Abstract
Background:
Up to 31% of kidney transplant (KT) recipients experience early hospital readmission (EHR). We hypothesized that EHR among older KT recipients is higher than younger recipients due to increased comorbidities and higher prevalence of frailty.
Methods:
We identified 22,458 older (age≥) and 86,372 younger (18 to <65) first-time KT recipients (12/1/1999-12/31/2014) using USRDS data. We estimated the association between patient-level characteristics and EHR (30 days post-KT discharge) with modified Poisson regression among older and younger KT recipients, separately. We estimated the association between graft loss and mortality and EHR using Cox proportional hazards.
Results:
EHR was more common in older KT recipients (30.1% vs 27.6%;p<0.001). Risk factors for EHR that differed by recipient age included female sex, African American race, diabetes, smoking, dialysis vintage, donor age, and length of stay. Risk of graft loss associated with EHR was greater among older KT recipients (aHR: 1.64, 95% CI: 1.51-1.77, p<0.001) than younger KT recipients (aHR: 1.43, 95%CI: 1.38-1.48, p<0.001)(interaction p<0.01). However, risk of mortality associated with EHR was greater among younger recipients (aHR:1.52, 95%CI:1.47-1.57, p<0.001) than older recipients (aHR:1.40, 95%CI:1.34-1.47, p<0.001)(interaction p<0.01).
Conclusions:
Older KT recipients are more likely to experience EHR and are at a higher risk of graft loss after EHR than younger recipients. Targeted interventions to prevent EHR and subsequent graft loss in this population should be identified.
Keywords: kidney transplantation, older adults, hospital readmission
INTRODUCTION
Approximately one-third of all kidney transplant (KT) recipients experience early hospital readmission (EHR) within 30 days of discharge after transplantation [1,2]; known risk factors include age, frailty, race, obesity, comorbidities, post-operative complications, extended criteria donors, and lack of induction immunosuppression [1-3]. EHR in KT recipients is associated with adverse outcomes such as graft loss and mortality along with an increased risk for subsequent hospital readmissions [4-6]. However, EHR may be preventable in many KT recipients [3]. While older age is associated with EHR [1], we hypothesized that specific risk factors for EHR in older KT recipients would differ from risk factors in younger recipients due to a comorbidity burden, a higher prevalence of frailty, and a senescent immune system [7-10], and that the impact of EHR on post-transplant outcomes such as graft loss and mortality might also be augmented in older recipients who have less physiologic reserve.
Risk factors for EHR have been investigated in older surgical patients in other specialties (trauma, breast, surgical oncology, and orthopedic) and include age-specific factors such as higher number of comorbidities, increasing age, and post-operative complications [11-15]; however, inferences from surgical patients in these populations may not be generalizable to older KT recipients because of immunosuppressive regimens, rejection, and transplant-specific infectious complications which often require readmission. Additionally, over the past decade, graft and overall survival in older KT recipients have significantly improved [16], but EHR after KT may modify and attenuate the improvement in outcomes. As the proportion of older adults undergoing KT increases, it is important to understand the risk factors for EHR and its impact on post-transplant outcomes in order to inform targeted EHR prevention strategies in this unique population [16].
Understanding the burden, risk factors, and sequelae of EHR in older KT recipients is imperative for the care of this population. In this study, we used national registry data to (1) quantify the burden of EHR in older versus younger recipients, (2) identify recipient and transplant factors for EHR in older versus younger recipients, (3) quantify the effect of EHR on graft loss and mortality in older versus younger KT recipients, and (4) identify center-level variation in EHR for older recipients.
METHODS
Data Source
This study used data from the Organ Procurement and Transplantation Network (OPTN) and linked to Medicare claims data by the United States Renal Data System (USRDS). USRDS release data was available through 12/31/2014; therefore, this was the censoring date for death and graft loss analyses. Donor, recipient, and transplant factors were obtained from 2728 Chronic Renal Disease Medical Evidence form, the OPTN registrant form and Center for Medicare and Medicaid Services (CMS) claims. Mortality and graft loss were augmented through linkage with the Social Security Master Death File, CMS data, and waitlist data.
