Abstract
The aim of the present study was to evaluate the short- and long-term outcomes of secondary metastasis to the pancreas in terms of overall survival (OS) and disease-free survival (DFS) after pancreatectomy.
This retrospective study included 29 patients who underwent pancreatectomy for secondary metastasis to the pancreas between December 1995 and August 2016.
The study group was divided into renal cell carcinoma (RCC) (17 patients) and non-RCC (12 patients). The non-RCC group had 5 cases of colorectal cancer and 7 of another primary origin. The OS for the whole cohort was 86.2% at 1 year, 63.2% at 3 years, and 46.7% at 5 years. There was no significant difference between the 2 groups at 1, 3, and 5 years survival and OS. In subgroup analysis of patients who underwent curative resection, there was no significant difference in OS between the 2 groups at 1, 3, and 5 years. However, there was a significant difference in recurrence rate at 3 years (P = .035). Pathologic analysis showed that the non-RCC group had significantly more positive lymph node metastasis than the RCC group (P = .002).
Pancreatectomy for secondary metastasis has promising short- and long-term outcomes in terms of OS and DFS.
Keywords: pancreatectomy, secondary pancreas metastasis
1. Introduction
Secondary pancreatic metastasis is a rare disease, representing 2%– to 5% of all pancreatic malignancies.[1–3] The most common primary cancer that metastasizes to the pancreas is renal cell carcinoma (RCC), comprising 62.6%– to 70% of all secondary pancreatic metastases.[4,5] Other metastatic primary cancers organs include melanoma (9.1%), colorectal cancer (8.8%), sarcoma (4.5%), breast cancer (4.3%), and lung cancer (3.1%).[5]
Surgical treatment of secondary pancreatic metastasis is feasible and safe and confers some survival benefit. Its use in pancreatic metastasis from RCC is well-established[4] but there are contradictory results for other primary pancreatic metastases because of the rarity of the disease and because most studies are case reports or case series.[2,4–9] However, with improvements in perioperative management and decreased mortality and morbidity after pancreatic resection, there are more reports with promising results on pancreatectomy for metastasis from various primary organs. Furthermore, only a few reports have compared RCC with another primary pancreatic metastasis.[6,7,10] Accordingly, in this present study, we report the oncologic outcomes of pancreatic resection in patients with secondary pancreatic metastasis.
2. Methods
A retrospective analysis was conducted of all consecutive pancreatic resections due to secondary pancreatic metastasis that took place between December 1995 and August 2016 at Asan Medical Center using our database information center and electronic medical file. Eligibility criteria include: adult patients (>18 years old) who had pancreas resection due to secondary distant metastasis to pancreas but pancreas resection due direct invasion to pancreas from the primary tumor were excluded. Baseline characteristic data, including age, sex, primary cancer organ, pattern of metastasis, site of pancreatic metastasis, type of pancreatectomy, tumor size, number of tumors, resection margin, lymph node metastasis, lymphovascular invasion, surrounding structure invasion, adjuvant chemotherapy, and neoadjuvant chemotherapy, were reviewed for all patients. Outcome measures were recorded as overall survival (OS) and recurrence rate. OS was recorded for all cohort studies and for patient who underwent resection with curative intent. Recurrence was recorded for patients who underwent resection with curative intent. Palliative resection was defined as pancreatic resection with R1/R2 margin or distant metastasis not planned for resection. The follow-up time (1, 3, and 5 years) was defined as the time elapsed from pancreatic resection until the end of the follow-up period.
Because the pancreatic resections were carried out by different hepatobiliary and pancreatic surgeons, it was sometimes difficult to determine the clear indication for surgery. Palliative resection was performed if the other organ metastasis could be controlled by another treatment modality or for symptomatic palliation. Patients who did not complete the planned staged curative resection for metastasis due to disease progression after pancreatic resection or who were unfit to undergo the second stage were also considered to have undergone palliative resection. This study was approved by Asan Medical Center Institutional Review Board. Our institutional review board waived the need for written informed consent from the participants.
2.1. Statistical analysis
Prospectively collected data were retrospectively analyzed using IBM SPSS statistic program for Macintosh, version 23 (IBM Corp, Armonk, NY). Continuous variables are expressed as median and standard deviation. Continuous variables were analyzed using the Mann–Whitney test and categorical variables were analyzed using the Chi-squared test or Fisher exact test as appropriate. OS and DFS were analyzed using Kaplan–Meier method. P < .05 was considered statistically significant.
