Abstract
Introduction
Several histologic features have been identified in the upper-extremity arteries and veins of patients with advanced chronic kidney disease (CKD), which may affect arteriovenous fistula maturation. However, it is unclear whether these CKD vascular features are abnormal.
Methods
We obtained upper-extremity arterial and venous specimens from 125 advanced CKD patients undergoing AVF creation, and from 15 control subjects. We quantified medial fibrosis, micro-calcification, and intimal hyperplasia with appropriate histology stains. We characterized medial collagen fiber configuration in second-harmonic-generation microscopy images for the fiber anisotropy index and the dominant fiber direction.
Results
The advanced CKD patients were significantly younger than control subjects (53±14 vs 76±11 yr, p<0.001). After controlling for age, the CKD patients had greater arterial medial fibrosis (69±14 vs 51±10%, p<0.001) and greater arterial micro-calcification (3.03±5.17 vs 0.01±0.03%, p=0.02), but less arterial intimal thickness (30±25 vs 63±25 μm, p<0.001), as compared to control subjects. The anisotropy index of medial collagen fibers was lower in both arteries (0.24±0.10 vs 0.44±0.04, p<0.001) and veins (0.28±0.09 vs 0.53±0.10, p<0.001) in CKD patients, indicating that orientation of the fibers was more disordered. The dominant direction of medial collagen fibers in CKD patients was greater in the arteries (49.3±23.6° vs 4.0±2.0°, p<0.001) and the veins (30.0±19.6° vs 3.9±2.1°, p<0.001), indicating that the fibers in general were aligned more perpendicular to the lumen.
Conclusions
Advanced CKD is associated with several abnormalities in vascular histology and collagen fiber configuration. Future research is needed to investigate whether these abnormalities affect the maturation outcomes of arteriovenous fistulas.
Keywords: Chronic kidney disease, collagen, hemodialysis arteriovenous fistula, intimal hyperplasia, vascular calcification, vascular fibrosis
Introduction
Patients with advanced chronic kidney disease (CKD) have a high prevalence of atherosclerosis and medial calcification affecting the coronary and femoral arteries, leading to ischemic heart disease, congestive heart failure, and ischemia of the lower extremities (1–3). Less is known about the vascular histology and ultra-structures, especially those in the upper extremities, in patients with advanced CKD. Several studies have examined specimens of upper-extremity arteries or veins used to create an arteriovenous fistula (AVF) from patients with advanced CKD, and described heterogeneous histologic features, including arterial and venous intimal hyperplasia, medial fibrosis, and micro-calcification (4–11), with the notion that these features may play a role in determining whether the AVF will mature for adequate hemodialysis. It is not clear, however, which of these histologic features are indeed abnormal, compared to individuals with normal kidney function.
A single study compared the cephalic vein histology in 20 patients with advanced kidney failure (mean age, 44±16 yr) undergoing AVF creation to three normal controls (ages 20–27) undergoing surgery to repair vascular trauma to the upper-extremity arteries (12). As compared to normal controls, the cephalic veins from patients with CKD exhibited more intimal hyperplasia, muscular atrophy, and inflammatory cell infiltration. That study was limited by the small sample size, the lack of quantification of histologic abnormalities, the absence of information on arterial histology, and the age difference between the two groups. Better understanding of the vascular histology in patients with advanced CKD may provide novel insights into its role in AVF maturation. To achieve this goal, we compared quantitatively upper-extremity arterial and venous histology in a cohort of patients with advanced CKD to that observed in upper-extremity vessels procured from embalmed cadavers (anatomic donations) of patients with co-morbidities that did not include end stage renal disease (ESRD).