Study population
The study population included 108,830 adult (age ≥18) first-time kidney-only transplant recipients between 12/1/1999 and 12/31/2014, of whom 22,458 were older (age≥65). EHR was defined as at least one hospital readmission (to any acute hospital, based on Medicare claims) within 30 days of discharge from initial KT hospitalization. To allow for appropriate longitudinal follow-up, the population was limited to recipients with Medicare as their continuous primary insurer over the 30-day period post-KT and recipients who did not die within 30 days of KT. We included recipients who died after EHR. Because of mechanistic differences, we excluded recipients who were initially discharged to a skilled nursing facility or nursing home to examine specific characteristics that lead to EHR from home. Also, we excluded patients with initial length of stay (LOS)>30 days after initial KT hospitalization.
EHR factors specific to older KT recipients
Recipient and transplant factors considered to be potential factors of EHR are listed in Table 1. Body mass index (BMI) was classified by WHO classification. All other comorbidities were categorized as reported on 2728 or as reported by OPTN. Recipients with missing HCV serostatus were considered to be HCV negative. Use of an induction agent was categorized as interleukin-2 receptor antagonists (Zenapax or Simulect), anti-CD3 or anti-CD52 agents (OKT3, OKT4, Thymoglobulin, ALG, ATG, Alemtuzumab), or no induction. Donor type was classified into three categories (live donor, deceased donor after brain death, deceased donor after cardiac death).
Table 1.
Characteristics of older kidney transplant (KT) recipient study population stratified by early hospital readmission (EHR).
| No HER (n= 15,696) |
EHR (N=6,762) |
p-value | |
|---|---|---|---|
| Recipient Factors | |||
| Age, years¥ | 68 (66-71) | 69 (66-72) | <0.01 |
| Female (%) | 37.3 | 35.9 | 0.04 |
| African American (%) | 17.6 | 21.7 | <0.001 |
| BMI (%) | <0.001 | ||
| Underweight | 1.5 | 1.3 | |
| Normal | 29.1 | 26.0 | |
| Overweight | 40.0 | 39.0 | |
| Obese | 29.5 | 33.8 | |
| Hypertension (%) | 86.3 | 87.2 | 0.07 |
| Diabetes (%) | 40.9 | 48.4 | <0.001 |
| Cancer (%) | 5.0 | 5.7 | 0.04 |
| Congestive heart failure (%) | 13.1 | 16.8 | <0.001 |
| Chronic obstructive pulmonary disease (%) | 2.6 | 3.3 | 0.02 |
| Hepatitis C virus positive (%) | 2.4 | 2.7 | 0.2 |
| Dialysis vintage, years+ | 2.5 ±2.5 | 2.9 ±2.6 | <0.001 |
| Smoker (%) | 2.2 | 2.0 | 0.3 |
| Panel reactive antibody (%) | 0.1 | ||
| 0 | 57.9 | 57.4 | |
| 1-79 | 32.2 | 31.7 | |
| 80-97 | 3.3 | 3.5 | |
| 98-100 | 6.6 | 7.4 | |
| Transplant factors | |||
| Donor age, years+ | 44.8±15.7 | 46.6±15.9 | <0.001 |
| Donor diabetes (%) | 6.2 | 7.8 | <0.001 |
| Donor hepatitis C virus (%) | 1.2 | 1.2 | 0.8 |
| African American donor (%) | 10.4 | 13.1 | <0.001 |
| Donor type (%) | <0.001 | ||
| Deceased after brain death | 63.6 | 68.5 | |
| Live donor | 28.8 | 22.8 | |
| Donation after cardiac death | 7.6 | 8.7 | |
| Extended criteria donor (%) | 22.8 | 30.3 | <0.001 |
| Received induction therapy (%) | 77.5 | 75.5 | <0.001 |
| 0 HLA mismatches (%) | 9.0 | 8.2 | 0.03 |
| Length of stay, days¥ | 5 (4-7) | 7 (5-10) | <0.001 |
reported as (mean ±SD)
reported as median (interquartile range)
Relative risk (RR) of EHR by recipient and transplant factors was estimated using modified Poisson regression, as described previously [17]. Modified Poisson regression allows reporting of more interpretable RRs instead of odds ratios with logistic regression. To test whether the risk of EHR associated with each factor differed between older and younger KT recipients, interactions between recipient age (older and younger) and each factor were explored. We ascertained the stratified associations from the modified Poisson models and estimated the stratified associations using the lincom command in Stata. Functional forms of continuous variables were based on the exploratory data analysis. The final multivariate model was selected for optimal parsimony by minimizing the Akaike Information Criteria (AIC).
