Abstract
Background:
Alendronate has been used to prevent or treat glucocorticoid-induced osteoporosis (GIO), data regarding its efficacy are inconsistent. We conducted the current systematic review and meta-analysis to evaluate both efficacy and safety of alendronate in the treatment of GIO.
Methods:
PubMed, Embase, the Cochrane Controlled Trials Registry, and the China Academic Journal Network Publishing Databases were searched up through March 1, 2018. Randomized controlled trials (RCTs) involving patients which received alendronate treatment were included. Outcome measures were bone mineral density (BMD) changes, bone fractures, and adverse reactions. Data from the individual studies were pooled using random or fixed effect models based on heterogeneity. Effect size was reported as standardized mean differences (SMD) for continuous outcomes and pooled odds ratios (OR) for dichotomous outcomes, with 95% confidence interval (CI).
Results:
Overall, 10 studies involving 1002 patients were included in the present investigation. Alendronate treatment significantly increased BMD of the lumbar spine and femoral neck during 6 to 24 months. These beneficial effects were apparent at 12 months after treatment for the lumbar spine but not the femoral neck BMD. Alendronate treatment did not significantly change fracture risk nor induce significant differences in adverse gastrointestinal effects.
Conclusion:
Alendronate significantly increases BMD of the lumbar spine and femoral neck in patients with GIO, but does not appear to reduce the risk of fractures. As relatively insufficient data regarding the GIO fracture incidence has been reported, more RCTs need to be carried out to determine the efficacy of alendronate in the prevention of GIO fracture.
Keywords: alendronate, glucocorticoid, meta-analysis, osteoporosis
1. Introduction
Osteoporosis is a persistent public health problem caused by aging, predominately in females. Osteoporosis is also a debilitating complication of long-term glucocorticoid therapy.[1,2] As high as 26% of the patients taking glucocorticoid had presumed glucocorticoid-induced osteoporosis (GIO),[3] among which 30% to 50% of patients eventually suffer fractures.[4–6] Common fracture sites include trabecular bone, predominantly in the lumbar spine and the femoral neck, within months after therapy initiation.[7,8] Because of the common use of glucocorticoids to treat diverse conditions such as rheumatoid arthritis, polymyalgia rheumatica, inflammatory bowel disease, and chronic obstructive pulmonary disease, GIO is a serious clinical concern worldwide. Specifically, about 3% of patients over 50 years of age have been treated with glucocorticoids, and 5.2% of patients over 80 years of age have used this therapy.[9]
Glucocorticoids inhibit bone development by decreasing osteoblasts and hampering their function and increase the rate of bone resorption by stimulating osteoclast formation and activity.[10,11] Furthermore, glucocorticoids decrease intestinal calcium absorption and increase renal calcium excretion.[12–14] Oral prednisone (starting at 6.8 mg/day) is associated with a dose-dependent increase in fracture risk. Patients using prednisone for <6 months do not experience this increased risk of fractures; however, those with longer than 6 months therapy experience this side effect[15] No significant fracture risk differences have been observed between men and women.[9]
Bisphosphonates are widely used to prevent or to treat osteoporosis via inducing osteoclast apoptosis and inhibiting bone resorption.[11] In particular, alendronate has been reported to be an effective and well-tolerated drug for prevention and treatment of GIO, offering sustained treatment benefits up to 2 years, especially with respect to increased BMD.[7,16] Sawka's group previously described that alendronate can decrease the risk of male vertebral fractures.[17] Katayama and Matsuno[18] reported that it also could prevent the fractures of patients with rheumatoid arthritis. Okada concluded that alendronate protects premenopausal women from bone loss and fracture associated with high-dose glucocorticoid therapy.[19] However, Compston[20] regarded alendronate as a drug which can increase BMD but could not reduce new fractures along with GIO.
We, therefore, conducted the present systematic review and meta-analysis to characterize the effects of alendronate on BMD changes of the lumbar spine and femoral neck, and fracture risk. We also assessed the risks of adverse reactions associated with alendronate treatment.
2. Materials and methods
2.1. Search strategy
RCTs involving alendronate treatment of individuals with GIO were searched in PubMed, EMBASE, the Cochrane Controlled Trials Registry and China Academic Journal Network Publishing Database from inception to March 1, 2018, with the following keywords: osteoporosis, alendronate, glucocorticoids/glucocorticoid, and corticoids/corticosteroids.
2.2. Study selection
Studies were included according to the following criteria. Adult patients with GIO taking alendronate for at least 6 months. Included diseases were rheumatoid arthritis, systemic lupus erythematous, or autoimmune disease. Any RCT, controlled clinical trial, or open label trial (OLT) designed with a control group providing calcium supplements and vitamin D3 (600–1000 IU) simultaneously, and another group receiving alendronate also with calcium supplements and vitamin D3. Data of fracture rate and BMD clearly presented and side effects observed. The language was limited to English and Chinese, while there was no restriction by country in which the trial occurred. Trials were excluded if they included children, patients with fatal diseases, or organ transplant recipients. If other drugs which could increase the BMD were used in addition to alendronate, calcium and vitamin D3, these trials were excluded. In total, 10 trials met our inclusion criteria. Corresponding authors of RCTs with incomplete data presentation (e.g., missing means, standard deviations of pretest and post-test data, or standard deviations of change scores) were contacted when necessary.
