Supplemental Digital Content is available in the text
Keywords: ACE2, enzymatic activity, essential hypertension, gender, single nucleotide polymorphisms
Abstract
Angiotensin-converting enzyme 2 (ACE2) plays an important role in the development of essential hypertension (EH). The aim of this study was to investigate the relationship of ACE2 gene polymorphisms and enzymatic activity with EH in the northeastern Chinese Han population. 34 single-nucleotide polymorphism (SNP) loci of ACE2 were detected in 1024 EH patients and 956 normotensive (NT) controls by Sequenom Mass-ARRAY RS1000. Five SNPs (rs1514283, rs4646155, rs4646176, rs2285666, and rs879922) in ACE2 gene were determined to significantly associate with EH in female participants, while no SNP locus was linked to male group. Specifically, it was the first time to report that rs4646155 was significantly associated with EH in females. Furthermore, the correlation between ACE2 activity and clinical parameters were performed by Pearson correlation analysis in EH patients. We found that the ACE2 activity level was negatively correlated with body mass index (BMI), DBP, and pulse pressure, and significantly positively with ACE2 concentration, blood glucose and estrogen level in female EH patients. These results demonstrated that the genetic variants of ACE2 played vital roles in the development of EH. And the serum ACE2 activity can predict the development of cardiac dysfunction in EH patients.
1. Introduction
Essential hypertension (EH) is a major risk factor for myocardial infarction, stroke and renal failure.[1] The prevalence of EH is increasing rapidly and it is estimated that the morbidity of EH will reach 29% of the global population by 2025.[2] As a major threat to human health worldwide, the incidence of EH in China has been an upward trend in the past several years. EH is a polygenetic and complex disease, caused by the interactions of genetic and environmental factors. Around 30% of the individual variability is considered to be genetically determined. A lot of studies have been performed to investigate the genetic variation in genes of renin–angiotensin system (RAS), especially the angiotensin-converting enzyme 2 (ACE2).
The RAS plays a vital role in the occurrence and development of EH. ACE2 is a key regulator in the RAS, which could convert Ang I to Ang 1 to 9 and degrade Ang II to the Ang 1 to 7.[3] ACE2 locates on the X chromosome in human genome. It encodes a protein of 805 amino acids, including 18 exons. In humans, ACE2 mainly expresses in the cardiovascular, renal and gastrointestinal tissues.[4] Moreover, ACE2 also has been found in the brain, lung and testis.[5] It has been mapped to a defined quantitative trait locus (QTL) on the X chromosome in human.[1]
The single nucleotide polymorphisms (SNPs) of ACE2 play an important role in cardiovascular diseases. Up to now, several studies focused on the association of ACE2 SNPs with EH.[6–10] But some of these results were inconsistent or even contradictory. For example, Fan et al reported that the TT genotype frequency of rs2106809 was significantly higher in female hypertensive patients than age- and gender-matched normotensive subjects.[6] Nevertheless, other results showed the rs2106809 was no significant deviation in either hypertensive or normotensive women in Chinese Han population.[11,12] The possible reasons may be attributed to that the observed population have different ethnicity and race, and come from regions with different natural environment. Therefore, to more precisely elucidate the role of ACE2 SNPs in EH, further research is necessary to determine a wider range of ACE2 polymorphic loci associated with EH in population who come from different regions and ethnic groups. In addition, few studies reported the serum ACE2 activity level between EH patients and healthy individuals, and the relation between the serum ACE2 activity and EH also need to be uncovered. In this study, the association between ACE2 polymorphism and EH was investigated in a large number of Han population of the northeast Chinese. The serum ACE2 activity was also determined to analyze its relation with clinical baseline characteristics and biochemical parameters in EH patients.
2. Methods
2.1. Study population
The studied cohort included 1024 (male, 510; female, 514) EH patients and 956 (male, 296; female, 660) NT controls. A case-control study was performed, in which all of the participants were Han Chinese from the Lan Xi of Heilongjiang Province in China. The EH patients were recruited from 2012 to 2015, in accordance with the Guidelines for Prevention and Treatment of Hypertension in China, and needed to meet the following criteria:
-
(1)
all subjects had no consanguinity at enrolment;
-
(2)
aged < 60;
-
(3)
systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 100 mmHg;
-
(4)
absence of secondary causes of hypertension based on extensive biochemical and clinical examinations; and
-
(5)
absence of pharmacological treatment for hypertension.
The NT subjects were screened from a physical examination and matched for age and ethnicity with the EH patients from the same area. The inclusion criteria for the NT subjects were as follows: age ≥ 40 and mean SBP < 120 mmHg and mean DBP < 80 mmHg. The study was approved by the Ethics Committee of Qiqihar Medical University, China. Written informed consent was obtained from each participant or their legal guardian. Blood pressure was measured 3 times using a mercury sphygmomanometer with patient in a seated position by a standard auscultatory method. The average measured value of blood pressure including systolic and diastolic pressure levels was used for quantitative analyses. Body mass index (BMI) was calculated as follows: BMI = Weight (kg)/[Height (m) × Height (m)].
2.2. DNA extraction
Four millilitres of peripheral blood was collected in an EDTA tube from each subject for DNA extraction. Then genomic DNA was extracted from leukocytes using CWBIO blood genomic DNA mini kit (CWBIO, Beijing China). Finally, the DNA specimens were stored at −20 °C.
