Abstract
Benign prostatic hyperplasia is the most common proliferative abnormality of the prostate. All men experience some prostatic growth as they age, but the rate of growth varies among individuals. Steroid 5α-reductase 2 (SRD5A2) is a critical enzyme for prostatic development and growth. Previous work indicates that one-third of adult prostatic samples do not express SRD5A2, secondary to epigenetic modifications. Here we show that the level of oestradiol is dramatically elevated, concomitant with significant upregulation of oestrogen response genes, in prostatic samples with methylation at the SRD5A2 promoter. The phosphorylation of oestrogen receptor-α in prostatic stroma is upregulated when SRD5A2 expression is absent. We show that tumour necrosis factor (TNF)-α suppresses SRD5A2 mRNA and protein expression, and simultaneously promotes expression of aromatase, the enzyme responsible for conversion of testosterone to oestradiol. Concomitant suppression of SRD5A2 and treatment with TNF-α synergistically upregulate the aromatase levels. The data suggest that, in the absence of prostatic SRD5A2, there is an androgenic to oestrogenic switch. These findings have broad implications for choosing appropriate classes of medications for the management of benign and malignant prostatic diseases.
Keywords: androgenic to oestrogenic switch, prostate, epigenetic silencing, methylation, steroid 5α-reductase 2
Introduction
The prostate is the only solid organ that grows continuously throughout adulthood [1]. All men experience some prostatic growth as they age, but the rate of growth varies significantly among different men. Despite the widespread use of medical therapy, there is no completely effective medical treatment for benign prostatic hyperplasia (BPH). The current management of BPH with lower urinary tract symptoms includes watchful waiting, medical therapy, or surgery [2,3]. The medication regimen includes daily treatment with an α-adrenergic blocker (e.g. doxazosin, terazosin, tamsulosin, or alfuzosin) and/or a 5α-reductase inhibitor (5ARI) (i.e. finasteride or dutasteride). Steroid 5α-reductase 2 (SRD5A2) is necessary for prostatic development [4,5] and growth [6–8]. As a result, 5ARIs have played a major role in treatment of bladder outlet obstruction secondary to BPH. However, one-third of men are resistant to 5ARI therapies [2,3,9].
It has been generally accepted that SRD5A2 is expressed in all human adult prostatic tissues. However, we have demonstrated that 30% of adult human prostatic tissues do not express SRD5A2 mRNA and protein [10]. We have shown that the somatic suppression of SRD5A2 during adulthood depends on epigenetic changes in the promoter region of the SRD5A2 gene [11]. As with the neoplastic initiation and progression of many cancers [12], epigenetic changes and variable expression of SRD5A2 in benign prostatic tissue constitute a plausible molecular mechanism of variable prostatic growth [11,13]. Epigenetic modifications of SRD5A2 are dependent on the DNA methyltransferase (DNMT) family, and methylation of the SRD5A2 promoter region is regulated by the upstream inflammatory mediators tumour necrosis factor (TNF)-α, nuclear factor-κΒ (NF-κB), and interleukin (IL)-6 [11].
Although the prostate is commonly thought of as an androgen target tissue, it is also an important target of oestrogens. Androgens and oestrogens exert similar, but distinct, effects on the prostate, and it is becoming clear that a finely tuned balance between the effects mediated by androgen receptor, by oestrogen receptor-α (ERα) and by oestrogen receptor-β (ERβ) is required for the maintenance of prostatic health [14]. Oestrogens directly and indirectly affect the growth and differentiation of the prostate [15–17]. Testosterone can be metabolized via CYP19/aromatase into the potent oestrogen, i.e. oestradiol-17β. In humans, the correlations of oestrogen levels with prostatic volume and other features of BPH remain unclear [18,19].
We have previously shown that clinical conditions associated with increased levels of inflammatory mediators (obesity and age) affect the methylation and expression of SRD5A2. In the present study, we examined the regulation of oestrogenic pathways when the prostatic conversion from testosterone to dihydrotestosterone (DHT) was blocked by SRD5A2 downregulation.
Materials and methods
Patient specimens
With institutional review board approval, 59 prostatic specimens were collected from patients who underwent transurethral resection of the prostate (TURP) at Massachusetts General Hospital (n = 57) or radical prostatectomy of the prostate for symptomatic BPH at the University of Texas Southwestern Medical Center between November 2011 and December 2016. Retrospective clinical and pathological data for each patient were collected at both institutions. All prostatic samples were from the transition zone and were collected post-surgically in cold saline after pathological examination to exclude malignancy. One portion was formalin-fixed for paraffin embedding, one portion was frozen and stored at −80 °C for protein, DNA and RNA extraction, and another portion was processed for cell digestion and isolation [20–22]. Thirty-five of 57 TURP tissues were processed for immunohistochemistry, or homogenized for steroid sex hormone determination and aromatase evaluation. Clinical characteristics of the subjects are summarized in supplementary material, Tables S1 and S2.
High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS)
The levels of testosterone, DHT and oestradiol were determined by HPLC-MS. Prostatic transition zone samples were homogenized, total protein concentrations were measured, and testosterone, DHT and oestradiol were then extracted by solid-phase extraction, followed by elution with high-performance liquid chromatography. The determination was performed by mass spectrometry with an electrospray ionization source. A deuterated stable isotope was utilized for the assay calibration. Testosterone test precision: intra-assay variation <2% relative standard deviation (RSD); interassay variation <8% RSD. DHT test precision: intra-assay variation <7% RSD; interassay variation <8% RSD. Oestradiol test precision: intra-assay variation <5% RSD; interassay variation <12% RSD.
