Supplemental Digital Content is available in the text
Keywords: diuresis, intensive care, lactate, microcirculation, mottling, pulmonary embolism, tissue perfusion
Abstract
We aimed to assess the relationship between alterations of tissue perfusion parameters at admission (highly predictive of mortality in septic shock) and outcome in patients admitted to the intensive care unit (ICU) for acute pulmonary embolism (PE). We conducted a retrospective study to analyze the association between arterial lactate level, skin mottling and urinary output, and 28-day mortality.
Over a 22-year period, 317 patients with PE were identified but we finally analyzed 108 patients whose main diagnosis for ICU admission was acute PE. At admission, the sequential organ failure assessment score was 2 (0–6) and the simplified acute physiology score II was 29 (16–43). Thirty patients (28%) received vasopressors and 37 patients (34%) received thrombolytic therapy. Day 28 mortality rate was 25% (n = 27). When compared to 28-day survivors, nonsurvivor patients had higher lactate level (4.5 [2.3–10.3] mmol/L vs 1.4 [1–2.9] mmol/L, P < .0001), more frequent mottling around the knee area (56% vs 25%, P = .003) and a lower urinary output (during the first 6 hours) (0.35 [0–1] mL/kg/h vs. 0.88 [0.62–1.677] mL/kg/h, P = .0002). Mortality increased with the number of tissue perfusion alterations present upon admission, 8% for none, 21% for 1, 28% for 2, and finally reached 85% for 3 tissue perfusion alterations (P < .0001). In a multivariate analysis, the relationship between the number of tissue perfusion alterations and 28-day mortality was maintained after adjustment on the presence of shock and right ventricular dilation at admission.
In ICU patients admitted for acute PE, tissue perfusion alterations correlated with 28-day mortality independently of blood pressure and right ventricular dilation.
1. Introduction
Pulmonary embolism (PE) is the 3rd cause of cardiovascular death worldwide. More than 600,000 patients suffering from PE are admitted to the hospital every year in the United States but <5% of them die within 3 months.[1–5] Based on these epidemiologic data, experts emphasize the importance of early stratification to identify patients with PE at high risk of death, who may benefit from more intensive monitoring or aggressive therapy.[6,7]
Recent international guidelines defined “high risk” population in the presence of persistent arterial hypotension (<90 mm Hg) or shock. However, hemodynamic parameters defining shock status in the context of PE remain unclear and their correlation to outcome has never been evaluated. In critically ill patients admitted in the intensive care unit (ICU) for septic shock or myocardial infarction-related cardiogenic shock, several groups have reported that alterations of microcirculation blood flow are strongly predictive of mortality independently of arterial blood pressure or cardiac output.[8–10] Microcirculatory perfusion could be evaluated using computer-assisted devices but these tools are not available at bedside. Tissue perfusion could also be assessed with more simple tools including arterial lactate level or noninvasive clinical parameters such as skin mottling or urinary output. In our department, we are interested in clinical evaluation of organ perfusion and we have showed that skin mottling, reflecting skin hypoperfusion, correlated with organ failure and predicted mortality in sepsis[11] and septic shock patients.[12] In this study, we aimed at investigating the relationship between 28-day mortality and tissue perfusion parameters, that is, arterial lactate level, skin mottling and urinary output, in patients admitted to ICU for acute PE.
2. Materials and methods
We conducted a retrospective observational study in a 16-bed ICU in a tertiary teaching hospital. All consecutive patients older than 18 years admitted for acute PE from January 1993 to December 2015 were included. Patients were identified by querying the electronic health records with the following keywords (in French): “pulmonary embolism,” “acute respiratory failure,” “anti-coagulant,” “deep venous thrombosis,” “cardiac arrest,” “heparin,” “vitamin K antagonists,” “cava filter,” or “thrombolysis.”
General characteristics of patients were recorded: demographic data, diagnoses, severity of illness evaluated by the sequential organ failure assessment (SOFA) score and simplified acute physiology score II (SAPS II). Global hemodynamic variables, right ventricle (RV) dilation and tissue perfusion parameters were collected at ICU admission. RV dilation was defined as a right to left ventricle diameter ratio superior or equal to 1 on echocardiography or computed tomography (CT) scan. We recorded 3 tissue perfusion parameters at admission: arterial lactate level, the presence/absence of skin mottling around the knee area, and mean urinary output (within the first 6 hours).