2.3. Outcome measurements
The primary outcome measures were BMD changes in lumbar spine and femoral neck, bone fracture, and adverse effects. BMD percent changes of the lumbar spine (L1–L4) and the femoral neck during the RCT period were assessed. Subgroup analysis was used to evaluate lumbar spine and femoral neck data at 6, 12, 24 months. The presence of a vertebral fracture or nonvertebral fracture was used as another stratifying factor. Secondary outcomes were adverse effects of alendronate, especially gastrointestinal symptoms.
2.4. Data extraction
Data extraction and quality scoring were performed by two independent researchers (CZ and JBG). When disagreements occurred, a third author (YKW) was consulted to reach a consensus. Mean values were measured from data tables or figures, if no direct data were available from the paper or the corresponding author. According to the handbook.cochrone.org, standard deviations within a group were obtained using the equation: 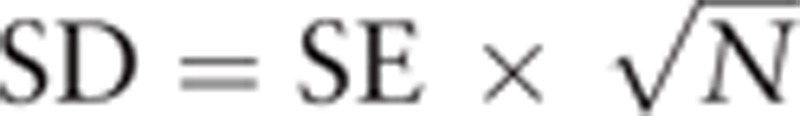 in treatment or placebo groups with standard errors. Standard deviations between groups were obtained according to the standard error of the mean differences with the following formula:
in treatment or placebo groups with standard errors. Standard deviations between groups were obtained according to the standard error of the mean differences with the following formula:
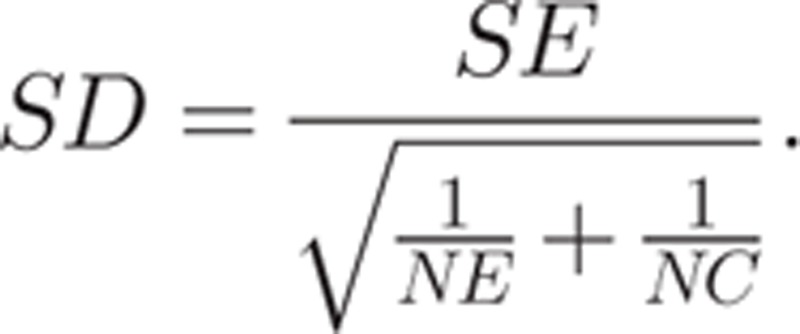 |
As the t value is the ratio of the difference in means to the standard error of the difference in means, the standard error of the difference in means was obtained by dividing the difference in means (MD) by the t value:
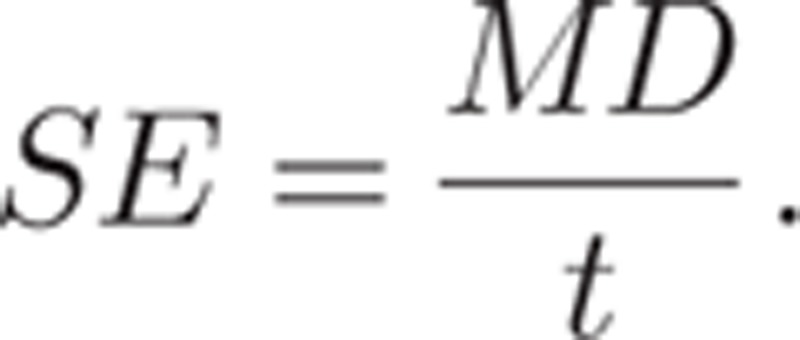 |
2.5. Assessment of study quality
Jadad scores were used to determine methodological quality.[21] Study quality evaluations were performed by 2 reviewers (SQQ and TM) using these categories: “Was the study described as randomized?”, “Was the method used to generate the sequence of randomization described and appropriate (random numbers, computer-generated, etc.)?”, “Was the study described as double-blind?”, “Was the method of double-blinding described and appropriate (identical placebo, active placebo, dummy, etc.)?”, and “Was there a description of withdrawals and drop-outs?”. A score of 4 to 5 was considered to be a study of high methodological quality (Table 1).
Table 1.
Jadad score.

2.6. Statistical analysis
Effect sizes for the continuous outcome (i.e., change in BMD) was quantified as standardized mean difference (SMD) with 95% confidence interval (CI) while pooled odds ratio (OR) with 95% CI were calculated for dichotomous outcomes (i.e., incidence of bone fractures and adverse effects).
Heterogeneity was assessed using the Q-test. A random-effects model was used to pool data if there was evidence of significant heterogeneity (I2 > 50%). Otherwise, the fixed-effects model was used. Publication bias was assessed with Funnel plots. Data were analyzed using the Cochrane Collaboration software Review Manager 5.2.
