Abstract
The monocyte-to-high density lipoprotein ratio (MHR) has recently been implemented as an indicator of inflammation and oxidative stress. The present study characterized MHR in patients with diabetic polyneuropathy (DPN), in which oxidative stress and microvascular damage play a role in pathogenesis, relative to patients with non-DPN, diabetic patients without polyneuropathy, and healthy individuals. We further aimed to evaluate the association between MHR and the decreased compound muscle action potential (CMAP) amplitude of patients with diabetic axonal polyneuropathy.
We enrolled 90 patients with DPN, 75 patients with nonDPN, 92 diabetic patients without polyneuropathy, and 67 healthy individuals; The monocyte, high-density lipoprotein cholesterol (HDL-C) values were obtained for all participants and MHR was calculated for each individual. Intergroup comparison was performed. The relationship between MHR and the posterior tibial nerve CMAP amplitudes was examined.
Statistically significant negative correlation was observed between MHR and the posterior tibial nerve CMAP amplitudes of patients with DPN. The MHR values of the patients with DPN were significantly higher than those of the patients with non-DPN, diabetic patients without polyneuropathy and the control group.
This study demonstrated that diabetic patients with higher MHR values may be more likely to develop polyneuropathy.
Keywords: axonal polyneuropathy, diabetic polyneuropathy, monocyte/high-density lipoprotein ratio, oxidative stress
1. Introduction
Diabetic neuropathy is one of the most common causes of peripheral neuropathies and is a major cause of low quality of life and morbidity in patients with diabetes.[1] The most commonly observed type is the length-dependent pattern of distal symmetric polyneuropathy, which is associated with progressive distal axonopathy.[2] Although the cause of diabetic polyneuropathy (DPN) is not known, metabolic and ischemic causes, autoimmune factors, oxidative stress, and inflammation account for its etiology.[1,3–6]
The monocyte/high-density lipoprotein ratio (MHR) indicates inflammation and oxidative stress due to the proinflammatory effect of the monocytes, as well as the anti-inflammatory and antioxidant effect of the high-density lipoprotein cholesterol (HDL-C). Several studies have used these metrics to determine whether inflammation and atherosclerosis contribute to the etiopathogenesis of cardiovascular and cerebrovascular diseases.[7–14] However, such indicators have not been employed by research to elucidate the cause of DPN, despite ischemia, and inflammation frequently referenced as factors in its etiopathogenesis.
The present study sought to address this dearth in the literature by investigating the relationship between the monocyte/high-density lipoprotein ratio and the decreased compound muscle action potential (CMAP) amplitude in patients with diabetic axonal polyneuropathy.
2. Materials and methods
2.1. Study design and patients
We retrospectively analyzed the consecutive electroneuromyography (ENMG) results of patients who were referred to our electrophysiology laboratory for electrodiagnostic examination from January 2017 to June 2018 because of the following clinical symptoms and signs that suggested polyneuropathy: pain, numbness, tingling, ataxia, muscle weakness, autonomic symptoms, reflex loss, and loss of sensation. Ethical approval was not necessary because this was a retrospective study.
The medical records and neurologists’ examination notes of diabetic patients whose electrodiagnostic examinations were normal, as well as those of all diabetic and nondiabetic patients with electrophysiologically diagnosed axonal polyneuropathy, were evaluated to determine the etiological cause of polyneuropathy. The patients were classified into 2 groups for analysis according to whether they had been previously diagnosed with diabetes or not. The patients with diabetes mellitus who were referred for suspected polyneuropathy were further subdivided into 2 groups according to whether or not they had electrophysiologically axonal polyneuropathy: patients with DPN and diabetic patients without polyneuropathy. Non-diabetic patients with axonal polyneuropathy were classified into a separate group. This group consisted of the following:
-
(1)
the patients who attended follow up due to a diagnosis of chronic renal failure had sub-clinical neuropathy screening for hemodialysis indication, or who were diagnosed with possible uremic polyneuropathy,
-
(2)
patients who had continuously consumed too much alcohol and who were subsequently diagnosed with alcoholic polyneuropathy after having been referred to us for having demonstrated clinical findings of polyneuropathy,
-
(3)
patients who were examined due to the neuropathic complaints that started when they received chemotherapy for cancer and were considered to have polyneuropathy associated with a chemotherapeutic agent,
-
(4)
patients who had an uncertain etiology of polyneuropathy and who were consequently diagnosed with idiopathic polyneuropathy. Patients who were diagnosed with axonal polyneuropathy as a result of electrodiagnostic examination but who featured comorbidities that could account for their polyneuropathy and patients with newly diagnosed diabetes were excluded from the study. The healthy controls did not have diabetes or any other chronic disease, nor did they have any symptoms or signs associated with polyneuropathy.