2.3. SNP selection and genotyping assay
The SNPs were selected based on the SNP genotype information from the GenBank database. Equidistant SNPs in the ACE2 gene regions were identified as common SNPs in our study. These SNPs were chosen based on a minor allele frequency (MAF) of at least 1%. Multiplexed SNP Mass EXTEND assay was designed using the Sequenom Mass ARRAY Assay Design 4.0 software (Agena Bioscience, San Diego, CA).[13] SNP genotyping was performed using the Sequenom Mass ARRAY RS1000 platform following the manufacturer's protocols. The corresponding primers included in this project are listed in Supplementary table S1. Sequenom Typer 4.0 software was used for data analyses.[13]
2.4. Plasma ACE2 measurement
The plasma ACE2 concentration was determined by Human Angiotensin Converting Enzyme 2 (ACE2) Enzyme Immunoassay Kit (MLBIO, Shanghai China) according to the manufacturer's instructions. ACE2 enzymatic activity was determined by a spectrophotometric mono-reagent assay. The serum (50 μL) and the substrate solution (50 μL) were reacted at 37°C for 60 minutes in ACE2 activity enzyme-linked immunosorbent assay (ELISA) specific plate reader (MLBIO, Shanghai China) and the optical density value was monitored at 450 nm. The serum ACE2 activity was expressed as unit/L.
2.5. Statistical analysis
All of the database management and statistical calculations were performed with SPSS v.20.0 software (PASW Statistics, SPSS Inc., Chicago, IL). Continuous variables were shown as mean ± SD. Anthropometric indices and genotype/allele frequencies were compared between the EH and NT subjects using chi-squared (χ2) tests. Hardy–Weinberg equilibrium was tested by Fisher's exact test (P > .05) using gene frequencies of the healthy individuals. For each odds ratio (OR), we calculated the 2-tailed probability value and 95% confidence interval (CI). A 2-tailed P < .05 was accepted as statistically significant. Haplotype distribution was analysed using the SHEsis software (http://analysis.bio-x.cn/SHEsisMain.htm).[14,15] Pearson correlation analysis was used to calculate the correlation between ACE2 concentration and enzymatic activity and the clinic data in all subjects.
3. Results
3.1. Baseline characteristics
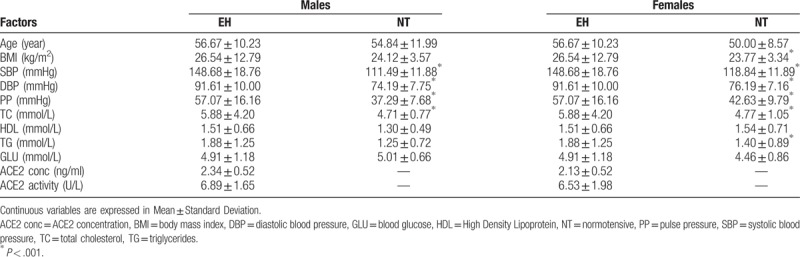
The clinical characteristics in formation of the EH patients and NT controls are presented in Table 1. The significantly higher levels of SBP, DBP, pulse pressure, and total cholesterol were found in EH patients, as compared to controls in both male and female groups (all P < .001). As for BMI and triglycerides levels, the significant deviations between the 2 groups were determined only in females. Hardy–Weinberg equilibrium for ACE2 gene was tested only in control group, and was consistent with the expected result.
Table 1.
Clinical characteristics of the study population (mean ± SD).
3.2. ACE2 gene polymorphisms
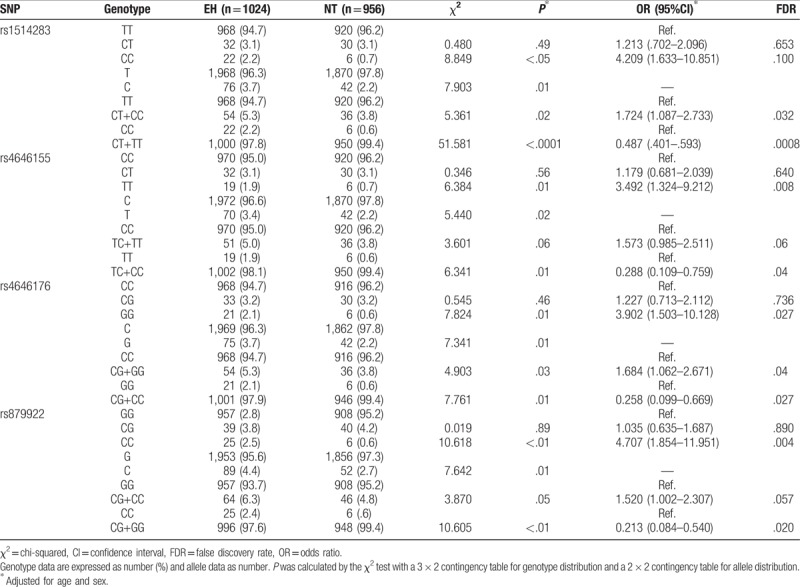
The genotype and allele frequencies at SNP loci rs1514283, rs4646155, rs4646176, and rs879922 in ACE2 are shown in Table 2. At the rs1514283 locus, the frequencies of the CC genotype and C allele were significantly higher in the EH patients than in the NT controls (CC: 2.2% vs 0.7%, P < .05, OR = 4.209, 95% CI 1.633–10.851; C: 3.7% vs 2.2%, P = .01). A significant association was found between the rs1514283 variant and EH risk under the dominant genetic model (TT vs CC+CT: OR = 1.724, 95% CI 1.087–2.733, P = .02). For rs879922, there were also significant differences in the frequencies of the CC genotype and the C allele between EH patients and the NT