Abstract
In gastric cancer, HER2 protein overexpression is considered to be conducive to the higher proliferation activity of the tumor cells. Tumor formation is associated with angiogenesis in order to secure an abundant supply of oxygen and glucose to cancer cells. The aim of the study was to assess if HER2 overexpression is related to higher microvessel density (MVD) in the tumor stroma.
The archival samples of primary tumor from 144 consecutive patients that underwent gastric resection for cancer between August 1, 2006 and December 31, 2013 in the Department of Oncological Surgery of Medical University of Gdańsk were analyzed. CD34 was used as a marker of MVD in the tumor stroma. Both CD34 and HER2 protein expressions were tested by immunohistochemistry.
The assays were unsuccessful to estimate HER2 in 10 cases and CD34 in 14 cases due to technical reasons. The results were obtained for 128 patients. HER2 0 and HER2 1+ were considered negative, while HER2+ and HER2 3+ were recognized as positive. Mean MVD (mean number of vessels in the visual field) was 32.4 (median 29.5). Microvessel density was insignificantly higher in HER2 positive tumors. The slight difference was also seen between IHC 2+ and 3+ groups. The differences did not reach the level of statistical significance.
Statistical analysis performed in our study did not reveal the significant relationship between HER2 overexpression on the tumor cells and MVD in the tumor stroma.
Keywords: CD34, ErbB-2, neovascularization, stomach neoplasms
1. Introduction
In spite of substantial progress in medical oncology and surgery in the last decades, gastric cancer remains a difficult oncological challenge and is still one of the most common lethal malignancy worldwide.[1] The disease is biologically heterogeneous and the mechanisms of tumorigenesis remain poorly understood.[2]
The most important prognostic factor is the stage of the disease according to the TNM system. However, different clinical outcomes are observed among patients of identical TNM stage.[3] Moreover, potential response to standard treatment is still a matter of uncertainty. Therefore the search for new prognostic and predictive factors is currently in the spotlight.[3]
In gastric cancer, HER2 protein overexpression is considered to increase proliferation activity and suppress apoptosis of the tumor cells.[4] It is found in 6% to 29.5% of gastric cancers.[4] Angiogenesis is the process of new capillary vessels formation and plays a central role in tumor growth and metastasis formation.[3] The numerical value of tumor angiogenesis is defined as microvessel density (MVD).[5] It is measured by immunohistochemical (IHC) staining using antibodies such as CD 34 and others that are specific for vessel endothelium.[5]
The aim of the study was to determine if HER2 overexpression is related to higher MVD in the tumor stroma. Demonstration of the relation between HER2 overexpression and the intensity of angiogenesis could have impact on patient selection to antiangiogenic therapy.
2. Methods
The study was approved by the Independent Ethic Committee at the Medical University of Gdańsk (NKBBN/427/2014). The archival samples of primary tumor from 144 consecutive patients that underwent major gastric resection for adenocarcinoma between August 1, 2006 and December 31, 2013 in the Oncological Surgery Clinic of Medical University of Gdańsk were analyzed. CD34 was used as a marker of MVD in the tumor stroma. Both CD34 and HER2 protein expressions were tested by IHC.
IHC staining was carried out on representative 4 μm sections which were obtained from routine tissue blocks, placed in silanized glasses and deparaffined in a routine way. The glasses were incubated in 36oC for 24 hours. For HER2 staining, anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody (ROCHE) was used in an automatic machine Roche Benchmark GX, according to the manufacturer's instructions. For MVD evaluation, Monoclonal Mouse Anti-human CD34 Class II Clone QBEND-10 (DAKO) was applied. The procedure was performed on automatic machine DAKO Autostainer Link-48 according to the manufacturer's protocol. For evaluation of both HER2 expression and MVD, light microscope Olympus BX43 was used. For HER2 evaluation, the criteria recommended by Hoffmann were applied.[6] The images of IHC HER2 staining are presented in Figure 1 . MVD was evaluated by counting anti-CD34 positive microvessels and calculated by the method described by Weidner et al.[7]
Figure 1.
