Abstract
To investigate the relationship between glaucoma severity and intraocular pressure (IOP) reduction after cataract surgery in patients with medically controlled primary open-angle glaucoma (POAG).
Retrospective case series.
This study included glaucoma suspects (GS) and POAG patients who underwent cataract surgery and continued to use the same glaucoma medications during the postoperative period of 4 months. The main outcomes were percent and absolute IOP changes calculated using the preoperative IOP and the postoperative IOP at 3 months. Preoperative glaucoma medications, preoperative IOP, demographic information, biometric parameters and variables for glaucoma severity were evaluated as potential predictors of IOP change.
The average IOP reduction was 3.3 ± 2.4 mmHg (20.0%) and 2.2 ± 2.5 mmHg (13.1%) from the preoperative mean of 16.0 ± 2.9 mmHg and 15.2 ± 3.3 mmHg in the GS and POAG groups, respectively. Preoperative IOP, preoperative IOP/anterior chamber depth (preoperative IOP/ACD [PD ratio]) and preoperative IOP/retinal nerve fiber layer (RNFL) thickness (preoperative IOP/RNFL [PNFL ratio]) and preoperative IOP score x MD score x number of glaucoma medications (glaucoma index) predicted absolute IOP change in the POAG group, whereas preoperative IOP, PD ratio, PNFL ratio, and axial length (AL) did in the GS group. Preoperative IOP, PD ratio, and PNFL ratio predicted %IOP change in the POAG group, whereas only AL did in the GS group.
In medically controlled POAG eyes, structural or functional parameters for glaucoma severity did not independently predict IOP change following phacoemulsification. However, novel severity indices obtained by addition of preoperative IOP and/or glaucoma medications to the structural or functional parameter predicted IOP changes.
Keywords: cataract surgery, glaucoma, intraocular pressure, phacoemulsification
1. Introduction
Elevated intraocular pressure (IOP) is a major modifiable risk factor for development or progression of glaucoma.[1,2] Among various therapeutic approaches, lowering IOP is the only proven one to prevent or slow down progression of glaucomatous optic neuropathy.[3] Cataract surgery has been shown to decrease IOP in eyes with or without glaucoma.[4–8] In eyes with angle-closure glaucoma, phacoemulsification is well established as an effective IOP-lowering procedure.[6,9] Although not as much as in ACG eyes, phacoemulsification has also been shown to provide a decrease in IOP in eyes with open-angle glaucoma (OAG).[10,11]
Higher preoperative IOP level, smaller anterior chamber depth (ACD) and greater ratio of preoperative IOP/ACD (PD ratio) were found to be predictors of greater IOP drop after surgery.[12,13] In addition to these biometric parameters, recent studies found axial length (AL), lens thickness (LT), lens vault, and lens position (LP) to be associated with the IOP change following cataract surgery.[14–17] However, the association of glaucoma severity to the IOP change after phacoemulsification remains unclear.
Slabaugh and co-workers evaluated the association of various demographic and biometric variables to IOP changes in eyes with medically controlled OAG, and demonstrated that higher IOP, older age, and deeper ACD were associated with lower postoperative IOP.[18] In their study, the severity of glaucomatous damage was found not to be associated with IOP change when assessed using mean deviation (MD) and pattern standard deviation (PSD) of visual field testing as the metric. However, the structural loss of glaucoma may precede the functional loss at an earlier stage of glaucoma, and the MD or VFI may not reflect the functional damage in an eye with early glaucoma.
Thus, we conducted the present study to investigate the glaucoma severity, assessed by structural and functional tests, as a predictor of IOP reduction after cataract surgery in patients with medically controlled primary open-angle glaucoma (POAG) and glaucoma suspects (GS).
2. Methods
Institutional review board approval for this retrospective study was obtained from the University of California-San Francisco (UCSF) Committee on Human Research (CHR). The study followed the tenets of the Declaration of Helsinki. This cross-sectional study enrolled consecutive POAG and GS patients who met the inclusion criteria and underwent phacoemulsification and intraocular lens implantation as a sole procedure at UCSF between January 1, 2014 and January 31, 2016.