controls (CC: 2.5% vs 0.6%, P < .01, OR = 4.707, 95% CI 1.854–11.951; C: 4.4% vs 2.7%, P = .01). The dominant (GG vs CC+GC) distribution of C allele of rs879922 revealed a significant association (OR = 1.520, 95% CI 1.002–2.307, P = .05) with EH risk. At the rs4646155 locus, the frequencies of the TT genotype and the T allele were significantly higher in the EH patients than the NT controls (TT: 1.9% vs 0.7%, P = .01, OR = 3.492, 95% CI 1.324–9.212; T: 3.4% vs 2.2%, P = .02). A logistic regression analysis shown that the dominant effect (CC vs TT + TC) was associated with EH risk (OR = 1.573 95% CI 0.985–2.511), but it did not reach statistical significance (P = .06). At the rs4646176 locus, the rates of the GG genotype and the G allele were significantly higher in the EH patients than in the NT participants (GG: 2.1% vs 0.6%, P < .01, OR = 3.902, 95% CI 1.503–10.128; G: 3.7% vs 2.2%, P = .01). The association between rs4646176 and the risk of EH was investigated under both the dominant and recessive genetic model. The dominant (CC vs GG + GC) genetic model of G allele showed a significantly association with EH risk (OR = 1.684, 95% CI 1.062–2.671, P = .03) in the Chinese Han population.
Table 2.
Distribution of genotypes and alleles for each of the ACE2 gene polymorphisms in all subjects.
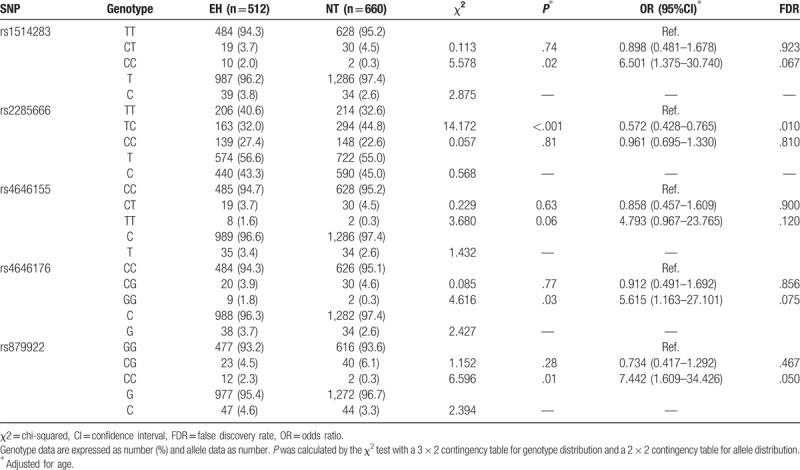
At the same time, the gene polymorphisms of ACE2 were analysed according to different groups of male and female in all the participants. None of the SNPs was significantly associated with EH in the male group. In the female group, the statistical results demonstrated that the genotype and allele distributions frequencies of 5 SNP loci (rs1514283, rs2285666, rs4646155, rs4646176, and rs879922) located in the introns of the ACE2 gene were significantly different between the EH and NT groups (Fig. 1). The distribution frequencies of genotype and allele for ACE2 SNPs in the female group are shown in Table 3. At the rs1514283 locus, the frequency of the CC genotype was significantly higher in the EH patients than in the NT subjects (CC: 2% vs 0.3%, P = .02, OR = 6.501, 95% CI 1.375–30.740). And at the rs879922 locus, there was also a significant difference between the EH patients and the NT controls regarding to the CC genotype (CC: 2.3% vs 0.3%, P = .01, OR = 7.442, 95% CI 1.609–34.426). These findings indicated that CC genotype of rs1514283 and rs879922 were high-risk factors for the progression of EH in females. Interestingly, a frequency of heterozygous genotype TC was significantly higher in NT group as compared with EH group for the rs2285666 locus (TC: 32.0% vs 44.8%, P < .001, OR = 0.572, 95% CI 0.428–0.765). The data implicated that the TC genotype of rs2285666 carriers had a reduced risk of EH, and it was a potential genetic resistance factor in the development of EH in Chinese female Han population. As for the rs4646176, the frequencies of the GG genotype in the EH patients were significantly higher than that in the NT controls (GG: 1.8% vs 0.3%, P = .03, OR = 5.615, 95% CI 1.163–27.101). The GG genotype of rs4646176 was a risk factor for EH in women. Moreover, the TT genotype frequencies of rs4646155 in the EH group were also higher than in the NT group (TT: 1.6% vs 0.3%, OR = 4.793, 95% CI 0.967–23.765), and no significant difference was observed between the locus and EH (P = .06). This was a limitation of our study, which the sample size was still relatively small and not provide sufficient information to precisely estimate the association between rs4646155 and EH in the Chinese female Han population.
