Abstract
Rationale
Botulinum toxin A (BTX-A) injection is effective in treating focal dystonia. However, there are no prior reports regarding the treatment of progressive focal dystonia by a single BTX-A injection that affect a distant muscle.
Patient concerns
A 19-year-old male was referred to the rehabilitation clinic with a complaint of involuntary movement in his left big toe. The involuntary movement pattern was initially observed in the abduction direction only; however, it progressed to irregular mixed patterns in the flexion and abduction directions.
Diagnoses
In needle electromyography, abnormal dystonic patterns were observed in the left abductor hallucis (AH), flexor hallucis longus, and flexor hallucis brevis muscles.
Interventions and outcomes
These symptoms resolved with a single BTX-A injection to the AH muscle.
Lessons
In this case, a single BTX-A injection to 1 muscle for treating progressive focal dystonia was effective on a distant noninjected muscle.
Keywords: botulinum toxin A, distant effect, progressive focal dystonia, single injection
1. Introduction
Dystonia is a neurological disorder characterized by involuntary and repetitive movements or by abnormal postures. Dystonia is an idiopathic process caused by genetics or by environmental factors such as trauma, infection, or reaction to pharmaceutical drugs.[1,2] Focal dystonia can affect any part of the body. The most common site of involvement is the face or cervical region.[3,4] However, focal dystonia involved in the lower extremities is rare.
Botulinum toxin A (BTX-A) injection is safe and effective in treating focal dystonia.[1,5] However, in clinical practice, it is difficult to inject BTX-A to each of the affected muscles due to the pain caused by multiple injections and because of technical difficulties that prevent injections on precise targets, especially in small muscles. Accordingly, BTX-A injection to multiple muscles may be cost-prohibitive and could cause adverse effects when the affected muscles are small and adjacent, such as intrinsic foot muscles.
We experienced an interesting case of focal dystonia that progressed from the abductor halluces (AHs) muscle to the flexor halluces longus (FHL) and flexor halluces brevis (FHB) muscles and effectively treated progressive focal dystonia involving multiple muscles by a single BTX-A injection to the AH muscle.
2. Method
Informed consent was obtained from the patient, and this report was approved by the Institutional Review Board of Hanyang University Guri Hospital, Guri, Korea.
2.1. Case presentation
A 19-year-old male was referred to the rehabilitation clinic with a complaint of involuntary movement in his left big toe. Two months before referral to the rehabilitation clinic, the patient underwent a Brostrom operation on his left ankle for chronic instability of the lateral ankle. Repetitive and involuntary movements of the left big toe occurred 2 weeks after surgery. The patient was then admitted to the department of neurology 2 weeks before referral to the rehabilitation clinic. The patient was taking oral clonazepam and baclofen as prescribed by the department of neurology but was showing no improvement. There was no prior history of trauma or suspicious signs of infection. The patient did not take medicine that might cause dystonia before the symptoms began. There was no personal or family history of neurologic disorder.
Upon physical examination, there were no motor or sensory abnormalities on the left lower extremity, and the deep tendon reflexes were normoactive and symmetric. Also, there were no signs of upper motor neurons. In the early phase of focal dystonia, involuntary movement pattern was observed only in the abduction direction of the left big toe. The symptom worsened in intensity and frequency within 1 month. The pattern of focal dystonia progressed to the flexion direction as well as to abduction of the big toe. At rest, the left big toe moved involuntarily, with irregular mixed patterns of flexion and abduction. The dystonia pattern was not associated with postural changes. The patient had a gait disturbance due to focal dystonia in the left big toe.
A simple X-ray and magnetic resonance imaging on the left ankle were obtained, and the results were normal except for postoperative changes in the soft tissue of the lateral malleolar region. An electrodiagnostic test was also performed and demonstrated abnormal dystonic patterns in the left AH (Fig. 1) and in the FHL and FHB muscles. The patient received physical therapy for 1 month and continued to take oral clonazepam and baclofen. However, the symptoms did not improve.
Figure 1.
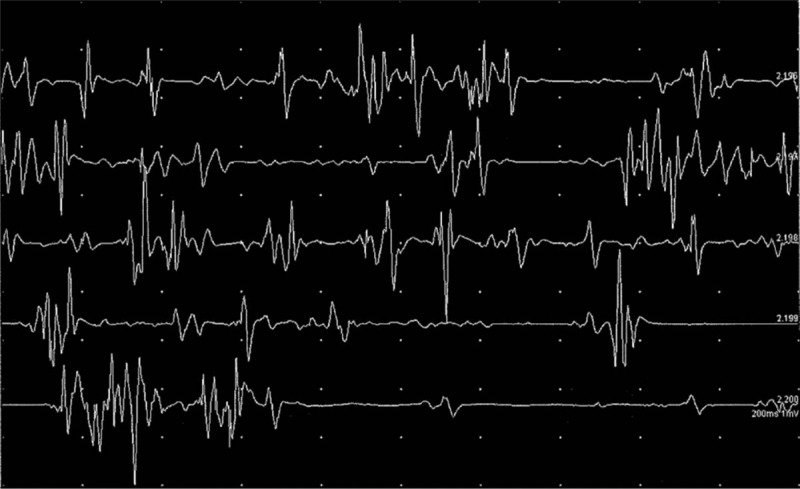
The results of needle electromyography in the left AH muscle demonstrated dystonic patterns at rest.