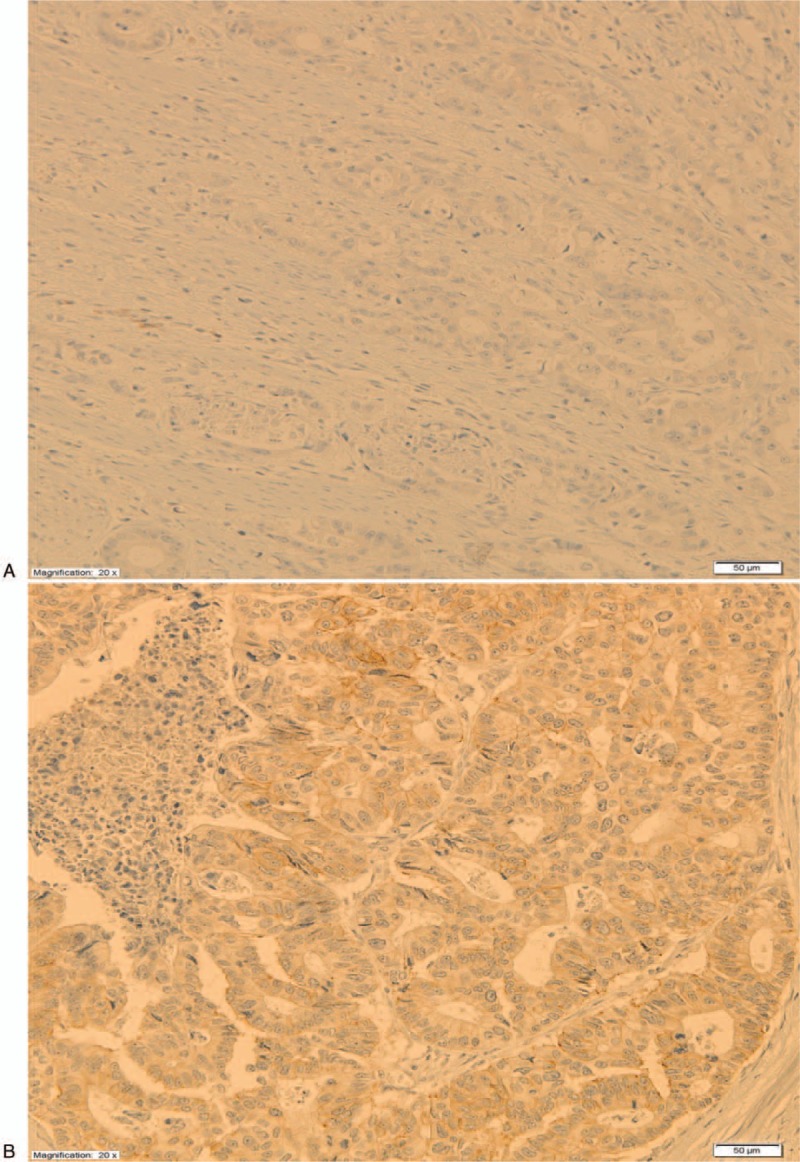
Immunohistochemistry HER2 stainings. (A) HER2 0. (B) HER2 1+. (C) HER2 2+. (D) HER2 3+.
The assays were unsuccessful to estimate HER2 in 10 cases and CD34 in 14 cases due to technical reasons. The results were obtained for 128 patients. HER2 0 and HER2 1+ were considered negative, while HER2+ and HER2 3+ were recognized as positive. Additionally, all HER2 2+ cases were further analyzed in fluorescence in situ hybridization (FISH) method and additional analysis has been performed for standard HER2 negative and positive groups.