Inclusion criteria included:
-
1)
adult patients (18 years or older);
-
2)
uncomplicated cataract surgeries without adjunctive procedures (e.g., pupil stretching, use of iris hooks or Malyugin rings);
-
3)
preoperative diagnosis of POAG;
-
4)
reliable spectral domain optical coherence tomography (SDOCT) of peripapillary retinal nerve fiber layer (RNFL) and visual field test results within 6 months of cataract surgery; and
-
5)
use of the same anti-glaucoma medication during the 4-month postoperative period.
POAG was determined if there was use of glaucoma medications plus 1 of the following scenarios:
-
(1)
VF loss consistent with glaucoma and cup-to-disc ratio ≥ 0.7, or
-
(2)
cup-to-disc ratio ≥ 0.9 if the patient was unable to perform reliable visual field examination in the affected eye.
Exclusion criteria included:
-
(1)
intraoperative or postoperative complications related to the cataract surgery (e.g., posterior capsule rupture, vitreous loss);
-
(2)
history of trabeculectomy or other intraocular glaucoma surgeries;
-
(3)
uveitis, retinal disease such as macular degeneration, or congenital anomalies;
-
(4)
history of ocular trauma or any prior intraocular surgery;
-
(5)
history of intraocular laser treatment;
-
(6)
contact lens use;
-
(7)
pseudoexfoliation or pigment dispersion;
-
(8)
history of any change in medication regimen within 3 months before surgery;
-
(9)
not having at least 3 prior IOP measurements; or
-
(10)
not having at least 6 months of follow-up after surgery. Both eyes of each participant were included, except in cases in which an eye did not meet inclusion and exclusion criteria.
We defined glaucoma suspect as
-
(1)
a patient having an optic disc with a vertical cup-to-disc ratio of 0.7 or more but with no focal neuroretinal rim thinning or disc hemorrhage, and normal visual fields on standard automated perimetry; or
-
(2)
a patient having ocular hypertension (IOP of 22 mmHg or greater), normal appearance of the optic disc and RNFL, and normal visual field test results.
Pertinent clinical information before phacoemulsification and in the year subsequent to surgery was recorded. This included baseline demographics including age and sex, disease severity indices such as visual field index (VFI) and peripapillary RNFL thickness, and ophthalmic biometry obtained by the LENSTAR LS 900 (Haag-Streit, Inc., Koeniz, Switzerland). In the preoperative assessment, which occurred 1 to 3 weeks before surgery, ocular biometry, BCVA testing, IOP measurement and complete slit-lamp and fundus examination were performed. IOP was measured using Goldmann applanation tonometry by an ophthalmologist. For ocular biometry, the LENSTAR LS 900 (Haag-Streit, Inc.) was used to measure AL, ACD, LT, and central corneal thickness (CCT). Five readings were taken for each eye. Preoperative IOP was defined as the IOP reading at the most recent examination within 1 week before cataract surgery. Three-month postoperative IOP was considered to be the single-visit reading obtained at the first routine visit at least 3 months postoperatively. The PD ratio was defined as the ratio of preoperative IOP to ACD.
Topical or sub-Tenons anesthesia was used. A temporal clear corneal incision was followed by injection of viscoelastic, continuous tear capsulorhexis, and standard phacoemulsification with placement of an acrylic intraocular lens in the lenticular bag. Patients were prescribed topical antibiotics, 0.5% ketorolac tromethamine, and 1% prednisolone acetate and tapered over 1 month. POAG patients were prescribed the same anti-glaucoma medication before and at least 3 months after phacoemulsification.
All eyes included in the VF analysis had at least 2 reliable tests and consistent VF defects corresponding to the optic disc changes. VF tests with a fixation loss rate ≤ 20% and false-negative and false-positive rates ≤ 20% were determined as reliable VF tests. Mean deviation (MD), PSD, and VFI were recorded from the most recent VF testing within 6 months from the cataract surgery. Peripapillary RNFL thickness was measured with RTVue (software V.6.1.0.4, Optovue, Fremont, CA) SDOCT using the 3.4 mm scan circle around the optic disc. Average peripapillary RNFL thickness was recorded from the SDOCT scans taken within 6 months from the cataract surgery. The preoperative IOP/RNFL (PNFL ratio) was defined as the ratio of preoperative IOP to the average peripapillary RNFL thickness.
