Abstract
The present study aims to analyze the risk factors for metabolic bone disease (MBD) of prematurity.
A total of 238 preterm infants who were born at <34 weeks of gestation and were hospitalized for at least 6 weeks in the Department of Neonatology, Fujian Maternity and Children Hospital between January 1, 2011 and November 30, 2015 were enrolled in the study. Sixteen preterm infants diagnosed with MBD were selected as the case group, and 32 non-MBD preterm infants were matched 2:1 at admission into the study. The 2 groups were compared to examine the differences in maternal obstetric conditions, conditions during parturition, neonatal conditions, and neonatal diseases and treatments. The risk factors for MBD of prematurity were analyzed using t tests, χ2 tests, and a logistic regression model.
The mean gestational age and birth weight of the case group were significantly lower (P < .05) than those of the control group. Compared with the control group, the case group had a significantly higher ratios of small-for-gestational-age infants, antenatal maternal corticosteroids use, sedative use, ventilator use, aminophylline use, diuretic use, liver function impairment, vitamin D (VitD) supplementation at more than 14 days of age, achievement of total enteral nutrition (TEN) beyond 28 days of age, and feeding intolerance.
Logistic regression analysis showed that birth at <30 weeks of gestation, VitD supplementation at >14 days of age, and achievement of TEN beyond 28 days of age were independent risk factors for MBD (P < .05).
Level of Evidence: IV
Keywords: infants, metabolic bone disease, nutrition, premature, risk factors
1. Introduction
Metabolic bone disease (MBD) of prematurity is a metabolic disease in preterm infants due to disorders in calcium and phosphorus metabolism. The clinical manifestations include abnormal bone mineral content, decreased trabecular bone, cortical thinning, and other skeletal changes. Severe cases may present with rickets-like symptoms and even fractures. MBD occurs mainly in extremely preterm infant, especially in very-low- or ultra-low-birth-weight infants. The condition often occurs in preterm infants 6 to 8 weeks after birth.[1] MBD is a serious complication in the late neonatal period, and it has a negative impact on the growth and development of preterm infants. Short-term, this disease can cause ventilator dependence and increase the risk of fractures, and it can result in increased risk of myopia, kidney failure, and abnormal bone development, which impairs respiratory function, affects adult height or lead to senile osteoporosis in the long term.[1–3] Early clinical diagnosis of MBD is difficult, and clinical manifestations often lag far behind blood biochemical changes or bone changes seen on x-ray. Therefore, a comprehensive diagnosis of MBD is largely made based on declines in serum calcium and phosphorus, elevation in alkaline phosphatase (ALP) and parathyroid hormone (PTH), and osteopenia or fractures revealed by x-ray, among other characteristics.
The understanding of MBD has grown in European and American countries in recent years. These developed countries have improved the nutrient management strategy for preterm infants, modified the clinical intervention methods, and strengthened the monitoring of high-risk infants, all of which help to gradually decrease the incidence of MBD and improve the quality of life for preterm infants. However, in-depth research and a systematic understanding of MBD of prematurity remain lacking in developing countries, from which too few relevant reports contribute to insufficient awareness and prevention of MBD. Owing to the rise and development of perinatal medicine, as well as the markedly improved technical levels in neonatal intensive care units (NICUs) in developing countries, the medical treatment and diagnosis capacity for preterm infants has significantly improved, and the survival rate of preterm infants has substantially increased. Cases of prematurity with MBD are not uncommon, and these require urgent attention. Preterm infants have insufficient innate reserves of minerals, and it is difficult to supplement mineral elements through the gastrointestinal tract in the early postnatal stage. Moreover, the primary disease treatment often requires extensive medical intervention. Together, these factors frequently lead to disorders in calcium and phosphorus metabolism and to bone loss, which together initiate the development of MBD. The present study analyzed the risk factors for MBD of prematurity to provide some suggestions for the prevention and control of this condition.