Molecular cytogenetic analysis was performed at the Molecular Oncology and Genetics Department, IFM, the Łukaszczyk Oncology Centre in Bydgoszcz. For FISH analysis, we used 4 and 6 μm section from formalin-fixed paraffin-embedded (FFPE) tissue. Commercially validated Vysis PathVysion HER2 FISH test was used to evaluate amplification or negative or equivocal result. A minimum of 60 cells in interphase were scored for each sample using a fluorescence microscope (Nikon Eclipse 80i) and ASCO/CAP 2013 HER2 standard recommendations.[8] FISH test was interpreted as positive in case of ratio of HER2 signals to CEP17 signals ≥ 2 and negative in case of ratio < 2. In case of average 3 or more copies of CEP17 (CEP 17 polysomy) and ratio < 2 the presence of more than 6 HER2 signals was interpreted as a positive result, the presence of <4 HER2 signals was interpreted as a negative result, and the presence between 4 and 6 HER2 signals was interpreted as an equivocal result. Photographic documentation was performed via the Lucia Cytogenetics software and presented in Figure 2.
Figure 1 (Continued).
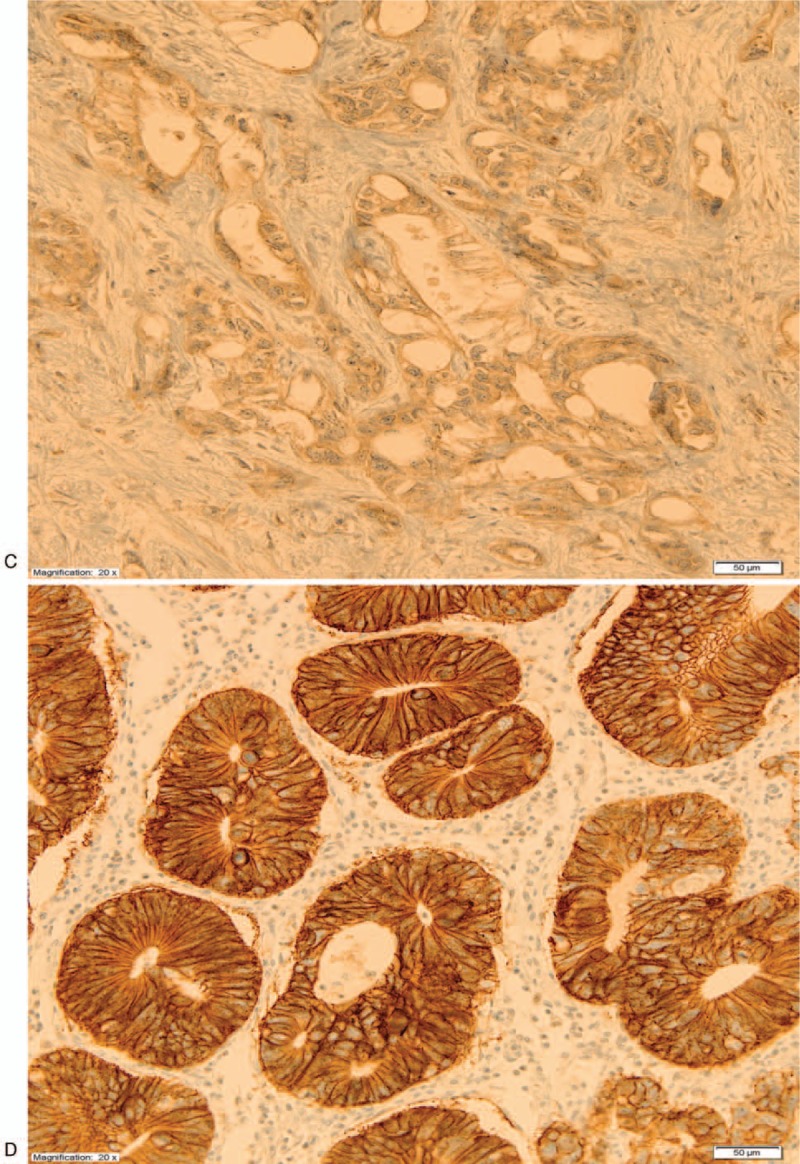
Immunohistochemistry HER2 stainings. (A) HER2 0. (B) HER2 1+. (C) HER2 2+. (D) HER2 3+.
Figure 2.