The preoperative IOP score x MD score x number of glaucoma medications (glaucoma index), GI, was calculated according to the definition described by Loewen et al.[18] Briefly, it was created as a variable based on visual field, number of preoperative medications, and preoperative IOP. Visual field was separated into 4 categories: up to mild, up to moderate, up to advanced, and more than advanced visual field damage,[19] which were assigned 1, 2, 3, and 4 points, respectively. Preoperative number of medications were divided into 4 categories: 0-1, 2, 3, or 4+, and assigned with a value of 1 to 4, respectively. Preoperative IOP was divided into 3 categories: <20 mmHg, 20 to 29 mmHg, 30 to 39 mmHg, and greater than 40 mmHg and assigned with 1 to 4 points, respectively. These categories were chosen based on IOP distribution and designed not to underrate low-pressure glaucoma. GI was then defined as preoperative IOP × preoperative number of medications × VF. GI was separated into 4 groups: <6 (Group 1), 6 to 12 (Group 2), >12 to 18 (Group 3) and >18 (Group 4).
2.1. Statistical analysis
For glaucoma suspect and glaucoma groups, we characterized the study population by calculating mean and standard deviation for continuous variables and number and percentages for categorical variables. We also tested statistical differences between both groups using the generalized estimating equations for continuous and categorical variables because some patients were repeatedly measured in the right and left eyes.
To separately assess the effects of preoperative IOP, age, sex, and other ocular parameters for the dependent variables such as %IOP change or absolute IOP change, we considered general linear mixed models with unstructured covariance matrix. When we fitted the general linear mixed models, we adjusted for the only effect of laterality in univariate analysis. However, we obtained the estimates of all predictors except PD ratio and PNFL ratio after adjusting for the effects of laterality, preoperative IOP, age, and sex in multivariate analysis (Tables 2 and 3). For PD ratio and PNFL ratio, we corrected for the effects of laterality, age, and sex in multivariate analysis because of very strong correlations of both variables and preoperative IOP.
Table 2.
Association of various predictors of intraocular pressure (IOP) change (using % IOP change at 3 months as the dependent variable).
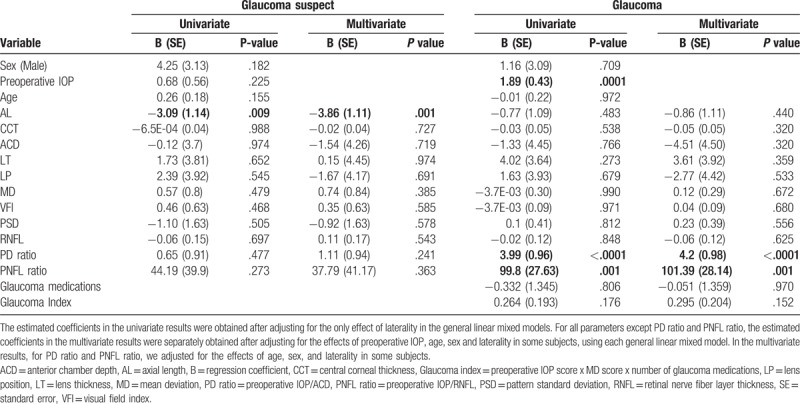
Table 3.
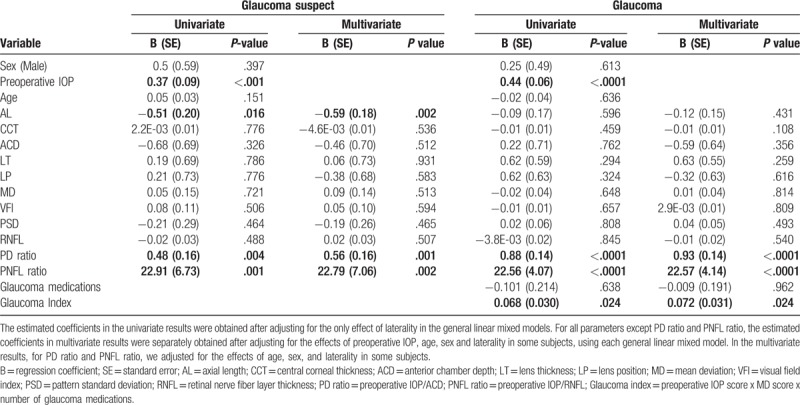
Association of various predictors of intraocular pressure (IOP) change (using absolute IOP change at 3 months as the dependent variable).