Methods
Patient population and procedure for obtaining vascular specimens
One hundred and twenty-five advanced CKD patients who were receiving hemodialysis or anticipated to initiate hemodialysis in the near future, and who were scheduled to undergo AVF creation, were enrolled in a prospective study previously reported by us (7). The patients provided informed written consent during their preoperative visit, under a protocol approved by the University of Alabama at Birmingham Institutional Review Board and adherent to the Declaration of Helsinki. At the time of AVF creation, the surgeon obtained a circumferential specimen from the vein and an elliptical section of the artery used to create the AVF. The specimens were immediately fixed in formaldehyde for subsequent processing. Vascular samples from CKD patients were obtained from a newly created AVF that was created in the side that did not have any previous access. 44 patients (35%) received a forearm AVF and their samples were from radial arteries and cephalic veins. 81 patients (65%) received an upper-arm AVF, and their samples were from brachial arteries and cephalic or basilic veins.
The control group consisted of deceased individuals whose bodies were donated for educational purposes. The cadavers were embalmed with phenol and formaldehyde and used for medical-school anatomy classes. At the end of the school semester, a pathologist (SHL) harvested upper-extremity arterial and venous samples from each cadaver, at the level of the elbow. We obtained brachial arteries from all cadavers; vein samples were mostly brachial but a few were cephalic. For this control group, the only clinical characteristics available were age, gender, and primary cause of death (Table 1).
Table 1.
Age, gender, and primary cause of death of the control subjects
| Subject ID | Age | Gender | Primary cause of death |
|---|---|---|---|
| 1 | 72 | M | Lung cancer |
| 2 | 80 | F | Dementia |
| 3 | 96 | F | Dementia |
| 4 | 84 | M | Cardiomyopathy without significant coronary artery disease |
| 5 | 87 | M | Esophageal carcinoma |
| 6 | 69 | M | Lung cancer |
| 7 | 81 | M | Cerebrovascular accident |
| 8 | 86 | M | Emphysema, respiratory failure |
| 9 | 83 | F | Dementia |
| 10 | 82 | M | Dysphagia |
| 11 | 64 | F | Metabolic encephalopathy |
| 12 | 54 | F | Chronic obstructive pulmonary disease |
| 13 | 67 | F | Protein calorie malnutrition |
| 14 | 67 | M | Brain tumor |
| 15 | 67 | M | Lung cancer |
Histologic staining of vascular specimens
Tissue samples fixed in formaldehyde were processed and cut into 5-μm thick sections. The arterial and venous tissue slides were stained with three different histological stains: Masson’s trichrome for identification of medial fibrosis and better delineation of the internal elastic lamina, von Kossa for identification of calcification, and hematoxylin and eosin for quantification of intimal hyperplasia. Masson’s trichrome stains smooth muscle red and collagen blue. Von Kossa stains calcium black. Using a Bioquant software program, medial fibrosis was quantified and expressed as the percent of the total medial area that stained blue (7). Similarly, calcification was quantified as the percent of total medial area that appeared black on von Kossa stain.4, 6 Intimal hyperplasia was defined as the greatest thickness between the internal elastic lamina and the lumen along the circumference (4, 13). To facilitate intimal hyperplasia and medial fibrosis quantification, elastic van Gieson stain was used to highlight elastic fibers, which appear black. This stain permitted us to better delineate the internal elastic lamina and reveal elastin in the medial layer. The pathologist was blinded to the clinical characteristics of the patients.
Collagen fiber configuration
Second-harmonic-generation (SHG) microscopy was used to image the individual collagen fibers in the media, as previously described by us (7). In brief, SHG signals of collagen fiber bundles in unstained paraffin-embedded tissue sections (5-μm thickness) were acquired at 850-nm excitation under a multi-photon microscope (Bruker Optics Inc., Billerica, MA). At least three fields per sample were acquired (by YTS) and then analyzed for fiber patterns and orientation by three independent observers (YTS, JCST, and CAS), who were blinded to the subjects’ clinical information. Qualitative fiber patterns were categorized as described by us previously (i.e., parallel to the lumen, perpendicular to the lumen, micro-perpendicular with macro-parallel, railroad track, honeycomb, random, and a mixed pattern) (7). Algorithms in the Fiber Analyzer and OrientationJ software were used to quantify the anisotropy index and orientation angles, respectively, of the collagen fibers in the media (14, 15). The anisotropy index is a measure of the randomness of the fiber network, ranging from “0” for totally random (i.e., no preferential directionality) to “1” for totally aligned in one direction. Each fiber’s orientation angle is calculated as the angle between the fiber’s main axis and the perimeter of the adjacent lumen, ranging from 0° (parallel to lumen or circumferential direction) to 90° (perpendicular to the lumen or radial direction).