An ethical approval was not necessary because it was a monocenter retrospective study using anonymous data.
2.1. Statistical analysis
Data were summarized as median (25th–75th percentiles) for skewed distributions and percentages as appropriate. Association of tissue perfusion parameters with 28-day mortality was tested using Mann–Whitney nonparametric test for continuous variables, Fischer exact test, and the Chi-squared test for categorical data according to sample size. Finally, we used logistic regression for multivariable analysis. All statistical analyses were performed using the R software (v 2.12.0; http://cran.r-project.org). Significance was defined as 2-sided P-value <.05.
3. Results
3.1. Studied population
Over a 22-year period, we identified 317 patients with PE admitted in our intensive care department by querying the electronic records. After reviewing medical charts, 122 patients were excluded because PE was not the main diagnosis for ICU admission and 87 patients were excluded because of missing data, leaving 108 patients for study (Supplemental Figure 1). PE was routinely diagnosed using nuclear planar V/Q-imaging during the 1993 to 2008 period, whereas CT pulmonary angiography became the diagnosis test of choice between 2008 and 2015. Echocardiography use in the context of acute severe PE also increased between the 2 time periods (Supplemental Figure 2).
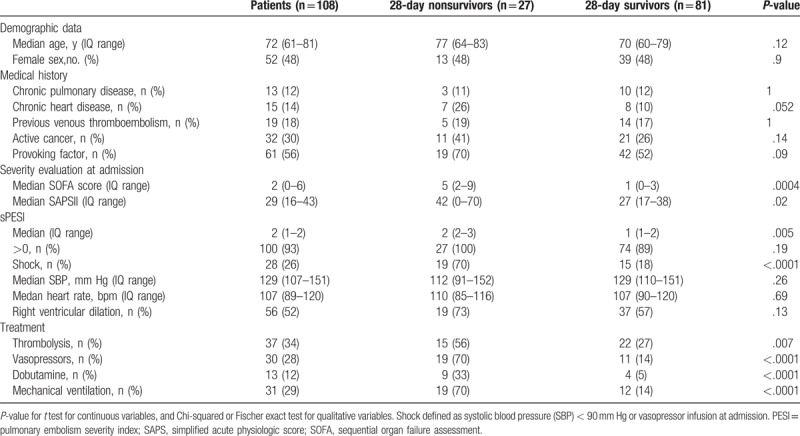
Characteristics of the studied population are summarized in Table 1. The median age was 72 (61–81) years with no gender predominance. The median length of stay was 4 (2–7) days in the ICU and 14 (8–21) days in the hospital. At admission, median SOFA score was 2 (0–6) and median SAPS II was 29 (16–43). Thirty-nine patients (36%) required support organ therapy including vasopressor infusion (n = 30, 28%) and mechanical ventilation (n = 31, 29%). Thirty-seven patients (34%) received thrombolytic therapy. Day 28 mortality rate was 25% (n = 27). Nonsurvivor patients had higher organ failure severity and received more frequently both support therapy and thrombolytic treatment.
Table 1.
General characteristics of the studied population.
3.2. Tissue perfusion parameters
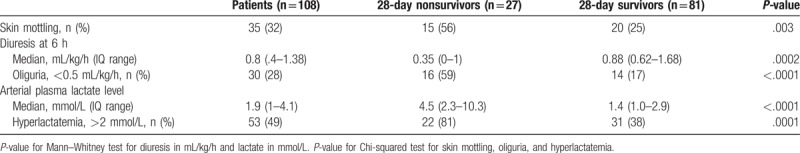
We analyzed tissue perfusion parameters at ICU admission according to 28-day outcome. When compared to survivors, nonsurvivor patients had higher arterial lactate level (4.5 [2.3–10.3] mmol/L vs 1.4 [1–2.9] mmol/L, P < .001), more frequent skin mottling around the knee area (56% vs 25%, P = .003) and lower mean 6-hour urinary output (0.35 [0–1] mL/kg/h vs 0.88 [0.62–1.68] mL/kg/h, P = .0002) (Table 2).
Table 2.
Analysis of tissue perfusion parameters at admission according to 28-day outcome.