In all models assessing PD ratio and PNFL ratio, preoperative IOP was not included because it is part of the calculation of PD ratio and PNFL ratio. We also assessed the interactions effects between the group and each of the ocular parameters such as AL, CCT, ACD, LT, LP, MD, VFI, PSD, RNFL, PD ratio, and PNFL ratio for each dependent variable (%IOP Change, Absolute IOP Change). For the predictors of AL, CCT, ACD, LT, LP, MD, VFI, PSD, and RNFL, we corrected for the effects of preoperative IOP, age, sex, glaucoma diagnosis, and laterality in the general linear mixed models. For PD ratio and PNFL ratio, we adjusted for the effects of laterality, age, group, and sex (Table 4). Finally, we fitted general linear mixed models including covariates such as laterality, sex, group, age, CCT, LT, LP, AL, MD, PSD, PD ratio, and PNFL ratio and obtained some significant predictors. In this analysis, we did not include the predictors with strong correlation higher than 0.8. Therefore, we tried to fit the models including VFI and PSD instead of MD and PSD among the covariates. All of statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC). P values less than .05 were considered to be significant.
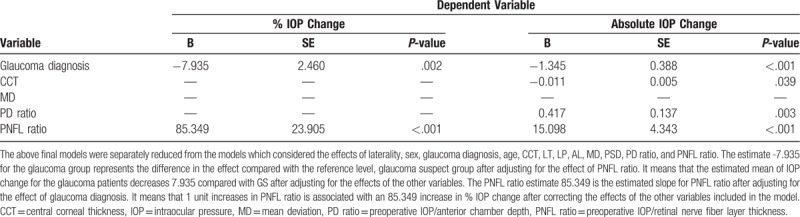
Table 4.
The final models reduced from each model considering the effects of 12 predictors as covariates in the general linear mixed models.
3. Results
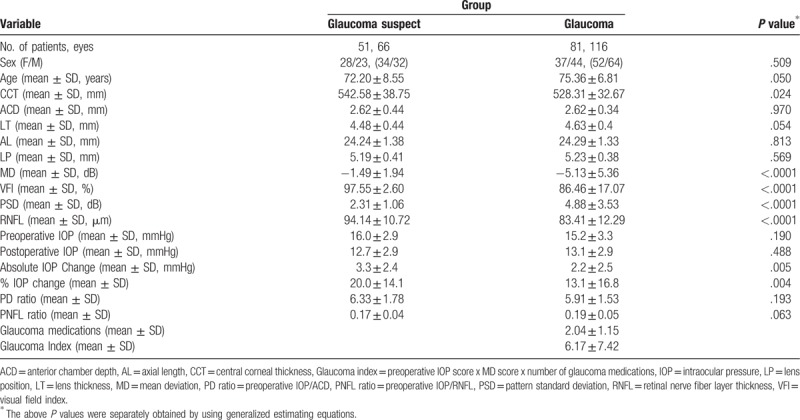
The study included 66 eyes of 51 GS and 116 eyes of 81 POAG patients. The mean age of the GS and POAG groups was 72.2 ± 8.6 and 75.4 ± 6.8 years, respectively. The GS group was 55% female compared to 46% of the POAG group. Compared to the GS group, the POAG group had thinner CCT and RNFL thickness, and worse visual field indices. Postoperative IOP reduction was greater in the GS group (3.3 ± 2.4 mmHg, 20%) than in the POAG group (2.2 ± 2.5 mmHg, 13%) (Table 1).
Table 1.
Demographic and clinical characteristics of the study patients.