3. Results
The results of the present investigation revealed a total of 194 relevant articles. Two trials which were not double-blind studies were included,[22,23] as they contained data of BMD and fracture rates. It has previously been reported that alendronate is therapeutically equivalent at a dose of 70 mg/week to a daily dose of 10 mg in the treatment of osteoporosis.[24,25] So three additional trials were also included, even though in these studies alendronate was administrated at a dose of 70 mg/week.[22,26,27] Dual energy x-ray absorptiometry was used in all trials. Overall, 9 trials in 10 papers met the inclusion criteria and were assessed (Fig. 1).[16,22,23,26–32]Table 2 depicts characteristics of the included studies. Quality appraisal of the studies based on the Jadad score are shown in Table 1. For evaluation of publication bias, one study[32] showed evidence of bias towards positive results at the lumbar spine, another study[23] showed evidence of bias towards positive results at the femoral neck, both suggesting selective reporting (Fig. 2).
Figure 1.
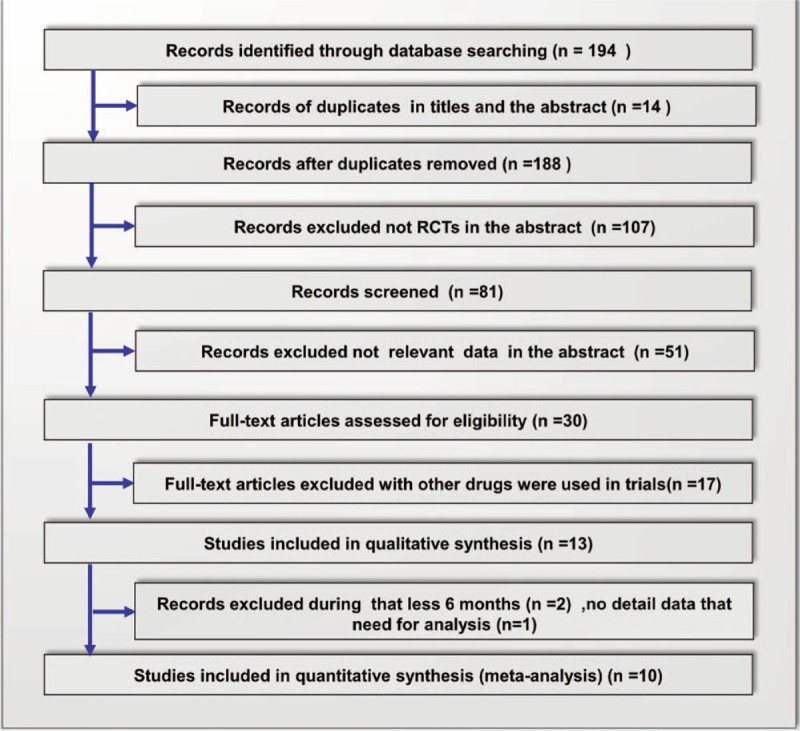
Flowchart of database search and selection process.
Table 2.
Characteristics of the trials of alendronate in the treatment of glucocorticoid-induced osteoporosis.
Figure 2.
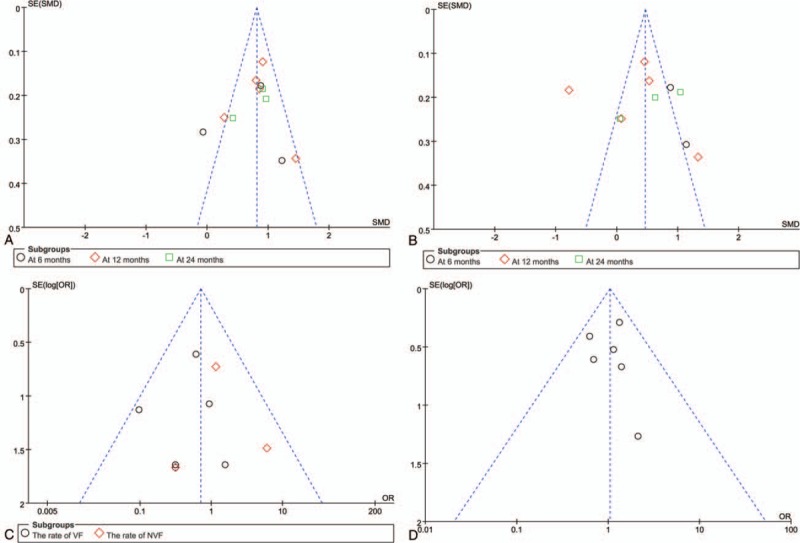
Publication bias was assessed with Funnel plots.
We first evaluated the percentage change in the lumbar BMD in patients receiving alendronate for 6 to 24 months using a random-effects model (Fig. 3). The pooled estimates showed that alendronate treatment significantly increased lumbar spine BMD of patients with GIO. However, there was significant heterogeneity between studies. In subgroup analysis, the increase in BMD after alendronate treatment was statistically significant at 12, and 24 months, but not at 6 months after alendronate administration (Fig. 3).