2. Materials and methods
2.1. Participants
A total of 249 preterm infants were retrospectively analyzed. All infants were born at <34 weeks of gestation and remained hospitalized in the Department of Neonatology of the authors’ hospital, for at least 6 weeks between January 1, 2011 and November 30, 2015. We excluded patients who were admitted at >1 day of age and preterm births complicated by a congenital chromosomal abnormalities or an inherited metabolic disease. A total of 238 cases were included. Sixteen preterm infants (9 boys and 7 girls) with the following features during hospitalization were chosen for the case group[4]: rickets-like clinical manifestations; serum phosphorus <1.3 mmol/L and/or ALP >800 mmol/L, and/or PTH>130 pg/mL; x-ray findings including loss of metaphyseal sclerotic line; diaphyseal cortical thinning; thinning of the vertebral endplates with an “empty vertebral body”; evidence of osteopenia plus metaphyseal cupping, fraying or irregularity; physeal widening especially of the wrist or knee; cupping or enlargement of the anterior rib ends at the costochondral junction; and/or acute or healing fracture.[5] Thirty-two non-MBD preterm infants were included in the control group and were matched 2:1 at admission. The development of MBD of prematurity or hospital discharge was the outcome. The study was approved by the Ethical Committee of the authors’ hospital. Informed consent was obtained from all individual participants (from their parents or guardians) included in the study.
2.2. Methods
Medical records were examined retrospectively. The following data were recorded: maternal conditions, including the number of births, pregnancy complications (e.g., gestational hypertension and gestational diabetes), endocrine disorders (e.g., parathyroid dysfunction), prenatal abnormalities of calcium and phosphorus metabolism, and prenatal steroid hormone use; conditions during parturition, including the mode of delivery and the presence of birth asphyxia; neonatal conditions, including gestational age, sex, birth weight, and the presence of small for gestational age (SGA); and neonatal disease and treatment, including the use of a ventilator, parenteral nutrition, sedatives, corticosteroids, aminophylline, and diuretics, as well as early supplementation of calcium, phosphorus, and vitamin D (VitD). The case and control groups were compared to analyze the differences in the above characteristics.
2.3. Statistical analysis
Data acquisition and data analysis were accomplished by different individuals. Data analysis was performed using SPSS 16.0, by one of the authors, who was blinded to data acquisition and grouping. Data with a normal distribution are expressed as ¯x ± sd; intergroup comparison was performed using the 2 independent samples t test. Count data are described using frequencies and percentages; the intergroup comparison of count data was performed using the χ2 test. Multifactorial analysis was conducted using forward stepwise logistic regression analysis. P < .05 was considered to indicate statistical significance.
3. Results
3.1. Incidence of MBD
The incidence of MBD in preterm infants was 6.7% (16/238). In the case group, 3 of 16 cases suffered fracture and x-ray examination indicated there was rickets-like changes in 13 cases. Their mean gestational age was 28.8 ± 1.5 weeks, and their mean birth weight was 1023.8 ± 192.5 g.
3.2. Unifactorial analysis of risk factors for MBD
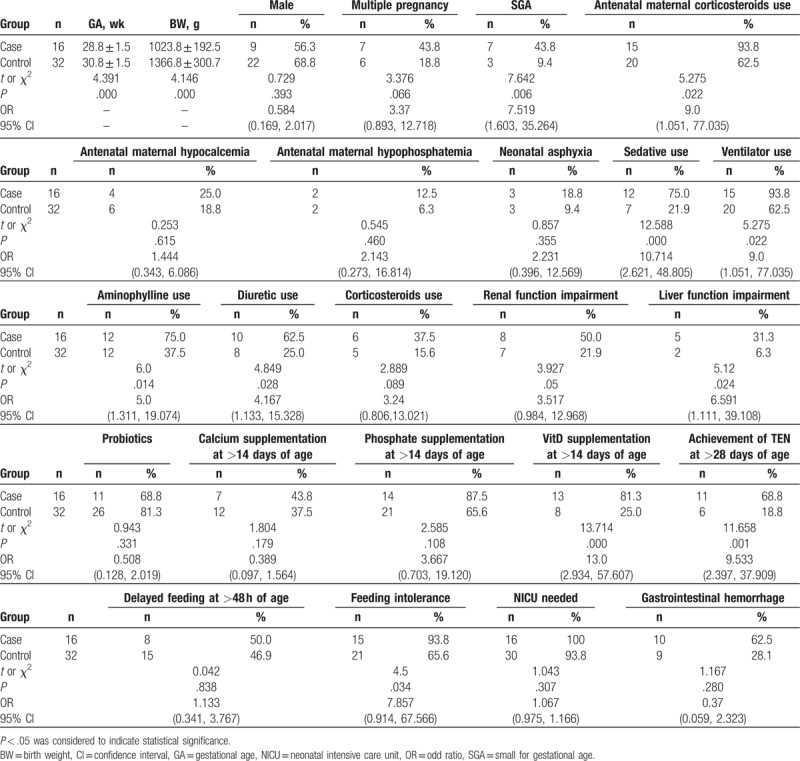
The case group had a significantly lower gestational age and birth weight than the control group, and they also had significantly higher use of antenatal maternal corticosteroids, sedatives, ventilators, aminophylline, and diuretics. In addition, the case group had a significantly higher ratio of SGA infants as well as significantly greater liver function impairment, VitD supplementation at >14 days of age, achievement of total enteral nutrition (TEN) at >28 days of age, and feeding intolerance (Table 1).