To assess the cumulative predictive value of tissue perfusion parameters, we analyzed the relationship between 28-day mortality and the number of tissue perfusion abnormalities at admission, that is, skin mottling, hyperlactatemia (>2 mmol/L), and oliguria (<0.5 mL/kg/h). Interestingly, 28-day mortality increased with the number of tissue hypoperfusion parameters at 8%, 21%, 28%, and 85% in the presence of 0, 1, 2, and 3 alterations, respectively (P < .0001, Chi-squared for a trend test) (Fig. 1).
Figure 1.
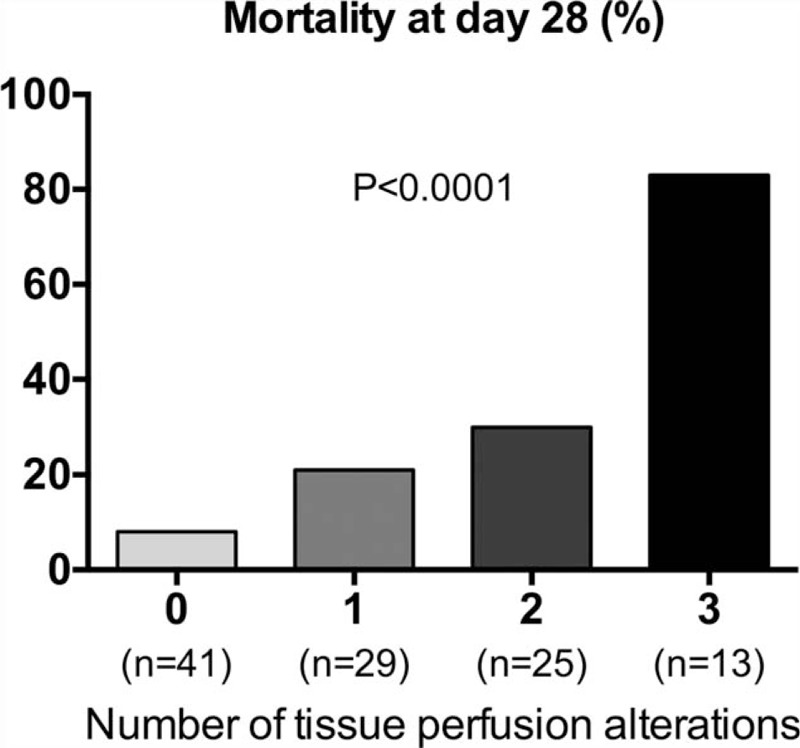
Day 28 mortality according to the number of tissue perfusion alterations. Tissue perfusion alterations were defined as the presence of skin mottling around the knee area, arterial hyperlactatemia >2 mmol/L and oliguria (mean 6-hour urinary output <0.5 mL/kg/h). Chi-squared test for a trend.
In a multivariate analysis, the relationship between the number of tissue perfusion alterations and 28-day mortality was maintained after adjustment on the presence of shock and RV dilation at admission (Table 3)
Table 3.
Multivariable logistic regression analysis of risk factors for 28-day mortality, including tissue perfusion alterations, shock, and right ventricular dilation.

4. Discussion
In this study, we found that 3 tissue perfusion parameters measured at admission, arterial lactate level, skin mottling, and urinary output were synergistically associated with 28-day mortality in ICU patients admitted for PE, independently of global hemodynamic status and right ventricular dilation.
In patients with PE, current guidelines recommend the evaluation of systolic blood pressure and RV injury to identify patients at high risk of death.[7] However, both parameters fail to fully identify patients who would benefit from more aggressive treatment (thrombectomy or fibrinolysis) with a substantial reduction on mortality without a major incidence of hemorrhagic complications.[13,14] Recently, the pathophysiology of severe PE has been extended beyond simple RV obstruction. Several studies highlighted the role of inflammation[15] and endothelial dysfunction[16] in the alteration of organ perfusion at the microcirculation level in the context of acute circulatory failure related to infection[17] or myocardial infarction.[18] In this study, we evaluated microcirculatory hypoperfusion parameters available at bedside and found these “easy to use and easy to learn” tools are helpful to identify high-risk patients in acute PE.
First, we investigated arterial lactate level the measurement of which is widely available in routine laboratories. Hyperlactatemia is a hallmark characteristic of shock states[19] and predicts organ failure and mortality[20] in the ICU, even more than refractory hypotension.[21] In patients with PE, Vanni et al reported that arterial lactate level measured in the emergency department was significantly associated with in-hospital mortality independently of systolic blood pressure and right ventricular dysfunction.[22,23] Here we confirmed these results in critically ill patients admitted to the ICU. We found that nonsurvivor patients had significantly higher lactate levels when compared to survivors. Mortality rate was higher in our study when compared to Vanni's study probably because ICU patients had more frequent comorbidities and more severe organ failure.