2.2. Demographic, medical, and laboratory data
Participants’ medical records were reviewed and the following clinical data were collected: age, gender, duration of diabetes mellitus, insulin use, history of hypertension, statins use, previous medical history. Peripheral venous blood samples of the patients were collected from veins at the time of their admission to the outpatient clinic. Peripheral blood count parameters, the percentage of glycosylated Hemoglobin A1c (HbA1c), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), HDL-C, and triglyceride (TG) levels were recorded. The MHR was calculated for each individual by dividing their monocyte count with their HDL cholesterol level.
2.3. Electrophysiology and axonal polyneuropathy diagnosis
Motor and sensory conduction studies were performed in all patients following receipt of approval for testing. Sensory transmission studies were conducted in the unilateral median, ulnar, and sural nerves. Motor nerve transmission studies were performed in the unilateral median and ulnar nerves, and the bilateral posterior tibial and peroneal nerves by using a Keypoint electromyography (EMG) machine. Observation of decreased action potential amplitudes in 2 or more nerves without the existence of criteria for demyelination, such as a significant slowdown in the nerve conduction rates or conduction blocks, was indicative of axonal polyneuropathy.
Intergroup comparison was performed. The relationship between the posterior tibial nerve CMAP amplitudes and the aforementioned parameters was examined. The posterior tibial nerve was selected for analysis because the majority of polyneuropathies are length dependent. The extensor digiti brevis muscle was misleading for measuring the fibular nerve amplitude due to atrophy in the years due to the shoe pressure, and the sural nerve amplitude decreased with age.
2.4. Statistical analysis
SPSS 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) statistics software was used for the evaluation of the data. The mean ± standard deviation, number, and percentage values were used as the variables. The homogeneity of the variances, which is one of the prerequisites of the parametric tests, was checked with the “Levene” test. The normality hypothesis was checked with the “Shapiro-Wilk” test. To identify differences between the 2 groups, the Student t test was used when parametric tests satisfied the pre-conditions, and the Mann–Whitney U test was used when parametric tests did not. One-way analysis of variance and Tukey honestly significant difference test were used for comparisons of 3 or more groups. “Kruskal Wallis” and “Bonferroni-Dunn” tests were used when the parametric test prerequisites were not fulfilled. The relationship between 2 variables was evaluated by Pearson correlation coefficient when parametric tests satisfied the pre-conditions and by Spearman correlation coefficient when parametric tests did not fulfill the pre-conditions. The relationships between categorical variables were analyzed using the Chi-square test and Fisher exact test. The Monte Carlo simulation method was used when the expected frequency values were smaller than 20% for inclusion of these frequencies in the analysis. Univariate and multivariate logistic regression analyses were used to characterize the relationship between independent and dependent variables (diabetic patients with and without polyneuropathy) and to present multivariate risk factors. The statistical significance level was set to P < .05 and P < .01.