Figure 1.
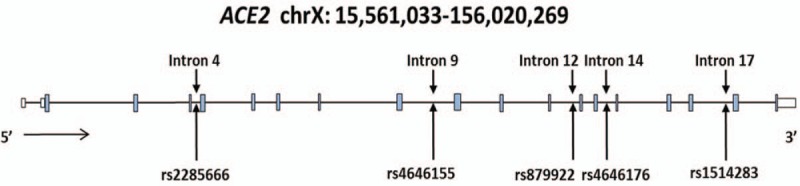
Schematic representation of ACE2 gene structure. Introns are shown as horizontal black line, exons are shown as blue boxes and white boxes represent the 5′ and 3′-UTRs.
Table 3.
Distribution of genotypes and alleles for each of the ACE2 gene polymorphisms in females.
3.3. Linkage disequilibrium (LD) and haplotype analysis
LD analysis showed that the 4 ACE2 gene polymorphism loci (rs1514283, rs4646155, rs4646176 and rs879922) were in strong LD. rs1514283 and rs4646155: D’ = 0.992, r2 = 0.924; rs1514283 and rs4646176: D’ = 0.992, r2 = 0.977; rs1514283 and rs879922: D’ = 1.000, r2 = 0.812; rs4646155 and rs4646176: D’ = 1.000, r2 = 0.932; rs4646155 and rs879922: D’ = 1.000, r2 = 0.86; rs4646176 and rs879922: D’ = 1.00, r2 = 0.819. Unfortunately, the SHEsis haplotype analysis demonstrated that no haplotype associated with EH was found in the 5 ACE2 SNPs in this cohort.
3.4. ACE2 concentration and enzymatic activity assay
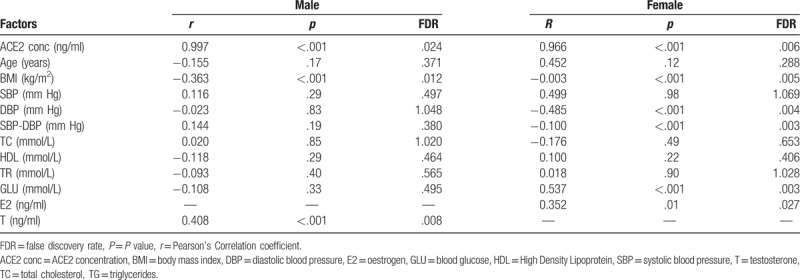
In order to further elucidate the relationship between ACE2 activity level and EH, we examined the concentration and activity of ACE2 for all subjects. The Pearson correlation analysis between ACE2 activity and clinical characters results are shown in Table 4. A strong positive correlation was found between ACE2 activity and concentration level in both male and female EH patients (males: r = 0.997, P < .001, females: r = 0.966, P < .001). Meanwhile, serum ACE2 activity had a negative correlation with BMI in the male and female groups (male: r = −0.363, P < .001, female: r = −0.003, P < .001). Interestingly, in female EH patients, ACE2 activity level were significant negatively correlated with DBP and pulse pressure (DBP: r = −0.485, P < .001; PP: r = −0.100, P < .001), and positively correlated with blood glucose (GLU: r = 0.537, P < .001). By contrast, no statistical correlations of these clinical parameters with ACE2 activity were found in male group, which further documented the specificity of our assay for ACE2. However, ACE2 activity levels were extremely low or even undetectable in the plasma from normotensive subjects (data not shown). Some previous reports also showed that the ACE2 activity levels were very low in normal healthy persons, and increased in patients with cardiovascular disease.[16,17]
Table 4.
The result of Pearson correlation analysis between ACE2 level and clinical characters.
4. Discussion
At present, EH is 1 of the most common cardiovascular diseases, and it is also a major public health problem around the world. At least 970 million people worldwide are suffering from elevated blood pressure (hypertension), and about 66% of them live in developing countries. EH is a complex disease, which is considered to the result of the genetic and environmental interaction. The RAS plays a vital role in the occurrence and development of EH. As an important member of the RAS, ACE2 is a dipeptidyl carboxydipeptidase, which counterbalances the action of angiotensin-converting enzyme. The over-expression of ACE2 may decrease RAS activation. The significant associations between ACE2 gene polymorphisms and EH have been reported for people of different race and ethnicity.[6,18–20]
In the present study, we found that the 4 SNPs of ACE2 (rs1514283, rs4646155, rs4646176, and rs879922) were associated with EH in northeastern Han populations of China. As a tag SNP in the CHB (Han Chinese in Beijing) SNP database for HapMap project, rs4646155 can capture the information of 4 ACE2 SNPs (rs4646140, rs4646144, rs4646155, and rs61433707) with minor allele frequencies equal to or greater than 3% in the HapMap CHB database. Up to now, only a few studies focused on the correlation between rs4646155 and hypertension, but these results showed that no significant difference in the rs4646155 genotypes/alleles frequencies between hypertensive patients and normotensive health volunteers.[6,17] In this study, it was the first time to report that the TT genotype of rs4646155 was a significant association with EH (OR = 3.492), especially in females (OR = 4.793). Prolonged exposure to low temperature may be the main cause of this inconsistency. Low temperature was a well-known risk factor for high blood pressure.[21] Under the condition of low temperature, the body maintains normal body temperature by sympathetic activity and vasoconstriction, which prevents heat loss and increases blood pressure.[22] The cold pressor test (CPT), which is a standard test for characterization of sympathetic function, also make certain that the blood pressure variations in the response to cold stimulation.[23,24] And CPT has been documented to predict the subsequent risk of hypertension in normotensive persons.[25] In this study, all the participants came from the northeastern Han Chinese within 3 generations reside in Lan Xi (46° 20′0″ N, 126° 16′0″ E) of Heilongjiang Province. The annual average temperature in this area is about 5 °C and the mean temperature is below 0 °C about 6 months of 1 year. The subjects of previous studies were resident in Henan[6] (the annual average temperature 14.3 °C) and Guangdong province[17] (the annual average temperature 21.8 °C). The prevalence of hypertension was consistently higher in northern than in southern residents (32.4% in Heilongjiang, 26.1% in Henan, 15.8% in Guangdong).[26] Therefore, the cold environmental and genetic factors may be the risk factors associated with EH for the population of the cold region. We will further confirm the relationship between the ACE2 SNP locus and ACE2 protein activity in subsequent experiments.
