Abstract
Background and Purpose
In 1948, Paul Yakovlev described an additional limbic circuit located basolateral to James Papez’s circuit (1937) and included orbitofrontal cortex, amygdala and dorsomedial nucleus of thalamus. This circuit is shown to be an important component of subcortical cognitive abilities. We aimed to demonstrate this circuit in a multiple sclerosis (MS) cohort, using diffusion tensor imaging (DTI) and evaluate its role in MS related cognitive impairment (CI).
Methods
We enrolled cognitively intact (n=10) and impaired (n=36) MS patients who underwent a comprehensive cognitive assessment; the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) and structural magnetic resonance imaging (MRI). Correlation analyses between volumetric and DTI derived values of the orbitofrontothalamic (OFT), amygdalothalamic tracts (ATT) and dorsomedial nucleus of thalamus and CI index derived from MACFIMS were computed after adjustment for age, education and lesion load.
Results
We observed a consistent trend between CI index and bilateral dorsomedial nucleus’ mean diffusivity (MD) (r=0.316; p: 0.02), left OFT FA (r:−0.302; p:0.02), MD (r=0.380; 0.006), and radial diffusivities (RD) (r=0.432; p:0.002), also with right ATT FA (r=−0.475; p:0.0006) and left ATT FA (r=−0.487; p:0.0005). After Bonferroni correction, correlations of left OFT RD, right and left ATT FA with CI were found to be significant.
Conclusions
Our study provides in vivo DTI delineation of Yakovlev’s historical basolateral limbic circuit and establishes a role in MS related CI. These findings may potentially pave the way for future clinical studies using targeted invasive and non-invasive neurostimulation modalities for cognitive impairment in MS.
Keywords: cognitive impairment, multiple sclerosis, diffusion tensor imaging, limbic
Introduction
In 1878, Paul Broca was the first neuroanatomist who described the limbic system as “le grand lobe limbique (the great limbic lobe)” which mainly included cingulate and hippocampal gyri and subcallosal frontal areas.1 Afterwards, in 1937, James Papez proposed for the first time the limbic system as a circuit in his work titled “A proposed mechanism of emotion”. Papez proposed the limbic circuits as hippocampus to fornix to mammillary body to mammillothalamic tract to anterior nucleus of thalamus to the thalamocingulate tract to the cingulate gyrus to the cingulum to the hippocampus.2 Eleven years later in 1948, Paul Yakovlev described an additional circuit involving orbitofrontal cortex, dorsomedial nucleus of thalamus and amygdala. Amygdala connects to orbitofrontal cortex through two loops; 1) directly by uncinate fasciculus 2) indirectly by the amydalothalamic tract projecting to dorsomedial nucleus of the thalamus then orbitofrontothalamic tract to orbitofrontal cortex.3 Historically the limbic system has been thought to be responsible for olfaction, the visceral functions, emotion and its driving force, learning and memory.4 However growing evidence suggests that it has a crucial role in various aspects of cognition.5–6 More recently, connections between the dorsomedial thalamus and orbitofrontal areas were found to be critical for subcortical cognitive abilities such as working memory, attention, and cognitive flexibility.7
Cognitive symptomatology in MS is more suggestive of the subcortical dementia spectrum as it lacks typical cortical symptoms such as agnosia, apraxia, neglect and aphasia.8,9 Patients with MS usually describe their cognitive symptoms as difficulty retaining instructions which is reflective of slowing in information processing speed, difficulty concentrating and focusing, impairment in verbal fluency, short memory issues such as acquiring, retaining and retrieving new information, and deficits in visual perception and constructional abilities. Recent studies have revealed the involvement of limbic structures in MS-related cognitive impairment (CI).10,11
The goal of this study was to 1) demonstrate the feasibility of delineating the elements of the Yakovlev’s indirect loop; orbitofrontothalamictract (OFT), amygdalothalamic tract (ATT) and dorsomedial nucleus of thalamus in an MS cohort using mainly diffusion tensor imaging (DTI) native space and 2) determine whether microstructural damage determined by DTI-derived values of this loop, relates to MS specific CI assessed by a comprehensive behavioral assessment; MACFIMS.