3. Findings
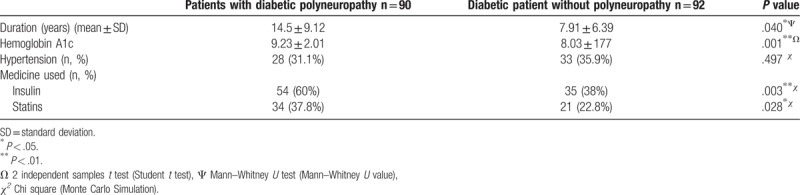
The present study included 90 patients with DPN, 75 patients with non-DPN, 92 diabetic patients without polyneuropathy, and 67 healthy individuals. The basic characteristics and laboratory results concerning the patients are shown in Table 1. The average disease duration of patients with DPN was significantly longer than that of diabetic patients without polyneuropathy (P = .040). Relative to diabetic patients without polyneuropathy, DPN patients featured higher HbA1c values, as well as insulin and statin use rates (P = .001, P = .003, P = .028, respectively) (Table 2). While the mean posterior tibial nerve CMAP amplitude of patients with DPN was 1.55 mv (0.1–5 mv), the mean posterior tibial nerve CMAP amplitude of patients with non-DPN was 2.04 mv (0.1–5 mv). Of the 75 patients who did not have diabetes, 35 had chronic renal failure, 9 chronically used excessive alcohol for more than 18 years, 27 patients received treatment with antineoplastic chemotherapeutic agents due to various cancers, and 5 patients were diagnosed with idiopathic polyneuropathy.
Table 1.
The characteristics, lipid profiles, and peripheral blood count parameters of the patients and healthy individuals.
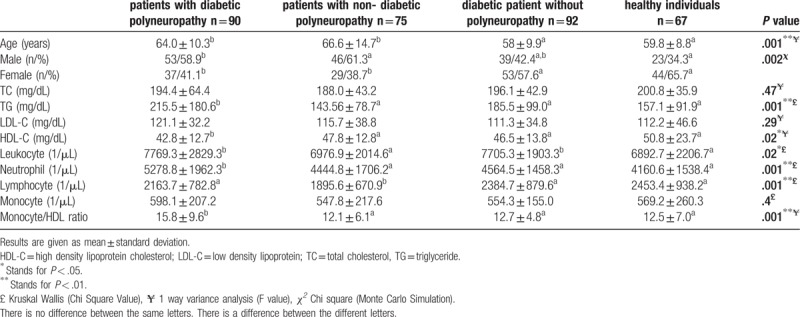
Table 2.
Characteristics of diabetic patients with and without polyneuropathy.
TG levels of the patients with DPN were significantly higher than those of the non-DPN group, diabetic patients without polyneuropathy, and healthy individuals (P = .001). There was no significant difference between the groups in terms of the LDL-C and TC levels (P = .29 and P = .47, respectively). HDL-C levels of the patients with DPN were significantly low compared to those in the non-DPN group, diabetic patients without polyneuropathy, and the control group (P = .02). While there was no significant difference between the groups in terms of monocyte count, neutrophil counts were higher in the patients with DPN relative to the patients in the 3 other groups (P = .4, P = .001, respectively). Lymphocyte counts of the patients with non-DPN were significantly lower than those of all other patients (P = .001) (Table 1). MHR was not different among the patients with non-DPN, diabetic patients without polyneuropathy, and healthy individuals. The MHR values of the patients with DPN were significantly higher than those of the patients with non-DPN, diabetic patients without polyneuropathy, and the control group (P = .001) (Table 1).
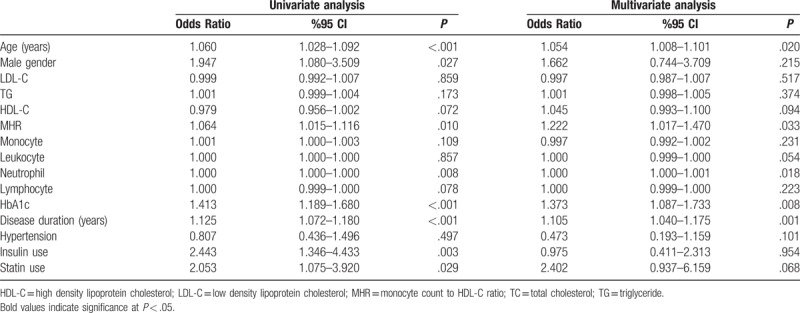
In the univariate analysis, age, sex, MHR, neutrophil, HbA1c, disease duration, insulin use, and statin use were demonstrated to be possible confounding factors for the polyneuropathy. After multivariate logistic regression analysis, age, MHR, neutrophil, HbA1c, and disease duration emerged as independent predictors of DPN. Univariate and multiple logistic regression analysis indicated that age (OR = 1.054, 95% CI = 1.008–1.101, P = .020), MHR (OR = 1.222, 95% CI = 1.017–1.470, P = .033), neutrophil (OR = 1.000, 95% CI = 1.000–1.001, P = .018), HbA1c (OR = 1.373, 95% CI = 1.087–1.733, P = .008) and disease duration (OR = 1.105, 95% CI = 1.040–1.175, P = .001) are independent predictors of DPN (Table 3).
