The Suzuki-Miyaura cross-coupling of organoboron nucleophiles with aryl halide electrophiles is one of the most widely used carbon-carbon bond-forming reactions in organic and medicinal chemistry1,2. A key challenge associated with these transformations is that they generally require the addition of an exogenous base, whose role is to enable transmetalation between the organoboron nucleophile and the metal catalyst3. This requirement limits the reaction’s substrate scope because the added base promotes competitive decomposition of many organoboron substrates3–5. As such, significant research has focused on strategies for mitigating base-mediated side reactions6–12. Prior efforts have primarily focused on (i) designing strategically masked organoboron reagents (to slow base-mediated decomposition)6–8 or (ii) developing highly active palladium pre-catalysts (to accelerate cross-coupling relative to base-mediated decomposition pathways)10–12. An attractive alternative approach involves identifying catalyst/electrophile combinations that enable Suzuki-Miyaura-type reactions to proceed without an exogeneous base12–14. The current report leverages this approach to develop a nickel-catalysed coupling of aryl boronic acids with acid fluorides15–17 (formed in situ from readily available carboxylic acids)18–22. This catalyst/electrophile combination enables a mechanistic manifold in which a ‘transmetalation active’ aryl-nickel-fluoride intermediate is generated directly in the catalytic cycle13,16. As such, this transformation does not require an exogenous base and is applicable to a wide range of base sensitive boronic acids and biologically active carboxylic acids.
The traditional Suzuki-Miyaura reaction involves the Pd-catalyzed coupling of an aryl halide (Ar-X) with a boronic acid in the presence of exogeneous base (MX*). The role of the base (Fig 1b, cycle I) is to convert the ‘transmetalation-inactive’ [Ar–Pd–X] intermediate (where X = chloride, bromide, or iodide) to a ‘transmetalation-active’ intermediate [Ar–Pd–X*] (where X* = hydroxide or fluoride). [Ar–Pd–X*] then participates in fast transmetalation with a boronic acid23–25. However, the base also mediates the off-cycle formation of organoboronate intermediates that competitively decompose via protodeboronation, oxidation, and/or homo-coupling4,5. Inspired by several literature reports13,16, we hypothesized that the combination of a nickel catalyst and an acid fluoride electrophile would directly form a ‘transmetalation-active’ intermediate [Ar–Ni–F] via oxidative addition and subsequent decarbonylation (Fig 1b, cycle II). Importantly, Ni0 is well-known to participate in oxidative addition reactions with carboxylic acid derivatives15,26–30. Furthermore, with appropriate selection of supporting ligands, the resulting NiII-acyl intermediates are known to undergo decarbonylation26–30. This approach offers the advantages that it: (1) eliminates the requirement for exogenous base; (2) uses highly electrophilic ArC(O)F substrates, which should undergo rapid oxidative addition under mild conditions (compared to, for example, the corresponding aryl fluorides13,31–33, esters26,28, or amides27); and (3) leverages readily available and inexpensive carboxylic acid derivatives as coupling partners. Notably, a similar strategy was recently applied to the palladium-catalyzed decarbonylative coupling of acid fluorides with triethyltrifluoromethylsilane16.
Figure 1 |. Suzuki-Miyaura reaction and mechanistic design for direct generation of transmetalation-active [Ar–M–X*] intermediates.
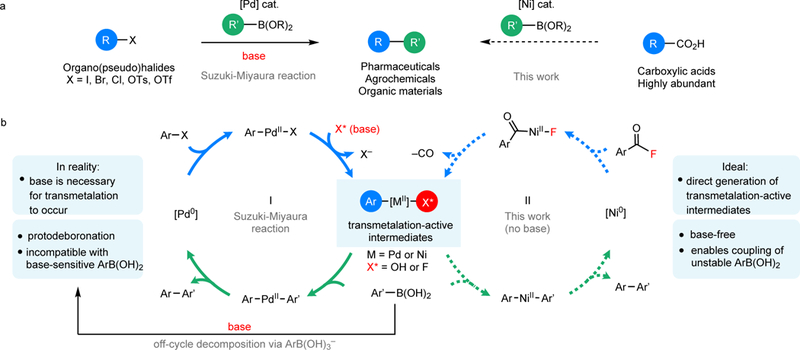
a, Cross-coupling reactions with organoboron reagents. b, Mechanistic design for directly accessing transmetalation-active intermediates for the base-free decarbonylative coupling of acid fluorides with organoboron reagents. R or Ar, an alkyl or aryl group.