Death-censored graft loss and mortality after EHR
Kaplan-Meier methods were used to evaluate 1-, 3-, and 5- year death-censored graft loss and mortality in older KT recipients with EHR compared to those without EHR. Cox proportional hazards models were used to calculate adjusted hazard ratio (aHR) of death-censored graft loss and mortality associated with EHR. For risk adjustment, recipient factors (sex, race, diabetes, BMI, dialysis vintage, history of cancer, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), smoking, LOS, and panel reactive antibody [PRA; 0, 1-79, 80-97, 98-100]), donor factors (age, donor type [live donor, deceased donor after brain death, deceased donor after cardiac death], and extended criteria donor), and transplant factors (year of KT) were included in the models. Additionally, we stratified survival models by age of KT recipient (younger or older). To test whether the risk of EHR associated with graft loss and mortality differed between older and younger KT recipients, interactions between recipient age (older and younger) and EHR were explored. For all models, proportional hazards assumptions were confirmed by visual complementary log-log plots and Schoenfeld residuals.
Center level variation in EHR in older KT recipients
To determine whether EHR in older recipients varied significantly across transplant centers, we used a multilevel model with a random intercept framework and reported the intraclass correlation coefficient (ICC). In the context of this study, the ICC quantifies the variance in EHR explained by transplant center. This model was adjusted for recipient (sex, age, BMI, race, history of cancer, diabetes, smoking, COPD, CHF, LOS, dialysis vintage, PRA, LOS) and transplant factors (donor age, donor type, extended criteria donor, HCV donor status, donor diabetes, and donor race) and used to test the association of center level factors and baseline rates of admission.
Statistical analysis
All analyses were two-tailed and α was set at 0.05. Analyses were performed using STATA 14.2/MP (College Station, Texas).
RESULTS
Study population
Among the 22,458 older KT recipients, the median (interquartile range [IQR]) at KT was 69 (66-71) years, 36.8% were female, and 18.9% were African American (Table 1). There were 6,762 (30.1%) older recipients with EHR. Of older recipients with EHR, 17.5% had readmission within 2 days of discharge, 45.2% within 7 days, and 70.0% within 14 days. The median (IQR) length of readmission after initial KT was 9 (4-16) days. The median (IQR) length of follow-up in our study was 1,471 (642-2,521) days for older KT recipients.
Factors associated with EHR
Risk factors for EHR that differed between younger and older recipients included female sex, African American race, diabetes, smoking, PRA 1-79, dialysis vintage, donor age, and LOS (Table 2). Almost all risk factors were equally associated or more strongly associated with EHR in younger KT recipients. For example, younger female recipients had a higher risk of EHR than younger male recipients, but older female recipients had a lower risk of EHR than older male recipients (interaction p<0.001); younger female recipients had a 5% increased risk of EHR (aRR: 1.05, 95%CI: 1.02-1.07, p<0.001), whereas older female recipients had similar risk of EHR compared to older males (aRR: 0.96, 95%CI: 0.92-1.00, p=0.07). Both older and younger recipients who with diabetes had a higher risk of EHR, but this association was stronger in younger recipients (interaction p=0.03). Younger recipients with diabetes had a 23% higher risk of EHR (aRR: 1.23, 95%CI: 1.20-1.26, p<0.001), whereas older recipients with diabetes had a 17% higher risk of EHR (aRR: 1.17, 95%CI: 1.12-1.21, p<0.001) compared to older recipients without diabetes. LOS at transplantation was more strongly associated with EHR in older recipients (interaction p<0.001): older recipients had a 24% higher risk of EHR for each 5-day increase in LOS (aRR: 1.24, 95%CI: 1.22-1.27, p<0.001), whereas younger recipients had a 20% higher risk of EHR for each 5-day increase in LOS (aRR: 1.20, 95%CI: 1.18-1.21, p<0.001). Prior cancer, obesity, COPD, CHF, PRA 98-100, African American donor, donor diabetes, extended criteria donors, and DCDs were associated with increased risk of EHR that did not differ between older and younger KT recipients (Table 2).
Table 2.