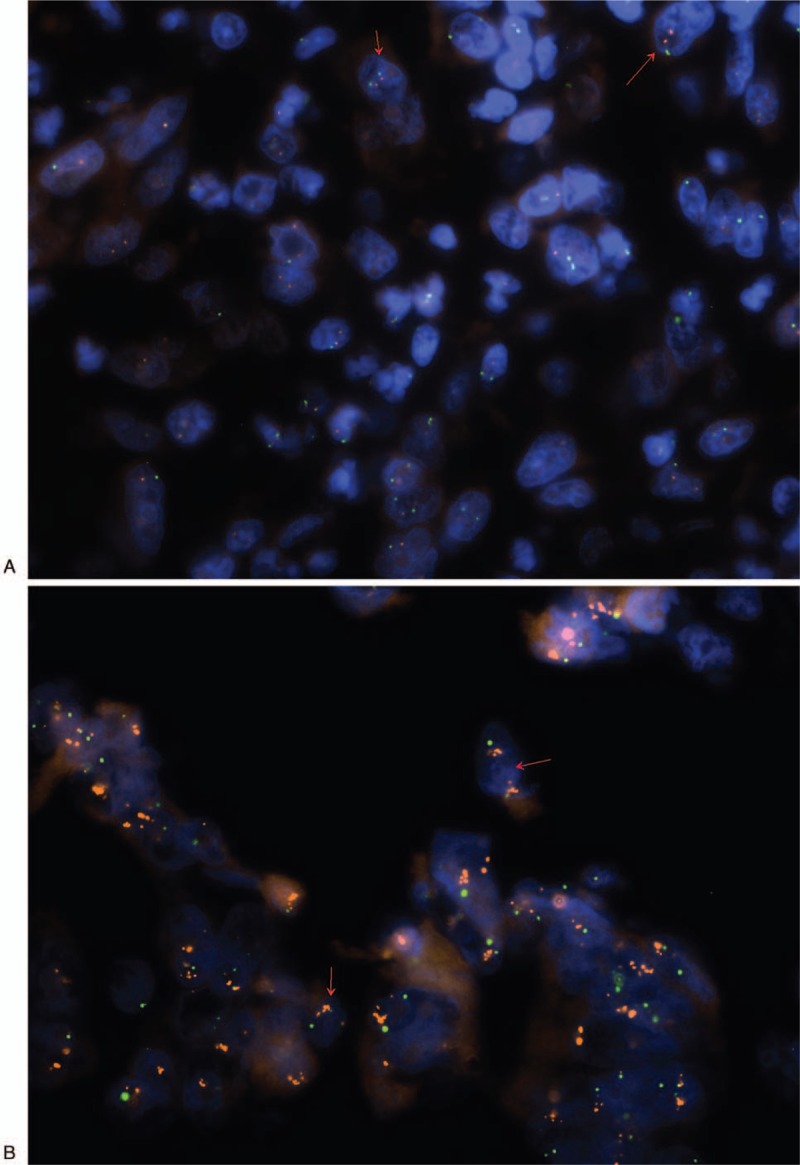
A dual-color fluorescent in situ hybridization assay demonstrating HER2 gene copies (red) and CEP17 (green). (A) FISH negative result (no amplification). (B) FISH positive result (amplification). FISH = fluorescence in situ hybridization.
For statistical reasons, we combined patients with TNM stages I and II into 1 group and III and IV into the second group. Similarly patients with Lauren type II and III were concerned as one group. Concerning WHO pathological types we combined tubular and papillary cases into one group and other types into the second group.
2.1. Statistical analysis
Statistical analysis was carried out with use of Statistica data analysis software system, StatSoft Inc. version 12 (2014). Mann Whitney U and Kruskal–Wallis tests were used to determine the association between pathoclinical variables, including HER2 receptor expression, and MVD as appropriate. Chi2-Pearson test was used to detect the relationship between HER2 status and pathoclinical characteristics. For all test, P-value ≤.05 was considered as significant.