In the glaucoma suspect group, AL was found to be a significant predictor of absolute and percent IOP change; PD ratio and PNFL ratio were significant predictors of only absolute IOP change. In the POAG group, preoperative IOP, PD ratio, and PNFL ratio were significant predictors of both percent and absolute IOP change, whereas GI was a significant predictor of absolute IOP change only. Age, sex, AL, ACD, LT, LP, MD, VFI, and RNFL were not associated with IOP reduction (P >.05) in the POAG group. The Spearman correlation coefficients describing the association of PD ratio and PNFL ratio with IOP change after phacoemulsification were different between the POAG and GS groups (P <.05).
Tables 2 and 3 list the regression coefficients, standard errors, and P values describing the associations of predictors with percent and absolute IOP change in all patients with POAG. Table 4 reports the regression coefficients, standard errors, and P values of these relationships from the final models.
4. Discussion
Recent years saw an increase in publications over the efficacy of phacoemulsification surgery in eyes with OAG.[5,7,11,14–16] There seem to be 2 major reasons accounting for such growing interest in this issue. First, the proven efficacy of phacoemulsification as a surgical treatment for angle-closure glaucoma generated clinical interest in its potential efficacy in other types of glaucoma.[6,9] Although small in its magnitude, most studies have demonstrated that phacoemulsification effectively reduces IOP in eyes with POAG.[10,11] Another reason is a growing popularity of minimally invasive glaucoma surgeries (MIGS).[20–23] Many of those MIGS procedures are performed concurrently with cataract surgery. Thus, it is mandatory for obtaining an approval to compare the IOP-lowering efficacy of their combined procedure with that of their stand-alone cataract surgery.[23] The findings of the present study agree with prior works where IOP was found to be reduced after phacoemulsification and the preoperative IOP was correlated with the amount of postoperative IOP reduction.[10,11,23]
When planning for phacoemulsification or choosing between the stand-alone procedure of phacoemulsification and the combined procedure of phacoemulsification and glaucoma surgery in eyes with POAG, the severity of glaucoma is 1 of the factors considered in judging the risk-benefit balance. In eyes with visually significant cataract and very advanced glaucoma, phaco-trabeculectomy may be preferred to the stand-alone cataract surgery because of the postoperative IOP spike risk and its potentially greater harmful impact on the already severely damaged optic nerve head. Although the IOP-lowering effect of cataract surgery has been extensively studied in eyes with early to moderate glaucoma, it is not clearly understood how the glaucoma severity itself affects the IOP-lowering efficacy of phacoemulsification in such eyes. In the present study, we found that preoperative IOP, PD ratio and PNFL ratio were significant predictors of absolute IOP change following cataract surgery in our patients with or at risk of POAG.
Previously, Slabaugh et al explored this issue in eyes with medically controlled OAG.[7] In their study, only higher IOP, older age, and deeper ACD were found to be associated with lower postoperative IOP, whereas the VF parameters (MD and PSD) reflecting the functional aspect of glaucomatous damage were not. The number of glaucoma medications, which may also indirectly indicate the glaucoma severity or resistance to glaucoma treatment, was not found to be associated. However, the functional loss of glaucoma may be preceded by the structural loss at an earlier stage of glaucoma, and the MD or VFI may not reflect the functional damage in an eye with early glaucoma. Recently, Loewen et al created a glaucoma index (i.e. GI) that combines preoperative IOP, number of preoperative medications and visual field damage to capture relative glaucoma severity and resistance to treatment, and they found that a higher GI was associated with a larger IOP reduction in trabectome surgery.[18] In line with their finding, the GI in the present study was shown to predict absolute IOP change after cataract surgery in the POAG group. Taken together, these findings suggest that a combination of factors relevant to relative glaucoma severity or resistance to glaucoma treatment or aqueous outflow may help us estimate the potential IOP-lowering efficacy of a surgical procedure in treated POAG patients.