3. Results
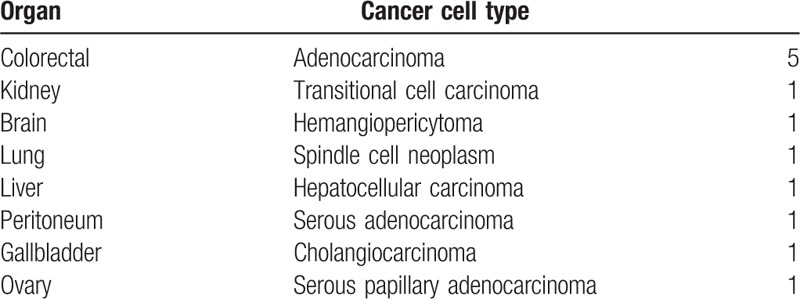
In total, 29 patients were included in our analysis: 29 were followed up for 1 year, 19 were followed up for 3 years, and 15 were followed up for 5 years. These subgroups were analyzed separately. We divided our cohort into RCC (n = 17) and non-RCC (n = 12) groups. The non-RCC group comprised 5 cases of colorectal cancer and seven cases of another primary origin (transitional cell carcinoma, kidney; hemangiopericytoma, brain; spindle cell neoplasm, lung; hepatocellular carcinoma, liver; serous adenocarcinoma, peritoneum; cholangiocarcinoma, gallbladder; and serous papillary adenocarcinoma, ovary) (Table 1). Seventeen patients underwent distal pancreatectomy, 10 underwent pancreaticoduodenectomy, 1 underwent central pancreatectomy, and 1 underwent total pancreatectomy.
Table 1.
Organ and cancer cell type in non-renal cell carcinoma group.
The baseline characteristics of the 2 groups are presented in Table 2. There were significant differences in age (RCC vs non-RCC: 62 ± 4.41 vs 61 ± 8.1 years, P = .027) and neoadjuvant (RCC vs non-RCC: 0% vs 50%, P = .001) and adjuvant (RCC vs non-RCC: 0% vs 66.7%, P = .005) chemotherapy before and after pancreatic resection. The pattern of metastasis was not significantly different for isolated metastasis to the pancreas (RCC vs non-RCC: 64.7% vs 75%) and concomitant distant metastasis (RCC vs non-RCC: 35.3% vs 25%) between the 2 groups. All patients with concomitant pancreatic and distant metastasis received palliative pancreatectomy. Three patients in the RCC group and 5 patients in the non-RCC group who underwent curative resection for local recurrence or distant metastasis to other organs before they developed pancreatic metastasis were considered to have had isolated pancreatic metastasis.
Table 2.
Baseline clinical and pathologic characteristic data for the patients in this study who underwent pancreatectomy due to secondary metastasis to the pancreas.
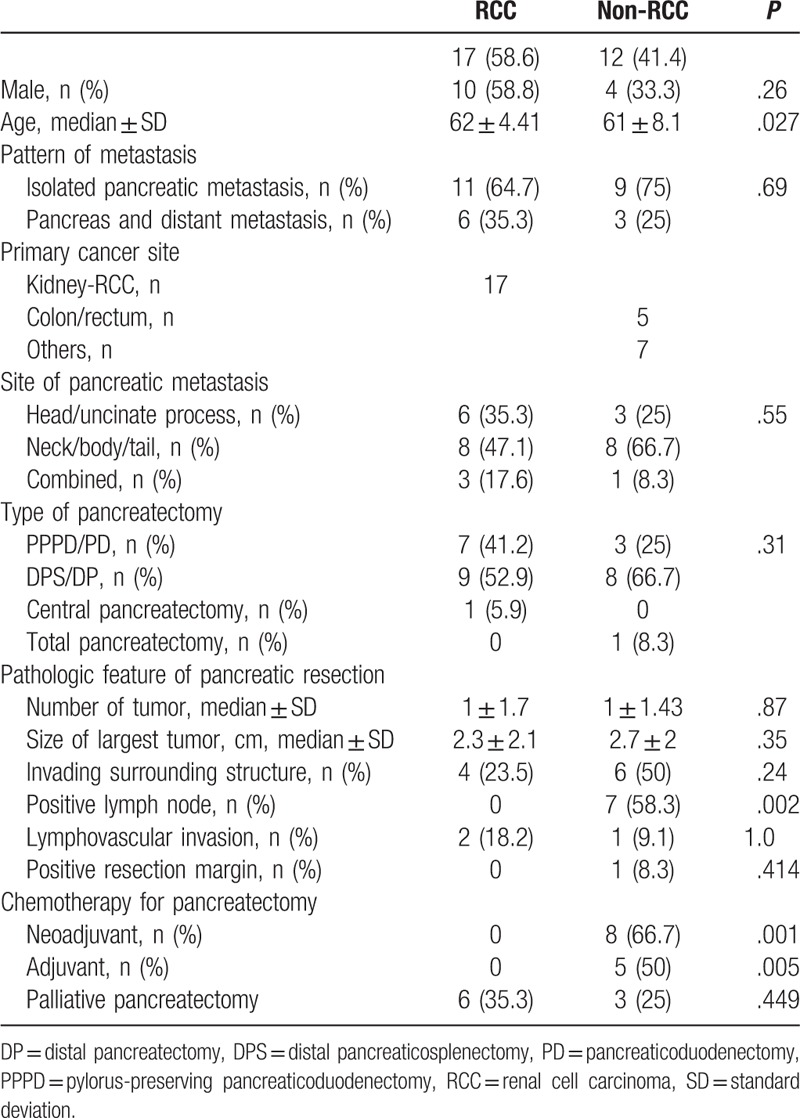
Pathologic analysis of pancreatic resection showed that there were no statistically significant differences between the 2 groups except that the non-RCC group had significantly more positive lymph node metastasis (RCC vs non-RCC: 0% vs 58.3%, P = .002) compared with the RCC group (Table 2).