Relative risk (RR) of early hospital readmission for older (N=22,458) and younger (N=86,372) kidney transplant (KT) recipients.
| Younger KT recipients |
Older KT recipients |
Interaction | |
|---|---|---|---|
| RR (95%CI) | RR (95%CI) | p value | |
| Female | 1.05 (1.02-1.07) | 0.96 (0.92-1.00) | <0.001 |
| African American | 1.13 (1.10-1.16) | 1.02 (0.97-1.07) | <0.001 |
| Body mass index | |||
| Underweight | 1.07 (0.99-1.15) | 0.94 (0.77-1.14) | 0.3 |
| Normal | REF | REF | |
| Overweight | 1.00 (0.97-1.03) | 1.04 (0.99–1.10) | 0.2 |
| Obese | 1.06 (1.03-1.09) | 1.12 (1.06-1.18) | 0.07 |
| Diabetes | 1.23 (1.20-1.26) | 1.17 (1.12-1.21) | 0.03 |
| Smoker | 1.09 (1.03-1.15) | 0.92 (0.78-1.07) | 0.04 |
| Prior cancer | 1.10 (1.00-1.20) | 1.18 (1.08–1.28) | 0.3 |
| COPD | 1.13 (1.03-1.23) | 1.14 (1.02-1.28) | 0.9 |
| Congestive heart failure | 1.09 (1.06-1.14) | 1.10 (1.04-1.16) | 0.9 |
| Dialysis vintage, per year | 1.04 (1.04-1.05) | 1.03 (1.02-1.04) | <0.01 |
| Panel reactive antibody | |||
| 0 | REF | REF | |
| 1-79 | 1.04 (1.02-1.07) | 0.98 (0.94-1.03) | 0.02 |
| 80-97 | 1.12 (1.06-1.18) | 1.05 (0.93-1.17) | 0.3 |
| 98-100 | 1.07 (1.02-1.12) | 1.11 (1.03-1.21) | 0.3 |
| Donor age, per 10 years | 1.00 (1.00-1.00) | 1.01 (1.00-1.01) | 0.047 |
| Donor diabetes | 1.06 (1.01-1.12) | 1.08 (1.00-1.16) | 0.7 |
| Donor hepatitis C virus | 1.26 (1.18-1.35) | 1.04 (0.86-1.25) | 0.06 |
| African American donor | 1.10 (1.07-1.14) | 1.10 (1.03-1.16) | 0.9 |
| Donor type (%) | |||
| Deceased after brain death | REF | REF | |
| Live donor | 0.97 (0.94-1.00) | 1.00 (0.95-1.06) | 0.3 |
| Donation after cardiac death | 1.07 (1.03-1.12) | 1.04 (0.96-1.12) | 0.4 |
| Extended criteria donor | 1.14 (1.09-1.18) | 1.15 (1.10-1.21) | 0.7 |
| Transplant length of stay, per 5 days | 1.20 (1.18-1.21) | 1.24 (1.22-1.27) | <0.001 |
Death-censored graft loss after EHR
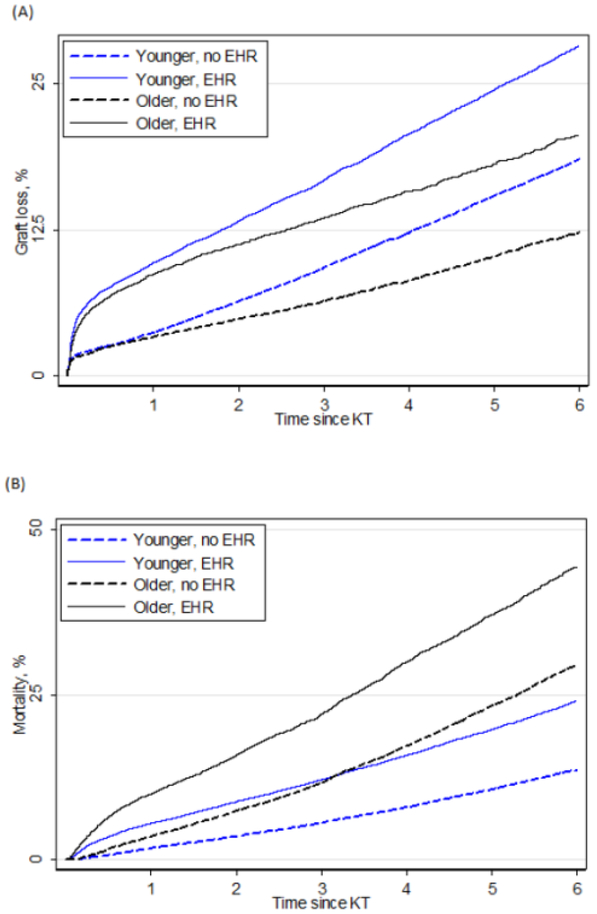
Death-censored graft loss in older KT recipients with EHR was higher than no EHR (Figure 1A). In older KT recipients, 1-, 3-, and 5- year death-censored graft loss was 5%, 8%, and 12% respectively. For older KT recipients, 1-, 3-, and 5- year death-censored graft loss for EHR versus no EHR was 9% vs. 3%, 14% vs. 6%, and 18% vs. 10%, respectively. The risk of graft loss associated with EHR varied by recipient age (interaction p<0.01): Older KT recipients with EHR were at a 1.64-fold increased risk of graft loss compared to older recipients without EHR (aHR: 1.64, 95% CI: 1.51-1.77, p<0.001). However, among younger KT the strength of this association was diminished; younger recipients with EHR were at a 1.43-fold (aHR: 1.43, 95%CI: 1.38-1.48, p<0.001) increased risk of graft loss compared to younger recipients without EHR.
Figure 1.
(A) Death-censored graft loss and early hospital readmission (EHR) by age of kidney transplant (KT) recipient. (B) Mortality and EHR by age of KT recipient. Older KT recipients were age ≥65.