Methods
Subjects
We enrolled ten cognitively non-impaired MS patients (MSNI) and 36 patients with diagnosed CI (MSCI) age 40.80 ± 11.26 years, education 14.17 ± 2.34 years, disease duration 13.29 ± 9.21 years, and EDSS 3.51 ± 2.03 (0–7). Each subject provided written informed consent following the University of Texas Health Science Center Institutional Review Board’s approval of the protocol. All subjects underwent the MACFIMS battery and an MR imaging. Cognitive testing was performed in the morning to avoid fatigue, prior to and within 2 weeks of the imaging session. Inclusion criteria specified meeting 2010 McDonald Criteria for MS. Exclusion criteria included history of psychiatric disorders or current depression, relapse within 3 months of enrollment, recent drug or alcohol abuse, other brain pathology, claustrophobia, or positive urine pregnancy test prior to MRI.
Cognitive Assessment
The MACFIMS is designed to quantify cognitive function with neuropsychological testing.12 It is composed of the following assessments: processing speed and working memory assessed by Paced Auditory Serial Addition Test (PASAT) and Symbol Digit Modality Test (SDMT), memory and learning by California Verbal Learning Test Second Edition (CVLT-II) and Brief Visuospatial Memory Test-Revised (BVMT-R), executive function by Delis-Kaplan Executive Function System (D-KEFS) sorting test, visual perception/spatial processing by Judgment of Line Orientation test (JLO), and verbal fluency measured by the controlled oral word association test (COWAT). In order to perform MACFIMS scoring, twenty MACFIMS parameters were identified in an a priori manner by literature review as most pertinent in the measurement of MS-related cognitive deficits.13 Based on previously validated methodology, a CI index was derived. In this cohort, patients were classified as cognitively impaired as previously described.11,14
Magnetic Resonance Imaging Acquisition
Whole brain MRI data were acquired on a Philips 3.0T Intera scanner using a SENSE receive head coil. The MRI protocol included conventional and non-conventional MRI sequences [dual echo turbo spin echo, fluid attenuation by inversion recovery (FLAIR) and 3D T1-weighted magnetization prepared rapid acquisition with gradient echo (MPRAGE)]. The T1-weighted sequence spatial resolution was 1 mm × 1 mm × 1 mm and field-of-view was 256 mm × 256 mm. Diffusion-weighted image (DWI) data were acquired axially from the same graphically prescribed conventional MRI volumes using a single-shot multi- slice 2-D spin-echo diffusion sensitized and fat-suppressed echo planar imaging (EPI) sequence, with the balanced Icosa21 tensor encoding scheme.15 The b-factor = 1,000 s mm–2, TR/TE = 7,100/65 ms, FOV = 256 mm × 256 mm, and slice thick-ness/gap/#slices = 3 mm/0 mm/44. The EPI phase encoding used a SENSE k-space undersampling factor of two, with an effective k-space matrix of 128 × 128, and an image matrix after zero-filling of 256 × 256. The constructed image spatial resolution for the DWI data was = 1 mm × 1 mm × 3 mm.
Lesion Load Segmentation
Whole brain lesion load was mapped and quantified in all patients using the co-registered multispectral dual FSE and the FLAIR volumes. The lesion probability masks were computed in MRIcron (http://www.nitrc.org/projects/mricron/).16 The lesion volumes were saved as binary masks to enable fusion with other multimodal volumes acquired from the same subject. We obtained both T1 and T2 lesion load to adjust for in the correlation analyses.
Atlas Based Thalamic Nuclei Segmentation
The analysis was conducted using DSI Studio (http://dsi-studio.labsolver.org). Thalamic nuclei were obtained from ICBM (International Consortium for Brain Mapping) (http://www.loni.usc.edu/atlases/) warped into DTI space thru DSIstudio. ICBM atlases provides probability maps of thalamic nuclei. As the DSIstudio software allows on editing regions, dorsomedial thalamus is verified and edited with neuroanatomy guidance on slice by slice basis on DTI maps. DTI derived mean diffusivities were obtained for dorsomedial thalamus.