3.1. Recurrence and overall survival
The survival rates for the whole cohort were 86.2% at 1 year, 63.2% at 3 years, and 46.7% at 5 years. There were no statistically significant differences in survival rate between the 2 groups at 1, 3, and 5 years (RCC vs non-RCC: 94.1% vs 75%, P = .28; 81.8% vs 37.5%, P = .075; 50% vs 42.9%, P = 1.0, respectively) and OS (Fig. 1). In subgroup analysis of patients who underwent curative resection, there was no statistically significant difference in survival rate between the 2 groups at 1, 3, and 5 years (Fig. 2). However, there was a statistically significant difference in recurrence (RCC vs non-RCC: 100% vs 40%, P = .035) (Fig. 3) at 3 years but no significant difference in DFS between the 2 groups at 1, 3, and 5 years (Fig. 4 ).
Figure 1.
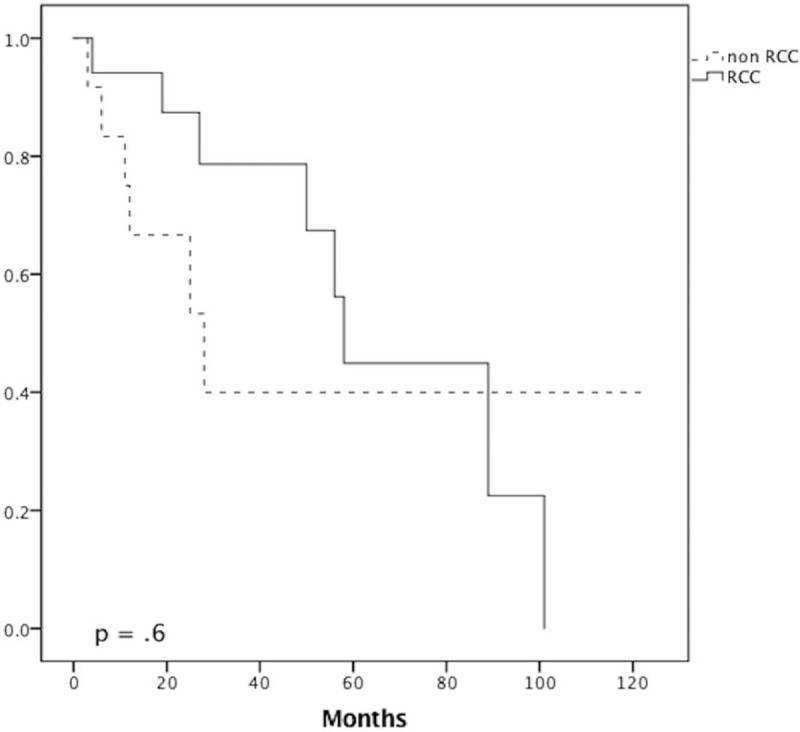
Survival curve analysis. Overall survival for the renal cell carcinoma (RCC) and non-RCC groups.
Figure 2.
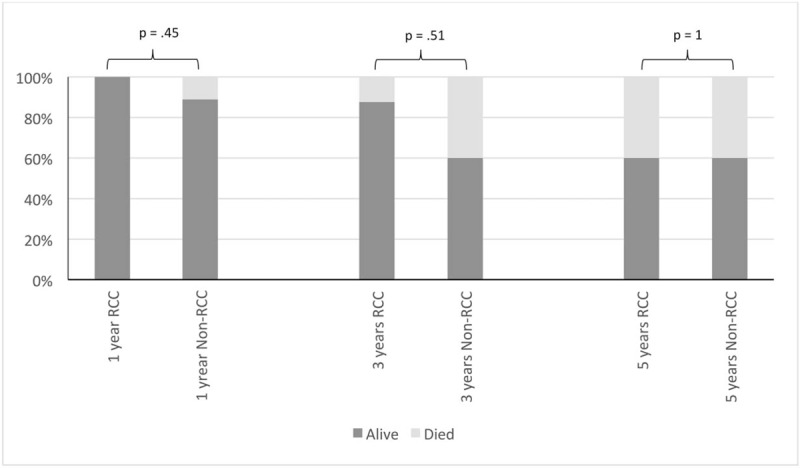
Bar chart. Survival rate for renal cell carcinoma (RCC) and non-RCC group after curative pancreatectomy.
Figure 3.