Microarray analysis
Prostatic specimens obtained after TURP from 22 patients were characterized by SRD5A2 promoter methylation status (12 methylated; 10 unmethylated). Total RNAs were extracted from each specimen with the RNeasy mini-kit (Qiagen, Valencia, CA). RNAs were then hybridized to Illumina (San Diego, CA) Human HT-12 v4 microarrays for performance of gene expression profiling according to the manufacturer’s protocol. The microarray data were normalized by computing the mean background-subtracted signal for each probe and then performing log2 transformation with the Illumina GenomeStudio (version 2011) and the GenePattern software suite [23]. The microarrays were run in two batches, and batch effects were corrected for by ComBat with the non-parametric method [24]. The processed dataset was then analysed for differential expression between SRD5A2-methylated and SRD5A2-unmethylated samples by the use of Gene Set Enrichment Analysis (GSEA) [25]. A collection of expert curated ‘hallmark’ gene sets from the Molecular Signatures Database were tested for enrichment among SRD5A2-methylated or SRD5A2-unmethylated samples by GSEA [25]. Probe sets in the expression dataset were collapsed by gene symbol and ranked by weighted signal-to-noise ratios, and enrichment scores were compared with a null distribution generated by randomly permuting phenotype labels 1000 times. All microarray data were deposited in the NCBI GEO database (Accession Number: GSE101486).
Results
Molecular subtypes exist among BPH patients
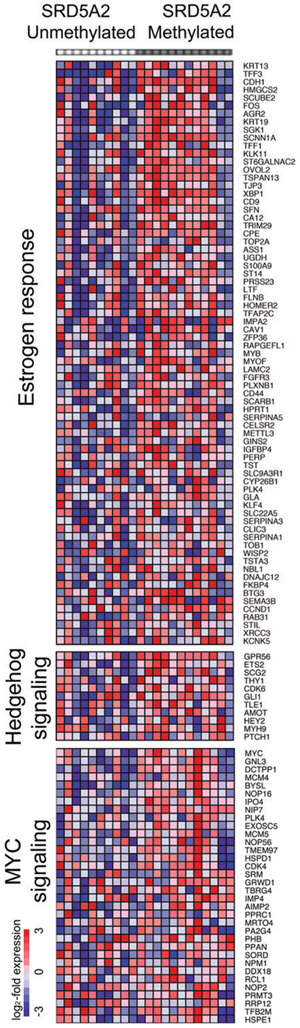
To determine whether epigenetic silencing of SRD5A2 leads to alternative molecular pathways driving growth in human prostatic tissues, we chose 22 patients from our cohort to explore gene expression signatures. Using GSEA to compare 12 SRD5A2-methylated with 10 SRD5A2-unmethylated samples [25], we identified three gene sets that were most significantly upregulated in SRD5A2-methylated samples: oestrogen response, sonic hedgehog signalling, and Myc transcription factor target genes (Figure 1). As oestrogens, like androgens, are known to regulate the development and growth of prostatic tissue [26], we wished to mechanistically assess whether oestrogen family members are differentially regulated in the absence of SRD5A2.
Figure 1.
Microarray gene expression analysis of human prostatic tissue, comparing samples with or without methylation at the SRD5A2 promoter. Oestrogen response, hedgehog signalling and MYC signalling are among the most significantly upregulated pathways in SRD5A2-methylated samples as compared with unmethylated samples.
Androgen and oestrogen levels are modified in human prostatic tissue in the absence of SRD5A2
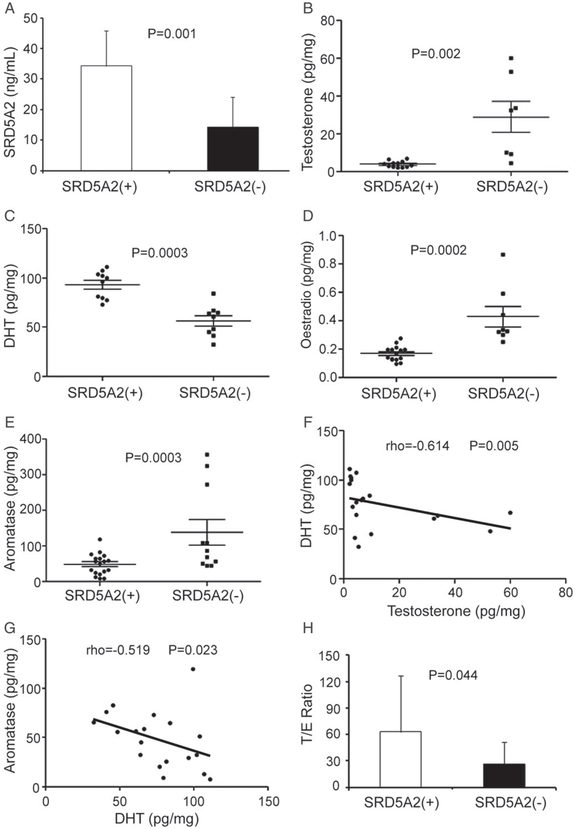
Although the importance of androgens in the induction and progression of prostate cancer is well established, the effect of metabolized byproducts of androgens and oestrogens on benign prostatic growth remains unclear [27]. As we found that lack of SRD5A2 transcription and translation is associated with upregulation of oestrogen response genes (Figure 1), we next wished to validate our findings. After immunohistochemical analysis of 35 subjects’ prostatic samples, we divided the cohort into two groups: SRD5A2-methylated with no SRD5A2 protein expression [designated the SRD5A2(−) group], and SRD5A2-unmethylated with SRD5A2 protein expression [designated the SRD5A2(+) group] (supplementary material, Figure S1). We first confirmed by enzyme-linked immunosorbent assay (ELISA) that the SRD5A2 level was higher in the SRD5A2(+) group than in the SRD5A2(−) group (P = 0.001; Figure 2A). We then investigated whether oestrogenic pathways are upregulated when androgenic pathways are blocked in the absence of prostatic SRD5A2. We found that the level of testosterone was higher in the SRD5A2(−) group than in the SRD5A2(+) group (P = 0.002; Figure 2B). As expected, DHT levels were lower in the SRD5A2(−) group (P = 0.0003; Figure 2C). The level of oestradiol in the SRD5A2(−) group was elevated as compared with the SRD5A2(+) group, which suggests preferential conversion of testosterone to oestradiol (P = 0.0002; Figure 2D). As conversion of testosterone to oestradiol is facilitated by CYP19/aromatase, we evaluated aromatase levels, which were elevated in prostatic samples lacking SRD5A2 (P = 0.003, Figure 2E). Furthermore, prostatic DHT levels were inversely correlated with testosterone levels (rho = −0.614, P = 0.005, Spearman rank correlation test; Figure 2F). Aromatase levels were also negatively correlated with DHT levels (rho = −0.519, P = 0.023; Figure 2G). Although we found a positive trend between oestradiol and aromatase, and a negative trend between oestradiol and DHT, these were not statistically significant (supplementary material, Figure S2).
Figure 2.
Androgen, oestrogen and aromatase levels in BPH prostatic samples. (A) SRD5A2 level, measured with ELISA. (B–D) Levels of testosterone (B), DHT (C) and oestradiol (D), measured by HPLC-MS. (E) Aromatase level, measured with ELISA. (F) DHT levels are negatively correlated with testosterone levels. (G) Aromatase levels are negatively correlated with DHT levels. (H) T/E ratio. Continuous variables were assessed with the Wilcoxon rank sum test. The association between two different parameters was assessed with Spearman rank correlation. The data in (A) and (H) represent means of average determinants ± standard error of the mean.
The ratio of androgen to oestrogen [testosterone/oestradiol (T/E) ratio] in the serum and prostatic tissue decreases with age [28]. We have found that ageing is associated with reduced SRD5A2 levels and increased methylation of the SRD5A2 promoter region [11]. To determine whether absence of SRD5A2 expression is associated with prostatic hormonal changes, we calculated T/E ratios, which were lower in the SRD5A2(−) samples (P = 0.04; Figure 2H). Collectively, these data suggest that, in the absence of SRD5A2 resulting from promoter methylation [11], there is an androgenic to oestrogenic switch in adult human prostatic tissue.
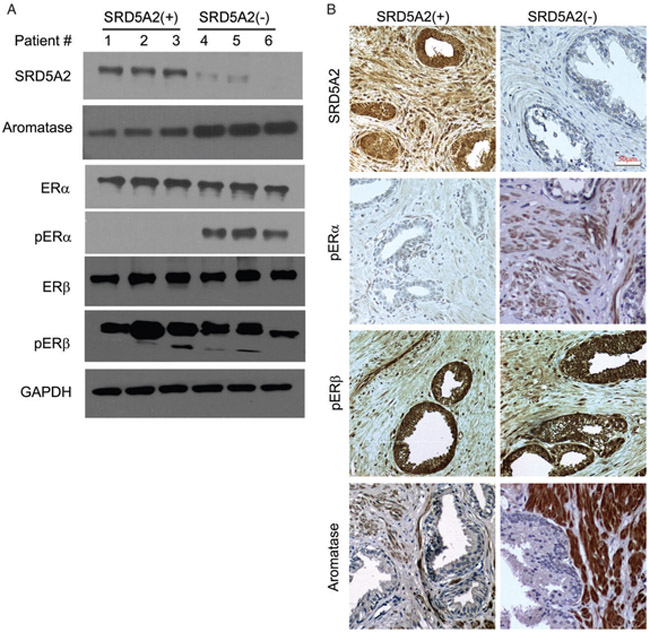
Because of its role in the conversion of androgens to oestrogens, we evaluated the level of aromatase in prostatic cellular compartments. Consistent with previous studies [29], aromatase was exclusively expressed in the stromal compartment, as assessed in primary cultured prostatic epithelial and stromal cells at the mRNA level, and in prostatic stromal tissue at the protein level (supplementary material, Figure S3). We next wished to evaluate the oestrogen response molecules that respond to an absence of SRD5A2. Phosphorylated ERα (pERα) was upregulated in human prostatic samples that lacked SRD5A2 protein expression, whereas protein levels of ERα, ERβ and phosphorylated ERβ (pERβ) were not affected (Figure 3A). These findings were confirmed by immunohistochemical analysis of prostatic tissue, which showed selective upregulation of aromatase and pERα in samples lacking SRD5A2 expression (Figure 3B).