Table 3.
Univariate and multivariate logistic regression analyses performed to identify possible confounding factors of diabetic polyneuropathy.
No correlation was observed between the levels of TC, TG, LDL-C, the counts of leukocyte neutrophils, lymphocytes, HbA1c, and the posterior tibial nerve CMAP amplitudes in the patients with DPN. There was a strong positive correlation between the posterior tibial nerve CMAP amplitudes and HDL-C values (P = .000, r = .360). The decrease in the posterior tibial nerve CMAP amplitudes was negatively correlated with the increase in both monocyte counts and MHR (P = .016, r = -.253; P = .008, r = -.279, respectively) (Table 4).
Table 4.
The relationship between lipid profile and peripheral blood count parameters of patients with diabetic polyneuropathy and their posterior tibial nerve compound muscle action potential amplitudes.

4. Discussion
The present study demonstrated that the increase in MHR levels of patients with diabetic axonal neuropathy was correlated with the decrease of the CMAP amplitude. To the best of our knowledge, this is the first study that demonstrates patients diabetic axonal neuropathy with exhibiting higher values of MHR relative to diabetic patients without polyneuropathy, with nondiabetic axonal neuropathy, and healthy controls.
Neuropathy is the most common microvascular complication of diabetes mellitus, and distal peripheral neuropathy is the most frequently observed form of neuropathy in patients with diabetes.[15] Despite being one of the major causes of morbidity in patients with diabetes,[16–18] prior research has not investigated it as and the mechanisms underlying DPN have yet to be elucidated. Monocyte-to-high density lipoprotein ratio (MHR) has recently been implemented as an indicator of inflammation and oxidative stress. The present study determined how MHR changed in patients with DPN, in which oxidative stress and microvascular damage play a role in pathogenesis, relative to the patients with non-DPN, diabetic patients without polyneuropathy, and healthy individuals. We aimed to evaluate the association between MHR and the decreased CMAP amplitude exhibited by patients with diabetic axonal polyneuropathy.
It is generally accepted that the pathogenesis of DPN is multifactorial: a combination of vascular and metabolic defects. The structural abnormalities of the endoneurial microvascular structure, defects in the vasoactive agents that regulate blood flow in the nerves, and changes to the autonomic innervation of the vascular structure of the nerves potentially contribute to the ischemia of the nerves. Deterioration of the polyol pathway, abnormalities in the lipid metabolism, increased end products of advanced glycation, and oxidative damage constitutes several of the metabolic factors associated with polyneuropathy.[19]
The change in the lipid metabolism is common in patients with diabetes and has been found to plays a key role in the pathogenesis of DPN.[16] The hepatic lipase activity, increased by the insulin resistance, hydrolyzes the phospholipids in the LDL and HDL particles and therefore causes smaller and denser LDL particles and decreased HDL.[20] Since the levels of LDL and triglyceride are usually high and the level of HDL is low in the patients with DPN, it was thought that there could be a relationship between these observations and the development of neuropathy. The altered plasma lipid profile induces neurovascular pathologies. The microangiopathic changes occurring in vasa nervorum associated with atherosclerosis can mediate the occurrence of polyneuropathy by affecting the feeding of peripheral nerves.[21] Research has further demonstrated that the size of the LDL particle, which is a marker of atherogenic dyslipidemia, is also an independent risk factor for the occurrence of neuropathy.[16,22] In addition, it was shown that increased levels of LDL and TG are associated with the quick progression of not only the final stage of renal failure and blindness but also peripheral neuropathy in patients with diabetes.[23] Hypertriglyceridemia contributes to the occurrence and progression of DPN on account of its atherogenic potential[24] and simultaneous changes caused by the Schwann cell myelin structure play a role in this.[25] The anti-inflammatory, antioxidant, and anti-thrombotic effects of HDL was evinced by prior research;[26,27] HDL behaves as an anti-atherogenic lipid by preventing the lipid transportation of macrophages with lipid loads to the arterial wall,[28] and prevents the adhesion of monocytes to the arterial wall by inhibiting the endothelial expression of the adhesion molecules through its inhibition of CD11b activation.[29]