Next, we analyzed skin mottling, an old clinical sign, readily available at bedside. Skin mottling is characterized by locally reduced blood flow[24] and decreased tissue oxygen content. In several prospective observational studies in sepsis patients, we have showed that mottling extension is strongly predictive of mortality.[11,12] Here, for the first time, we found that the presence of skin mottling is significantly associated with 28-day mortality in patients with PE, corroborating data from the severe infection context.
Urinary output can also be routinely measured. In sepsis, urinary output monitoring is recommended to assess hemodynamic status and to guide treatment.[25] Guidelines are based on seminal studies that reported an association between oliguria and pejorative outcome.[26,27] Here, we showed that in critically ill patients with PE, urinary output was significantly lower over the first 6 hours in nonsurvivors when compared to survivors.
We have previously reported a significant relationship between these tissue perfusion parameters. Indeed, in septic shock patients, mottling extension correlates negatively with urinary output and positively with arterial lactate levels.[12] Here, we found that these parameters could be combined as the risk of mortality increased with the number of tissue perfusion alterations. In light of all these data, we believe that combining arterial lactate level, mottling and urinary output could be helpful at the bedside to improve risk stratification of patients with PE.
Our study has several limitations. First, it is a retrospective study and the predictive value of these tissue perfusion parameters has to be confirmed prospectively in a larger cohort of patients with PE. The next step would be to evaluate if clinical detection of tissue perfusion disorders could have an impact on the therapeutic management of patients with PE. Second, we did not investigate the variations of tissue perfusion parameters during resuscitation, although a reduction of lactate arterial level or a decrease in mottling skin areas over time have been both reported to be correlated with better outcome.[28,29] Third, because a large number of patients were included before 2008 international guidelines, circulating markers of RV injury, including troponin and B-type Natriuretic peptide (BNP) were not measured and not included in the risk stratification for death.
5. Conclusion
In this retrospective study, we found that 3 tissue perfusion parameters, arterial lactate level, mottling, and urinary output are significantly associated with 28-day mortality in ICU patients admitted for acute PE independently of shock and right ventricular dilation. These tissue perfusion parameters could be helpful to improve risk stratification of patients with PE.
Acknowledgment
The authors thank R. Leboursicaud for her participation and help in recovering medical files.
Author contributions
Study concept and design, all authors. Data collection T.U., Y.N. Drafting of the manuscript, T.U., N.B., E.M., B.G. and H.A.O. Critical revision of manuscript, all authors. Statistical analysis, T.U., N.B., PYB and H.A.O.
Conceptualization: Tomas Urbina, Hafid Ait-Oufella.
Data curation: Pierre-Yves Boelle, Hafid Ait-Oufella.
Formal analysis: Tomas Urbina, Pierre-Yves Boelle, Bertrand Guidet, Hafid Ait-Oufella.
Investigation: Tomas Urbina, Naike Bige, Yann Nguyen, Vincent Dubee, Jeremie Joffre, Idriss Abdallah, Jean-Luc Baudel, Hafid Ait-Oufella.
Methodology: Pierre-Yves Boelle, Vincent Dubee, Eric Maury, Bertrand Guidet, Hafid Ait-Oufella.
Project administration: Hafid Ait-Oufella.
Software: Yann Nguyen, Hafid Ait-Oufella.
Supervision: Hafid Ait-Oufella.
Validation: Tomas Urbina, Naike Bige, Yann Nguyen, Pierre-Yves Boelle, Hafid Ait-Oufella.
Visualization: Jean-Luc Baudel, Hafid Ait-Oufella.
Writing – original draft: Tomas Urbina, Naike Bige, Hafid Ait-Oufella.
Writing – review & editing: Naike Bige, Vincent Dubee, Jeremie Joffre, Jean-Luc Baudel, Eric Maury, Bertrand Guidet, Hafid Ait-Oufella.