Figure 3.
Aromatase and pERα are upregulated in the absence of SRD5A2. (A) Representative figures for protein expression by immunoblot assay. Patients 1, 2 and 3 represent SRD5A2-unmethylated samples; patients 4, 5 and 6 represent 5RD5A2-methylated samples. (B) Representative pictures of immunohistochemical analyses demonstrating increased levels of pERα and aromatase when SRD5A2 expression was absent. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
TNF-α selectively activates aromatase in prostatic stromal cells but not in epithelial cells
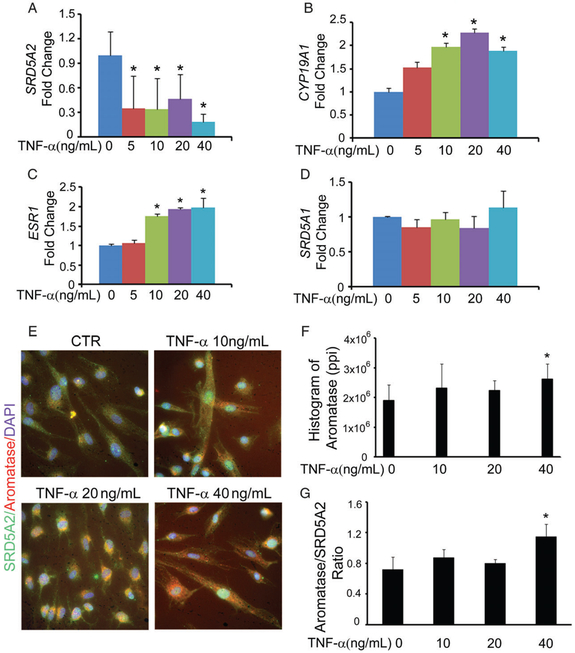
Inflammatory changes have long been associated with the histological changes seen in BPH, and autonomous regulation of prostatic epithelial cell proliferation has been shown to include upregulation of specific cytokines [22]. We have shown that methylation of the SRD5A2 promoter region is regulated by the inflammatory mediators TNF-α, NF-κB and IL-6 via DNMT1 [11]. Here, we investigated whether cytokines influence the activity of aromatase. We isolated primary prostatic stromal cells from fresh tissues that were harvested after debulking surgery for the management of bladder outlet obstruction secondary to BPH. Primary prostatic stromal cells were treated with TNF-α at 5, 10, 20 and 40ng/ml for 24 h. Administration of TNF-α resulted in reductions in SRD5A2 mRNA (Figure 4A) and protein levels [11]. More importantly, TNF-α upregulated the mRNA expression of aromatase (CYP19A1) and ERα (ESR1) in a dose-dependent manner (Figure 4B, C). In contrast, we found no change in SRD5A1 (Figure 4D) or ERβ (data not shown) expression when stromal cells were treated with TNF-α.
Figure 4.
TNF-α regulates aromatase activity in prostatic stromal cells. Primary prostatic stromal cells were treated with 5, 10, 20 and 40 ng/ml TNF-α for 24 h, and this was followed by RNA extraction or immunostaining. (A–D) Data of quantitative polymerase chain reaction analysis are presented as the fold change of mRNA expression for SRD5A2 (A), aromatase (CYP19A1) (B), ERα (ESR1) (C), and SRD5A1 (D). (E–G) Stromal cells were immunostained with anti-SRD5A2 and anti-aromatase primary antibodies. (E) Representative pictures of immunostained cells. (F) Histogram of image stained with anti-aromatase antibody. Ppi, pixels per inch. (G) The aromatase-positive/SRD5A2-positive ratio. The data represent means of average determinants ± standard error of the mean. All experiments were repeated independently at least three times, with similar results. *P < 0.05 as compared with the phosphate-buffered saline-treated vehicle control group (CTR). DAPI, 4′,6-diamidino-2-phenylindole.
As interactions between stromal and epithelial cells play an important role in maintaining prostatic homeostasis [30,31], we tested whether the effect of TNF-α on epithelial cells is associated with SRD5A2 expression. In benign prostatic epithelial (BPE) BPH-1 cells, increasing doses of TNF-α did not affect the levels of SRD5A2, aromatase, or ER-α (supplementary material, Figure S4). Our data suggest that TNF-α affects the prostatic androgen and oestrogen balance by targeting the stromal cells, with little effect on epithelial cells.
To further evaluate the role of TNF-α in oestrogen biosynthesis in the stroma at the post-transcriptional level, we treated primary prostatic stromal cells with different concentrations of TNF-α for 24 h, and then immunostained for SRD5A2 and aromatase (Figure 4E). Administration of TNF-α to stromal cells inhibited the expression of SRD5A2 (green), but simultaneously promoted the expression of aromatase (red), in a dose-dependent manner. Quantification of the immunofluorescent images suggested that aromatase levels were elevated with increasing concentrations of TNF-α, and there was a statistically significant difference from controls at 40 ng/ml (Figure 4F, G). Together, these data suggest that TNF-α not only suppresses SRD5A2 expression, but also promotes upregulation of aromatase.