In addition, we observed that the TC genotype of rs2285666 conferred a protective effect on EH in female group of all participants. Rs2285666 was 1 of the most frequently investigated loci in the studies of ACE2 gene polymorphism. Thus far, some prior studies have reported the relation between rs2285666 and blood pressure change, but these results were inconsistent.[11,18–20] Two studies reported that the T allele of rs2285666 were significant associations with hypertension or SBP or DBP in female groups of Chinese population.[18,19] And Zhong et al found that the C allele of the SNP associated with higher blood pressure.[20] However, another orthostatic blood pressure study including 3630 Chinese Han subjects indicated that the hypertension had no association with the rs2285666 locus.[11] In 2 meta-analyses, the results of rs2285666 polymorphism were also conflicting. One study combined 2528 hypertension patients and 2024 normotensive controls from Chinese Han population and reported no association between the locus polymorphism and hypertension.[27] The other study, which included 7251 hypertension patients comprising of Chinese Han, Chinese Dongxiang, Anglo-Celtic population, showed that the TT genotype of rs2285666 was a significant association with hypertension in Chinese Dongxiang and Anglo-Celtic females.[28] In the present study, we observed that the TC heterozygous genotype of rs2285666 was a protective factor for EH in females of Chinese Han subjects. The heterogeneity of the association between rs2285666 gene polymorphism and hypertension may be caused by the following reasons. First, several reports have shown that hormonal and sex chromosomes contributed to the blood pressure variations between the sexes.[29,30] Second, there was a much great difference in the frequency of distribution of ACE2 variants among different racial and ethnic lines.[19,28] For instance, the T allele frequencies of rs2285666 were 40.1% in Chinese Han, 32.4% in Chinese Dongxiang, 22.0% in Anglo Celtic.[19,31] At last, it is possible that the rs2285666 has characteristic of QTL for hypertension. A quantitative trait locus (QTL) is a region of DNA which is associated with a particular phenotypic trait, which varies in degree and which can be attributed to polygenic effects, that is, the product of 2 or more genes, and their environment.[26] Phenotypic variation for quantitative traits is the result of the segregation of alleles at quantitative trait loci (QTL). Environmental factors and other external influences can also play a role in phenotypic variation.[26] The previous study has reported that ACE2 gene maps to a QTL on the X chromosome in 3 different rat models of hypertension.[1]
Furthermore, we found that rs2285666 was a protein truncating variant (PTV) locus through checking GTEx database, which the type was splice disruption model. PTVs are genetic variants predicted to shorten the coding sequence of genes,[32] also commonly referred to as loss-of-function variants as they often result in a non-functional or unstable gene product.[33] PTVs are generally the strongest acting genetic variants in medical genetics and, as 1 functional copy of the gene is removed, may often provide insight into what is achievable pharmacologically via inhibition of the product of the gene.[32] Thus, the homozygous variants of rs2285666 may result in the product inhibition for ACE2 gene.
The National Center for Biotechnology Information (NCBI) Genotype-Tissue Expression (GTEx) database aims to evaluate the relationship between genetic variation and gene expression in normal human tissues to understand how this relationship contributes to disease susceptibility and development.[34,35] Expression quantitative trait locus (eQTL) browser in the GTEx database is a central resource that archives and displays results of a national research project for determining association between genetic variations and high-throughput molecular-level expression phenotypes, and this information can provide insight into the biological relevance of data from genome-wide association studies.[35] To characterize the functional relevance of the 5 SNP loci found in this study, we utilized this database resource to evaluate the relationship of these variants with the expression levels of ACE2 in human different tissues. The GTEx database indicated that the rs879922 polymorphism was a significant eQTL for ACE2. A significant association was observed between the expression level of ACE2 and different genotypes of rs879922 in 361 normal tibial nerve (P = 7.4 × 10−5), whereas the relative expression of ACE2 was significantly lower in subjects with the CC genotype of rs879922 compared with those carrying the GG/CG genotype (Fig. 2).