3. Results
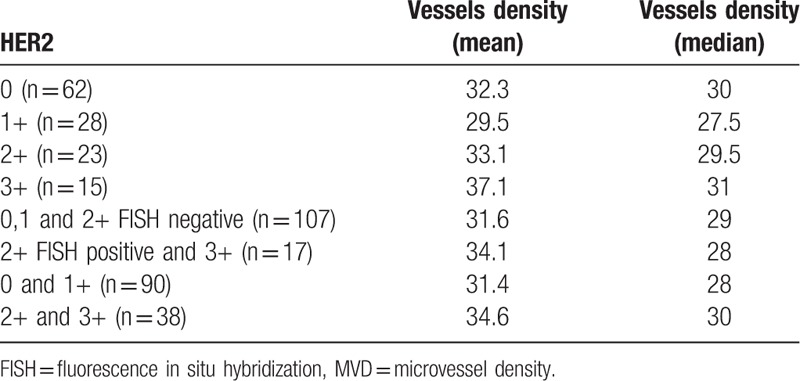
The median age of patients was 63. Mean number of harvested lymph nodes was 20 (range 1–71). Mean MVD (mean number of vessels in the visual field) was 32.4 (median 29.5). The main pathoclinical parameters of the studied cases are presented in Table 1. The relation between HER2 protein expression and MVD is presented in Table 2. The differences did not reach the level of statistical significance (P = .6).
Table 1.
Pathoclinical parameters of the studied cases.

Table 2.
The relationship between HER2 protein expression and MVD.
HER2 positive status was related to the intestinal Lauren type (63.2% vs 38.2%, P = .006), less advanced (I-II) stage of the disease according to TNM (57.9% vs 38.9%, P = .04) and tubular or papillary WHO pathologic types (55.2% vs 36.7%, P = .05).
HER2 positive status was not related to the median of metastatic lymph nodes (1.5 vs 4, P = .1), to total number of harvested lymph nodes (20 vs 21) and to cardiac location of the tumor (39.5% vs 26.7%, P = .1).
Among 23 HER2 2+ patients, only 2 were FISH positive (HER2:CEP17 ratio>2 or HER2:CEP17 ratio<2 with an average HER2 copy number ≥6 signals/cell) and 17 cases were FISH negative (HER2:CEP17 ratio < 2 with an average HER2 copy number <4 signals/cell. There were also 4 equivocal cases (HER2:CEP17 ratio <2.0, the presence of CEP17 polysomy and 4–6 HER2 copy signals).
MVD was not related to any studied pathological variables as: depth of tumor infiltration (T stage), the presence of lymph nodes metastasis (N stage), the presence of mucinous component in the primary tumor, Lauren type, tumor location in the stomach, the presence of distant metastases, TNM stage of the disease, and WHO pathological types of cancer.
4. Discussion
The relationship between HER2 overexpression and angiogenesis in gastric cancer has not been widely studied to date. According to many authors, HER2 overexpression is related to more aggressive course of the disease.[9] However not all the studies confirm this thesis.[10] In our present material, we found the relationship between HER2 positive status and well-recognized factors that indicate better prognosis (less advanced TNM stage, better histological differentiation, and intestinal type of the tumor). Previously, we also demonstrated similar results in the material from different centre.[11]
The relationship between HER2 overexpression and worse prognosis is well documented in breast cancer.[12] High MVD in breast cancer was also shown to be a significant and independent negative prognostic factor in majority of performed studies.[13] The relationship between HER2 overexpression and angiogenesis at the molecular level has been shown for breast cancer in both preclinical and clinical research.[12,14–17]
Ludovini et al[14] found the significant relationship between HER2 overexpression and MVD assessed using CD34 antibody in stages I and II breast cancer patients. Other authors demonstrated the relationship between HER2 positivity and VEGF expression.[15–17] However, there are also studies that do not confirm these findings. Turkish authors found the relationship between high MVD and increasing tumor size and lymph node involvement, but did not find any relationship between HER2 status and MVD in breast ductal carcinoma.[5]
The relationship between HER2 overexpression and angiogenesis was found by Zhang et al[18] in ovarian cancer. The authors studied the effect of 2 anti-HER2 monoclonal antibodies on angiogenesis in the ovarian cancer in a murine model. They found significant decrease in MVD after 21 days of treatment with trastuzumab alone (56% decrease), chA21 alone (54% decrease) and with trastuzumab plus chA21 (69% decrease).