Tractography
Yakovlev described the circuit of orbitofrontal cortex-dorsomedial thalamus-amygdala as basolateral limbic circuit in addition to Papez circuit.3 This circuit has two main white matter loops; 1) anteriorly uncinate fasciculus, previously studied in MS related CI10–11 connecting amygdala or temporal pole to orbitofrontal frontal cortex and 2) posteriorly bisynaptic orbitofrontoamygdalar loop including bidirectional and ipsilateral orbitfrontothalamic and amygdalothalamic projection pathways. From the cortex, this loop arises from OFC travels posteriorly through the anterior limb of the internal capsule and synapses in the dorsomedial nucleus of the thalamus.17 It then projects inferolaterally, travels adjacent to forniceal fibers and reaches the amygdala.18 For deterministic tractography methodology, we used brute force, ROI-based, the fiber assignment with continuous tractography (FACT) algorithm19,20 (DTI Studio, Johns Hopkins University, Baltimore, MD) and our ROI based approach illustrated and described in figure 1.
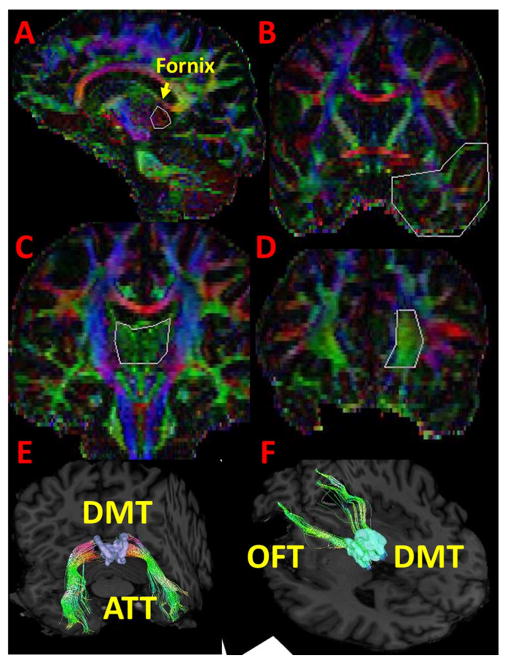
Figure 1.
Illustration of the deterministic tractography used for the analyses. For amygdalothalamic tract (E), we seeded our first region of interest (ROI) (OR operator in DTIstudio) in the commissural fibers (red), best seen in approximately 13 mm off-midline sagittal, inferior to the antero-inferior tips of the fornix fimbria (pointed by arrow), posterior to projection fibers (blue) and anteroinferior to corpus callosum isthmus and splenium parts (A) and second ROI (AND operator) in temporal lobe coronal section at the level of anterior commissure (B). For orbitofrontothalamic tract (F) we seeded our first ROI (OR operator) (C) on the midline association fibers (green) coronally which is dorsolateral thalamus, bordered by a triangle; projection fibers bilaterally and corpus callosum and fornix at the top and second ROI (AND operator) in medial frontal pole where orbitofrontal cortex located (D). NOT operator was used to clean the contaminations of other pathways and it was performed in varying degree in subjects by neuroanatomy guidance. The seeds were placed on one slice for each operator. DTIStudio and DSIStudio (http://dsi-studio.labsolver.org/) were used to generate this figure. Illustration maps are color-coded map (A–D) and T1weighted (E–F) maps. Abbreviations: ATT: amygdalothalamic tract, DMT: dorsolateral thalamus, OFT: orbitofrontothalamic tract.
Statistical Analyses
We obtained residual values for diffusion derived values and CI by using the general linear model multivariate analysis controlled for age, T1w and T2w lesion load and education. These residual values were then used in a Spearman rank analysis as normal distribution could not be assured by Kolmogorov Smirnov analyses. Significance defined as p<0.05. We also computed Bonferroni correction for multiple comparisons (calculated by diving 0.05 by number of comparisons (n=20), p value defined as p<0.003.