Stoichiometric studies were first conducted to interrogate the viability of each step of the proposed catalytic cycle. To probe oxidative addition and decarbonylation, benzoyl fluoride 1 was reacted with Ni(cod)2/PCy3 (Fig. 2a). The benzoyl-nickel-fluoride intermediate 2 was formed rapidly (within 10 min at room temperature), and this complex underwent decarbonylation to afford phenyl-nickel-fluoride complex 3 in 90% yield after 15 h. To confirm that 3 is ‘transmetalation-active’, this complex was treated with 4-fluorophenyl boronic acid. As predicted, biaryl 5 (the product of transmetalation and subsequent C–C bond-forming reductive elimination) was formed in 90% after 1 h at room temperature. An analogous reaction with 2,4,6-trifluorophenylboronic acid (which is known to undergo rapid protodeboronation under basic conditions)4,10 provided 6 in >95% yield, indicating that transmetalation with 3 is faster than protodeboronation. Notably, the analogous phenyl-nickel-chloride 7 and -bromide 8 do not react with aryl boronic acids to form 5/6 even when heated at 100 °C.
Figure 2 |. Discovery of transmetalation-active nickel fluoride intermediates generated from decarbonylation enables Suzuki-Miyaura reaction of carboxylic acids and aryl boronic acids.
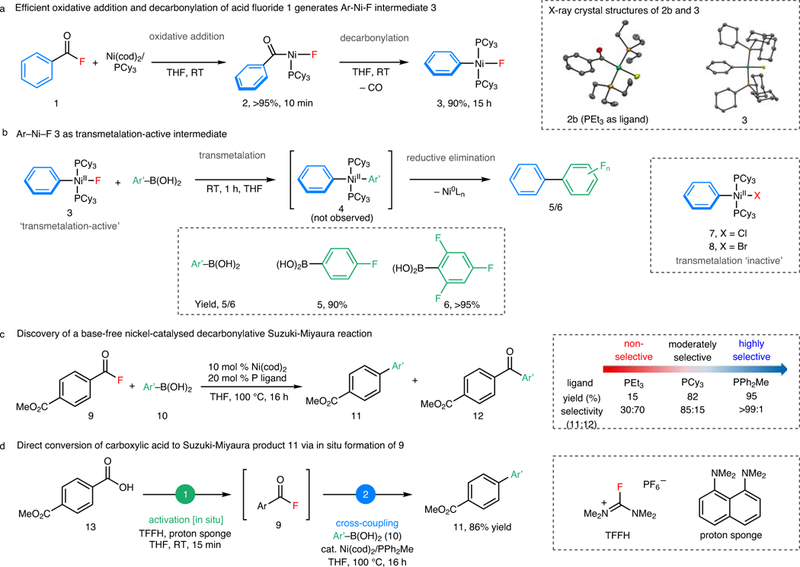
a, Oxidative addition/decarbonylation of 1 at room temperature. b, Transmetalation/reductive elimination of 3 and aryl boronic acids. c, Base-free Ni-catalysed decarbonylative Suzuki-Miyaura-type reaction. d, Direct conversion of aryl carboxylic acid to biaryl product via in situ generation of acid fluoride. Yields are based on 19F NMR spectroscopy (a and b) and gas chromatography (c). Cod, 1,5-cyclooctadiene; Cy, cyclohexyl; THF, tetrahydrofuran; RT, room temperature; Ar, aryl; Ph, phenyl; Me, methyl; Et, ethyl; TFFH, tetramethylfluoroformamidinium hexafluorophosphate. See Supplementary Information (sections III, VI and VII) for details on reaction conditions.