Changes to lipid metabolism are concurrent with low-grade inflammation in patients with diabetes. Past investigations have demonstrated that the levels of C-reactive protein, interleukin (IL)-1, IL-6, tumor necrosis factor-a, and inflammatory cytokines are high in patients with diabetes.[30,31] The proinflammatory changes have a direct role in the pathogenesis of complications such as atherosclerosis, nephropathy, and neuropathy.[32–34] The main type of cells that play a role in the occurrence of atherosclerosis, a lipid-guided inflammatory disease that induces proinflammatory cytokine secretion,[35,36] is the monocyte. Hyperglycemia in diabetic patients activates them[37]: hyperglycemia causes oxidative stress, which further damages the nerve cells through lipid peroxidation, induction of the proinflammatory factors, waste of the cellular antioxidants, and pathologic activation of the repair mechanisms.[38] It is therefore thought that MHR can indicate inflammation due to the proinflammatory effect of the monocytes, as well as the anti-inflammatory and antioxidant effects of the HDL cholesterol.
Previous studies have observed that the neutrophil/lymphocyte ratio (NLR), as an indicator of systemic inflammation, increases in patients with diabetes,[39] diabetic retinopathy,[40] nephropathy[41]— the latter 2 are complications of diabetes—and in diabetic patients with coronary artery disease.[42] In a recent study conducted by Liu et al, the relationship between diabetic peripheral neuropathy and NLR was examined; it was observed that patients with high NLR exhibited a lower nerve conduction velocity and that such patients were more likely to develop polyneuropathy.[43]
As a new vascular inflammatory marker, MHR was investigated in a series of clinical studies: Kanbay et al demonstrated that an increase in MHR was associated with a decreased glomerular filtration rate in chronic kidney disease;[10] Canpolat et al found that high MHR was associated with the slow coronary phenomenon.[12]; Kundi et al[9] determined the relationship between MHR and the SYNTAX score in the context of coronary artery disease; Bolayir et al[14] demonstrated that the high MHR was an independent risk factor for 30-day mortality in patients with ischemic stroke; and You et al[7] showed that MHR was associated with the increased disability and mortality in patients with intracerebral hemorrhage.
The white blood cells and their subtypes, which are thought to be the potential predictors of clinical findings, morbidity, and mortality in a series of diseases, are commonly used markers of inflammation. IL-1, IL-6, IL-18, tumor necrosis factor-a1, interferon-1, transforming growth factor-1, and C-reactive protein are the inflammatory factors associated with the pathogenesis of diabetes.[44–46] These circulating factors reflect low-grade chronic inflammation and are associated with the diabetic complications.[47] However, their detection entails high costs, prohibiting their use in daily clinical practice. Laboratory indexes such as MHR, which features a lower cost and ease of measurement, have increasingly been adopted as indicators of systemic inflammation.
To the best of our knowledge, the present study is the first to assess MHR in diabetic patients with and without axonal polyneuropathy. We found that the MHR value was higher in patients with diabetic axonal polyneuropathy than in diabetic patients without polyneuropathy. The MHR values of both diabetic patients without polyneuropathy and patients with non-DPN were similar to those of healthy people. The group of patients with non-DPN consisted of individuals with chronic renal failure, those with malignancy who used chemotherapeutic agents, and patients with alcoholic neuropathy. In fact, the last group exhibited systemic as well. Despite this, the relatively higher MHR in patients DPN indicates that inflammation and oxidative stress were much more acute and suggests that the decreased HDL value may affect the pathogenesis of polyneuropathy more than we had expected.