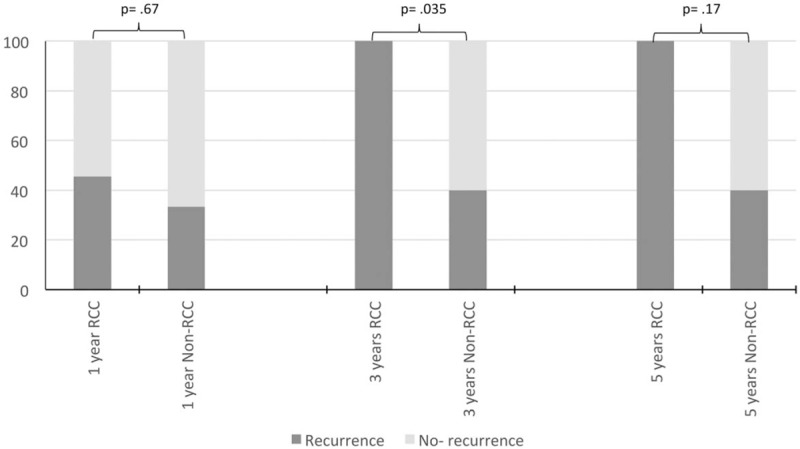
Bar chart. Recurrence rate for renal cell carcinoma (RCC) and non-RCC group after curative pancreatectomy.
Figure 4.
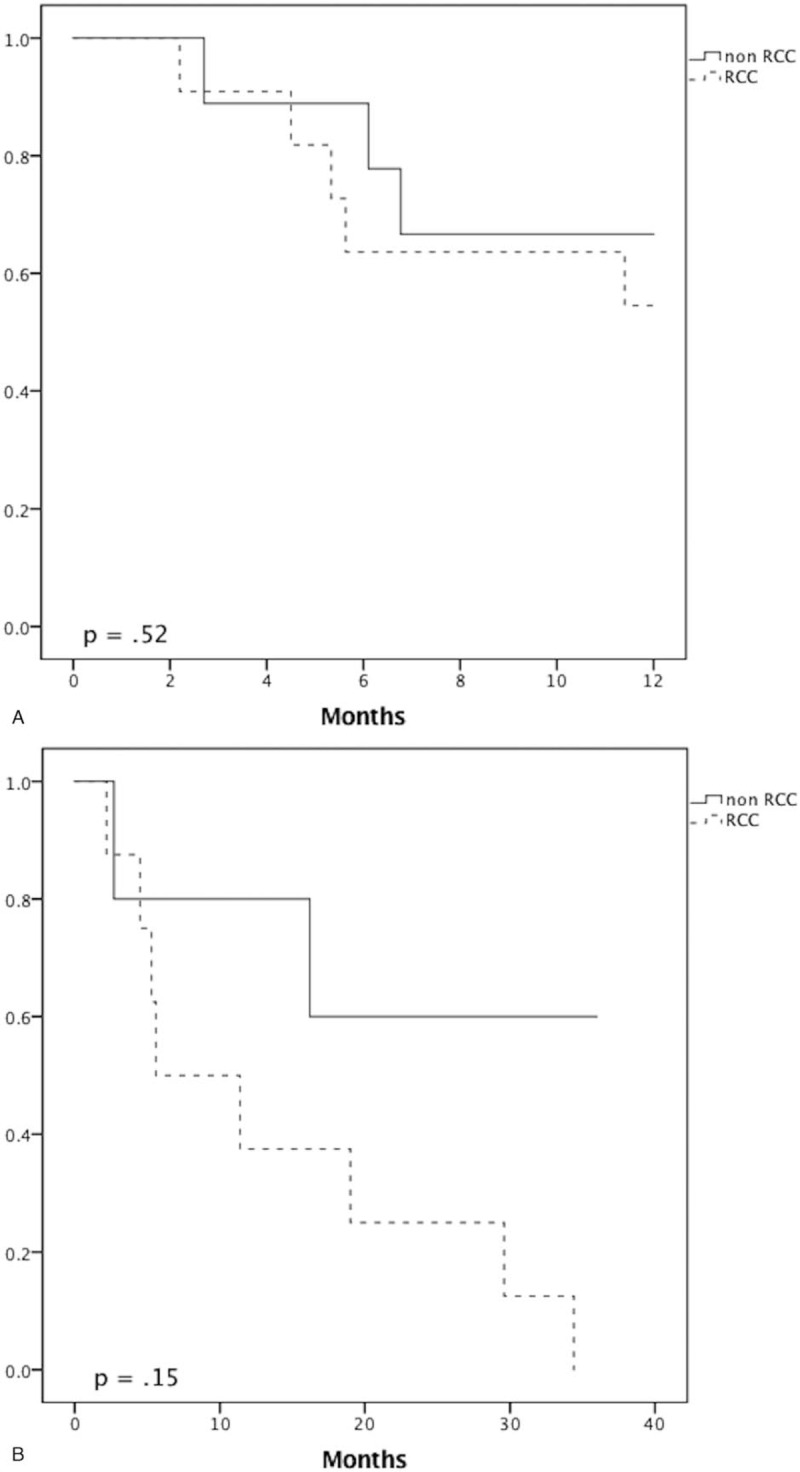
Recurrence curve analysis. Disease-free survival for the renal cell carcinoma (RCC) and non-RCC groups at 1 year (A), 3 years (B), and 5 years (C).