Results
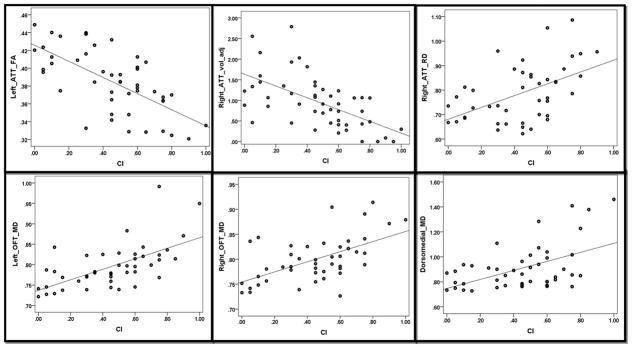
Correlation analyses are summarized in the table and important correlations are highlighted as scatter plots in figure 2. A consistent trend between CI index and bilateral dorsomedial nucleus’ MD (r=0.316, p=0.016), right OFT FA (r=−0.280, p=0.035) and MD (r=0.284, p=0.032), left OFT FA (r=−0.302, p=0.025), MD (r=0.380, p=0.006), and RD (r=0.432, p=0.002), right ATT adjusted volume (r=−0.354, p=0.01), FA (r=−0.475, p=0.0006) and RD (r=0.336, p=0.014), left ATT FA (r=−0.487, 0.0005) and RD (r=0.306, p=0.023). After Bonferroni correction, correlations of left OFT RD, right and left ATT FA with CI index remained significant.
Table.
The results of Spearman’s correlation analyses (r and p values) of cognitive impairment index to fractional anisotropy, mean, axial and radial diffusivities and adjusted volumes of orbitofrontothalamic tract, amygdalothalamic tract and dorsomedial nucleus of thalamus.
| CI | ||
|---|---|---|
| r | p | |
| Dorsomedial_Nucleus_MD | .316* | .016 |
| Dorsomedial_Nucleus_vol_adj | −.068 | 0.30 |
| Right_OFT_vol_adj | −.144 | .178 |
| Right_OFT_FA | −.280* | .035 |
| Right_OFT_MD | .284* | .032 |
| Right_OFT_RD | .254 | .050 |
| Right_OFT_AD | .166 | .144 |
| Left_OFT_vol_adj | −.034 | .413 |
| Left_OFT_FA | −.302* | .025 |
| Left_OFT_MD | .380** | .006 |
| Left_OFT_RD | .432** | .002 |
| Left_OFT_AD | .237 | .063 |
| Right_ATT_vol_adj | −.354** | .010 |
| Right_ATT_FA | −.475** | 0.0006 |
| Right_ATT_MD | .210 | .088 |
| Right_ATT_RD | .336* | .014 |
| Right_ATT_AD | .072 | .322 |
| Left_ATT_vol_adj | −.155 | .160 |
| Left_ATT_FA | −.487** | 0.0005 |
| Left_ATT_MD | .231 | .068 |
| Left_ATT_RD | .306* | .023 |
| Left_ATT_AD | .153 | .164 |
Abbreviations: AD: Axial Diffusivity, ATT: amygdalothalamic tract, CI: cognitive impairment, MD: Mean diffusivity. OFT: orbitofrontothalamic tract, RD: radial diffusivity, vol_adj: adjusted volume.
Figure 2.
Highlights of scatter plots of cognitive impairment index and fractional anisotropy, mean and radial diffusivities and adjusted volumes of orbitofrontothalamic tract, amygdalothalamic tract and dorsomedial nucleus of thalamus. Abbreviations: amygdalothalamic tract, CI: cognitive impairment, MD: Mean diffusivity. OFT: orbitofrontothalamic tract, RD: radial diffusivity, vol_adj: adjusted volume.
Discussion
This work describes Yakovlev’s basolateral limbic connections; ATT and OFT, in an MS cohort with a robust and reproducible deterministic tractography methodology. Our results show that this circuit plays a crucial role in MS related CI even after adjustments for age, education, lesion load and correction for multiple comparisons. The other important pathway; uncinate fasciculus was recently investigated by our group and showed a similar trend.11 Also for the first time, we report on specific thalamic nuclei to the circuit, i.e. the dorsomedial nucleus rather than the entire thalamus, which showed a parallel trend of correlation to CI along with the OFT and ATT.