TNF-α promotes upregulation of aromatase while suppressing SRD5A2 levels
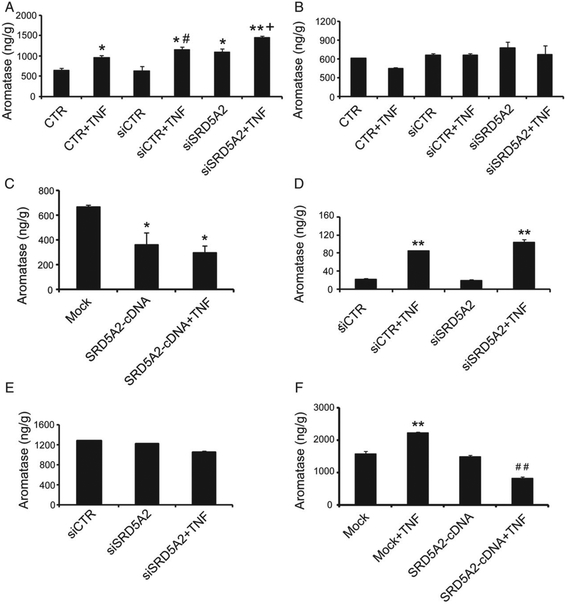
To further examine the effect of TNF-α on aromatase in the presence and absence of SRD5A2, primary cultured prostatic stromal cells were treated with TNF-α (20ng/ml, 24 h). The aromatase level was increased with TNF-α treatment, and also when SRD5A2 expression was suppressed by small interfering RNA transfection (Figure 5A). Concomitant suppression of SRD5A2 and treatment with TNF-α synergistically increased aromatase levels (Figure 5A). On the other hand, ectopic expression of SRD5A2 in prostatic stromal cells did not affect intracellular aromatase levels (data not shown).
Figure 5.
TNF-α and SRD5A2 synergistically regulate aromatase activity in prostatic stromal cells. (A—C) Primary prostatic stromal cells were transfected with SRD5A2 siRNA (A and B) or SRD5A2-cDNA plasmids (C) for 48 h; this was followed by 20 ng/ml TNF-α treatment for 24 h, and then cell lysate (A) or culture medium (B and C) was analysed to evaluate aromatase with ELISA. (D) Primary prostatic epithelial BPE cells were transfected with SRD5A2 siRNA for 48 h, subjected to 20 ng/ml TNF-α treatment for 24 h, and then lysed for evaluation of aromatase with ELISA; the medium was used for stromal cell culture. (E and F) Stromal cells were harvested for aromatase evaluation after culture with BPE-conditioned medium (E) or BPH-1-conditioned medium (F) for 24 h. Prostatic epithelial BPE/siSRD5A2 cells (E) or BPH-1/SRD5A2 cells (F) were reseeded and treated with 20 ng/ml TNF-α for 24 h, and conditioned medium was collected. Stromal cells were cultured with conditioned medium for 24 h, and cell lysate was analysed. The data represent means of average determinants ± standard error of the mean. All experiments were repeated independently at least three times, with similar results. *P < 0.05 and **P < 0.01 as compared with mock or phosphate-buffered saline-treated control group (CTR). #P < 0.05 and ##P < 0.01 as compared with siCTR (A) or SRD5A2-cDNA (C and F). +P < 0.05 as compared with siSRD5A2 (A). Mock: control for SRD5A2 cDNA.
In addition to assessing intracellular aromatase levels, we determined whether TNF-α affects paracrine secretion of aromatase. Unlike intracellular levels, aromatase levels in culture medium were not significantly affected after treatment with TNF-α or suppression of SRD5A2 (Figure 5B). However, after ectopic expression of SRD5A2 in prostatic stromal cells, the level of secreted aromatase in culture medium was reduced (Figure 5C).
Paracrine signalling and mesenchymal–epithelial cell interactions are essential components of androgenic control of the prostate gland [30]. We have previously shown that stromal cells can regulate the proliferation of epithelial cells [31,32]. Here, we examined whether aromatase levels were modified in epithelial cells with modifications in SRD5A2, and whether this change in epithelial cells affects stromal cells. We chose prostatic primary BPE cells, which express SRD5A2 and are not methylated at the SRD5A2 promoter [10]. As aromatase is predominantly expressed in stromal cells (supplementary material, Figure S3), we detected only trace levels of aromatase (20 ng/g tissue) in prostatic epithelial cells at baseline when SRD5A2 was silenced (Figure 5D). Addition of TNF-α dramatically stimulated aromatase production (Figure 5D). Our findings are in line with previous reports that increased production of prostatic and circulating aromatase is associated with inflammatory change [33].
Finally, to test whether epithelial cell secretion affects aromatase levels in stromal cells, we cultured prostatic stromal cells with BPE cell-conditioned medium. Aromatase levels were unchanged in stromal cells after treatment with the conditioned medium of control cells, of cells subjected to SRD5A2 suppression, or cells subjected to SRD5A2 suppression plus TNF-α treatment (Figure 5E). However, when we used conditioned medium from BPH-1 cells, which do not express SRD5A2 [10], treatment with TNF-α alone increased the stromal cell aromatase expression (Figure 5F). However, conditioned medium from BPH-1 cells with restored SRD5A2 expression plus TNF-α treatment suppressed stromal cell aromatase expression (Figure 5F).