Figure 3.
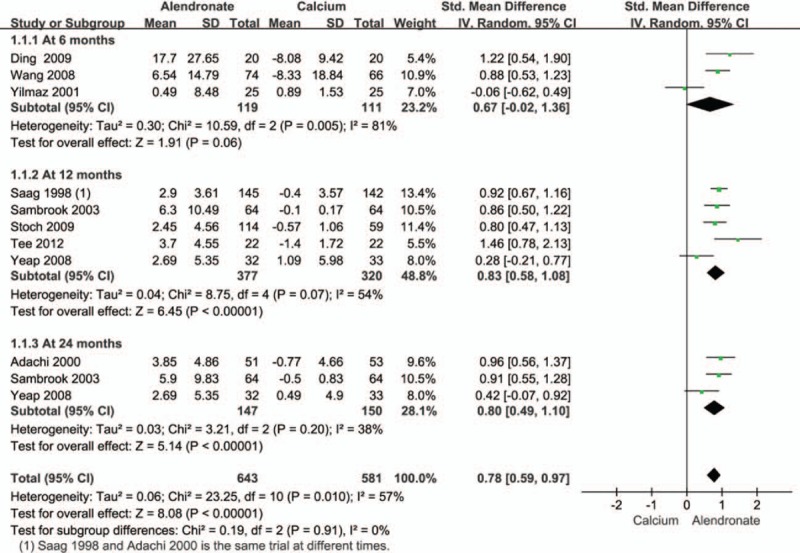
Pooled effect size of treatment group (alendronate) compared to controls (calcium) on lumbar spine BMD in all patients receiving an average daily dose of at least 7.5 mg of prednisone. BMD = bone mineral density.
In the present investigation, a total of eight studies evaluated changes in femoral neck BMD.[16,22,23,26,28–30,32] The pooled data demonstrated a statistically significant increase in femoral neck BMD (Fig. 4). Subgroup analysis for this outcome showed significant differences at 6 and 24 months but not at 12 months after alendronate administration (Fig. 4).
Figure 4.
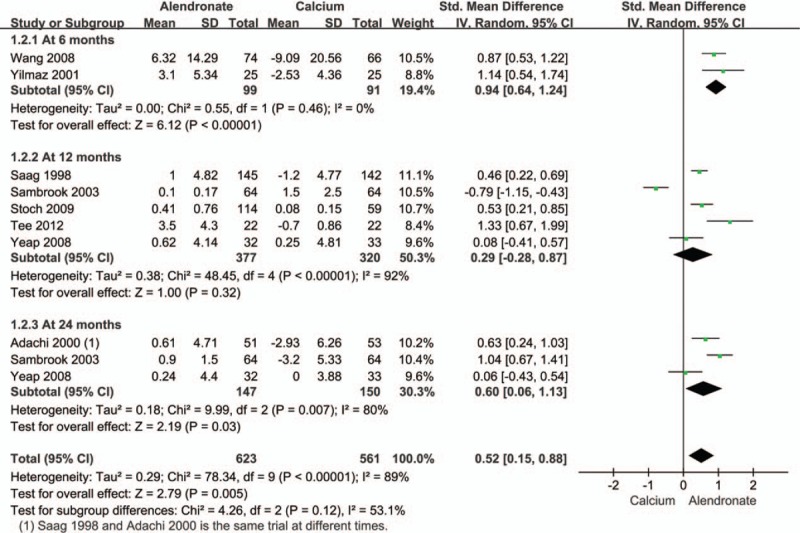
Pooled effect size of treatment group (alendronate) compared to controls (calcium) on femoral neck BMD in all patients receiving an average daily dose of at least 7.5 mg of prednisone. BMD = bone mineral density.
The effect of alendronate on bone fracture in patients with GIO after alendronate treatment from 6 to 24 months was reported in 5 studies.[16,23,26–28,31] The pooled data showed no statistically significant difference in vertebral and nonvertebral fractures in patients treated with alendronate (Fig. 5).
Figure 5.
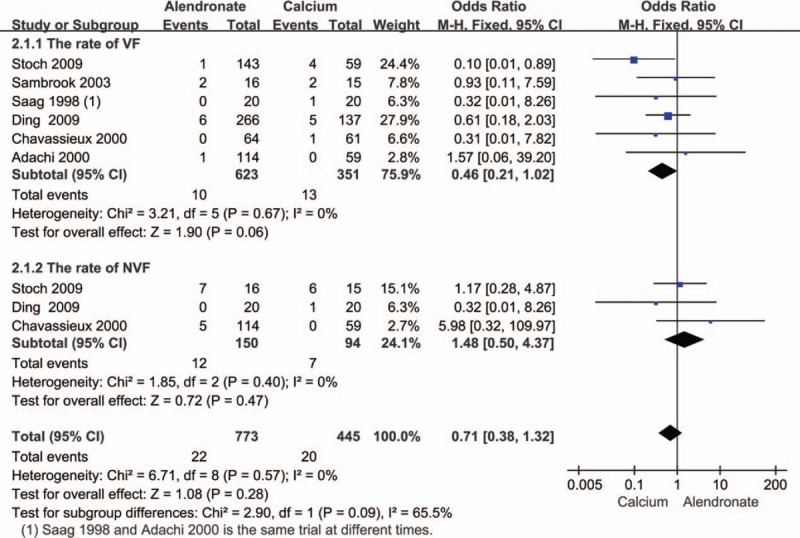
Pooled estimate of the OR for fracture incidence: treatment group (alendronate) compared to controls (calcium) in all patients receiving an average daily dose of at least 7.5 mg of prednisone. OR = odds ratio.