Initially, Yakovlev’s circuit was thought to be related to the visceral aspects of emotional processing,3 its damage leading to loss of insight, inability to recognize socially inappropriate behaviors in oneself.21 However, later it was shown to contribute to the neural basis of learning and memory.4 Additionally, recent MS studies showing the importance of the amygdala and OFC10,22 in cognition support our findings.
The anterior thalamic radiations (ATR) were shown to have the greatest yearly percentage atrophy in an MS cohort.23 The thalamus has numerous projections to prefrontal cortex arising from its different nuclei with different functions and even the dorsomedial nucleus projects into the dorsolateral prefrontal cortex (DLPFC), the ventrolateral prefrontal cortex (VLPFC), and the orbitofrontal cortex (OFC), separately.17 Thus investigating ATR, composed of the different pathways, alone remains quite non-specific for exploring the function of these projections. Functionally, the projections from the dorsomedial thalamus to the orbitofrontal and prefrontal areas are an important component of subcortical regulation of cognitive abilities such as working memory, attention, and cognitive flexibility.7 As hypothesized, the OFT showed strong correlation with MS related CI, which is more characterized by subcortical dementia clinical picture.8,9
Kamali and colleagues described a robust methodology to track the ATT in a healthy cohort based on a high resolution DTI dataset.18 We translated the methodology to our MS cohort and demonstrated that it is also feasible with a relatively standard acquisition protocol used in clinical trials involving MS patients. Functionally this tract was implicated in the rapid shifting of attention to emotional stimuli,24 assessment in purposefulness and social appropriateness of emotional stimuli, and social cognition.25 As part of the orbitofrontal-amygdalar loop, similar to OFT, this tract also revealed a significant association with MS related subcortical dementia like CI.
Previously thalamic atrophy was shown as a reliable marker of neurodegeneration in MS (Hasan 2011), and related to CI in MS.26 However the thalamus, as the major relay center in the brain, has numerous unconnected nuclei with different functions and connections with the rest of the brain. Similar to ATR, thalamic nuclei need to be investigated separately to better delineate their projections and understand function. Recent studies in healthy subjects27,28 provided a road map for thalamus segmentation which was also feasible in our MS cohort. Similar to the other component of the basolateral limbic circuit, it showed a similar trend in the correlation analyses but not as strong as OFT or ATT.
In conclusion, our study explores Yakovlev’s historical basolateral limbic circuit in MS and provides an alternative neural correlate for CI in MS. Clearly the study has several limitations such as small sample size, lack of healthy controls, and cross sectional design. Also, the acquired resolution of 2×2×3 mm and final resolution of 1×1×3 mm voxels are non-isotropic and may introduce bias into tracography. Thus our DTI results need to be interpreted with caution. However, given adjustment for age, education, lesion load and correction for multiple comparisons, our statistical analyses were conservative enough to reduce the likelihood of false positive correlations. Larger studies are needed to confirm these findings as the role of the limbic system in MS related CI should continue to be investigated.
Acknowledgments
This study was funded by a K-23 (the project # K23 NS072134) training award to FN. DUNN research foundation to KH. We wish to thank Vipul Kumar Patel for helping in data acquisition.
Footnotes
Disclosure: The authors have no potential conflict of interest to disclose.