Supplementary Material
Footnotes
Abbreviations: ICU = intensive care unit, PE = pulmonary embolism, RV = right ventricle, SAPS II = simplified acute physiologic score II, SOFA = sequential organ failure assessment, sPESI = simplified pulmonary embolism severity index.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756–64. [DOI] [PubMed] [Google Scholar]
- [2].Burge AJ, Freeman KD, Klapper PJ, et al. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol 2008;63:381–6. [DOI] [PubMed] [Google Scholar]
- [3].Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585–93. [DOI] [PubMed] [Google Scholar]
- [4].Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002;121:877–905. [DOI] [PubMed] [Google Scholar]
- [5].Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Haemost 2016;41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tapson VF. Acute pulmonary embolism. N Engl J Med 2008;358:1037–52. [DOI] [PubMed] [Google Scholar]
- [7].Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–69. [DOI] [PubMed] [Google Scholar]
- [8].De Backer D, Creteur J, Dubois M-J, et al. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 2004;147:91–9. [DOI] [PubMed] [Google Scholar]
- [9].Sakr Y, Dubois M-J, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825–31. [DOI] [PubMed] [Google Scholar]
- [10].Hernandez G, Bruhn A, Luengo C, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med 2013;39:1435–43. [DOI] [PubMed] [Google Scholar]
- [11].Preda G, Bourcier S, Joffre J, et al. Mottling score is associated with 28-day mortality in critically ill patients with sepsis. Minerva Anestesiol 2017;83:664–6. [DOI] [PubMed] [Google Scholar]
- [12].Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med 2011;37:801–7. [DOI] [PubMed] [Google Scholar]
- [13].Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11. [DOI] [PubMed] [Google Scholar]
- [14].Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414–21. [DOI] [PubMed] [Google Scholar]
- [15].Watts JA, Gellar MA, Obraztsova M, et al. Role of inflammation in right ventricular damage and repair following experimental pulmonary embolism in rats: cardiac damage, inflammation and healing. Int J Exp Pathol 2008;89:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kurtipek E, Büyükterzi Z, Büyükterzi M, et al. Endothelial dysfunction in patients with pulmonary thromboembolism: neutrophil to lymphocyte ratio and platelet to lymphocyte ratio: early endothelial dysfunction in thromboembolism. Clin Respiratory J 2017;11:78–82. [DOI] [PubMed] [Google Scholar]
- [17].De Backer D, Orbegozo Cortes D, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014;5:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation 2003;107:2998–3002. [DOI] [PubMed] [Google Scholar]
- [19].Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haas SA, Lange T, Saugel B, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 2016;42:202–10. [DOI] [PubMed] [Google Scholar]
- [21].Gotmaker R, Peake SL, Forbes A, et al. Mortality is greater in septic patients with hyperlactatemia than with refractory hypotension. Shock 2017;48:294–300. [DOI] [PubMed] [Google Scholar]
- [22].Vanni S, Socci F, Pepe G, et al. High plasma lactate levels are associated with increased risk of in-hospital mortality in patients with pulmonary embolism. Acad Emerg Med 2011;18:830–5. [DOI] [PubMed] [Google Scholar]
- [23].Vanni S, Jimenez D, Nazerian P, et al. Short-term clinical outcome of normotensive patients with acute PE and high plasma lactate. Thorax 2015;70:333–8. [DOI] [PubMed] [Google Scholar]
- [24].Ait-Oufella H, Bourcier S, Alves M, et al. Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care 2013;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vincent J-L, De Backer D. Finfer SR, Vincent J-L. Circulatory Shock. N Engl J Med 2013;369:1726–34. [DOI] [PubMed] [Google Scholar]
- [26].Vaara ST, Parviainen I, Pettilä V, et al. FINNAKI Study Group Association of oliguria with the development of acute kidney injury in the critically ill. Kidney Int 2015;doi: 10.1038/ki.2015.269. [DOI] [PubMed] [Google Scholar]
- [27].Macedo E, Malhotra R, Bouchard J, et al. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 2011;80:760–7. [DOI] [PubMed] [Google Scholar]
- [28].Liedl G, Nazerian P, Pepe G, et al. Different time course of plasma lactate, troponin I and Nt-proBNP concentrations in patients with acute pulmonary embolism. Thromb Res 2017;156:26–8. [DOI] [PubMed] [Google Scholar]
- [29].Galbois A, Bigé N, Pichereau C, et al. Exploration of skin perfusion in cirrhotic patients with septic shock. J Hepatol 2015;62:549–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