Gastrointestinal adverse effects were reported in 6 trials.[16,23,26–29] Pooled data from a fixed-effect model revealed no statistically significant differences with alendronate treatment (Fig. 6).
Figure 6.
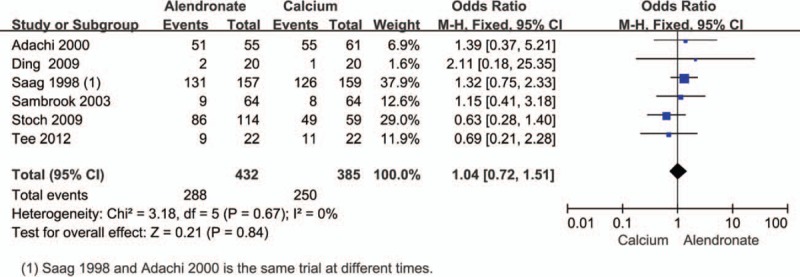
Pooled estimate of the OR for adverse effects, treatment group (alendronate) compared to controls (calcium) in all patients receiving an average daily dose of at least 7.5 mg of prednisone. OR = odds ratio.
For the regions and demographics heterogeneity of BMD, we conducted a further regional subgroup analysis of bone density changes, which was divided into American[16,26,28] and Asian populations.[22,27,29,30,32] We found that alendronate significantly increased BMD in the spine and the femoral neck of the Asia region, except of the United States (Fig. 7). For fractures and adverse reactions, we can see from Figures 5 and 6 that there is no obvious heterogeneity, and we also performed subgroup analysis by the review manage 5 software, and found no regional difference and I2 value are all <50%.
Figure 7.
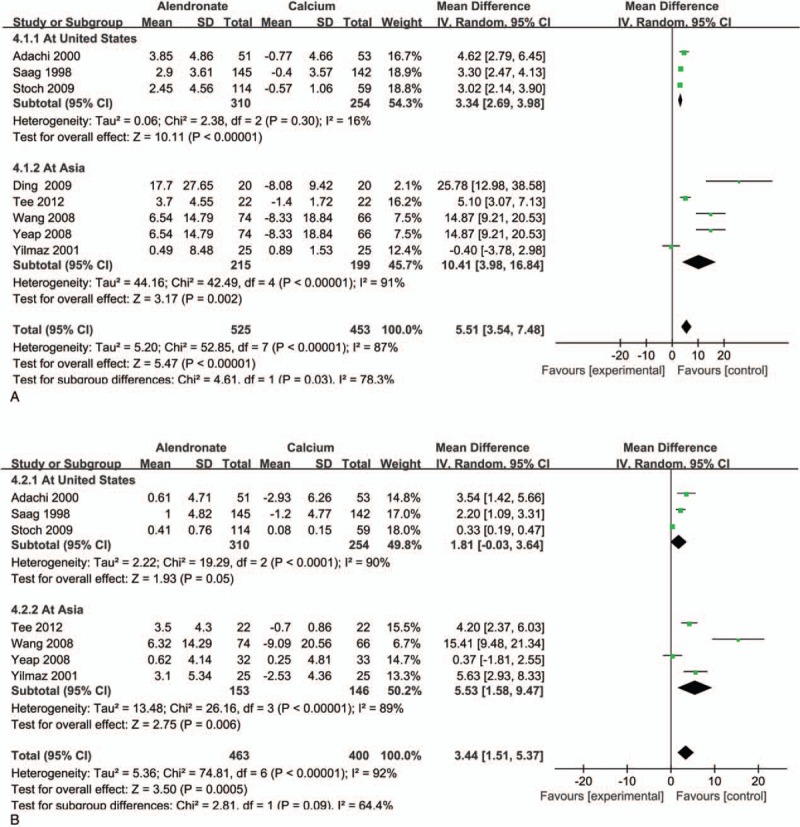
Treatment group (alendronate) versus controls (calcium) for lumbar spine BMD (A) and femoral neck BMD (B): Subgroup analyses stratified by regions. BMD = bone mineral density.