Figure 2.
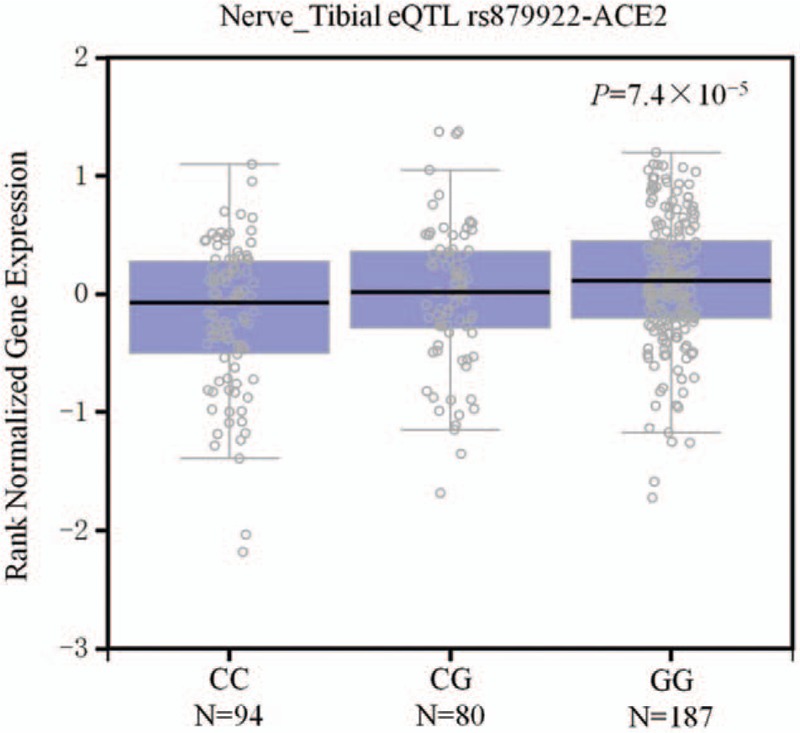
The genotype of rs879922 was correlated with expression of ACE2 in the tibial nerve on GTEx Portal.
The LD analysis showed that rs1514283, rs4646155, rs4646176, and rs879922 were in strong LD. And in haplotype analysis, 3 haplotypes were found in these 4 SNPs. But all the 3 haplotypes were not significantly different between EH patients and controls (all P > .05) (LFT = 0.01, data not shown). Only when lowest frequency threshold (LFT) was set at 0.001, the frequency of the T-C-C-G haplotype was significantly higher in controls than in EH patients (OR 0.733, 95% CI 0.544–0.988, P = .04). Yet haplotype with frequency less than LFT will not be considered in analysis. Therefore, it is necessary to further confirm the correlation between the haplotype and EH in large sample size. To sum up, only when more ACE2 SNP locus associated with EH were revealed, we can fully understand the role of ACE2 in EH. The rich SNPs information can predict the risk of hypertension, and provide a theoretical basis for the prevention and treatment of hypertension. And abundant gene polymorphism information can also lay a solid foundation for personalized medicine. Personalized medicine is becoming a medical reality, as important phenotype-genotype relationships are uncovered. In addition, the discovery of a large number of SNP loci associated with hypertension could provide new targets for the research of anti-hypertensive drugs and the possibility of obtaining more effective anti-hypertensive drugs. Thus, new SNP locus associated with hypertension are important for the prevention, diagnosis, treatment of EH and the development of new drugs, and also a key element of individualized care.
ACE2 undergoes “shedding” from endothelial cells, which result in the release of the ectodomain with catalytic active into the circulation.[36] The activity of ACE2 can be measured in serum by ELISA. In reviewing the literature, a number of studies focused on the association between ACE2 activity and EH in animal models.[37–39] However, only few reports noted that the serum activity level of ACE2 was associated with cardiovascular disease,[40] specifically EH. Thus, in this study, we evaluated the correlation of ACE2 activity with clinical characters between the different genders in Chinese Han EH patients. The results indicated that ACE2 activity was inversely related to DBP in females EH patients. Previously, only 1 research reported the association between ACE2 activity and DBP response to cold pressor test in women.[24] And a study in animal model showed that the elevated diastolic pressure in the spontaneously hypertensive rat was associated with the reduction in ACE2 activity.[37] Moreover, a research about ACE2 activity in human atrial fibrillation (AF) demonstrated that elevated plasma ACE2 activity level was associated with impaired left ventricular (LV) diastolic function.[41] In addition, the association between pulse pressure and ACE2 activity was found in female EH patient for the first time in this study. The pulse pressure is an important parameter represented heart function, and a high pulse pressure tends to accelerate the normal aging of heart. The increased pulse pressure has been considered as a marker of arterial stiffness and an important predictor of new-onset AF.[42] These results suggested that ACE2 activity could be a potential biomarker for the variations of blood pressure, providing the useful information for the prediction and prevention of cardiac dysfunction in EH patients.