Table 1.
Unifactorial analysis of risk factors for metabolic bone disease of prematurity between the case and control groups.
3.3. Multiple logistic regression analysis of risk factors for MBD
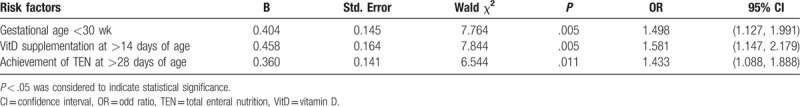
Relevant factors selected by the unifactorial analysis were subjected to nonconditional multiple logistic regression analysis, and an α value of 0.05 and a β value of 0.10 were obtained. The results showed that gestational age of <30 weeks, achievement of TEN beyond 28 days of age, and VitD supplementation after 14 days of age were retained in the stepwise regression equation. These 3 factors were therefore identified as the main factors influencing the development of MBD in preterm infants (Table 2).
Table 2.
Multiple logistic regression analysis of risk factors for metabolic bone disease of prematurity.
4. Discussion
The development of MBD of prematurity is associated with gestational age at birth. The incidence of MBD of prematurity increases at younger gestational ages and lower birth weights.[1] The incidence of MBD is up to 30% in preterm infants born at <28 weeks of gestation, and approximately 10% of preterm infants suffer fractures by a corrected gestational age of 36 to 40 weeks.[1,6,7] Our results also show that young gestational age was a risk factor for the development of MBD of prematurity. Premature birth leads to MBD mainly because premature birth results in deficient mineral reserves in the fetus. The last 3 months of pregnancy are important because the fetus acquires 80% of calcium and phosphorus reserves between 25 and 40 weeks of gestation.[8,9] In this period, the mean deposition rates of calcium and phosphorus are 100 to 120 and 50 to 65 mg/kg/day, which provide the newborn with 20 and 10 g of calcium and phosphorus reserves. If preterm birth occurs in this period, the newborn will partially or completely miss the optimal stage of acquiring calcium and phosphorus reserves.[10] A study by Henriksen et al[11] showed that serum 25-(OH)D3 levels are significantly affected by gestational age in preterm infants born at 25 to 35 weeks of gestation; suggesting that preterm birth can also affect VitD reserves in newborns. In addition, the younger the gestational age, the less the organ systems have matured, and the likelihood of requiring drug intervention increases. The use of aminophylline, diuretics, and steroid hormones can also lead to the loss of bone minerals as well as to disorders in calcium and phosphorus metabolism.[9,12]
The present study found that late achievement of TEN was a risk factor for MBD of prematurity. Preterm infants, particularly those with very-low-birth weight or ultra-low-birth weight, are often unable to feed in the early postnatal period because of illness or have difficulties achieving TEN in the short term, and thus requiring long-term parenteral nutrition. However, the formulation of parenteral nutrition often fails to provide a sufficient or available mineral supply because of various factors, including a deficiency of corresponding mineral formulations, poor solubility of minerals, mutual antagonism of nutrients, impact of the pH value, and limit of the volume of intravenous fluid for preterm infants because of illness.[13] Consequently, calcium and phosphorus depositions in preterm infants during the early postnatal period cannot meet the requirements of the intrauterine bone growth rate.[10,14] Furthermore, it has been reported that aluminum contamination of parenteral nutrition can cause MBD.[15] A study reported that the bone aluminum content of preterm infants receiving parenteral nutrition for >3 weeks was 10-fold that of the control group.[16,17] Aluminum contamination of parenteral nutrition can result in excessive aluminum deposition at the mineralized surface of bone, which affects osteoblast activity and hinders bone formation, ultimately leading to osteomalacia. This seriously affects the bone health of infants in both the short and long terms.
Fortified formula has a suitable proportion of minerals and balanced nutrients, and the proportion of calcium and phosphorus is similar to that of breast milk. Thus, fortified formula can provide an optimal calcium and phosphorus deposition rate during intrauterine growth. For example, the gastrointestinal absorption rate of phosphorus can exceed 90% during enteral feeding.[18] Therefore, enteral nutrition has unparalleled advantages for ensuring the absorption efficiency of minerals, including calcium and phosphorus, in preterm infants during the early postnatal period.