These stoichiometric studies were next translated to a Ni-catalysed decarbonylative coupling between acid fluoride 9 and 4-methoxyphenyl boronic acid (10). The use of 10 mol % Ni(cod)2 and 20 mol % PCy3 as catalyst afforded biaryl 11 along with a ketone byproduct 12 (11:12 = 85:15). PEt3 afforded poorer selectivity (11:12 = 30:70), while PPh2Me provided 11 as a single detectable product in 95% yield. These changes in selectivity as a function of phosphine arise from ligand effects on the decarbonylation step (see Supplementary Information section VIII for details).
A key advantage of acid fluoride electrophiles is that they are directly accessible from carboxylic acids via deoxyfluorination. Evaluation of various deoxyfluorinating reagents and bases revealed that the combination of tetramethylfluoroformamidinium hexafluorophosphate (TFFH) and proton sponge converts carboxylic acid 13 to acid fluoride 9 within 15 min at room temperature. The subsequent addition of Ni-catalyst and boronic acid 10 to the same pot and heating for 16 h at 100 °C then affords biaryl product 11 in 86% yield. A variety of aromatic and heteroaromatic carboxylic acids participate in this one-pot Ni-catalysed coupling with arylboronic acids (Fig. 3). Esters, nitriles, trifluoromethyl groups, methyl/phenyl ethers, sulfonamides, amides, alkenes, imidazoles, oxazoles, and pinacolboronate esters are tolerated. Aryl chlorides and phenyl esters26,28, common electrophiles in other Ni-catalysed cross-coupling reactions, are also compatible, demonstrating the orthogonality of the current method. Moderate yields were obtained with acid fluorides bearing electron-donating substituents (products 17-23) as well as those with ortho-substituents (products 20–23). For the former, GCMS analysis of the crude reaction mixtures showed ketone side-products, indicating that decarbonylation is relatively slow with electron-rich substrates. With the latter, unreacted starting material remained, suggesting that oxidative addition is sluggish when the acid fluoride is sterically hindered. Heteroaromatic carboxylic acids, including thiophene, benzofuran, indole, pyridine, and quinoline derivatives, are also effective coupling partners. Finally, a variety of carboxylic acid-containing bioactive molecules, including probenecid, bexarotene, tamibarotene, telmisartan, flavone, and febuxostat, participate in this one-pot decarbonylative cross-coupling.
Figure 3 |. Scope of Ni-catalysed decarbonylative Suzuki-Miyaura reaction with various carboxylic acids.
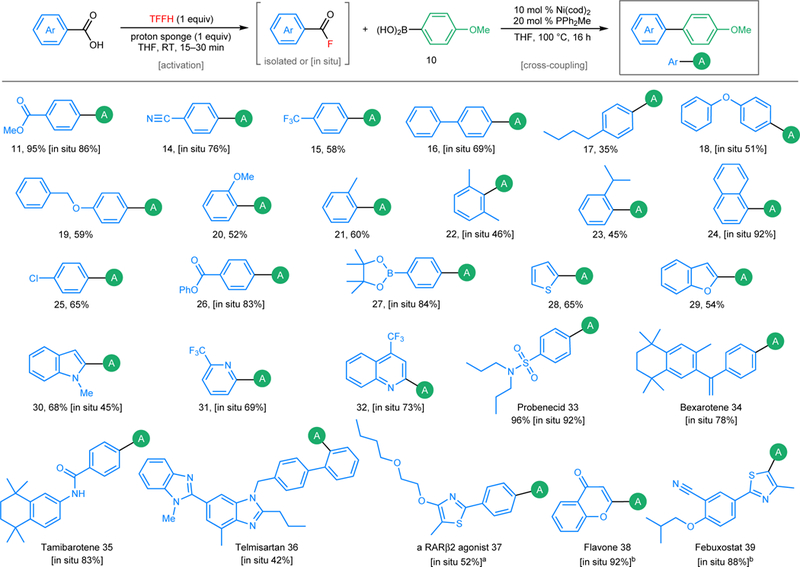
A (in green), p-anisyl; Bu, butyl. aContains inseparable protodecarbonylated carboxylic acid fluoride (7%) as impurity. bTributyl(4-methoxyphenyl)stannane was used as the coupling partner. See the Supplementary Information (section X) for details on reaction conditions and for examples of acid fluorides (Fig. S7) that did not undergo high-yielding decarbonylative coupling.