4. Discussion
In our meta-analysis of 9 randomized controlled trials, the efficacy of alendronate in treating GIO was evaluated among individuals over 18 years at different time points during treatment. With a random effect model, meta-analysis was performed and showed that alendronate treatment significantly increased lumbar spine BMD when compared with treatment with calcium during 6 to 24 months. As statistical heterogeneity was observed, the subgroup analyses were used to obtain more robust estimates at 6, 12, 24 months, respectively. The results indicate that alendronate administration significantly increased BMD of the lumbar spine. In addition, fixed effects model analysis was carried out to evaluate the effects of alendronate after 24 months administration. The same positive result was identified: pooled SMD was: 0.82 (95% CI 0.58 to 1.05, P < .00001, I2 = 38%). The femoral neck BMD increased significantly when the alendronate was taken for 6 months but no statistical difference was observed after alendronate administration for 12 or 24 month. As for nonvertebral fracture or vertebral fracture rate, no statistical differences were observed between alendronate and calcium treatments. There were also no significant differences in terms of gastrointestinal or other side effects. To our knowledge, this investigation is the first systematic analysis to evaluate the effect of alendronate on GIO as well as its side effects.
Alendronate, a second generation bisphosphonate, inhibits osteoclast activity, reduces bone resorption, and maintains the balance of bone resorption and formation.[33,34] Alendronate may also stimulate osteoblast differentiation, and prevent or mitigate bone cell and osteoblast apoptosis.[35–37] Previous studies have shown that, in postmenopausal women with or without established osteoporosis, alendronate administration over 2 to 3 years could increase bone density, while reducing vertebral fracture rate. Further, nonvertebral fractures rates were significantly reduced when treated with alendronate among postmenopausal women without prevalent fractures and have BMD levels below the World Health Organization threshold for osteoporosis.[38]
Glucocorticoid use for more than 3 months can cause bone loss and decrease BMD.[7] However, the efficacy of alendronate on the BMD as well as the relative risk of fracture in GIO patients has been reported from several studies with varying magnitude. A 48-week research study carried out by Saag et al[28] indicated that 10 mg of alendronate administration daily significantly increased the BMD of the lumbar spine (2.9 ± 0.3[28]) and the femoral-neck (1.0 ± 0.4%) in patients who were receiving glucocorticoid therapy, while in the placebo group, BMD of both lumbar spine and femoral-neck were significantly decreased. A previous meta-analysis assessed the effects of bisphosphonates of any type in the prevention and treatment of glucocorticoid induced osteoporosis.[39] Compared with placebo groups, treatment with bisphosphonates for 6 to 12 months significantly improved the BMD both at the lumbar spine and the femoral neck. However, it should be noted that after 12 months of treatment, no significant differences were observed for femoral neck BMD. Patients who used alendronate for 6 to 24 months had greater femoral neck BMD and these studies were heterogeneous, but subgroup analysis suggested that these increases were not statistically significant. These results indicate that improvement of BMD of the femoral-neck was not as significant as that of the lumbar spine.[20,28] The reason for this phenomenon may be that the lumbar spine contains more trabecular bone and corticosteroid associated bone loss is greater in bones with higher trabecular bone ratio.[32] To some extent, similar results were obtained in our study compared with Kan's study. However, Kan's paper focuses on the population of rheumatoid patients with long-term glucocoticoid-induced osteoporosis, while we included other patients who suffered rheumatoid arthritis, systemic lupus erythematous, or autoimmune disease. Alendronate has a significant effect on the increase in bone mineral density in the spine and femoral neck, but there is no significant difference on the fracture and the adverse reaction compared with the control group.[40] In Allen's paper, the meta-analysis aimed at Bisphosphonate effects, including not only alendronate, but also pamidronate, etidronate, and other drug was summarized and analyzed. At the lumbar spine, there is an absolute increase in BMD of 3.5% with bisphosphonates. At the femoral neck, the absolute difference in BMD is 2.06% higher in the bisphosphonate group compared to the control group. There was high-certainty evidence that bisphosphonates are beneficial in reducing the risk of vertebral fractures with data extending to 24 months of use. There was low-certainty evidence that bisphosphonates may make little or no difference in preventing nonvertebral fractures, which was different with our results.[41]
It is important to evaluate the effects of alendronate on fracture prevention. In postmenopausal women, previous system review studies have indicated that alendronate administration could reduce vertebral and nonvertebral fractures.[38,42] The same meta-analysis found that in GIO patients, although there was a 24% reduction in odds of spinal fractures after treatment with bisphosphonate, the results were not statistically significant.[39]
Although all the meta-analysis of bone density results are relatively robust, the number of fractures in the prevention trials were very small and the CIs very wide. As a result, our analysis had very little power to detect differences in relative risk reduction of fracture with alendronate.[38,39] Whether alendronate administration can reduce the rate of both vertebral and nonvertebral fractures in GIO patients, due to a dearth of large number RCT evidence, this conclusion requires further investigation in the future.