Discussion
Prostatic development and growth depend largely on androgens. Despite advances in our understanding of prostatic development during gestation [34] and the pathogenesis of prostatic neoplasia [35–37], very little is known about prostatic growth during adulthood. We have shown that SRD5A2 is absent in 30% of adult human prostatic tissues [11], owing to methylation of the SRD5A2 promoter, regulated by the DNMT1 family of genes (a process that is enhanced by conditions associated with increased inflammatory mediators), ageing, and obesity. Here, we demonstrate that absence of SRD5A2 expression is associated with an androgenic to oestrogenic switch, which may explain the variable growth pattern of human adult prostates, and provides a rationale for alternative pathways for the management of BPH in patients who are resistant to 5ARIs.
Two distinct oestrogen receptors, ERα and ERβ, act as hormone-inducible transcription factors. In the normal prostate and BPH, ERα is localized to stromal cells, and oestrogen mediates its effects on the prostatic epithelium through paracrine pathways [14,38], although epithelial cells in prostatic periurethral ducts also express ERα [39]. The action of oestrogens can be complex, and they can have both proliferative and inhibitory effects via ERα and ERβ. The role of ERα has been associated with increased proliferation and inflammatory changes in the prostate [27]. Here, we found that pERα was activated in human prostatic samples that lack SRD5A2 protein expression, although protein levels of ERα, ERβ and pERβ were not affected (Figure 3A). As the balance between ERα and ERβ plays a major role in modulating prostatic growth [15,17,40], our findings suggest that, in the absence of SRD5A2, alternative oestrogenic pathways are activated that may affect prostatic proliferative capacity [27], and that may serve as alternative targets for the treatment of BPH in selected patients who lack SRD5A2 expression [41].
With advancing age, oestrogen levels remain constant in the epithelium, but increase in the stroma [42]. In elderly men, the ratio of free testosterone to free oestradiol in plasma declines by up to 40% [43], reflecting an androgenic to oestrogenic switch. Here, we found that human prostatic tissues lacking SRD5A2 expression have a lower T/E ratio than prostatic tissues that express SRD5A2 (Figure 2H). Our data suggest that, in the absence of SRD5A2, there is an androgenic to oestrogenic switch in the human adult prostatic tissue, owing to methylation of the SRD5A2 promoter region [11].
Aromatase encoded by the CYP19A1 gene catalyses the conversion of androgens to oestrogen [44]. Associations between aromatase gene polymorphisms and the risks of prostatic hyperplasia and malignancy have been identified [45–48]. Aberrantly expressed aromatase in prostatic epithelial cells and infiltrating inflammatory cells is also associated with the progression of prostate cancer [27]. However, the molecular mechanism and actions of aromatase in human benign prostatic disease remain largely unknown. Aromatase plays a key role in maintaining prostatic homeostasis. Loss of aromatase expression causes decreased oestrogen-induced prostatic proliferation [29,49], although, in clinical trials, the selective aromatase inhibitor atamestane had no effect on established BPH [50,51], which may have been a result of inadequate selection of patients who have elevated aromatase levels in the setting of absent SRD5A2. The proinflammatory cytokines IL-1, IL-6 and TNF-α stimulate aromatase activity and induce the conversion of androgens to oestrogens. In the breast tissue of overweight and obese women, clinical data suggest that inflammation associated with obesity promotes aromatase activity [52]. These findings suggest that low androgen levels and disease states associated with increased levels of inflammatory mediators promote aromatase activity. In concert with these studies, we found that a significant increase in aromatase level is associated with absence of SRD5A2 expression and methylation of the SRD5A2 promoter. More importantly, increased aromatase levels are negatively correlated with DHT levels (Figure 2G), suggesting that increased oestrogenic levels in the absence of SRD5A2 may serve as an alternative pathway to promote prostatic growth.
We have shown that TNF-α regulates aromatase activity in an SRD5A2-dependent manner. TNF-α increases aromatase levels in primary cultured prostatic stromal cells from BPH patients. Furthermore, suppression of SRD5A2 in stromal cells increased aromatase activity, and TNF-α and SRD5A2 suppression synergistically promoted aromatase activity (Figure 5A). Interestingly, in ectopic SRD5A2-overexpressing stromal and epithelial BPH-1 cells, TNF-α did not increase aromatase activity as expected, but reduced aromatase production both in cells and in culture media (Figure 5B, D, G).
Our findings have broad implications for the chronic use of 5AR2 inhibitors for the management of BPH [9]. With activated oestrogenic pathways that modulate prostatic growth, alternative treatment strategies for the management of BPH in carefully selected patients who lack SRD5A2 expression may be more scientifically sound [41].
Beyond the management of benign prostatic diseases, variable expression of SRD5A2 has implications for the use of 5ARIs for prostate cancer chemoprevention [53,54], and even the treatment of patients with castration-resistant prostate cancer (CRPC). In CRPC managed with the CYP17A1 inhibitor abiraterone, addition of 5ARI has been shown to block the production of tumour-promoting metabolites and permit accumulation of Δ4-abiraterone, which has stronger antitumour activity [55]. Therefore, the presence of SRD5A2 may be crucial for efficacy when combinations of CYP17A1 and 5ARIs are considered for patients with advanced prostate cancer.