Because HER2 receptor overexpression in gastric cancer has significantly different biological nature than the one observed in breast and other cancers, the results achieved in different carcinomas cannot be used for gastric cancer patients without any restraint.
In gastric cancer, the relationship between HER2 overexpression on the tumor cells and MVD in the tumor stroma is not as well documented as it is in case of breast cancer.
In our material, MVD was insignificantly higher in HER2 positive tumors. The slight difference is also seen between 2+ and 3+ groups. Similar relation was found in the study by Badescu et al,[19] but their study was performed on very small group of 28 patients.
Singh et al[20] proved that administration of VEGF-Trap or trastuzumab into nude mice bearing HER2-overexpressing gastric cancer xenografts for 28 days is similarly effective in both tumor volume reduction and decrease of intratumoral vascular density. In trastuzumab alone group, the 74.5% reduction of intratumoral MVD was observed. Moreover, both tumor volume and MVD in the peritumoral region were decreased twice as much when both agents were given simultaneously.
In clinical studies to date, antiangiogenic agents have shown limited efficacy against gastric cancer. However, biomarkers for selecting patients who benefit from anti-angiogenic therapy are still lacking.[21] To date, HER2 is the only established evidence-based biomarker predictive of tumor response to targeted agents.[2] In spite of this, the enrolled patients have never been stratified according to HER2 status. The AVAGAST trial showed, that adding anti-VEGF antibody bevacizumab to chemotherapy improved progression-free survival, but not overall survival.[22] The results of the REGARD and RAINBOW trials demonstrated an overall survival benefit in patients with advanced gastric and gastro-oesophageal junction adenocarcinoma with another anti-angiogenic monoclonal antibody, VEGF-2 receptor antagonist, and ramucirumab. Both trials recruited patients with the disease progression after first line chemotherapy.[23,24] In the REGARD trial patients received ramucirumab monotherapy vs placebo and the survival benefit was 5.2 month versus 3.8 months.[23] In the RAINBOW trial patients received ramucirumab plus paclitaxel or paclitaxel plus placebo and the survival benefit was 9.6 vs 7.4 months.[24] Unfortunately in all 4 mentioned trials the authors considered the treatment efficacy in many different subgroups of patients but not with HER2 positive vs HER2 negative tumors.
The main limitation of our study was a relatively small number of patients. If it could be proven, that in human gastric cancer, HER2 overexpression had significantly increased the intensity of angiogenesis, the combined targeting of HER2 and angiogenesis in case of HER2 positive gastric cancer would be the option to consider in future clinical trials.
Acknowledgements
The authors wish to thank Prof. Wojciech Biernat and Prof. Janusz Jaśkiewicz for the assistance in the study.
Author contributions
Conceptualization: Maciej Ciesielski, Mariusz Szajewski, Wiesław Janusz Kruszewski.
Data curation: Mariusz Szajewski, Rafał Pęksa, Marzena Anna Lewandowska, Jacek Zieliński, Jakub Walczak, Jarosław Szefel.
Formal analysis: Maciej Ciesielski, Mariusz Szajewski.
Funding acquisition: Wiesław Janusz Kruszewski.
Investigation: Maciej Ciesielski, Mariusz Szajewski, Rafał Pęksa, Jakub Walczak, Jarosław Szefel.
Methodology: Maciej Ciesielski, Rafał Pęksa, Marzena Anna Lewandowska.
Project administration: Wiesław Janusz Kruszewski.
Resources: Rafał Pęksa, Marzena Anna Lewandowska, Jacek Zieliński.
Software: Mariusz Szajewski.
Supervision: Marzena Anna Lewandowska, Wiesław Janusz Kruszewski.
Validation: Jacek Zieliński, Wiesław Janusz Kruszewski.
Writing – original draft: Maciej Ciesielski.
Writing – review & editing: Marzena Anna Lewandowska, Wiesław Janusz Kruszewski.
Footnotes
Abbreviations: FISH = fluorescence in situ hybridization, IHC = immunohistochemistry, MVD = microvessel density.