Figure 4 (Continued).
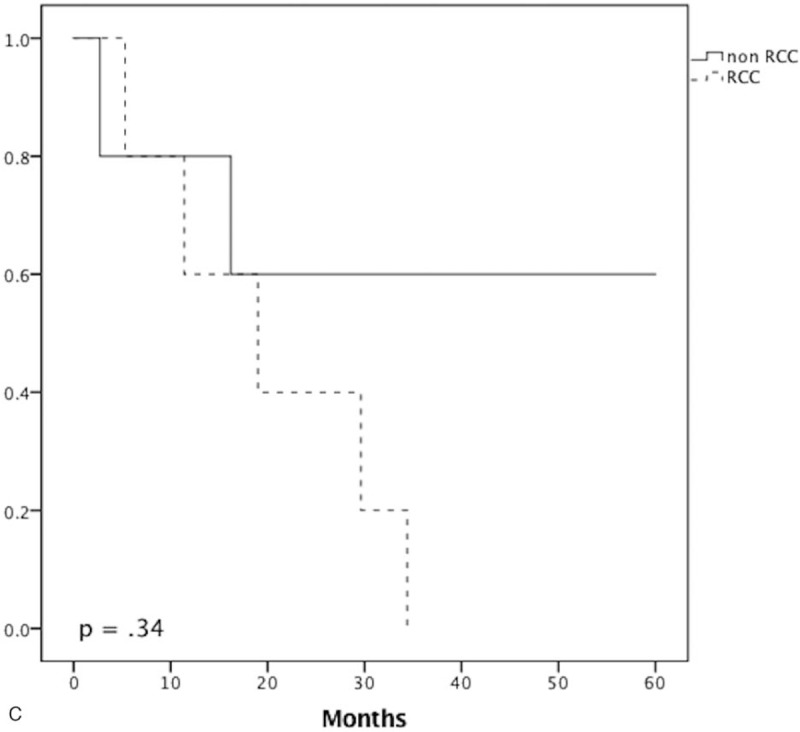
Recurrence curve analysis. Disease-free survival for the renal cell carcinoma (RCC) and non-RCC groups at 1 year (A), 3 years (B), and 5 years (C).
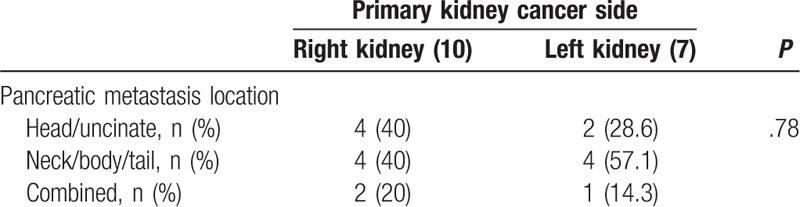
In subgroup analysis of the RCC group, there was no significant difference between the side of the primary kidney cancer and the side of the pancreatic metastasis (head/uncinate process: left vs right, 28.6% vs 40%; neck/body/tail: left vs right, 57.1% vs 40%; combined, left vs right, 14.3% vs 20%; P = .78) (Table 3). There was no significant difference in survival rate between pancreatic resection for curative intent and that for palliative intent at 1, 3, and 5 years (100% vs 83.3%, P = .353; 87.5% vs 66.7%, P = .447; 60% vs 33.3%, P = 1.0, respectively).
Table 3.
Correlation between the primary kidney cancer side and pancreatic metastasis location in the renal cell carcinoma renal cell carcinoma group.
4. Discussion
Secondary metastasis to the pancreas is an uncommon disease and RCC is considered the most common primary cancer to metastasize to the pancreas.[4] In our current study, RCC accounted for 58.6% of all metastatic cancers to the pancreas. Compared with the non-RCC group, the RCC group was older and none had received chemotherapy before or after pancreatic resection (RCC vs non-RCC: 62 vs 61 years, P = .027; 0% vs 50%, P = .001; 0% vs 66.7%, P = .005, respectively) (Table 2). This is because chemotherapy is not used to treat surgically resectable RCC metastasis but is recommended for the metastases of other cancers. The pattern of secondary metastasis to the pancreas was similar in the 2 groups. It presented as an isolated metastasis to the pancreas in 64.7% of the RCC group and in 75% of the non-RCC group and as metastasis to the pancreas with concomitant distant metastasis in 35.3% of the RCC group and in 25% of the non-RCC group.