The generality of this method with respect to the boronic acid coupling partner was explored using probenecid as the substrate (Fig. 4a). Aryl boronic acids containing fluorine, ester, and methyl ketone substituents proved compatible. Alkenyl boronic acids underwent coupling to generate 45 and 46. Cyclopropyl, allyl, and benzyl boronic acids reacted under the optimized conditions to afford moderate yields of 47-49. Additionally, without any modification on the conditions, arylstannane nucleophiles afforded the flavone and febuxostat analogues 36 and 37 (Fig. 3). Base-sensitive α-heteroaryl boronic acids, including furans, thiophenes, and pyrroles, also underwent coupling (Fig. 4b). Finally, highly base sensitive ortho-difluorophenyl boronic acids4,10, underwent high yielding coupling with probenecid acid fluoride.
Figure 4 |. Scope of Ni-catalysed decarbonylative Suzuki-Miyaura coupling with various organoboron reagents.
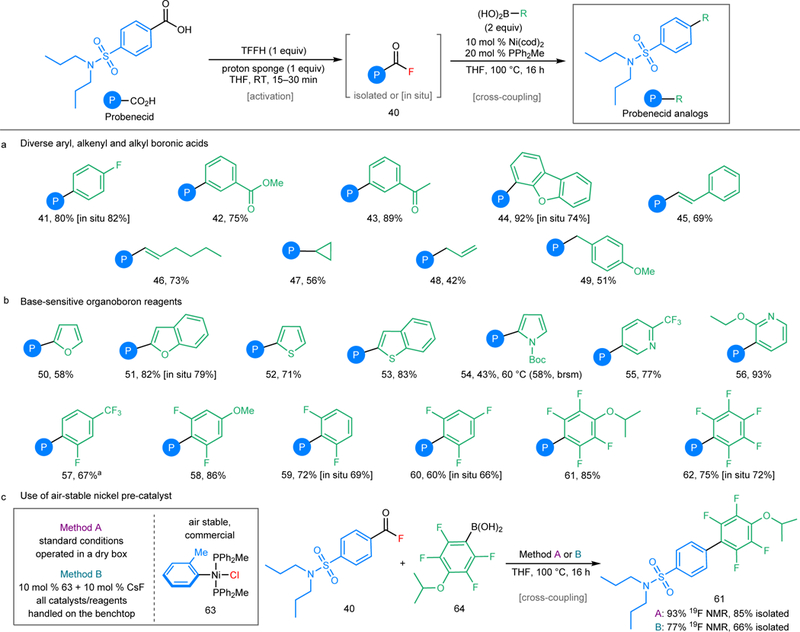
a, (Hetero)aryl, alkenyl, and alkyl boronic acids. b, Organoboron reagents that undergo facile protodeboronation. c, Use of a commercial, air-stable precatalyst 63. Method B conditions: 10 mol % of 63, 10 mol % of CsF, all catalysts/reagents handled on the benchtop. P (in blue), probenecid aryl fragment; Boc, tert-butoxycarbonyl. aIsolated product contains inseparable ketone byproduct (5%). See the Supplementary Information (section XII) for details on reaction conditions and for examples of organoboron reagents (Fig. S8) that did not undergo high yielding decarbonylative coupling.