This work was financially supported by Medical University of Gdańsk (ST 02/130/07/308). The APC was financially supported by the “Fundacja na Rzecz Rozwoju Chirurgii VIRIBUS UNITIS im. Oskara Jankaua.”
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:359–86. [DOI] [PubMed] [Google Scholar]
- [2].Baniak N, Senger J-L, Ahmed S, et al. Gastric biomarkers: a global review. World J Surg Oncol 2016;14:212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jomrich G, Schoppmann SF. Targeted therapy in gastric cancer. Eur Surg 2016;48:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sener E, Sipal S, Gundogdu C. Comparison of microvessel density with prognostic factors in invasive ductal carcinomas of the breast. Turk J Pathol 2016;32:164–70. [DOI] [PubMed] [Google Scholar]
- [6].Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797–805. [DOI] [PubMed] [Google Scholar]
- [7].Weidner N, Semple JP, Welch WR, et al. Tumour angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- [8].Bartley AN, Washington MK, Colasacco C, et al. HER2 testing and clinical decisions making in gastroesophageal adenocarcinoma: guideline from the College of American Pahologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017;35:446–66. [DOI] [PubMed] [Google Scholar]
- [9].Park DI, Yun JW, Park JH, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 2006;51:1131–9. [DOI] [PubMed] [Google Scholar]
- [10].Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: rare, heterogenous and of no prognostic value – conclusions from 924 cases of two independent series. Cell Oncol 2010;32:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ciesielski M, Kruszewski WJ, Śmiałek U, et al. The HER2 gene and HER2 protein status and chromosome 17 polysomy in gastric cancer cells in own material. Appl Immunohistochem Mol Morphol 2015;23:113–7. [DOI] [PubMed] [Google Scholar]
- [12].Alameddine RS, Otrock ZK, Awada A, et al. Crosstalk between HER2 signalling and angiogenesis in breast cancer: molecular basis, clinical applications and challenges. Curr Opin Oncol 2013;25:313–24. [DOI] [PubMed] [Google Scholar]
- [13].Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist 2000;5suppl 1:37–44. [DOI] [PubMed] [Google Scholar]
- [14].Ludovini V, Sidoni A, Pistola L, et al. Evaluation of the prognostic role of vascular endothelial growth factor and microvessel density in stages I and II breast cancer patients. Breast Cancer Res Treat 2003;81:159–68. [DOI] [PubMed] [Google Scholar]
- [15].Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 2004;10:1706–16. [DOI] [PubMed] [Google Scholar]
- [16].Schoppmann SF, Tamandl D, Roberts L, et al. HER2/neu expression correlates with vascular endothelial growth factor-C and lympangiogenesis in lymph node-positive breast cancer. Ann Oncol 2010;21:955–60. [DOI] [PubMed] [Google Scholar]
- [17].Yang W, Klos K, Yang Y, et al. ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer 2002;94:2855–61. [DOI] [PubMed] [Google Scholar]
- [18].Zhang A, Shen G, Zhao T, et al. Augmented inhibition of angiogenesis by combination of HER2 antibody chA21 and trastuzumab in ovarian carcinoma xenograft. J Ovarian Res 2010;3:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Badescu A, Georgescu CV, Vere CC, et al. Correlations between Her2 oncoprotein, VEGF expression, MVD and clinicopathological parameters in gastric cancer. Rom J Morphol Embryol 2012;53:997–1005. [PubMed] [Google Scholar]
- [20].Singh R, Kim WJ, Kim PH, et al. Combined blockage of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockage. Exp Mol Med 2013;45:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Obermannova R, Lordick F. Insights into next developments in advanced gastric cancer. Curr Opin Oncol 2016;28:367–75. [DOI] [PubMed] [Google Scholar]
- [22].Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76. [DOI] [PubMed] [Google Scholar]
- [23].Fuchs CS, Tomasek J, Yong CJ. REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9. [DOI] [PubMed] [Google Scholar]
- [24].Wilke H, Muro K, Van Cutsem E, et al. RAINBOW Study Group Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]