Statistical analysis
Two sample t-tests were used to compare the vascular histology characteristics (medial fibrosis, calcification, intimal thickness, medial anisotropy index, and medial dominant directionality), assuming unequal variance between the advanced CKD patients and the control subjects. In addition, linear regressions adjusting for patient age were performed for each comparison. Chi-square test was used to compare gender frequencies between the two groups. Linear regressions were used to examine the association of age with medial fibrosis, calcification, and intimal thickness in advanced CKD patients. All data analyses were performed using Stata 13 Statistical Software (College Station, TX). Two-sided tests were performed, with a p-value < 0.05 considered statistically significant.
Results
Clinical features of the study population
There were 125 patients in the CKD group and 15 subjects in the control group. The proportion of males was similar in both groups (54% and 60%, respectively) (Table 2). The CKD patients were substantially younger than the control subjects (mean age 53 vs 76 yr). Co-morbidities in the patients with CKD included diabetes (51%), hypertension (90%), congestive heart failure (17%), coronary artery disease (13%), peripheral artery disease (10%), and cerebrovascular disease (10%).7 Information on co-morbidity other than the primary cause of death was not available for the control group, but none of them were dialysis patients.
Table 2.
Demographics and histologic features of arteries and veins in patients with advanced chronic kidney disease and control subjects
| Variables | Advanced CKD patients | Control subjects | Unadjusted p-value* (CKD vs. control) | Adjusted p-value* (CKD vs. control) |
|---|---|---|---|---|
| No. of subjects | 125 | 15 | ||
| Age, mean ± SD | 53 ± 14 | 76 ± 11 | < 0.001 | |
| Male gender, N (%) | 68 (54%) | 9 (60%) | 0.68 | |
| Vascular histology | ||||
| Arterial medial fibrosis (%), mean ± SD | 69 ± 14 | 51 ± 10 | < 0.001 | < 0.001 |
| Venous medial fibrosis (%), mean ± SD | 63 ± 12 | 59±10 | 0.12 | 0.12 |
| Arterial calcification (%), mean ± SD | 3.03 ± 5.17 | 0.01 ± 0.03 | < 0.001 | 0.02 |
| Arterial intimal thickness (μm), mean ± SD | 30 ± 25 | 63 ± 25 | < 0.001 | < 0.001 |
| Venous intimal thickness (μm), mean ± SD | 37 ± 40 | 14 ± 6 | < 0.001 | 0.06 |
| Collagen fiber configuration | ||||
| Arterial medial anisotropy index, mean ± SD | 0.24 ± 0.10 | 0.44 ± 0.04 | < 0.001 | < 0.001 |
| Venous medial anisotropy index, mean ± SD | 0.28 ± 0.09 | 0.53 ± 0.10 | < 0.001 | < 0.001 |
| Arterial medial dominant directionality (°), mean ± SD | 49.3 ± 23.6 | 4.0 ±2.0 | < 0.001 | < 0.001 |
| Venous medial dominant directionality (°), mean ± SD | 30.0 ± 19.6 | 3.9 ± 2.1 | < 0.001 | < 0.001 |
t-tests were used for comparing the continuous variables assuming unequal variance between groups, and unadjusted p-values were reported.
Linear regressions were used to obtain the adjusted p-values after controlling for age. Chi-squared test was used for comparing gender between the two groups.