The pathologic features of resected pancreatic metastasis showed a trend toward local aggressive behavior in the non-RCC group. Compared with the RCC group, the non-RCC group tended to have larger tumors (non-RCC vs RCC: 2.7 ± 2 cm vs 2.3 ± 2.1 cm, P = .35) and were more likely to invade the surrounding structure (non-RCC vs RCC: 50% vs 23.5%, P = .24) and to have positive lymph node metastasis (non-RCC vs RCC: 58.3% vs 0%, P = .002), but the differences were not significant except for the positive lymph node finding. These results may indicate the possibility of lymphatic spread to the pancreas through the local lymphatic system, particularly in intraabdominal primary cancer. Furthermore, most of these primary cancers have high potential for lymph node metastasis. On the contrary, RCC usually metastasizes to the pancreas through homogenous spread and has low potential for lymph node metastasis.[11] This result could also suggest a radical surgical treatment for non-RCC pancreatic metastasis with lymph node resection.
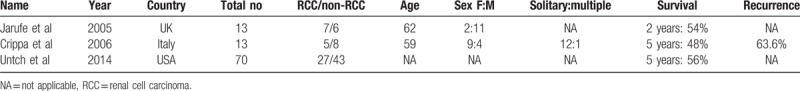
The reported recurrence and survival rates after pancreatectomy in secondary pancreatic metastasis differ in the literature and there are few reports comparing RCC and non-RCC. Crippa et al[6] reported higher recurrence for non-RCC than for RCC (83.5% vs 40%, respectively). In that same study, the 5-year survival was nonsignificantly better for RCC than for non-RCC (80% vs 22%, respectively) (Table 4). In our present study, we observed the opposite result, with the RCC group showing higher recurrence than the non-RCC group for patients who underwent curative resection. The difference was statistically significant at 3 years (RCC vs non-RCC: 100% vs 40%, P = .035) (Fig. 3). There were 4 local recurrences in the pancreas in the RCC group; one of these patients had undergone repeated resection and 3 had distant metastasis treated with palliative chemotherapy. However, this high recurrence did not adversely affect the survival rate. In fact, we noticed a trend toward better survival in the RCC group than in the non-RCC group at 1, 3, and 5 years (RCC vs non-RCC: 94.1% vs 75%, 81.8% vs 37.5%, and 50% vs 42.9%, respectively) and in the subgroup of patients who underwent curative resection (RCC vs non-RCC: 100% vs 88.9%, 87.5% vs 60%, and 60% vs 60%, respectively) (Fig. 2). However, the differences were not significant. Similar results have been reported by other studies. Untch and Allen[10] found no difference in OS between RCC and non-RCC after pancreatic resection (Table 4). In another study, Jarufe et al[7] reported no difference in OS between RCC and non-RCC at 1 and 2 years (Table 4).
Table 4.
Previous studies comparing RCC and non-RCC.
These findings could be interpreted as follows. First, although the heterogeneity in non-RCC primary tumor does not reflect the true recurrence and survival rates after pancreatectomy of each primary tumor, they needed to be analyzed together due to the rarity of the disease. In fact, Sperti et al,[5] who reviewed case series in the literature related to pancreatic resection in secondary pancreatic metastasis, concluded that surgical resection is recommended in RCC pancreatic metastasis with good outcomes, justified in selected cases of sarcoma pancreatic metastasis, and provides palliative benefit in other primary pancreatic metastases. In previous multivariate analysis, Masetti et al[12] found that lung cancer and melanoma pancreatic metastasis were associated with poorer survival outcomes compared with RCC. Second, RCC has an indolent nature and can metastasize after a long time. Rather than a true recurrence of inadequate oncologic resection, the recurrence in RCC could be widespread metastasis to the pancreas and other organs before pancreatic resection that was undetected on preoperative images. In fact, all RCC patients in our current study underwent typical pancreatic resection and none of them had positive margin or lymph node metastasis. Third, chemotherapy has been widely used for the treatment of resectable tumor recurrences of some cancers with oncologic outcome benefits,[13,14] but chemotherapy is used only as a palliative treatment in RCC. In our present study series, 50% of the non-RCC group received adjuvant chemotherapy and 66% received neoadjuvant chemotherapy, in contrast to no patients in the RCC group. This approach may have had a positive impact on oncologic outcomes in the non-RCC group. With the growing evidence supporting the effect of biologic therapy in RCC metastasis for palliative treatment, the use of biologic therapy, as adjuvant or neoadjuvant, in surgically resectable pancreatic metastasis may have a positive impact on oncologic outcomes and may need to be evaluated in future reports.