Univariate and multivariate logistic regression analyses in the present study showed that age, disease duration, high levels of HbA1c, and elevated MHR values were independent predictors of DPN. Patients with DPN had a longer disease duration than did diabetic patients without polyneuropathy and their HbA1c levels were higher, that is, they exhibited worse glycemic control.
These findings are in line with the known pathogenesis of DPN. A longer duration of diabetes, poor glycaemic control, age-related neuronal attrition, and recognised risk factors have been implicated.[4,15,48–50] Glycaemic control is the most important factor in the prevention and progression of DPN.[51] High blood glucose level induces nerve damage due to vascular and metabolic deterioration.[19] The study by Popescu et al[52] showed that age is an independent predictor of DPN development in diabetic patients. A study has previously shown that a disease that leads to a subclinical impairment that lasts for decades results in affected neurons that are more prone to the consequences of age-related neuronal impairment.[53] Therefore, age may be a factor that affects the development of polyneuropathy. As the person ages, the age-related neuronal deterioration will also increase.
Contrary to the study of Popescu et al,[52] we found that the duration of the disease as a risk factor is independent of glycemic control. In their study, they claimed that in spite of good glycemic control, the risk of developing neuropathy is similar in patients with and those without diabetes. However, as mentioned above, the development of neuropathy in diabetic patients is not only due to hyperglycemia. Our results support that long-term disease duration is an independent predictor of polyneuropathy development in diabetic patients. Other than these factors, MHR as a marker of oxidative stress and inflammation is an independent predictor of DPN. This association remained significant even after adjusting for DPN-related factors and is important because it shows that inflammation and oxidative stress play an important role in the development of polyneuropathy in diabetic patients.
In this study, a significant positive correlation was found between HDL-C and posterior tibial nerve CMAP amplitudes; negative correlations between posterior tibial nerve CMAP amplitudes and MHR, and between the latter and both monocyte counts; and no correlation between CMAP amplitudes and HbA1c levels.
In brief, our results demonstrated that diabetic patients with higher MHR levels tend to have lower CMAP amplitudes and are more likely to develop polyneuropathy. MHR could, therefore, be used as a predictor of polyneuropathy in diabetic patients.
Our study was subject to several limitations. First, our sample was small. Second, other inflammation factors were not measured in this research. Third, this study was designed as a retrospective study. Future research should adopt a prospective design to establish the relationship between progression of polyneuropathy and MHR.
5. Conclusion
MHR, an indicator of systemic inflammation, is a valuable index of the modified lipid profile. It, therefore, has potential as a predictor of the pathogenesis of diabetic neuropathy.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: Gönül Vural.
Data curation: Gönül Vural, Şadiye Gümüşyayla.
Investigation: Gönül Vural, Şadiye Gümüşyayla.
Methodology: Gönül Vural.
Project administration: Gönül Vural.
Writing – original draft: Gönül Vural, Şadiye Gümüşyayla.
Writing – review & editing: Gönül Vural, Şadiye Gümüşyayla.
Gönül Vural orcid: 0000-0002-1245-7273.
Footnotes
Abbreviations: CMAP = compound muscle action potential, DPN = diabetic polyneuropathy, HbA1c = Hemoglobin A1c, HDL-C = high density lipoprotein cholesterol, IL = interleukin, LDL-C = low-density lipoprotein cholesterol, MHR = monocyte-to-high density lipoprotein ratio, NLR = neutrophil/lymphocyte ratio, TC = total cholesterol, TG = triglyceride.
The authors have no conflicts of interest to disclose.