Correlation of age with vascular features in the CKD and control croups
We investigated the correlation of age with histological features (Table 3) and with collagen fiber configuration. Arterial medial fibrosis was directly associated with age in the patients with advanced CKD; specifically, for each one-year increase in age, the absolute medial fibrosis increased by 0.25% (95% CI 0.09 to 0.42, p=0.003). However, venous medial fibrosis was not associated with patient age (p=0.24). Age was not associated with arterial medial fibrosis (p=0.64) or venous medial fibrosis (p=0.06) in control subjects. Arterial calcification was not associated with age in CKD patients (p=0.45) or in control subjects (p=0.38). Arterial intimal hyperplasia was not associated with age in CKD patients (p=0.75) but was associated with age in control subjects; specifically, for each one-year increase in control subject age, the absolute intimal thickness increased by 1.42 μm (95% CI 0.40 to 2.44 μm, p=0.01). Venous intimal hyperplasia was not associated with age in CKD patients (p=0.88) or in control subjects (p=0.91). Finally, there was no significant correlation of age with the medial anisotropy index or the medial dominant directionality in the arteries or the veins of the CKD patients or control subjects (data not shown).
Table 3.
Correlation of age with histologic features of arteries and veins in patients with advanced chronic kidney disease and control subjects
| Variables | P-value for advanced CKD patients | P-value for control subjects |
|---|---|---|
| Vascular histology | ||
| Arterial medial fibrosis | 0.003 | 0.64 |
| Venous medial fibrosis | 0.24 | 0.06 |
| Arterial calcification | 0.45 | 0.38 |
| Arterial intimal thickness | 0.75 | 0.01 |
| Venous intimal thickness | 0.88 | 0.91 |
Arterial and venous medial fibrosis in the CKD and control groups
While there was a broad range of medial fibrosis in the arterial samples from the CKD patients (32% – 96%) and control subjects (30% – 64%), elastin was minimal in the medial layers of all samples (Figure 1). The mean arterial medial fibrosis was significantly higher in the patients with advanced CKD than in the control group (69% vs 51%), and this difference remained significant even after controlling for subject age (Table 2). Similar to the arteries, the veins also had a broad range of medial fibrosis in the CKD patients (33% – 94%) and control subjects (41% – 72%), with minimal elastin in the medial layers. However, the mean venous medial fibrosis was similar in the CKD patients and the control group (63% vs 59%), before and after controlling for subject age (Table 2).
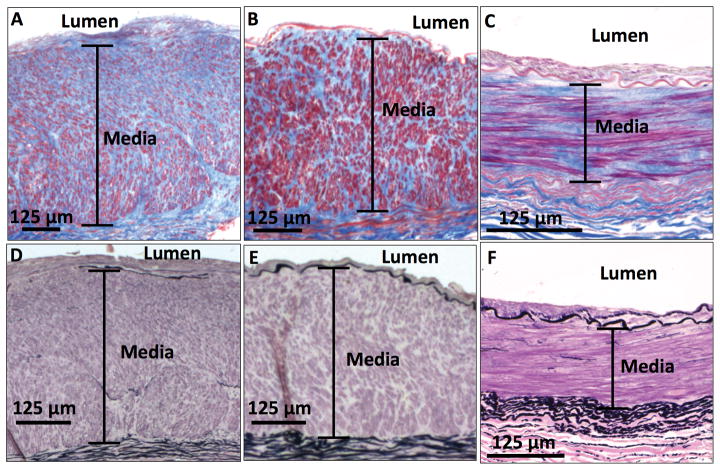
Figure 1. Medial fibrosis in the upper-extremity arteries.
Masson’s trichrome stain shows (A) an artery with severe medial fibrosis (86%) and (B) an artery with moderate medial fibrosis (65%) from patients with advanced CKD, as well as (C) an artery with representative medial fibrosis (50%) from a control subject. Elastic van Gieson stain of these samples shows that all arteries have very few elastic fibers in their respective medial layers (D, E, F). Collagen appears blue and smooth muscle cells appear red in Masson’s trichrome stain. Elastic fibers appear black in elastic van Gieson stain.