The BTX-A injection was considered an alternative treatment. However, it would be difficult to inject BTX-A to each of the AH, FHL, and FHB muscles due to the pain caused by multiple injections. There was also a technical problem that made it difficult to inject on precise targets because the AH and FHB muscles are small and adjacent to each other. Based on the progression pattern in the focal dystonia, we assumed that the origin of the focal dystonia was the AH muscle. For this reason, the target of the single BTX-A injection was determined to be the AH muscle. Twenty-five units of BTX-A mixed with 0.25 mL saline were injected to the mid-belly of the left AH muscle under electromyographic and ultrasonographic guidance. After injection, we confirmed that there was no leakage of injected fluid outside of the AH muscle using ultrasonography. One week after injection, the focal dystonia was completely resolved not only in abduction, but also in flexion of the left big toe. At the 6-month follow-up, there was no recurrence of dystonia or other complications in the patient.
3. Discussion
This case involved focal dystonia that progressed from the AH muscle to the FHL and FHB muscles. Although focal dystonia in the foot was reported, the majority of these types of cases of dystonia involved the extensor hallucis muscle rather than the AH muscle. Rosenberg et al reported deformity and dystonia of the AH muscle; however, it was limited to the AH muscle.[6] To the best of our knowledge, no case of dystonia that progressed from the AH muscle to the FHL and FHB muscles has been reported.
The BTX-A injection is the first line treatment of focal dystonia despite some adverse effects.[1,7] In the clinical setting, BTX-A is usually injected under ultrasonographic or electromyographic guidance. However, in an injection to the small and deep muscles in the hand or foot, it is a difficult procedure even with ultrasonographic or electromyographic guidance. An inaccurate injection or local diffusion of BTX-A can cause unexpected paralysis of the adjacent muscles. In this case, however, there were no adverse effects from the BTX-A injection. The patient's symptoms were completely resolved with a single injection.
The exact neurophysiologic mechanism of BTX-A effects on noninjected muscles is unknown. Ramirez-Castaneda et al reported local and systemic distribution of BTX depending on the spread, diffusion, and migration.[8] Spread refers to the physical movement of the toxin and is dependent on injection technique, volume, needle size, and other physical factors. In this case, we confirmed that injection fluid entered the target muscle without any leakage using ultrasonography and an electromyography-guided injection technique. Also, the volume of injection was small. Therefore, we did not expect spread to be the major mechanism of the BTX-A effect on the noninjected muscle.
Diffusion refers to more of a microscopic phenomenon in which a soluble molecule is dispersed by a passive transport. Shaari et al demonstrated that BTX can penetrate the muscle fascia.[9] It is reasonable to assume that the effect of BTX on the noninjected adjacent muscle was due to diffusion of the unbound toxin through extracellular space. The diffusion was driven by the concentration gradient and the dynamics of the injection. Therefore, the dose of BTX is an important factor in determining the extent of local diffusion. Although a small dose of BTX-A was injected in this case, the effects of BTX-A on the FHB muscle might have been induced by the diffusion mechanism. Nevertheless, it is unlikely that the effects of BTX-A on the FHL muscle were caused by local diffusion only, because the FHL muscle is relatively far from the AH muscle.
Migration of BTX can occur via hematogenous or neuroaxonal transport. The effects of BTX to the distal muscle can be explained by migration of the toxin via blood vessels. Also, peripheral BTX injection can affect the central circuits via retrograde transport and transcytosis.[8] According to this mechanism, BTX can spread to distant sites. Antonucci et al demonstrated that BTX-A can be retrogradely transported by central neurons and motoneurons and can be then transcytosed to afferent synapses.[10] Restani et al demonstrated that BTX-A can undergo anterograde axonal transport and transcytosis.[11] Also, Giladi proposed that BTX-A acts on focal dystonia through a dual effect on efferent and afferent pathways.[12] Furthermore, many studies reported the role of BTX regarding anti-nociceptive action by inhibiting the release of several neurotransmitters.[13–16] According to these previous studies, BTX can prevent vesicular-mediated release of neurotransmitters from the central nervous system and thus can decrease sensitized nociception.[13–16] These findings suggest that BTX can be axoplasmically transported centrally. As such, the distant BTX-A effects on the noninjected FHL muscle might be explained by migration mechanisms.