Although there were no differences in serious adverse effects, such as requiring hospitalization, life-threatening events or mortality among placebo, 5 or 10 mg alendronate treated GIO patients, a small but significant increase in nonserious upper gastrointestinal effects was found in the 10 mg treated group.[28] More patients receiving bisphosphonate treatment withdrew because of adverse effects (OR: 6.01, 95% CI 1.58–22.93, P = .0087), and there was a statistical significance.[39] As gastrointestinal adverse effects are one of the main adverse effects of alendronate, our investigation mainly focused on gastrointestinal adverse effects, as well as other serious adverse effects. There were no significant differences observed between alendronate and placebo treated GIO patients. As for other bisphosphonate agents, such as ibandronate and pamidronate, their side effects in treating GIO patients require further investigation. It should be noted that some patients were excluded in RCTs if they had a certain disease history, and therefore, the participants tended to be healthier with fewer co-morbid diseases, and the evaluated adverse effects may not be generalized to clinical practice.[38]
4.1. Limitations of study
Although our results do provide evidence of an association between alendronate and the BMD of lumbar spine and femoral neck, some limitations of our meta-analysis need to be addressed. All adults of GIO were incorporated into our meta-analysis, but they were not selectively included and no stratified analysis based on sex, ethics and comorbidity was performed. However, all these stratifying factors may result in selective bias on the analysis of BMD. Additionally, some of the included data were extracted by measurements extracted from figures; therefore information bias may be a limitation in this investigation.
In general, when alendronate is taken for more than 6 months or 1 year, many adverse reactions may potentially occur, including nausea, vomiting, abdominal pain, diarrhea, peptic ulcer, esophagitis, upper digestive symptoms and calcium hyperlipidemia or other complications. Because adverse reactions caused by the digestive system are the main adverse reactions of the drug, this likely would result in many patients potentially ceasing their medication. There have been previous data and summarizes about these GI related adverse effects in the literature, and therefore, our meta-analysis was mainly focused on digestive symptoms. As for the control group, our investigation did not study in a separate analysis whether calcium or vitamin D has any significant side effects.
5. Conclusions
In summary, for patients with GIO, alendronate treatment can significantly increase BMD of the lumbar spine and femoral neck compared with calcium treatment, but did not show superiority over calcium in reducing vertebral fracture or nonvertebral fracture incidences or related to adverse effects. The efficacy and safety of long-term alendronate in treating GIO should be further investigated in carefully designed future trials.
Author contributions
Conceived, designed the experiments and wrote the paper: Ya-Kang Wang. Study selection: Jian-bin Guo. Analyzed the data: Chao Zhu. Jian-bin Guo, Chao Zhu, and Zhuojing Luo contributed equally to this work.
Formal analysis: Si-Qing Qin.
Investigation: Ya-Kang Wang, Chao Zhu.
Methodology: Tao Ma.
Software: Xu Wang.
Supervision: Yu-min Zhang, Zhuojing Luo.
Writing – original draft: Ya-Kang Wang.
Writing – review & editing: Ya-Kang Wang, Jian-bing Guo.
Footnotes
Abbreviations: BMD = bone mineral density, CI = confidence interval, GIO = glucocorticoid-induced osteoporosis, OR = odds ratio, RCTs = randomized controlled trials, SMD = standardized mean differences, WMD = weighted mean difference.
JBG, CZ, and ZL contributed equally to this work.
This project was supported by the Medjaden Academy & Research Foundation for Young Scientists and the science and technology project of Xi ’an Health Bureau (J2014032).
The authors have no conflicts of interest to disclose.
References
- [1].Sambrook P, Birmingham J, Kempler S, et al. Corticosteroid effects on proximal femur bone loss. J Bone Miner Res 1990;5:1211–6. [DOI] [PubMed] [Google Scholar]
- [2].Saag KG. Low-dose corticosteroid therapy in rheumatoid arthritis: balancing the evidence. Am J Med 1997;103:31S–9S. [DOI] [PubMed] [Google Scholar]
- [3].Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis 2002;61:32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohen S, Levy RM, Keller M, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 1999;42:2309–18. [DOI] [PubMed] [Google Scholar]
- [5].Wallach S, Cohen S, Reid DM, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 2000;67:277–85. [DOI] [PubMed] [Google Scholar]
- [6].Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 2006;39:253–9. [DOI] [PubMed] [Google Scholar]
- [7].van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002;13:777–87. [DOI] [PubMed] [Google Scholar]
- [8].Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am 2005;34:341–56. viii-ix. [DOI] [PubMed] [Google Scholar]
- [9].Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 2004;19:893–9. [DOI] [PubMed] [Google Scholar]