Accumulated evidence proves that estrogen participates in the upregulates of ACE2 expression and activity level.[43] Our results showed the positive association between ACE2 activity and estrogen level in EH patients. Estrogen can regulate the components of the renin-angiotensin-aldosterone system, which increases synthesis of angiotensinogen, ACE2 and AT2R and decreases the expression of renin, ACE and AT1R.[17] Moreover, estrogen can impair the increment of aldosterone. Therefore, normal estrogen level can protect female from being harmed by high blood pressure caused by renin-angiotensin-aldosterone system. These results provided evidence that genetic variant of ACE2 plays a vital role in the development of EH. And the serum ACE2 activity can be used as a potential biomarker for the identification of blood pressure variation, providing the useful information for the prediction and prevention of cardiac dysfunction in EH patients.
In summary, here we observed that 5 SNPs (rs1514283, rs4646155, rs4646176, rs2285666, and rs879922) of ACE2 gene were significantly associated with EH in women of Chinese Han population. It is the first time to report that the susceptibility of rs4646155 for EH in female group. In addition, we found the ACE2 activities were significantly correlated with DBP, pulse pressure and blood glucose in female EH patients. The finding may provide a novel insight into the clinical diagnosis of EH and the development of antihypertensive drugs.
Acknowledgment
We thank the subjects for participating in this study. We gratefully acknowledge the assistance of clinical, field and laboratory staff that made this work possible.
Author contributions
Conceptualization: Qi Zhang, Changchun Qiu.
Formal analysis: Jingping Li.
Investigation: Keyong Zhang.
Methodology: Hao Zhang.
Resources: Mingyu Cong.
Software: Ningning Wang.
Validation: Xueyan Li, Ming Jin, Nan Wu.
Writing – original draft: Qi Zhang.
Supplementary Material
Footnotes
Abbreviations: ACE2 = angiotensin-converting enzyme 2, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, EH = essential hypertension, eQTL = expression quantitative trait locus, FDR = false discovery rate, LD = linkage disequilibrium, LFT = lowest frequency threshold, OR = odds ratio, PTV = protein truncating variant, RAS = renin–angiotensin system, SBP = systolic blood pressure, SNPs = single nucleotide polymorphisms.
QZ and C-CQ designed the study. X-YL, MJ, and NW conducted the experiment. QZ, N-NW, and HZ analysed the results. K-YZ, M-YC, and J-PL collected the control and case samples. QZ drafted the manuscript. J-PL and C-CQ made the same contributions to this study.
This work was supported by a grant from the National Natural Science Foundation of China (31171146, 31371208, and 31440054) and the Scientific Research Foundation of the Education Bureau of Heilongjiang Province, China (2016-KYYWF-0847, 2016-KYYWF-0864).
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
References
- [1].Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417:822–8. [DOI] [PubMed] [Google Scholar]
- [2].Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- [3].Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 2002;277:14838–43. [DOI] [PubMed] [Google Scholar]
- [4].Mary Donoghue, Frank Hsieh, Elizabeth Baronas, et al. A novel angiotensin-converting enzyme–related carboxypeptidase (ace2) converts angiotensin i to angiotensin 1-9. Circ Res 2000;87:E1–9. [DOI] [PubMed] [Google Scholar]
- [5].Harmer D, Gilbert M, Borman R, et al. Quantitative mRNA expression pro¢ling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002;532:107–10. [DOI] [PubMed] [Google Scholar]
- [6].Fan X, Wang Y, Sun K, et al. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of Captopril in women. Clin Pharmacol Ther 2007;82:187–96. [DOI] [PubMed] [Google Scholar]
- [7].Song SB, Hs J, Kw H, et al. Association between renin-angiotensin-aldosterone system-related genes and blood pressure in a Korean population. Blood Press 2011;20:204–10. [DOI] [PubMed] [Google Scholar]
- [8].Patnaik M, Pallabi P, Surendra N Swain, et al. Association of angiotensin-converting enzyme and angiotensin-converting enzyme-2 gene polymorphisms with essential hypertension in the population of Odisha, India. Ann Hum Biol 2013;41:145–52. [DOI] [PubMed] [Google Scholar]
- [9].Malard L, Kakinami L, Loughlin JO, et al. The association between the angiotensin-converting enzyme-2 gene and blood pressure in a cohort study of adolescents. BMC Med Genet 2013;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao Q, Hixson JE, Rao DC, et al. Genetic variants in the apelin system and blood pressure responses to dietary sodium interventions: a family-based association study. J Hypertens 2010;28:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fan XH, Wang YB, Wang H, et al. Polymorphisms of angiotensin-converting enzyme (ACE) and ACE2 are not associated with orthostatic blood pressure dysregulation in hypertensive patients. Acta Pharmacol Sin 2009;30:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li J, Feng M, Wang Y, et al. The relationship between three X-linked genes and the risk for hypertension among northeastern Han Chinese. J Renin Angiotensin Aldosterone Syst 2015;16:1321–8. [DOI] [PubMed] [Google Scholar]
- [13].Gabriel S, Ziaugra L, Tabbaa D. SNP Genotyping Using the Sequenom MassARRAY iPLEX Platform. Curr Protoc Hum Genet 2009;1:1–8. [DOI] [PubMed] [Google Scholar]
- [14].Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 2005;15:97–8. [DOI] [PubMed] [Google Scholar]
- [15].Li Z, Zhang Z, He Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell Res 2009;19:519–23. [DOI] [PubMed] [Google Scholar]