Mortality after EHR
Mortality in older KT recipients with EHR was higher than in those with no EHR (Figure 1B). In older KT recipients, 1-, 3-, and 5- year patient mortality was 5%, 15%, and 28%, respectively. For older KT recipients, 1-, 3-, and 5- year patient mortality for EHR versus no EHR was 10% vs. 4%, 22% vs. 12%, and 37% vs. 23%, respectively. The risk of mortality associated with EHR varied by recipient age (interaction p<0.01): Older KT recipient with EHR were at a 1.40-fold increased risk of mortality compare to older recipients without EHR (aHR:1.40, 95%CI:1.34-1.47, p<0.001). However, among younger KT recipients the strength of this association was stronger; younger KT recipients were at a 1.52-fold (aHR:1.52, 95%CI:1.47-1.57, p<0.001) increased risk of mortality compared to younger recipients without EHR.
Center level factors associated with EHR among older KT recipients
Including transplant center in a multilevel model improved the fit (likelihood ratio test p<0.001). However, the ICC was 0.036, meaning that only 3.6% of the variation in EHR for older adults was explained by transplant center. However, 57 (21.0%) of 271 transplant centers had a statistically significantly higher odds ratio of EHR as compared to the national average, and 37 (13.7%) had statistically significantly lower odds of EHR as compared to the national average (Figure 2). The center-specific odds ratios of EHR ranged from 0.22 (95%CI: 0.14-0.33) to 1.96 (1.68-2.30).
Figure 2.
Relative odds of early hospital readmission (EHR) after kidney transplantation in older recipients by transplant center compared to national average. Each dot represents the relative risk of EHR for each transplant center in the United States with 95% confidence interval.
DISCUSSION
In this national study of 108,830 KT recipients, of which 22,458 were older KT recipients, 30.1% of older KT recipients experienced EHR. Risk factors for EHR that differed between younger and older recipients included female sex, African American race, diabetes, smoking, PRA 1-79, dialysis vintage, donor age, and LOS. Younger recipients who were female, were African American, and had diabetes were at increased risk of EHR compared to younger male, non-African American, non-diabetic recipients; these associations were weaker in older recipients. Older recipients with a longer LOS after KT were at an increased risk of EHR compared to younger recipients with longer LOS after KT. EHR is associated with an increased risk of mortality, and the association of mortality and EHR was stronger in younger recipients compared to older recipients (aHR: 1.52 vs. 1.40, p<0.01). However, the association of EHR and graft loss was significantly higher for older recipients compared to younger recipients (aHR: 1.64 vs 1.43 p<0.01). Importantly, there was little variation in EHR of older KT recipients across transplant centers.