The RCC metastasis to the pancreas has a favorable natural history that improves even further after surgical resection compared with no resection.[4,11] Curative resection is the primary goal of pancreatectomy in secondary pancreatic metastasis. However, palliative resection can also have survival benefits. A previous report showed that pancreatic resection provided better palliation and improved survival.[15] In our present study, palliative pancreatectomy was found to have a survival benefit at 1, 3, and 5 years (83.3%, 66.7%, and 33.3%, respectively), which are better rates than those reported in the literature after no resection. Tanis et al[16] reported a low survival rate at 2 and 5 years for patients who had secondary RCC metastasis to the pancreas and did not undergo pancreatic resection (41% and 14%, respectively). The survival benefit that we observed could also be related to palliative chemotherapy. In our current cohort, 5 out of 7 patients who underwent palliative resection received palliative chemotherapy, which could have a significant impact on patient survival. However, it is difficult to determine whether these results are related to the surgery, chemotherapy, or both because we do not have a no resection group in our study for comparison. Currently, biologic therapy shows promising outcomes in metastatic RCC to the pancreas compared with nonpancreatic metastasis.[17] Santoni et al[18] compared the oncologic outcomes of surgical resection to targeted therapy for RCC metastasis to the pancreas. They found no survival difference between tyrosine kinase inhibitor and surgery, although the DFS outcome was better with the surgical approach. Although chemotherapy is a widely accepted treatment option in the palliative setting, pancreatic resection may still be indicated in selected patients with low surgical risk and limited metastasis that can be controlled with another treatment modality, especially in symptomatic patients. However, these kinds of cases need to be discussed in multidisciplinary teams to improve outcomes.
The extent of pancreatectomy in secondary pancreatic metastasis from RCC is a debatable issue in the literature. An atypical resection, in the form of enucleation, central pancreatectomy, or limited pancreatic resection, is a nonmajor parenchyma-preserving procedure and usually does not include lymphadenectomy but is claimed to have a high risk of recurrence.[19] It also has a high risk of postoperative pancreas fistula compared with typical resection.[3,19] On the contrary, typical resection in the form of pancreaticoduodenectomy, distal pancreatectomy, or total pancreatectomy is a radical procedure that includes the resection of regional lymph nodes. It is believed to have a low recurrence rate due to the multifocal nature of RCC and lymphadenectomy compared with atypical resection.[19] However, it is a major procedure and carries risk of postoperative complications, including postoperative pancreas fistula, and risk of endocrine and exocrine insufficiency. The rate of reported multifocal pancreatic metastasis from RCC ranges from 20% to 45%.[3] Similarly, in our present study series, multifocal metastasis was observed in 20% of patients. Identification of multifocal metastasis to the pancreas is a critical step in surgical planning for pancreatic resection. It can be identified on preoperative images or intraoperatively (manual palpation of the entire pancreas and intraoperative ultrasound). Its identification helps to avoid unnecessary parenchyma resection and spare patients from the consequences of endocrine and exocrine pancreatic insufficiency, but routine generous resection should be avoided.[3,11] Some studies have reported a high recurrence rate after atypical resection[19] but other reports did not find any relationship between the type of resection and recurrence.[2,3,20] In our current analysis, all patients underwent typical resection but it did not have a positive impact on the incidence of recurrence. Lymph node metastasis in primary RCC is uncommon, reported to be 4.4%,[21] and regional lymphadenectomy during nephrectomy is recommended in positive lymph node enlargement on preoperative images or intraoperative identification.[22] Lymph node metastasis in RCC pancreatic metastasis is also uncommon in the literature[11] and none was found in our current patients, which puts in doubt the benefit of routine lymphadenectomy and typical pancreatic resection.
We believe, along with other authors, that the type of surgical resection should be individualized and that considerable effort should be exerted to identify multifocal disease.[3,11] Typical resection can be indicated by multifocal disease, suspicious enlargement of regional lymph nodes on preoperative images or their intraoperative identification, or a tumor location close to the pancreatic duct. Atypical resection can be indicated by localized metastasis to a small segment of the pancreas with no suspicious lymph node enlargement and to achieve a negative margin.
This study had several limitations of note. The first was the retrospective nature of the study with its inherit selection bias. Second, the heterogeneity of the non-RCC group may have affected the oncologic outcome because it comprised different kinds of cancer with different aggressive behaviors. It would be better to analyze the metastasis of each cancer type to the pancreas separately to identify their true impact on oncologic outcomes but we analyzed them together due to the rarity of the disease. Finally, a quite significant number of patients underwent their primary cancer treatment at another hospital, which precluded a true correlation between the primary cancer pathologic finding and the disease-free interval with oncologic outcomes.
In conclusion, pancreatic resection shows promising results in terms of curative intent in cases of secondary pancreatic metastasis. Due to an acceptable survival rate, it may also play some role in pancreatic resection performed with palliative intent in addition to chemotherapy, especially in RCC. Targeted chemotherapy for resectable RCC pancreatic metastasis may have a positive impact on oncologic outcomes and should be evaluated further in future studies.
Author contributions
Conceptualization: Song Cheol Kim, Sang Hyun Shin, Ki Byung Song, Kwang Min Park.
Data curation: Ahmad Abdullah Madkhali, Song Cheol Kim, Sang Hyun Shin, Jae Hoon Lee, Kwang Min Park, Young Joo Lee.
Formal analysis: Ahmad Abdullah Madkhali, Song Cheol Kim, Dae Wook Hwang.
Investigation: Ahmad Abdullah Madkhali, Young Joo Lee.