Arterial and venous calcification in the CKD and control croups
There was a broad range of micro-calcification in the arterial samples from the CKD patients (0.01% – 34%). Although a few control subjects had notable arterial micro-calcification, most had no or minimal arterial micro-calcification (Figure 2). The mean arterial micro-calcification was significantly higher in patients with CKD than in the control group, even after controlling for patient age (Table 2). There was negligible calcification in the veins from patients with advanced CKD and the control group.
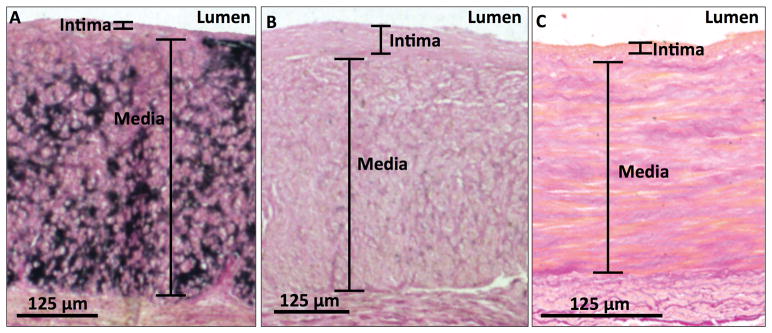
Figure 2. Micro-calcification in the upper-extremity arteries.
Von Kossa stain shows (A) an artery with significant micro-calcification (15%) and (B) an artery with mild micro-calcification (1%) in the medial layer from patients with advanced CKD, as well as (C) an artery with typical medial micro-calcification (0%) from a control subject. Calcification appears black in this stain.
Arterial and venous intimal hyperplasia in the CKD and control croups
There was a broad range of intimal hyperplasia in the arterial samples (1 – 184 μm in the CKD patients and 22 – 109 μm in the controls) (Figure 3) and venous samples (1 – 279 μm in the CKD patients and 6 – 27 μm in the controls). The mean arterial intimal thickness in the CKD group was approximately half the value of that in the control group, and this difference remained highly significant even after controlling for patient age (Table 2). In contrast, the age-adjusted venous intimal thickness was not different between the CKD patients and the controls.
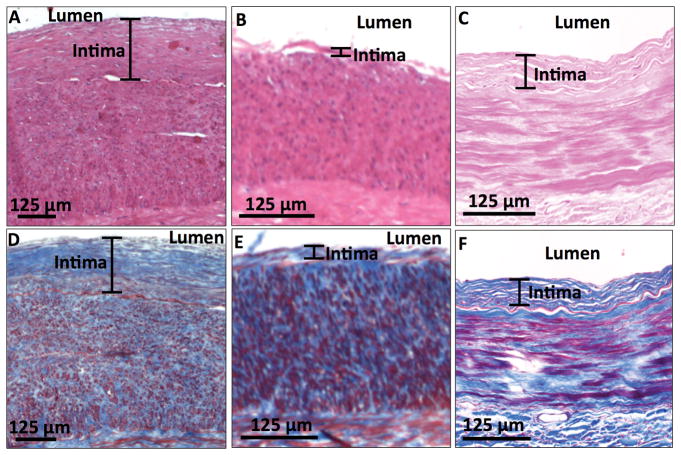
Figure 3. Intimal hyperplasia (IH) in the upper-extremity arteries.
Hematoxylin and eosin stain shows (A) an artery with significant IH (170 μm) and (B) an artery with mild IH (13 μm) from patients with advanced CKD, as well as (C) an artery with representative IH (63 μm) from a control subject. Masson’s trichrome stain of these samples shows their respective internal elastic lamina (D, E, F), which appears red and has the characteristic wavy-line structure.