A final set of studies focused on eliminating the need for air-sensitive Ni(cod)2 as the nickel source in these transformations. These investigations revealed that the combination of air-stable, commercially available Ni(o-tolyl)(PPh2Me)2Cl (63, 10 mol %) and CsF (10 mol %) affords a relatively comparable yield to the original Ni(cod)2/PPh2Me catalyst system in the formation of product 61 (Fig. 4c) as well as in related transformations (Figure S9). All of the catalysts and reagents for the Ni(o-tolyl)(PPh2Me)2Cl/CsF reactions were weighed on the benchtop, without the requirement for an inert atmosphere glove-box. As such, this advance should render these coupling reactions even more practical and accessible to a wide variety of synthetic and medicinal chemistry researchers.
Supplementary Material
Acknowledgements
We acknowledge financial support from NIH NIGMS (GM073836) and the Danish National Research Foundation (Carbon Dioxide Activation Center; CADIAC). We acknowledge J. W. Kampf for X-ray crystallographic analyses of 2b and 3.
Footnotes
Author Contributions:
C.A.M., J.R.B. and C.E.B. developed the stoichiometric reactions. C.A.M. discovered and developed the catalytic reactions. C.A.M., J.R.B. and M.S.S. conceived and designed the investigations. M.S.S. directed and supported the research. C.A.M., J.R.B. and M.S.S. wrote and revised the manuscript.
Data Availability The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. Additional data are available from the corresponding author upon request. Metrical parameters for the structures of complexes 2b and 3 (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC-1837039 and CCDC-1837038, respectively.
Author Information The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to M.S.S. (mssanfor@umich.edu).
References
- 1.Suzuki A Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 50, 6722–6737 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Brown DG & Boström J Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Lennox AJJ & Lloyd-Jones GC Transmetalation in the Suzuki-Miyaura coupling: the fork in the trail. Angew. Chem. Int. Ed. 52, 7362–7370 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Cox PA, Reid M, Leach AG, Campbell AD, King EJ & Lloyd-Jones GC Base-catalyzed aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Cox PA, Leach AG, Campbell AD & Lloyd-Jones GC Protodeboronation of heteroaromatic, vinyl, cyclopropyl boronic acids: pH–rate profiles, autocatalysis, and disproportionation. J. Am. Chem. Soc. 138, 9145–9157 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Molander GA & Biolatto B Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions of potassium aryl- and heteroaryltrifluoroborates. J. Org. Chem. 68, 4302–4314 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Knapp DM, Gillis EP & Burke MD A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates. J. Am. Chem. Soc. 131, 6961–6963 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins DW & Hartwig JF A C–H borylation approach to Suzuki–Miyaura coupling of typically unstable 2–heteroaryl and polyfluorophenyl boronates. Org. Lett. 14, 4266–4269 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Bulfield D & Huber SM Synthesis of polyflourinated biphenyls; pushing the boundaries of Suzuki–Miyaura cross coupling with electron-poor substrates. J. Org. Chem. 82, 13188–13203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinzel T, Zhang Y & Buchwald SL A new palladium precatalyst allows for the fast Suzuki–Miyaura coupling reactions of unstable polyfluorophenyl and 2-heteroaryl boronic acids. J. Am. Chem. Soc. 132, 14073–14075 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Francis H & Carrow BP An “on-cycle” precatalyst enables room-temperature polyfluoroarylation using sensitive boronic acids. ACS Catal. 8, 2989–2994 (2018). [Google Scholar]
- 12.Chen L, Sanchez DR, Zhang B & Carrow BP “Cationic” Suzuki–Miyaura coupling with acutely base-sensitive boronic acids. J. Am. Chem. Soc. 139, 12418–12421 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Ohashi M, Saijo H, Shibata M & Ogoshi S Palladium-catalyzed base-free Suzuki-Miyaura coupling reactions of fluorinated alkenes and arenes via a palladium fluoride key intermediate. Eur. J. Org. Chem. 443–447 (2013). [Google Scholar]