Methodology: Ahmad Abdullah Madkhali, Song Cheol Kim.
Project administration: Ahmad Abdullah Madkhali, Song Cheol Kim, Jae Hoon Lee.
Resources: Song Cheol Kim, Jae Hoon Lee.
Supervision: Song Cheol Kim, Sang Hyun Shin, Jae Hoon Lee, Dae Wook Hwang.
Validation: Sang Hyun Shin, Ki Byung Song, Jae Hoon Lee, Dae Wook Hwang, Kwang Min Park.
Visualization: Song Cheol Kim, Ki Byung Song, Dae Wook Hwang.
Writing – original draft: Ahmad Abdullah Madkhali, Song Cheol Kim, Sang Hyun Shin, Ki Byung Song, Dae Wook Hwang.
Writing – review & editing: Ahmad Abdullah Madkhali, Song Cheol Kim, Ki Byung Song, Dae Wook Hwang, Kwang Min Park, Young Joo Lee.
Footnotes
Abbreviations: DFS = disease-free survival, OS = overall survival, RCC = renal cell carcinoma.
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant number: HI14C2640).
The authors have no conflicts of interest to disclose.
References
- [1].Ballarin R, Spaggiari M, Cautero N, et al. Pancreatic metastases from renal cell carcinoma: the state of the art. World J Gastroenterol 2011;17:4747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Konstantinidis IT, Dursun A, Zheng H, et al. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg 2010;211:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zerbi A, Ortolano E, Balzano G, et al. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol 2008;15:1161–8. [DOI] [PubMed] [Google Scholar]
- [4].Adler H, Redmond CE, Heneghan HM, et al. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol 2014;40:379–86. [DOI] [PubMed] [Google Scholar]
- [5].Sperti C, Moletta L, Patane G. Metastatic tumors to the pancreas: the role of surgery. World J Gastrointest Oncol 2014;6:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crippa S, Angelini C, Mussi C, et al. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg 2006;30:1536–42. [DOI] [PubMed] [Google Scholar]
- [7].Jarufe N, McMaster P, Mayer AD, et al. Surgical treatment of metastases to the pancreas. Surgeon 2005;3:79–83. [DOI] [PubMed] [Google Scholar]
- [8].Pawlik TM, Vauthey JN, Abdalla EK, et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006;141:537–43. [DOI] [PubMed] [Google Scholar]
- [9].Sperti C, Pasquali C, Berselli M, et al. Metastasis to the pancreas from colorectal cancer: is there a place for pancreatic resection? Dis Colon Rectum 2009;52:1154–9. [DOI] [PubMed] [Google Scholar]
- [10].Untch BR, Allen PJ. Pancreatic metastasectomy: the Memorial Sloan-Kettering experience and a review of the literature. J Surg Oncol 2014;109:28–30. [DOI] [PubMed] [Google Scholar]
- [11].Sellner F, Tykalsky N, De Santis M, et al. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol 2006;13:75–85. [DOI] [PubMed] [Google Scholar]
- [12].Masetti M, Zanini N, Martuzzi F, et al. Analysis of prognostic factors in metastatic tumors of the pancreas: a single-center experience and review of the literature. Pancreas 2010;39:135–43. [DOI] [PubMed] [Google Scholar]
- [13].National Comprehensive Cancer Network. colon cancer (version 2017.2). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Accessed April 10, 2017. [Google Scholar]
- [14].National Comprehensive Cancer Network. Soft tissue sarcoma (Version 2017.2). Available at: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf Accessed April 10, 2017. [Google Scholar]
- [15].Minni F, Casadei R, Perenze B, et al. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology 2004;4:509–20. [DOI] [PubMed] [Google Scholar]
- [16].Tanis PJ, van der Gaag NA, Busch OR, et al. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg 2009;96:579–92. [DOI] [PubMed] [Google Scholar]
- [17].Grassi P, Verzoni E, Mariani L, et al. Prognostic role of pancreatic metastases from renal cell carcinoma: results from an Italian center. Clin Genitourin Cancer 2013;11:484–8. [DOI] [PubMed] [Google Scholar]
- [18].Santoni M, Conti A, Partelli S, et al. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann Surg Oncol 2015;22:2094–100. [DOI] [PubMed] [Google Scholar]
- [19].Bassi C, Butturini G, Falconi M, et al. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg 2003;90:555–9. [DOI] [PubMed] [Google Scholar]
- [20].Yazbek T, Gayet B. The place of enucleation and enucleo-resection in the treatment of pancreatic metastasis of renal cell carcinoma. JOP 2012;13:433–8. [DOI] [PubMed] [Google Scholar]
- [21].Blute ML, Leibovich BC, Cheville JC, et al. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol 2004;172:465–9. [DOI] [PubMed] [Google Scholar]
- [22].National Comprehensive Cancer Network. Kidney Cancer (version 2.2017). Available at: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf Accessed April 10, 2017. [Google Scholar]