References
- 1.Broca P. Anatomie comparée des circonvolutions cérébrales: le grande lobe limbique et la scissure limbique dans la série des mammifères. Rev D’Anthropol. 1878;1:385–498. [Google Scholar]
- 2.Papez J. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–44. [Google Scholar]
- 3.Yakovlev PI. Motility, behavior and the brain: stereodynamic organization and neural correlates of behavior. J Nerv Men Dis. 1948;107:313–35. doi: 10.1097/00005053-194810740-00001. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza J, Foundas A. Clinical Neuroanatomy: A Neurobehavioral Approach. New Orleans, LA: Springer; 2007. pp. 213–71. [Google Scholar]
- 5.Oishi K, Mielke MM, Albert M, et al. The fornix sign: a potential sign for Alzheimer’s disease based on diffusion tensor imaging. J Neuroimaging. 2012;22:365–74. doi: 10.1111/j.1552-6569.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove J, Alty JE, Jamieson S. Cognitive impairment in Parkinson’s disease. Postgrad Med J. 2015;91:212–20. doi: 10.1136/postgradmedj-2015-133247. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson BR, Gao WJ. Development of thalamocortical connections between the mediodorsal thalamus and the prefrontal cortex and its implication in cognition. Front Hum Neurosci. 2014;8:1027. doi: 10.3389/fnhum.2014.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–91. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Subcortical vascular cognitive impairment: similarities and differences with multiple sclerosis. J Neurol Sci. 2006;245:3–7. doi: 10.1016/j.jns.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Keser Z, Hasan KM, Mwangi B, et al. Quantitative limbic system mapping of main cognitive domains in multiple sclerosis. Front Neurol. 2018;9:132. doi: 10.3389/fneur.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keser Z, Hasan KM, Mwangi B, et al. Limbic pathway correlates of cognitive impairment in multiple sclerosis. J Neuroimaging. 2017;27:37–42. doi: 10.1111/jon.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reitan RM, Wolfson D. In: Theoretical, methodological, and validational bases of the Halstead-Reitan neuropsychological test battery, in comprehensive handbook of psychological assessment: intellectual and neuropsycological assessment. Goldstein G, Beers GS, Hersen M, editors. Hoboken, NJ: John Wiley & Sons; 2003. pp. 105–33. [Google Scholar]
- 13.Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80:201–6. doi: 10.1136/jnnp.2008.148403. [DOI] [PubMed] [Google Scholar]
- 14.Nelson F, Akhtar MA, Zúñiga E, et al. Novel fMRI working memory paradigm accurately detects cognitive impairment in multiple sclerosis. Mult Scler. 2017;23:836–47. doi: 10.1177/1352458516666186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasan KM, Halphen C, Sankar A, et al. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. Neuroimage. 2007;34:1497–505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan KM, Walimuni IS, Abid H, et al. Multimodal quantitative magnetic resonance imaging of thalamic development and aging across the human lifespan: implications to neurodegeneration in multiple sclerosis. J Neurosci. 2011;31:16826–32. doi: 10.1523/JNEUROSCI.4184-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang SH, Yeo SS. Thalamocortical connections between the mediodorsal nucleus of the thalamus and prefrontal cortex in the human brain: A diffusion tensor tractographic study. Yonsei Medical Journal. 2014;55:709–14. doi: 10.3349/ymj.2014.55.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamali A, Riascos R, Pillai J, et al. Mapping the trajectory of the amygdalothalamic tract in the human brain. J Neurosci Res. 2018 doi: 10.1002/jnr.24235. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keser Z, Yozbatiran N, Francisco GE, Hasan KM. A note on the mapping and quantification of the human brain corticospinal tract. Eur J Radiol. 2014;83:1703–5. doi: 10.1016/j.ejrad.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 22.Pravatà E, Rocca MA, Valsasina P, et al. Gray matter trophism, cognitive impairment, and depression in patients with multiple sclerosis. Mult Scler. 2017;23:1864–74. doi: 10.1177/1352458517692886. [DOI] [PubMed] [Google Scholar]
- 23.Kezele IB, Arnold DL, Collins DL. Atrophy in white matter fiber tracts in multiple sclerosis is not dependent on tract length or local white matter lesions. Mult Scler. 2008;14:779–85. doi: 10.1177/1352458507088106. [DOI] [PubMed] [Google Scholar]
- 24.Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci. 2012;32:5338–50. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–23. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- 27.Glaister J, Carass A, Stough JV, Calabresi PA, Prince JL. Thalamus parcellation using multi-modal feature classification and thalamic nuclei priors. Proc SPIE Int Soc Opt Eng. 2016;9784:97843J. doi: 10.1117/12.2216987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert C, Simon H, Colman J, Barrick TR. Defining thalamic nuclei and topographic connectivity gradients in vivo. Neuroimage. 2017;158:466–79. doi: 10.1016/j.neuroimage.2016.08.028. [DOI] [PubMed] [Google Scholar]