- 14.Graham TJA & Doyle AG Nickel-catalyzed cross-coupling of chromene acetals and boronic acids. Org. Lett. 14, 1616–1619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y & Rovis T A unique catalyst effects the rapid room-temperature cross-coupling of organozinc reagents with carboxylic acid fluorides, chlorides, anhydrides, and thioesters. J. Am. Chem. Soc. 126, 15964–15965 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Keaveney ST & Schoenebeck F Palladium-catalyzed decarbonylative trifluoromethylation of acid fluorides. Angew. Chem. Int. Ed. 57, 4073–4077 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogiwara Y, Sakurai Y, Hattori H & Sakai N Palladium-catalyzed reductive conversion of acyl fluorides via ligand-controlled decarbonylation. Org. Lett. 20, 4204–4208 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Gooßen LJ, Deng G & Levy LM Synthesis of biaryls via catalytic decarboxylative coupling. Science 313, 662–664 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG & MacMillan DWC Merging photoredox with nickel catalysis: coupling of α-carbonyl sp3-carbons with aryl halides. Science 345, 437–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Qin T, Chen T-G, Wimmer L, Edwards JT, Cornella J, Vokits B, Shaw SA & Baran PS Nickel-catalyzed cross-coupling of redox active esters with boronic acids. Angew. Chem. Int. Ed. 55, 9676–9679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards JT, Merchant RR, McClymont KS, Knouse KW, Qin T, Malins LR, Vokits B, Shaw SA, Bao D-H, Wei F-L, Zhou T, Eastgate MD & Baran PS Decarboxylative alkenylation. Nature 545, 213–218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fawcett A, Pradeilles J, Wang Y, Mutsuga T, Myers EL & Aggarwal VK Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Carrow BP & Hartwig JF Distinguishing between pathways for transmetalation in Suzuki-Miyaura reactions. J. Am. Chem. Soc. 133, 2116–2119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amatore C, Jutand A & Le Duc G The triple role of fluoride ions in palladium-catalyzed Suzuki-Miyaura reactions: unprecedented transmetalation from [ArPdFL2] complexes. Angew. Chem. Int. Ed. 51, 1379–1382 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Thomas AA & Denmark SE Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: the missing link. Science 352, 329–332 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Muto K, Yamaguchi J, Musaev DG & Itami K Decarbonylative organoboron cross-coupling of esters by nickel catalysis. Nature Commun. 6, 7508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi S, Meng G & Szostak M Synthesis of biaryls through nickel-catalyzed Suzuki-Miyaura coupling of amides by carbon–nitrogen bond cleavage. Angew. Chem. Int. Ed. 55, 6959–6963 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Guo L & Rueping M Decarbonylative cross-couplings: nickel catalyzed functional group interconversion strategies for the construction of complex organic molecules. Acc. Chem. Res. 51, 1185–1195 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Masson-Makdissi J, Vandavasi JK & Newman SG Switchable selectivity in the Pd-catalyzed alkylative cross-coupling of esters. Org. Lett. 20, 4094–4098 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Ichiishi N, Malapit CA, Wozniak L & Sanford MS Palladium- and nickel-catalyzed decarbonylative C–S coupling to convert thioesters to thioethers. Org. Lett. 20, 44–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaub T, Backes M & Radius U Catalytic C–C bond formation accomplished by selective C–F activation of perfluorinated arenes. J. Am. Chem. Soc. 128, 15964–15965 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Tobisu M, Xu T, Shimasaki T & Chatani N Nickel-catalyzed Suzuki-Miyaura reaction of aryl fluorides. J. Am. Chem. Soc. 133, 19505–19511 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Liu X-W, Echavarren J, Zarate C & Martin R Ni-catalyzed borylation of aryl fluorides via C–F cleavage. J. Am. Chem. Soc. 137, 12470–12473 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