References
- [1].Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J 2006;82:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Said G. Diabetic neuropathy-a review. Nat Clin Pract Neurol 2007;3:331–40. [DOI] [PubMed] [Google Scholar]
- [3].Bodman MA, Dulebohn SC. Neuropathy, Diabetic. [Updated 2017 Oct 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK442009/. [Google Scholar]
- [4].Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments and subtypes. Curr Neurol Neurosci Rep 2014;14:473(pp1-18) doi: 10.1007/s11910-014-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Charnogursky GA, Emanuele NV, Emanuele MA. Neurological complications of diabetes. Curr Neurol Neurosci Rep 2014;14:457(pp1-16). doi: 10.1007/s11910-014-0457-5. [DOI] [PubMed] [Google Scholar]
- [6].Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, et al. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res 2016;2016:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].You S, Zhong C, Zheng D, et al. Monocyte to HDL cholesterol ratio is associated with discharge and 3-month outcome in patients with acute intracerebral hemorrhage. J Neurol Sci 2017;372:157–61. [DOI] [PubMed] [Google Scholar]
- [8].Karataş MB, Çanga Y, Özcan KS, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med 2016;34:240–4. [DOI] [PubMed] [Google Scholar]
- [9].Kundi H, Kiziltunc E, Cetin M, et al. Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Herz 2016;41:523–9. [DOI] [PubMed] [Google Scholar]
- [10].Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 2014;46:1619–25. [DOI] [PubMed] [Google Scholar]
- [11].Canpolat U, Aytemir K, Yorgun H, et al. The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace 2015;17:1807–15. [DOI] [PubMed] [Google Scholar]
- [12].Canpolat U, Çetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost 2016;22:476–82. [DOI] [PubMed] [Google Scholar]
- [13].Balta S, Celik T, Ozturk C, et al. The relation between monocyte to HDL ratio and no-reflow phenomenonin the patients with acute ST-segment elevation myocardial infarction. Am J Emerg Med 2016;34:1542–7. [DOI] [PubMed] [Google Scholar]
- [14].Bolayir A, Gokce SF, Cigdem B, et al. Monocyte/high-density lipoprotein ratio predicts the mortality in ischemic stroke patients. Neurol Neurochir Pol 2017;52:150–5. [DOI] [PubMed] [Google Scholar]
- [15].Katulanda P, Ranasinghe P, Jayawardena R, et al. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr 2012;4:21(pp1–8). doi: 10.1186/1758-5996-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Perez-Matos MC, Morales-Alvarez MC, Mendivil CO. Lipids: a suitable therapeutic target in diabetic neuropathy. J Diabetes Res 2017;2017:6943851(pp1–9) doi: 10.1155/2017/6943851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ward JD. Abnormal microvasculature in diabetic neuropathy. Eye 1993;7:223–6. [DOI] [PubMed] [Google Scholar]
- [18].Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361:1545–51. [DOI] [PubMed] [Google Scholar]
- [19].Stevens MJ, Feldman EL, Greene DA. The aetiology of diabetic neuropathy: the combined roles of metabolic and vascular defects. Diabet Med 1995;12:566–79. [DOI] [PubMed] [Google Scholar]
- [20].Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004;27:1496–504. [DOI] [PubMed] [Google Scholar]
- [21].Çomoğlu S, Yardımcı S, Okçu Z. The alterations in plasma lipid profile of diabetic polyneuropathic patients. T Klin J Med Sci 2001;21:345–8. [Google Scholar]
- [22].Isomaa B, Henricsson M, Almgren P, et al. The metabolic syndrome influences the risk of chronic complications in patients with Type II diabetes. Diabetologia 2001;44:1148–54. [DOI] [PubMed] [Google Scholar]
- [23].Obrosova IG, Ilnytska O, Lyzogubov VV, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes 2007;56:2598–608. [DOI] [PubMed] [Google Scholar]
- [24].Tesfaye S, Harris N, Jakubowski JJ, et al. Impaired blood flow and arterio-venous shunting in human diabetic neuropathy: a novel technique of nerve photography and fluorescein angiography. Diabetologia 1993;36:1266–74. [DOI] [PubMed] [Google Scholar]
- [25].Thomas PK, Ward JD, Watkins PJ. Keen H, Jarrett J. Diabetic Neuropathy. Complications of diabetes. London: Edward Arnold; 1982. 109–36. [Google Scholar]
- [26].Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res 2004;95:764–72. [DOI] [PubMed] [Google Scholar]
- [27].Cockerill GW, Rye KA, Gamble JR, et al. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol 1995;15:1987–94. [DOI] [PubMed] [Google Scholar]