Unlike MD, VFI or PSD, the PNFL ratio was found to predict absolute and percent IOP change after phacoemulsification in this study. Given the fact that the POAG group of this study sample included predominantly early to moderate POAG, RNFL thickness may better serve as an indicator of glaucoma severity at this zone of the disease spectrum. Our finding of PNFL ratio as a positive predictor of IOP change after cataract surgery suggests that greater IOP reduction may be obtained in eyes with higher preoperative IOP and/or thinner RNFL, which reflects relatively greater resistance to medical treatment or greater structural damage, such as to the outflow tract. This assumption is supported by a positive association between a higher GI and IOP reduction after phacoemulsification. However, this issue warrants further studies with larger sample sizes including more severe glaucomatous damage.
The present study found AL to be associated with absolute and percent IOP changes after phacoemulsification in the GS. However, no significant association was found in the POAG eyes. These findings agree with the observations of Hsu et al,[16] Coh et al,[17] and Bilak et al,[24] although Moghimi et al[25] failed to find such association between AL and postoperative IOP change in nonglaucomatous eyes. The inconsistent results between the 2 groups may be explained by the use of glaucoma medications in the POAG group, which may have offset the potential association between the AL and the postoperative IOP change in the POAG group. The difference between the postoperative IOP and the unmedicated preoperative (instead of medicated preoperative) IOP may account for the different relationships with AL; however, lack of unmedicated preoperative IOP data precluded such assessment in the present study.
Previous studies associated LP with IOP changes after phacoemulsification in nonglaucomatous eyes[16,17] Coh and colleagues found the LP was associated with the control group but not with the POAG group, and presumed that the reason might be that the use of glaucoma medications may mask the true effect of LP by suppressing IOP. Another notable difference between their 2 groups (control group vs. POAG group) was AL (23.68 ± 1.30 mm vs 24.37 ± 1.43 mm, P = .001). LP was not found to be associated with postoperative IOP change in this study. Relatively longer AL (24.24 ± 1.38, GS; 24.29 ± 1.33 mm, POAG) of our study samples may be responsible for the differences between previous studies and ours.[16,17]
This study has some limitations. First, this is a retrospective study. Second, this study included only POAG patients whose preoperative IOP was medically controlled and whose glaucoma medication did not change during the postoperative period. Hence, the current findings may not reflect what happens in those POAG patients who are at relatively greater risk of IOP elevation after surgery. Third, one may criticize the IOP measurements performed in this study. However, we only selected those whose tonometry was performed using Goldman tonometer and during the similar time period of the day.
In conclusion, structural or functional parameters for glaucoma severity did not independently predict IOP change following phacoemulsification in medically controlled eyes with POAG. However, novel severity indices obtained by addition of preoperative IOP and/or number of glaucoma medications predicted IOP changes in eyes with POAG.
Author contributions
Conceptualization: Chungkwon Yoo.
Data curation: Chungkwon Yoo, Behzad Amoozgar.
Formal analysis: Chungkwon Yoo, Kyung-Sook Yang, Ji-Hye Park, Shan C Lin.
Investigation: Chungkwon Yoo, Behzad Amoozgar, Shan C Lin.
Methodology: Chungkwon Yoo, Behzad Amoozgar, Kyung-Sook Yang, Shan C Lin.
Project administration: Chungkwon Yoo, Behzad Amoozgar, Kyung-Sook Yang, Ji-Hye Park.
Supervision: Chungkwon Yoo.
Validation: Chungkwon Yoo, Kyung-Sook Yang, Ji-Hye Park, Shan C Lin.
Writing – original draft: Chungkwon Yoo.
Writing – review & editing: Chungkwon Yoo, Behzad Amoozgar, Kyung-Sook Yang, Ji-Hye Park, Shan C Lin.
Footnotes
Abbreviations: ACD = anterior chamber depth, AL = axial length, CCT = central corneal thickness, Glaucoma index = preoperative IOP score x MD score x number of glaucoma medications, GS = glaucoma suspects, IOP = intraocular pressure, LP = lens position, LT = lens thickness, MD = mean deviation, MIGS = minimally invasive glaucoma surgery, PD ratio = preoperative IOP/ACD, PNFL ratio = preoperative IOP/RNFL, POAG = primary open-angle glaucoma, PSD = pattern standard deviation, RNFL = retinal nerve fiber layer, SDOCT =spectral domain optical coherence tomography, VFI = visual field index.
The authors have no conflicts of interest to disclose.