Arterial and venous medial collagen configuration in the CKD and control croups
The most common medial collagen fiber pattern for both the arterial and venous samples from the control group was the parallel pattern, i.e., fibers largely aligned parallel to the lumen’s circumference (Figure 4). In contrast, advanced CKD patients exhibited a variety of medial collagen patterns, with the honeycomb, perpendicular to lumen, and random patterns being most common in the arteries; and railroad track, parallel to lumen, and mixed patterns most common in the veins (Figure 4). Both the arterial and venous medial anisotropy indices were significantly lower in the patients with CKD than in the control group, consistent with a more random configuration of the collagen fibers in the media. The dominant medial angle was close to zero in the controls (i.e., parallel to the lumen), and substantially higher (more perpendicular) in the patients with CKD (Table 2). All the comparisons between the CKD patients and controls of anisotropy index and dominant medial angle remained highly significant, after controlling for patient age.
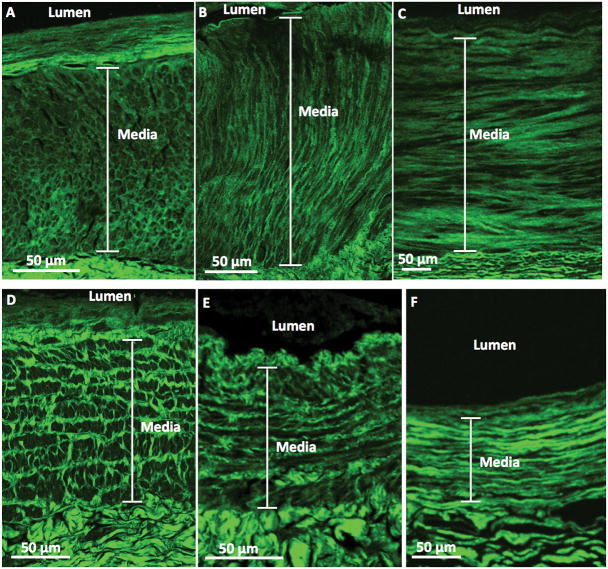
Figure 4. Representative second-harmonic-generation (SHG) images of the upper-extremity arteries and veins.
Upper panels: SHG images show (A) an artery whose medial collagen fibers had the honeycomb pattern and (B) an artery whose medial collagen fibers had the perpendicular to lumen pattern from patients with advanced CKD, as well as (C) an artery whose medial collagen fibers had the parallel to lumen pattern from a control subject. Lower panels: SHG images show (D) a vein whose medial collagen fibers had the railroad track pattern and (E) a vein whose medial collagen fibers had the parallel to lumen pattern from patients with advanced CKD, as well as (F) a vein whose medial collagen fibers had the parallel pattern from a control subject.
Discussion
The current study documented several striking differences in the histology of upper-extremity blood vessels in patients with CKD, as compared to the controls. Specifically, after controlling for age, patients with CKD had greater arterial medial fibrosis and arterial micro-calcification, but less arterial intimal hyperplasia. In addition, analysis of medial collagen fiber configuration using SHG observed a more random configuration in both arteries and veins, and a fiber orientation more perpendicular to the vascular lumen, in CKD patients compared to the control group.
Arterial medial fibrosis increases with age, presumably reflecting exposure of the arterial wall to higher blood pressures, inflammation and extracellular matrix remodeling (16, 17). Notably, the arterial medial fibrosis was greater in patients with advanced CKD than in control subjects, even though their mean age was over 20 years lower. This finding suggests that CKD accelerates the development of arterial medial fibrosis by a yet to be defined mechanism.