- [16].Varagic J, Ahmad S, Nagata S, et al. ACE2: angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Curr Hypertens Rep 2014;16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen YY, Zhang P, Zhou XM, et al. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J Clin Pharm Ther 2017;43:189–95. [DOI] [PubMed] [Google Scholar]
- [18].Niu W, Qi Y, Hou S, et al. Correlation of angiotensin-converting enzyme 2 gene polymorphisms with stage 2 hypertension in Han Chinese. Transl Res 2007;150:374–80. [DOI] [PubMed] [Google Scholar]
- [19].Yi L, Gu Y, Wang X, et al. Association of ACE, ACE2 and UTS2 polymorphisms with essential hypertension in han and dongxiang populations from north-western China. J Int Med Res 2006;34:272–83. [DOI] [PubMed] [Google Scholar]
- [20].Zhong J, Yan Z, Liu D, et al. Association of angiotensin-converting enzyme 2 gene A/G polymorphism and elevated blood pressure in Chinese patients with metabolic syndrome. J Lab Clin Med 2006;147:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Su D, Du H, Zhang X, et al. Season and outdoor temperature in relation to detection and control of hypertension in a large rural Chinese population. Int J Epidemiol 2014;43:1835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Youn JC, Rim SJ, Park S, et al. Arterial stiffness is related to augmented seasonal variation of blood pressure in hypertensive patients. Blood Press 2007;16:375–80. [DOI] [PubMed] [Google Scholar]
- [23].Mei H, Gu D, Rice TK, et al. Heritability of blood pressure responses to cold pressor test in a Chinese population. Am J Hypertens 2009;22:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huang J, Chen S, Lu X, et al. Polymorphisms of ACE2 are associated with blood pressure response to cold pressor test: the GenSalt study. Am J Hypertens 2012;25:937–42. [DOI] [PubMed] [Google Scholar]
- [25].Kasagi FMA, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension 1995;25:71–6. [DOI] [PubMed] [Google Scholar]
- [26].Yin M, Augustin B, Fu Z, et al. Geographic distributions in hypertension diagnosis, measurement, prevalence, awareness, treatment and control rates among middle-aged and older adults in China. Sci Rep 2016;6:37020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jian-Bo Zhou, Yang J-K. Meta-analysis of association of ACE2 G8790A polymorphism with Chinese Han essential hypertension. J Renin Angiotensin Aldosterone Syst 2009;10:13–4. [DOI] [PubMed] [Google Scholar]
- [28].Lu N, Yang Y, Wang Y, et al. ACE2 gene polymorphism and essential hypertension: an updated meta-analysis involving 11,051 subjects. Mol Biol Rep 2012;39:6581–9. [DOI] [PubMed] [Google Scholar]
- [29].Charchar FJ, Bloomer LDS, Barnes TA, et al. Inheritance of coronary artery disease in men: an analysis of the role of the Y chromosome. The Lancet 2012;379:915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17b-oestradiol- dependent and sex chromosome-independent. Biol Sex Differ 2010;1:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Benjafield AV, Wang WYS, Morris BJ. No association of angiotensin-converting enzyme 2 gene (ACE2) polymorphisms with essential hypertension. Am J Hypertens 2004;17:624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rivas MA, Pirinen M, Conrad DF, et al. Effect of predicted protein-truncating genetic variants on the human transcriptome. Science 2015;348:666–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].MacArthur DG, Balasubramanian S, Frankish A, et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science 2012;335:823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carithers LJ, Moore HM. The Genotype-Tissue Expression (GTEx) Project. Biopreserv Biobank 2015;13:307–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Consortium TG. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015;348:648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Patel SK, Velkoska E, Burrell LM. Emerging markers in cardiovascular disease: where does angiotensin-converting enzyme 2 fit in? Clin Exp Pharmacol Physiol 2013;40:551–9. [DOI] [PubMed] [Google Scholar]
- [37].da Silva JS, Gabriel-Costa D, Wang H, et al. Blunting of cardioprotective actions of estrogen in female rodent heart linked to altered expression of cardiac tissue chymase and ACE2. J Renin Angiotensin Aldosterone Syst 2017;18:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang Z, Yu X, Cheng L, et al. Effects of enalapril on the expression of cardiac angiotensin-converting enzyme and angiotensin-converting enzyme 2 in spontaneously hypertensive rats. Arch Cardiovasc Dis 2013;106:196–201. [DOI] [PubMed] [Google Scholar]
- [39].Kamilic J, Hamming I, Kreutz R, et al. Renal ACE2 expression and activity is unaltered during established hypertension in adult SHRSP and TGR(mREN2)27. Hypertens Res 2010;33:123–8. [DOI] [PubMed] [Google Scholar]
- [40].Uri K, Fagyas M, Kertesz A, et al. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angiotensin Aldosterone Syst 2016;17:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Walters TE, Kalman JM, Patel SK, et al. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace 2017;19:1280–7. [DOI] [PubMed] [Google Scholar]
- [42].Valbusa F, Bonapace S, Bertolini L, et al. Increased pulse pressure independently predicts incident atrial fibrillation in patients with type 2 diabetes. Diabetes Care 2012;35:2337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bukowska A, Spiller L, Wolke C, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med 2017;242:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