A lack of VitD supplementation in the early postnatal period is associated with the development of MBD.[18,19] The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends 800 to 1000 IU/day of VitD for preterm infants in the first week after birth. This dose can ensure more than 80 nmol/L of 25-(OH)D3 and satisfy the metabolic needs of preterm infants.[7] In the present study, 13 preterm infants in the case group received no VitD supplementation within 14 days of birth, and 4 cases only received attention and intervention after the development of MBD. The mechanism of VitD acts through 1, 25-(OH)2D3, a major hormone involved in maintaining the balance of calcium and phosphorus metabolism. The 1,25-(OH)2D3 hormone acts on the intestine, kidney, and bone, among other target organs to regulate the transport, deposition, and release of calcium and phosphorus; 1,25-(OH)2D3 is essential to ensure the bone conversion of calcium and phosphorus. Insufficient long-term intake of VitD can result in a series of pathophysiological changes, including decreased intestinal absorption of calcium and phosphorus, increased urinary excretion of calcium and phosphorus, and disorders in osteogenesis. The catch-up growth of preterm infants after birth requires a high nutrient demand. However, the VitD reserves of preterm infants are insufficient at birth, while a lack of sunlight due to long-term hospitalization also affects VitD production in the body. In addition, the primary disease condition often leads to delayed feeding or repeated fasting that also negatively affects the early administration of VitD. All of these factors can lead to a serious deficiency of VitD in preterm infants and thus harm the balance of calcium and phosphorus in vivo. This imbalance will cause a lack of calcium and phosphorus as well as disorders in bone metabolism, leading to the development of MBD.[19]
4.1. Limitations
This study has several limitations. First, our sample size seemed relatively small and that may influence the results. As we know, in general, the incidence of MBD is very low in newborns, but it increases at younger gestational ages and lower birth weights, particularly for the newborns with a gestational age <28 weeks. The level of technology in the NICU is markedly improved in China, but the expensive treatment cost and the possible sequel make many young couples suspend treatment of their extremely preterm infants. Moreover, due to the unbalanced development of the economy and society in China, it is still a common problem and cannot be solved in the short term. Therefore, long-term survival of extremely preterm infant is not always assured. However, the sample source in our study was collected from the authors’ hospital, which is a top neonate emergency center in Fujian Province and the vast majority of the extremely preterm infants of this region were treated here. Although the sample size seems small in our study the data from the infants was precious and representative because it likely reflects the current state of MBD in Fujian Province, China. Second, our study should also investigate factors which may mediate the incidence of MBD, yielding a certainty about the relative contribution of nonspecific factors in the outcome and identifying specific and nonspecific factors which may help us to formulate intervention. Third, follow-up data should be included. Longitudinal samples of high risk of MBD are ideal for this type of study, as it is possible to evaluate the subjects on a baseline before they are exposed to risk factors.
5. Conclusions
MBD is a serious disease affecting the short-term and long-term physical development of preterm infants. Premature birth, lack of TEN, and early VitD deficiency are the major risk factors for MBD. For the prevention and treatment of MBD, it is important to guarantee perinatal care, actively prevent premature birth, and appropriately increase the balanced intake of calcium, phosphorus, and VitD in the third trimester of pregnancy. In the early postnatal period, sufficient minerals should be supplied for preterm infants through parenteral nutrition, and make the transition to TEN as soon as possible. Oral supplementation of calcium, phosphorus, and VitD is needed when enteral nutrition is first established. For primary disease treatment, caution must be taken to avoid the use of drugs that may lead to decreased absorption or increased excretion of mineral elements and VitD, such as steroid hormones, aminophylline, diuretics, or sedatives. For preterm infants at risk, it is necessary to perform dynamic monitoring of blood biochemistry as well as to make an early diagnosis and undertake timely interventions to reduce complications and improve the prognosis, all of which improve the quality of life of preterm infants.
Acknowledgments
The authors appreciate the dedication of the study participants and their families.
Author contributions
Conceptualization: Wenhao Chen, Changyi Yang, Hanqiang Chen.
Data curation: Wenhao Chen, Changyi Yang.
Formal analysis: Baoquan Zhang.
Funding acquisition: Wenhao Chen.
Methodology: Changyi Yang.
Project administration: Changyi Yang.
Software: Baoquan Zhang.
Writing – original draft: Wenhao Chen, Changyi Yang, Baoquan Zhang.
Writing – review and editing: Hanqiang Chen.
Footnotes
Abbreviations: ALP = alkaline phosphatase, MBD = metabolic bone disease, NICU = neonatal intensive care unit, PTH = parathyroid hormone, SGA = small for gestational age, TEN = total enteral nutrition, VitD = vitamin D.