Our findings of an association between EHR and obesity, African American race, dialysis vintage, and longer LOS after KT are consistent with previous reports in KT recipients of all ages [1]. However, our study identified differences EHR in risk factors between older and younger KT recipients, such as sex, race, diabetes, and time on dialysis, and LOS. These associations are more strongly associated with risk of EHR in younger recipients compared to older recipients, except longer LOS, which is more strongly associated with EHR in older recipients. Furthermore, diabetes and smoking are associated with EHR in older and younger recipients, but more strongly in younger recipients, likely given that comorbidities play a larger role in EHR for younger patients.
Additionally, our group previously demonstrated that intermediate center level volume and average LOS after KT were center level factors associated with EHR [1]. Although, in this study, we found no center level variation in EHR for older KT recipients; this is consistent with findings in simultaneous pancreas-kidney transplant recipients that demonstrated no variation between centers [18]. The lack of center level variation in EHR for older KT recipients may be due to the higher medical complexity of older recipients or longer length of stay compared to younger recipients rather than poor quality of care.
Furthermore, EHR is associated with increased mortality and graft loss after KT, and the 30-day readmission window represents a high-risk window for KT recipients of all ages [4,6,19]. These previous studies are consistent with our findings that EHR is associated with mortality in older and younger KT recipients, and not surprisingly, we demonstrated that risk of graft loss associated with EHR in greater in older KT recipients than younger KT recipients [20]. However, risk of mortality associated with EHR is greater in younger KT recipients compared to older KT recipients. The weaker association of mortality, but stronger association of graft loss among older KT recipients may be due to the fact that older recipients are at increased risk due to a number of other causes and EHR may be a marker of overall health in this population.
The greatest strength of this study is the use of a national population with a large sample size. The most notable limitation is a potential selection bias: in order to assess the prevalence and risk factors for EHR, we limited our inclusion criteria to Medicare-primary patients. However, over three-fourths of older (age ≥65) KT recipients are primarily covered by Medicare and, given that all ESRD patients requiring dialysis and KT are eligible for Medicare, this is a common inclusion criterion in studies of ESRD patients including our own previously published studies [1,18]. Also, we excluded patients who were discharged to non-home facility. However, the mechanism for EHR from a non-home facility is quite different than from home, so we focused on EHR that occurs for patients at home. Another limitation is the lack of granular cause of death data available in USRDS and SRTR databases.
In conclusion, 30.1% of older KT recipients are readmitted within 30 days of transplantation, and older KT recipients have a greater risk of EHR than younger recipients. Risk factors for readmission are weaker among older recipients than younger recipients. However, the risk of graft loss associated with EHR is greater in older KT recipients, so strategies to prevent EHR in older KT recipients would decrease costs of readmission and potentially improve patient outcomes. As an important first step, we have identified risk factors in for EHR than differ between older and younger KT recipients; further work is needed to explore interventions, which could decrease potentially preventable readmissions.
ACKNOWLEDGEMENTS
The United States Renal Data System (USRDS) has supplied the data reported here. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the USRDS, or the US Government. Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the National Institute on Aging, grant numbers F32AG053025 (PI: Haugen), F32AG044994 (PI: King), F32DK109662 (PI: Holscher), K24DK101828 (PI: Segev), R01AG055781 (PI: McAdams-DeMarco), and K01AG043501 (PI: McAdams-DeMarco).
Funding: Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the National Institute on Aging, grant numbers F32AG053025 (PI: Haugen), F32AG044994 (PI: King), F32DK109662 (PI: Holscher), K24DK101828 (PI: Segev), R01AG055781 (PI: McAdams-DeMarco), and K01AG043501 (PI: McAdams-DeMarco).
ABBREVIATIONS
- aHR
adjusted hazard ratio
- aRR
adjusted relative risk
- BMI
body mass index
- CHF
congestive heart failure
- CI
confidence interval
- CMS
Center for Medicare and Medicaid Services
- COPD
chronic obstructive pulmonary disease
- EHR
early hospital readmission
- HRSA
Health Resources and Services Administration
- ICC
intraclass correlation coefficient
- KT
kidney transplantation
- LOS
length of stay
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- USRDS
United States Renal Data System
Footnotes
Conflict of interest: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Nephrology.
Contributor Information
Christine E. Haugen, Email: chaugen2@jhmi.edu.