In summary, lack of SRD5A2 expression in the prostate induces an androgenic to oestrogenic switch in human benign prostatic tissues, via increased stromal levels of aromatase and pERα. The proinflammatory mediator TNF-α suppresses SRD5A2, while simultaneously promoting the expression of aromatase. We conclude that, in the absence of prostatic SRD5A2 when androgenic pathways are blocked, the alternative oestrogenic pathways are upregulated to drive prostatic growth. Whereas anti-androgen pathways have been major targets for the treatment of patients with BPH [56], our data suggest that, in carefully selected patients who lack SRD5A2 expression, oestrogenic pathways may serve as effective treatment targets. We believe that the knowledge gained from this study will enable us to appropriately offer 5ARI therapies to those who express SRD5A2.
Supplementary Material
SRD5A2(−) and (+) immunohistochemistry
Aromatase, testosterone and estradiol correlations
Stromal expression of aromatase
Treatment of TNF-α in BHP-1 cells did not affect the mRNA expression of SRD5A2 (A), aromatase (CYP19A1, B) and ERα (ESR1, C)
Demographic and clinical characteristics of patients processed for genetic signature analysis
Cohort demographic and clinical characteristics
Acknowledgements
AFO gratefully acknowledges financial support from NIH/NIDDK (NIH/R01 DK091353). ZW was supported by the Urology Care Foundation/American Urological Association Research Scholar Award.
Footnotes
No conflicts of interest were declared.
SUPPLEMENTARY MATERIAL ONLINE
Supplementary materials and methods
References
- 1.Loeb S, Kettermann A, Carter HB, et al. Prostate volume changes over time: results from the Baltimore Longitudinal Study of Aging. J Urol 2009; 182: 1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapple CR. Pharmacological therapy of benign prostatic hyperplasia/lower urinary tract symptoms: an overview for the practising clinician. BJU Int 2004; 94: 738–744. [DOI] [PubMed] [Google Scholar]
- 3.Sarma AV, Jacobson DJ, McGree ME, et al. A population based study of incidence and treatment of benign prostatic hyperplasia among residents of Olmsted County, Minnesota: 1987 to 1997. J Urol 2005; 173: 2048–2053. [DOI] [PubMed] [Google Scholar]
- 4.Imperato-McGinley J, Guerrero L, Gautier T, et al. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science 1974; 186: 1213–1215. [DOI] [PubMed] [Google Scholar]
- 5.Walsh PC, Madden JD, Harrod MJ, et al. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med 1974; 291: 944–949. [DOI] [PubMed] [Google Scholar]
- 6.Livingstone DE, Barat P, Di Rollo EM, et al. 5alpha-Reductase type 1 deficiency or inhibition predisposes to insulin resistance, hepatic steatosis, and liver fibrosis in rodents. Diabetes 2015; 64: 447–458. [DOI] [PubMed] [Google Scholar]
- 7.Godoy A, Kawinski E, Li Y, et al. 5alpha-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate 2011; 71: 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stiles AR, Russell DW. SRD5A3: a surprising role in glycosylation. Cell 2010; 142: 196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998; 338: 557–563. [DOI] [PubMed] [Google Scholar]
- 10.Niu Y, Ge R, Hu L, et al. Reduced levels of 5-alpha reductase 2 in adult prostate tissue and implications for BPH therapy. Prostate 2011; 71: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 11.Bechis SK, Otsetov AG, Ge R, et al. Age and obesity promote methylation and suppression of 5-alpha reductase 2 – implications for personalized therapy in benign prostatic hyperplasia. J Urol 2015; 194: 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteller M Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 2007; 8: 286–298. [DOI] [PubMed] [Google Scholar]
- 13.Bechis SK, Otsetov AG, Ge R, et al. Personalized medicine for management of benign prostatic hyperplasia. J Urol 2014; 192: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol 2007; 39: 183–188. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson TM, Moses MA, Uchtmann KS, et al. Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol 2015; 193: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation 2011; 82: 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynder JL, Nicholson TM, DeFranco DB, et al. Estrogens and male lower urinary tract dysfunction. Curr Urol Rep 2015; 16: 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meikle AW, Stephenson RA, McWhorter WP, et al. Effects of age, sex steroids, and family relationships on volumes of prostate zones in men with and without prostate cancer. Prostate 1995; 26: 253–259. [DOI] [PubMed] [Google Scholar]
- 19.Miwa Y, Kaneda T, Yokoyama O. Association between lower urinary tract symptoms and serum levels of sex hormones in men. Urology 2008; 72: 552–555. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein AS, Drake JM, Burnes DL, et al. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat Protoc 2011; 6: 656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strand DW, Aaron L, Henry G, et al. Isolation and analysis of discreet human prostate cellular populations. Differentiation 2016; 91: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Kwon OJ, Henry G, et al. Non-cell-autonomous regulation of prostate epithelial homeostasis by androgen receptor. Mol Cell 2016; 63: 976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reich M, Liefeld T, Gould J, et al. GenePattern 2.0. Nat Genet 2006; 38: 500–501. [DOI] [PubMed] [Google Scholar]
- 24.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8: 118–127. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricke WA, Wang Y, Cunha GR. Steroid hormones and carcinogenesis of the prostate: the role of estrogens. Differentiation 2007; 75: 871–882. [DOI] [PubMed] [Google Scholar]
- 27.Ellem SJ, Risbridger GP. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer 2007; 7: 621–627. [DOI] [PubMed] [Google Scholar]