- [28].Zhang Y, Zanotti I, Reilly MP, et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 2003;108:661–3. [DOI] [PubMed] [Google Scholar]
- [29].Murphy AJ, Woollard KJ, Hoang A, et al. High density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 2008;28:2071–7. [DOI] [PubMed] [Google Scholar]
- [30].Garcia C, Feve B, Ferré P, et al. Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes Metab 2010;36:327–38. [DOI] [PubMed] [Google Scholar]
- [31].Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286–92. [DOI] [PubMed] [Google Scholar]
- [32].Skundric DS, Lisak RP. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: from glucose metabolism to neurodegeneration. Exp Diabesity Res 2003;4:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Navarro JF, Mora C, Muros M, et al. Urinary tumour necrosis factor-alpha excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial Transplant 2006;21:3428–34. [DOI] [PubMed] [Google Scholar]
- [34].Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep 2007;7:242–8. [DOI] [PubMed] [Google Scholar]
- [35].Ancuta P, Wang J, Gabuzda D. CD16 monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol 2006;80:1156–64. [DOI] [PubMed] [Google Scholar]
- [36].Hilgendorf I, Swirski FK, Robbins CS. Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:272–9. [DOI] [PubMed] [Google Scholar]
- [37].Nandy D, Janardhanan R, Mukhopadhyay D, et al. Effect of hyperglycemia on human monocyte activation. J Investig Med 2011;59:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Negi G, Kumar A, Joshi RP, et al. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem and Biophys Res Commun 2011;408:1–5. [DOI] [PubMed] [Google Scholar]
- [39].Yilmaz H, Ucan B, Sayki M, et al. Usefulness of the neutrophil-to-lymphocyte ratio to prediction of type 2 diabetes mellitus in morbid obesity. Diabetes Metab Syndr 2015;9:299–304. [DOI] [PubMed] [Google Scholar]
- [40].Wang RT, Zhang JR, Li Y, et al. Neutrophil-Lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complications 2015;29:245–9. [DOI] [PubMed] [Google Scholar]
- [41].Huang W, Huang J, Liu Q, et al. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf) 2015;82:229–33. [DOI] [PubMed] [Google Scholar]
- [42].Verdoia M, Schaffer A, Barbieri L, et al. Impact of diabetes on neutrophil-to- lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab 2015;41:304–11. [DOI] [PubMed] [Google Scholar]
- [43].Liu S, Zheng H, Zhu X, et al. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res Clin Pract 2017;130:90–7. [DOI] [PubMed] [Google Scholar]
- [44].Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care 2003;26:1619–23. [DOI] [PubMed] [Google Scholar]
- [45].Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- [46].Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012;2012:146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Blüher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia 2002;45:210–6. [DOI] [PubMed] [Google Scholar]
- [48].Morkrid K, Ali L, Hussain A. Risk factors and prevalence of diabetic peripheral neuropathy:a study of type 2 diabetic outpatients in Bangladesh. Int J Diabetes Dev Ctries 2010;30:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Valensi P, Giroux C, Seeboth-Ghalayini B, et al. Diabetic peripheral neuropathy: effects of age, duration of diabetes, glycemic control,and vascular factors. J Diabetes Complications 1997;11:27–34. [DOI] [PubMed] [Google Scholar]
- [50].Albers JW, Brown MB, Sima AA, et al. Nerve condition measures in mild diabetic neuropathy in the early diabetes intervention trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Tolrestat study group for the early diabetes intervention trial. Neurology 1996;46:85–91. [DOI] [PubMed] [Google Scholar]
- [51].Viswanathan V, Tilak P, Kumpatla S. Risk factors associated with the development of overt nephropathy in type 2 diabetes patients: a 12-year observational study. Indian J Med Res 2012;136:46–53. [PMC free article] [PubMed] [Google Scholar]
- [52].Popescu S, Timar B, Baderca F, et al. Age as an independent factor for the development of neuropathy in diabetic patients. Clin Interv Aging 2016;11:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Albers JW, Berent S. Issues and controversies involving the peripheral nervous system evaluation, Neurobehavioral Toxicology; Peripheral nervous system. 2005;London and New York: Taylor&Francis, 2:653-708. [Google Scholar]