Elizabeth A. King, Email: eking19@jmhi.edu.
Mara McAdams-DeMarco, Email: mara@jhu.edu.
REFERENCES
- 1.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL: Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant 2012;12:3283–3288. [DOI] [PubMed] [Google Scholar]
- 2.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL: Frailty and early hospital readmission after kidney transplantation. Am J Transplant 2013;13:2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubetzky M, Yaffe H, Chen C, Ali H, Kayler LK: Early Readmission After Kidney Transplantation: Examination of Discharge-Level Factors. Transplantation 2016;100:1079–1085. [DOI] [PubMed] [Google Scholar]
- 4.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL: Sequelae of early hospital readmission after kidney transplantation. Am J Transplant 2014;14:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King EA, Bowring MG, Massie AB, Kucirka LM, McAdams-DeMarco MA, Al-Ammary F, Desai NM, Segev DL: Mortality and Graft Loss Attributable to Readmission following Kidney Transplantation: Immediate and Long-Term Risk. Transplantation 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harhay M, Lin E, Pai A, Harhay MO, Huverserian A, Mussell A, Abt P, Levine M, Bloom R, Shea JA, Troxel AB, Reese PP: Early rehospitalization after kidney transplantation: assessing preventability and prognosis. Am J Transplant 2013;13:3164–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, Walston J, Segev DL: Frailty and mortality in kidney transplant recipients. Am J Transplant 2015;15:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danovitch GM, Gill J, Bunnapradist S: Immunosuppression of the elderly kidney transplant recipient. Transplantation 2007;84:285–291. [DOI] [PubMed] [Google Scholar]
- 9.Gruver AL, Hudson LL, Sempowski GD: Immunosenescence of ageing. J Pathol 2007;211:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman HM, McBride MA, Cors CS, Roza AM, Wynn JJ: Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation 2007;83:404–410. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein DN, Thirukumaran C, Saleh A, Molinari RW, Mesfin A: Complications and Readmission After Cervical Spine Surgery in Elderly Patients: An Analysis of 1786 Patients. World Neurosurg 2017;103:859–868 e858. [DOI] [PubMed] [Google Scholar]
- 12.Gibreel WO, Day CN, Hoskin TL, Boughey JC, Habermann EB, Hieken TJ: Mastectomy and Immediate Breast Reconstruction for Cancer in the Elderly: A National Cancer Data Base Study. J Am Coll Surg 2017;224:895–905. [DOI] [PubMed] [Google Scholar]
- 13.Joseph B, Orouji Jokar T, Hassan A, Azim A, Mohler MJ, Kulvatunyou N, Siddiqi S, Phelan H, Fain M, Rhee P: Redefining the association between old age and poor outcomes after trauma: The impact of frailty syndrome. J Trauma Acute Care Surg 2017;82:575–581. [DOI] [PubMed] [Google Scholar]
- 14.Yeo H, Mao J, Abelson JS, Lachs M, Finlayson E, Milsom J, Sedrakyan A: Development of a Nonparametric Predictive Model for Readmission Risk in Elderly Adults After Colon and Rectal Cancer Surgery. J Am Geriatr Soc 2016;64:e125–e130. [DOI] [PubMed] [Google Scholar]
- 15.Yeo HL, O'Mahoney PR, Lachs M, Michelassi F, Mao J, Finlayson E, Abelson JS, Sedrakyan A: Surgical oncology outcomes in the aging US population. J Surg Res 2016;205:11–18. [DOI] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL: Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc 2014;62:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 18.King EA, Kucirka LM, McAdams-DeMarco MA, Massie AB, Al Ammary F, Ahmed R, Grams ME, Segev DL: Early Hospital Readmission After Simultaneous Pancreas-Kidney Transplantation: Patient and Center-Level Factors. Am J Transplant 2016;16:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covert KL, Fleming JN, Staino C, Casale JP, Boyle KM, Pilch NA, Meadows HB, Mardis CR, McGillicuddy JW, Nadig S, Bratton CF, Chavin KD, Baliga PK, Taber DJ: Predicting and preventing readmissions in kidney transplant recipients. Clin Transplant 2016;30:779–786. [DOI] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, Kucirka LM, Desai NM, Dagher NN, Lonze BE, Montgomery RA, Walston J, Segev DL: Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg 2017;266:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]