiopathic” retroperitoneal fibrosis in the era of IgG4-related disease. Medicine 2013;92:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012;366:539–51. [DOI] [PubMed] [Google Scholar]
- [8].Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol 2015;67:2466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zen Y, Onodera M, Inoeu D, et al. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol 2009;33:1833–9. [DOI] [PubMed] [Google Scholar]
- [10].Vaglio A, Palmisano A, Alberici F, et al. Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomized controlled trial. Lancet 2011;378:338–46. [DOI] [PubMed] [Google Scholar]
- [11].van Bommel EF, Siemes C, Hak LE, et al. Long-term renal and patient outcome in idiopathic retroperitoneal fibrosis treated with prednisone. Am J Kidney Dis 2007;49:615–25. [DOI] [PubMed] [Google Scholar]
- [12].van der Bilt FE, Hendriksz TR, van der Meijden WA, et al. Outcome in patients with idiopathic retroperitoneal fibrosis treated with corticosteroid or tamoxifen monotherapy. Clin Kidney J 2016;9:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scheel PJ, Feeley N, Sozio SM. Combined prednisone and mycophenolate mofetil treatment for retroperitoneal fibrosis: a case series. Ann Int Med 2011;154:31–6. [DOI] [PubMed] [Google Scholar]
- [14].Alberici F, Palmisano A, et al. Methotrexate plus prednisone in patients with relapsing idiopathic retroperitoneal fibrosis. Ann Rheum Dis 2013;72:1584–6. [DOI] [PubMed] [Google Scholar]
- [15].Carruthers MN, Topazian MD, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 2015;74:1171–7. [DOI] [PubMed] [Google Scholar]
- [16].Wallace ZS, Mattoo H, Mahajan VS, et al. Predictors of disease relapse in IgG4-related disease following rituximab. Rheumatology 2016;55:1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet 2015;385:1460–71. [DOI] [PubMed] [Google Scholar]
- [18].Khosroshahi A, Carruthers M, Deshpande V, et al. Rituximab for the treatment of IgG4-related disease: lessons from ten consecutive patients. Medicine (Baltimore) 2012;91:57–66. [DOI] [PubMed] [Google Scholar]
- [19].KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012;2: doi:10.1038/kisup.2012.2. [Google Scholar]
- [20].Perugino CA, Wallace ZS, et al. Large vessel involvement by IgG4-related disease. Medicine 2016;95:e3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Deshpande V, Khosroshahi A. Diagnostic guidelines for IgG4-related disease with a focus on histopathological criteria. Diag Histopathol 2013;19:119–27. [Google Scholar]
- [22].Umehara H, Okazaki K, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21–30. [DOI] [PubMed] [Google Scholar]
- [23].Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goldoni M, Bonini S, Urban ML, et al. Asbestos and smoking as risk factors for idiopathic retroperitoneal fibrosis: A case-control study. Ann Intern Med 2014;161:181–8. [DOI] [PubMed] [Google Scholar]
- [25].Maritati F, Corradi D, et al. Rituximab therapy for chronic periaortitis. Ann Rheum Dis 2012;71:1262–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