- [10].Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci 2002;966:73–81. [DOI] [PubMed] [Google Scholar]
- [11].Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 2003;9:2643–58. [DOI] [PubMed] [Google Scholar]
- [12].Klein RG, Arnaud SB, Gallagher JC, et al. Intestinal calcium absorption in exogenous hypercortisonism. Role of 25-hydroxyvitamin D and corticosteroid dose. J Clin Invest 1977;60:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suzuki Y, Ichikawa Y, Saito E, et al. Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism 1983;32:151–6. [DOI] [PubMed] [Google Scholar]
- [14].Rubin MR, Bilezikian JP. Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 2002;87:4033–41. [DOI] [PubMed] [Google Scholar]
- [15].Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int 2008;82:249–57. [DOI] [PubMed] [Google Scholar]
- [16].Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001;44:202–11. [DOI] [PubMed] [Google Scholar]
- [17].Sawka AM, Papaioannou A, Adachi JD, et al. Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord 2005;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Katayama K, Matsuno T. Effects of bisphosphonates on fracture incidence and bone metabolism in rheumatoid arthritis patients in general practice taking long-term corticosteroid therapy: a retrospective study. Clin Drug Investig 2008;28:149–58. [DOI] [PubMed] [Google Scholar]
- [19].Okada Y, Nawata M, Nakayamada S, et al. Alendronate protects premenopausal women from bone loss and fracture associated with high-dose glucocorticoid therapy. J Rheumatol 2008;35:2249–54. [DOI] [PubMed] [Google Scholar]
- [20].Compston JE. Alendronate increased bone mineral density but did not reduce new fractures in glucocorticoid induced osteoporosis. Gut 1999;44:780–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [22].Yeap SS, Fauzi AR, Kong NC, et al. A comparison of calcium, calcitriol, and alendronate in corticosteroid-treated premenopausal patients with systemic lupus erythematosus. J Rheumatol 2008;35:2344–7. [DOI] [PubMed] [Google Scholar]
- [23].Sambrook PN, Kotowicz M, Nash P, et al. Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. J Bone Miner Res 2003;18:919–24. [DOI] [PubMed] [Google Scholar]
- [24].Schnitzer T, Bone HG, Crepaldi G, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 2000;12:1–2. [PubMed] [Google Scholar]
- [25].Simon JA, Lewiecki EM, Smith ME, et al. Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study. Clin Ther 2002;24:1871–86. [DOI] [PubMed] [Google Scholar]
- [26].Stoch SA, Saag KG, Greenwald M, et al. Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol 2009;36:1705–14. [DOI] [PubMed] [Google Scholar]
- [27].Ding XK, Yi L, Xu YL, et al. Compound of vitamin D and Calcium or combining with bisphosphonates in preventing bone loss induced by glucocorticoid. Chin J Clin Pharmacol Ther 2009;14:332–6. [Google Scholar]
- [28].Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 1998;339:292–9. [DOI] [PubMed] [Google Scholar]
- [29].Tee SI, Yosipovitch G, Chan YC, et al. Prevention of glucocorticoid-induced osteoporosis in immunobullous diseases with alendronate: a randomized, double-blind, placebo-controlled study. Arch Dermatol 2012;148:307–14. [DOI] [PubMed] [Google Scholar]
- [30].Wang QH, Wu HX, Huang YL, et al. Alendronate prevents steroid-induced osteoporosis in patients with rheumatic diseases. Zhonghua Yi Xue Za Zhi 2008;88:1888–91. [PubMed] [Google Scholar]
- [31].Chavassieux PM, Arlot ME, Roux JP, et al. Effects of alendronate on bone quality and remodeling in glucocorticoid-induced osteoporosis: a histomorphometric analysis of transiliac biopsies. J Bone Miner Res 2000;15:754–62. [DOI] [PubMed] [Google Scholar]
- [32].Yilmaz L, Ozoran K, Gunduz OH, et al. Alendronate in rheumatoid arthritis patients treated with methotrexate and glucocorticoids. Rheumatol Int 2001;20:65–9. [DOI] [PubMed] [Google Scholar]
- [33].Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003;11:1–4. [DOI] [PubMed] [Google Scholar]
- [34].Madore GR, Sherman PJ, Lane JM. Parathyroid hormone. J Am Acad Orthop Surg 2004;12:67–71. [DOI] [PubMed] [Google Scholar]
- [35].Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 1999;104:1363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].von KF, Jaquiery C, Kowalsky M, et al. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 2005;26:6941–9. [DOI] [PubMed] [Google Scholar]
- [37].Giuliani N, Pedrazzoni M, Negri G, et al. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 1998;22:455–61. [DOI] [PubMed] [Google Scholar]
- [38].Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 2002;23:508–16. [DOI] [PubMed] [Google Scholar]
- [39].Homik J, Cranney A, Shea B, et al. Bisphosphonates for steroid induced osteoporosis. Cochrane Database Syst Rev 2000;CD001347. [DOI] [PubMed] [Google Scholar]
- [40].Kan SL, Yuan ZF, Li Y, et al. Alendronate prevents glucocorticoid-induced osteoporosis in patients with rheumatic diseases: A meta-analysis. Medicine (Baltimore) 2016;95:e3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016;10:CD001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Karpf DB, Shapiro DR, Seeman E, et al. Prevention of nonvertebral fractures by alendronate. A meta-analysis. Alendronate Osteoporosis Treatment Study Groups. JAMA 1997;277:1159–64. [PubMed] [Google Scholar]