In this case, the symptoms completely resolved in the noninjected muscles by a single BTX-A injection. Erdal et al reported significant reduction in the quantitative electromyographic parameters of the noninjected muscle after long-term BTX treatments.[17] However, in that study, there were no significant changes in electromyographic activity of the noninjected muscle after the first BTX treatment. The cause of these findings is unknown. Erdal et al hypothesized that the long-term electromyographic changes in the noninjected muscle might be due to the downregulating activity of the noninjected muscle when the injected muscle was denervated by continuous BTX injections.[17] On the contrary, Wohlfarth et al demonstrated that slight F-wave changes in remote muscles occurred 1 week after the first injection of BTX.[18] Decreased excitability of alpha-motoneurons supplying noninjected muscles was considered as the cause after a single BTX injection.[18] Although further research is needed to determine the difference between the effects of a single injection as opposed to multiple injections, our case suggests that a single BTX-A injection can induce sufficient improvement of symptoms.
Focal dystonia treated with BTX-A injection was reported in previous studies. However, there is no report on focal dystonia that progressed from the AH muscle to the FHL and FHB muscles. In addition, in this case, the focal dystonia involved multiple muscles and was treated with a single BTX-A injection to 1 muscle. To the best of our knowledge, our case is the first to report these particular findings. Although further studies are needed to confirm the exact mechanism of BTX-A effects on noninjected muscles, we found that a single BTX-A injection to 1 muscle was effective on distant noninjected muscles. Our findings provide a promising new treatment strategy of focal dystonia involving multiple muscles in a clinical setting.
Author contributions
Investigation: Sung Young Lee, Hyeok Dong Lee, Young-Shin Cho, Seung Hoon Han.
Supervision: Seung Hoon Han.
Writing – original draft: Sung Young Lee, Hyeok Dong Lee, Young-Shin Cho.
Writing – review & editing: Sung Young Lee.
Footnotes
Abbreviations: AH = abductor hallucis, BTX-A = botulinum toxin A, FHB = flexor hallucis brevis, FHL = flexor hallucis longus.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Truong D. Botulinum toxins in the treatment of primary focal dystonias. J Neurol Sci 2012;316:9–14. [DOI] [PubMed] [Google Scholar]
- [2].Pont-Sunyer C, Martí MJ, Tolosa E. Focal limb dystonia. Eur J Neurol 2010;17(s1):22–7. [DOI] [PubMed] [Google Scholar]
- [3].Steeves TD, Day L, Dykeman J, et al. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord 2012;27:1789–96. [DOI] [PubMed] [Google Scholar]
- [4].Zoons E, Dijkgraaf MGW, Dijk JM, et al. Botulinum toxin as treatment for focal dystonia: a systematic review of the pharmaco-therapeutic and pharmaco-economic value. J Neurol 2012;259:2519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Klein AW. Contraindications and complications with the use of botulinum toxin. Clin Dermatol 2004;22:66–75. [DOI] [PubMed] [Google Scholar]
- [6].Rosenberg NS, Odderson IR. Diagnosis and treatment of abductor hallucis focal dystonia with botulinum toxin injection: a case presentation. PM R 2013;5:726–8. [DOI] [PubMed] [Google Scholar]
- [7].Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol 2011;18:5–18. [DOI] [PubMed] [Google Scholar]
- [8].Ramirez-Castaneda J, Jankovic J, Comella C, et al. Diffusion, spread, and migration of botulinum toxin. Mov Disord 2013;28:1775–83. [DOI] [PubMed] [Google Scholar]
- [9].Shaari CM, George E, Wu BL, et al. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope 1991;101:960–4. [DOI] [PubMed] [Google Scholar]
- [10].Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci 2008;28:3689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Restani L, Antonucci F, Gianfranceschi L, et al. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J Neurosci 2011;31:15650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giladi N. The mechanism of action of botulinum toxin type A in focal dystonia is most probably through its dual effect on efferent (motor) and afferent pathways at the injected site. J Neurol Sci 1997;152:132–5. [DOI] [PubMed] [Google Scholar]
- [13].Francisco GE, Tan H, Green M. Do botulinum toxins have a role in the management of neuropathic pain?: a focused review. Am J Phys Med Rehabil 2012;91:899–909. [DOI] [PubMed] [Google Scholar]
- [14].Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003;43(s1):9–15. [DOI] [PubMed] [Google Scholar]
- [15].Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005;26:785–93. [DOI] [PubMed] [Google Scholar]
- [16].Bach-Rojecky L, Šalković-Petrišić M, Lacković Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injection. Eur J Pharmacol 2010;633:10–4. [DOI] [PubMed] [Google Scholar]
- [17].Erdal J, Østergaard L, Fuglsang-Frederiksen A, et al. Long-term botulinum toxin treatment of cervical dystonia – EMG changes in injected and noninjected muscles. Clin Neurophysiol 1999;110:1650–4. [DOI] [PubMed] [Google Scholar]
- [18].Wohlfarth K, Schubert M, Rothe B, et al. Remote F-wave changes after local botulinum toxin application. Clin Neurophysiol 2001;112:636–40. [DOI] [PubMed] [Google Scholar]