The study was funded by the Provincial Natural Science Foundation of Fujian, China (grant 2015J05153). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
References
- [1].Vachharajani AJ, Mathur AM, Rao R. Metabolic bone disease of prematurity. NeoReviews 2009;10:e402–11. [Google Scholar]
- [2].Tosun Ö, Bayat M, Günes T, et al. Daily physical activity in low-risk pre-term infants: positive impact on bone strength and mid-upper arm circumference. Ann Hum Biol 2011;38:635–9. [DOI] [PubMed] [Google Scholar]
- [3].Chin LK, Doan J, Teoh YSL, et al. Outcomes of standardised approach to metabolic bone disease of prematurity. J Paediatr Child Health 2018;54:665–70. [DOI] [PubMed] [Google Scholar]
- [4].Cloherty JP, Eichenwald EC, Stark AR. Manual of Neonatal Care. 2007;UK: Lippincott Williams & Wilkins, 555-557. [Google Scholar]
- [5].Tkach EK, WhiteF AM, Dysart KC, et al. Comparison of intact parathyroid hormone, alkaline phosphatase, phosphate levels for diagnosing severe metabolic bone disease in infants with severe bronchopulmonary dysplasia. Am J Perinatol 2017;34:1199–204. [DOI] [PubMed] [Google Scholar]
- [6].Backström MC, Kuusela AL, Mäki R. Metabolic bone disease of prematurity. Ann Med 1996;28:275–82. [DOI] [PubMed] [Google Scholar]
- [7].Harrison CM, Johnson K, McKechnie E. Osteopenia of prematurity: a national survey and review of practice. Acta Paediatr 2008;97:407–13. [DOI] [PubMed] [Google Scholar]
- [8].Nehra D, Carlson SJ, Fallon EM, et al. American Society for Parenteral and Enteral Nutrition A. S. P. E. N. Clinical guidelines: nutrition support of neonatal patients at risk for metabolic bone disease. JPEN J Parenter Enteral Nutr 2013;37:570–98. [DOI] [PubMed] [Google Scholar]
- [9].Lothe A, Sinn J, Stone M. Metabolic bone disease of prematurity and secondary hyperparathyroidism. J Paediatr Child Health 2011;47:550–3. [DOI] [PubMed] [Google Scholar]
- [10].Mimouni FB, Mandel D, Lubetzky R, et al. Calcium, phosphorus, magnesium and vitamin D requirements of the preterm infant. World Rev Nutr Diet 2014;110:140–51. [DOI] [PubMed] [Google Scholar]
- [11].Henriksen C, Helland IB, Rønnestad A, et al. Fat-soluble vitamins in breast-fed preterm and term infants. Eur J Clin Nutr 2006;60:756–62. [DOI] [PubMed] [Google Scholar]
- [12].Viswanathan S, Khasawneh W, Mcnelis K, et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. JPEN J Parenter Enteral Nutr 2014;38:982–90. [DOI] [PubMed] [Google Scholar]
- [13].Abrams SA. Committee on Nutrition Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics 2013;131:e1676–83. [DOI] [PubMed] [Google Scholar]
- [14].Bozzetti V, Tagliabue P. Metabolic bone disease in preterm newborn: an update on nutritional issues. Ital J Pediatr 2009;35:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lima-Rogel V, Romano-Moreno S, De Jesús López-López E, et al. Aluminum contamination in parenteral nutrition admixtures for low-birth-weight preterm infants in Mexico. JPEN J Parenter Enteral Nutr 2014;40:1014–20. [DOI] [PubMed] [Google Scholar]
- [16].Sedman AB, Klein GL, Merritt RJ, et al. Evidence of aluminum loading in infants receiving intravenous therapy. N Engl J Med 1985;312:1337–43. [DOI] [PubMed] [Google Scholar]
- [17].Holland PC, Wilkinson AR, Diez J, et al. Prenatal deficiency of phosphate, phosphate supplementation, and rickets in very-low-birthweight infants. Lancet 1990;335:697–701. [DOI] [PubMed] [Google Scholar]
- [18].Taheri PA, Sajjadian N, Beyrami B, et al. Prophylactic effect of low dose vitamin d in osteopenia of prematurity: a clinical trial study. Acta Med Iran 2014;52:671–4. [PubMed] [Google Scholar]
- [19].Pieltain C, De Halleux V, Senterre T. Prematurity and bone health. World Rev Nutr Diet 2013;106:181–8. [DOI] [PubMed] [Google Scholar]