References
- [1].Leske MC, Heijl A, Hussein M, et al. Early manifest glaucoma trial group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- [2].Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13. [DOI] [PubMed] [Google Scholar]
- [3].Stamper RL, Lieberman MF, Drake MV. Medical treatment of glaucoma: general principles. In: Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. 8th ed. Philadelphia: Mosby; 2009:346. [Google Scholar]
- [4].Poley BJ, Lindstrom RL, Samuelson TW, et al. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: Evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg 2009;35:1946–55. [DOI] [PubMed] [Google Scholar]
- [5].Mansberger SL, Gordon MO, Jampel H, et al. Ocular Hypertension Treatment Study Group. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology 2012;119:1826–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology 2009;116:725–31. [DOI] [PubMed] [Google Scholar]
- [7].Slabaugh MA, Bojikian KD, Moore DB, et al. The effect of phacoemulsification on intraocular pressure in medically controlled open-angle glaucoma patients. Am J Ophthalmol 2014;157:26–31. [DOI] [PubMed] [Google Scholar]
- [8].Kim KS, Kim JM, Park KH, et al. The effect of cataract surgery on diurnal intraocular pressure fluctuation. J Glaucoma 2009;18:399–402. [DOI] [PubMed] [Google Scholar]
- [9].Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology 2008;115:1134–40. [DOI] [PubMed] [Google Scholar]
- [10].Chen PP, Lin SC, Junk AK, et al. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology 2015;122:1294–307. [DOI] [PubMed] [Google Scholar]
- [11].Armstrong JJ, Wasiuta T, Kiatos E, et al. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma 2017;26:511–22. [DOI] [PubMed] [Google Scholar]
- [12].Issa SA, Pacheco J, Mahmood U, et al. A novel index for predicting intraocular pressure reduction following cataract surgery. Br J Ophthalmol 2005;89:543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dooley I, Charalampidou S, Malik A, et al. Changes in intraocular pressure and anterior segment morphometry after uneventful phacoemulsification cataract surgery. Eye 2010;24:519–26. [DOI] [PubMed] [Google Scholar]
- [14].Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol 2011;129:1283–90. [DOI] [PubMed] [Google Scholar]
- [15].Huang G, Gonzalez E, Lee R, et al. Association of biometric factors with anterior chamber angle widening and intraocular pressure reduction after uneventful phacoemulsification for cataract. J Cataract Refract Surg 2012;38:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hsu CH, Kakigi CL, Lin SC, et al. Lens position parameters as predictors of intraocular pressure reduction after cataract surgery in nonglaucomatous patients with open angles. Invest Ophthalmol Vis Sci 2015;56:7807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coh P, Moghimi S, Chen RI, et al. Lens position parameters as predictors of intraocular pressure reduction after cataract surgery in glaucomatous versus nonglaucomatous eyes. Invest Ophthalmol Vis Sci 2016;57:2593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loewen RT, Roy P, Parikh HA, et al. Impact of a glaucoma severity index on results of trabectome surgery: larger pressure reduction in more severe glaucoma. PLoS One 2016;11:e0151926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ng M, Sample PA, Pascual JP, et al. Comparison of visual field severity classification systems for glaucoma. J Glaucoma 2012;21:551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Francis BA, Singh K, Lin SC, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011;118:1466–80. [DOI] [PubMed] [Google Scholar]
- [21].Bovee CE, Pasquale LR. Evolving surgical interventions in the treatment of glaucoma. Semin Ophthalmol 2017;32:91–5. [DOI] [PubMed] [Google Scholar]
- [22].Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol 2016;10:189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vold S, Ahmed II, Craven ER, et al. CyPass Study Group. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology 2016;123:2103–12. [DOI] [PubMed] [Google Scholar]
- [24].Bilak S, Simsek A, Capkin M, et al. Biometric and intraocular pressure change after cataract surgery. Optom Vis Sci 2015;92:464–70. [DOI] [PubMed] [Google Scholar]
- [25].Moghimi S, Abdi F, Latifi G, et al. Lens parameters as predictors of intraocular pressure changes after phacoemulsification. Eye 2015;29:1469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