- 28.Krieg M, Klotzl G, Kaufmann J, et al. Stroma of human benign prostatic hyperplasia: preferential tissue for androgen metabolism and oestrogen binding. Acta Endocrinol 1981; 96: 422–432. [DOI] [PubMed] [Google Scholar]
- 29.Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci 2009; 1155: 174–186. [DOI] [PubMed] [Google Scholar]
- 30.Cunha GR, Donjacour A. Stromal–epithelial interactions in normal and abnormal prostatic development. Prog Clin Biol Res 1987; 239: 251–272. [PubMed] [Google Scholar]
- 31.Li W, Wu CL, Febbo PG, et al. Stromally expressed c-Jun regulates proliferation of prostate epithelial cells. Am J Pathol 2007; 171: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olumi AF, Grossfeld GD, Hayward SW, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999; 59: 5002–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol 2010; 118: 246–251. [DOI] [PubMed] [Google Scholar]
- 34.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev 1987; 8: 338–362. [DOI] [PubMed] [Google Scholar]
- 35.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013; 155: 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014; 159: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hieronymus H, Schultz N, Gopalan A, et al. Copy number alteration burden predicts prostate cancer relapse. Proc Natl Acad Sci U S A 2014; 111: 11139–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellem SJ, Schmitt JF, Pedersen JS, et al. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab 2004; 89: 2434–2441. [DOI] [PubMed] [Google Scholar]
- 39.Schulze H, Claus S. Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate 1990; 16: 331–343. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson TM, Sehgal PD, Drew SA, et al. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation 2013; 85: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roehrborn CG, Spann ME, Myers SL, et al. Estrogen receptor beta agonist LY500307 fails to improve symptoms in men with enlarged prostate secondary to benign prostatic hypertrophy. Prostate Cancer Prostatic Dis 2015; 18: 43–48. [DOI] [PubMed] [Google Scholar]
- 42.Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab 1993; 77: 375–381. [DOI] [PubMed] [Google Scholar]
- 43.Stone NN, Fair WR, Fishman J. Estrogen formation in human prostatic tissue from patients with and without benign prostatic hyperplasia. Prostate 1986; 9: 311–318. [DOI] [PubMed] [Google Scholar]
- 44.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 1999; 84: 4677–4694. [DOI] [PubMed] [Google Scholar]
- 45.Azzouzi AR, Cochand-Priollet B, Mangin P, et al. Impact of constitutional genetic variation in androgen/oestrogen-regulating genes on age-related changes in human prostate. Eur J Endocrinol 2002; 147: 479–484. [DOI] [PubMed] [Google Scholar]
- 46.Roberts RO, Bergstralh EJ, Farmer SA, et al. Polymorphisms in genes involved in sex hormone metabolism may increase risk of benign prostatic hyperplasia. Prostate 2006; 66: 392–404. [DOI] [PubMed] [Google Scholar]
- 47.Grindstad T, Skjefstad K, Andersen S, et al. Estrogen receptors alpha and beta and aromatase as independent predictors for prostate cancer outcome. Sci Rep 2016; 6: 33114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miftakhova R, Hedblom A, Semenas J, et al. Cyclin A1 and P450 aromatase promote metastatic homing and growth of stem-like prostate cancer cells in the bone marrow. Cancer Res 2016; 76: 2453–2464. [DOI] [PubMed] [Google Scholar]
- 49.Ricke WA, McPherson SJ, Bianco JJ, et al. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J 2008; 22: 1512–1520. [DOI] [PubMed] [Google Scholar]
- 50.Dias JP, Melvin D, Shardell M, et al. Effects of transdermal testosterone gel or an aromatase inhibitor on prostate volume in older men. J Clin Endocrinol Metab 2016; 101: 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radlmaier A, Eickenberg HU, Fletcher MS, et al. Estrogen reduction by aromatase inhibition for benign prostatic hyperplasia: results of a double-blind, placebo-controlled, randomized clinical trial using two doses of the aromatase-inhibitor atamestane. Atamestane Study Group. Prostate 1996; 29: 199–208. [DOI] [PubMed] [Google Scholar]
- 52.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res 2011; 4: 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349: 215–224. [DOI] [PubMed] [Google Scholar]
- 54.Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology 1998; 52: 195–201; discussion 201–202. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Alyamani M, Li J, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature 2016; 533: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan SA, Lee JY, Meehan AG, et al. Time course of incident adverse experiences associated with doxazosin, finasteride, and combination therapy in men with benign prostatic hyperplasia: the Medical Therapy of Prostatic Symptoms (MTOPS) Trial. J Urol 2016; 195: 1825–1829. [DOI] [PubMed] [Google Scholar]
- *57.Ge R, Wang Z, Bechis SK, et al. DNA methyl transferase 1 reduces expression of SRD5A2 in the aging adult prostate. Am J Pathol 2015; 185: 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cited only in supplementary material.
- 58.Wang Z, Cheng Z, Cristofaro V, et al. Inhibition of TNF-alpha improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes 2012; 61: 2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Cited only in supplementary material.
- 59.Burgess A, Vigneron S, Brioudes E, et al. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 2010; 107: 12564–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Cited only in supplementary material.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SRD5A2(−) and (+) immunohistochemistry
Aromatase, testosterone and estradiol correlations
Stromal expression of aromatase
Treatment of TNF-α in BHP-1 cells did not affect the mRNA expression of SRD5A2 (A), aromatase (CYP19A1, B) and ERα (ESR1, C)
Demographic and clinical characteristics of patients processed for genetic signature analysis
Cohort demographic and clinical characteristics