Patients with CKD are known to have accelerated medial calcification of the coronary and femoral arteries (18, 19). We previously reported that arterial medial micro-calcification was also common in the upper extremities of advanced CKD patients, but was not associated with AVF maturation failure (4, 6). In the current study, we found that most control subjects had no or minimal arterial micro-calcification. Of note, even though aging is reported to be associated with arterial calcification in non-CKD subjects (20) and our CKD patient group was younger than the control group, arterial micro-calcification was higher in the CKD patient group. This finding suggests that CKD per se is associated with arterial micro-calcification.
In native arteries and veins, neointimal hyperplasia occurs frequently following vascular injury such as venipuncture, angioplasty (21) or after arteriovenous vascular access creation (22, 23). Animal studies have shown that CKD accelerates the formation of neointimal hyperplasia after AVF creation, in part by changing vascular smooth muscle cells from the contractile to the proliferative and mobile phenotype (24–27). In contrast, the mechanisms of de novo venous or arterial hyperplasia in patients with CKD are poorly understood. Preexisting venous intimal hyperplasia occupying at least 20% of the lumen was observed in 57% of CKD patients enrolled in the Hemodialysis Fistula Maturation (HFM) Study (11). However, veins from normal controls were not available for comparison in the HFM Study. Wali et al. described a qualitative increase in venous intimal hyperplasia in the cephalic veins of 20 patients with CKD, as compared to veins obtained from three normal controls (12). We have confirmed this finding in a larger cohort of patients (125 with advanced CKD and 15 controls) in whom the venous intimal thickness was quantified, but the difference was no longer significant after controlling for patient age.
Although it is tempting to speculate that preexisting venous intimal hyperplasia accelerates neointimal hyperplasia after AVF creation (manifesting as AVF stenosis in the postoperative ultrasound), a recent analysis of the HFM Study found limited evidence for such an association (28). In contrast to venous intimal thickness, arterial intimal thickness was significantly lower in patients with CKD than in the control group in the current study. This observation may be explained in part by the significantly younger age of the CKD patients than the control subjects and other factors that remain to be explored. Indeed, it has been previously reported that arterial intimal hyperplasia increases with age in patients without CKD (29, 30), and we also confirmed such a correlation in our cohort of control subjects.
Medial collagen fibers are organized into lamellar units and oriented circumferentially (parallel to the lumen) in healthy arteries and veins (31). We have confirmed this architectural pattern in a cohort of control subjects, despite their advanced age (mean 76 yrs) and that our samples were obtained from embalmed cadavers. Abnormal vascular collagen fiber organization has been found to be associated with several diseases, including aortic abdominal aneurysms and aneurysms in Marfan syndrome (32). We have previously described seven distinct medial collagen patterns in advanced CKD patients enrolled from a single center: parallel to the lumen, perpendicular to the lumen, micro perpendicular with macro parallel, railroad track, honeycomb, random, and a mixed pattern (7). Only a minority of the CKD patients in the present study exhibited the parallel pattern seen in normal controls; most had other collagen configuration patterns, suggesting that CKD disrupts and changes collagen fiber configuration. CKD is associated with progressive changes in central arterial extracellular matrix composition and collagen fiber configuration (33) and increased arterial stiffness (34–36). Changes in the matrix structures of venous walls, however, have not been studied in CKD patients until our recent report describing qualitative patterns of collagen configuration (7).
The current study has expanded on our previous qualitative description of collagen fiber patterns (7), by quantifying two components of upper-extremity vascular fiber configuration, namely anisotropy index and orientation angle. The medial collagen fiber configuration was more random (i.e., lower anisotropy index) in both the arteries and veins of patients with advanced CKD, as compared to the control group. Additionally, the medial collagen fiber orientation was more perpendicular to the vascular lumen (i.e., greater orientation angles) in both the arteries and veins of patients with advanced CKD. Taken together, these observations suggest that advanced CKD results in substantial changes in arterial and venous medial collagen fiber architecture, which may affect their mechanical and functional properties.
One should not necessarily assume that the vascular histologic abnormalities observed in patients with advanced CKD patients translate into inferior vascular access outcomes. In fact, several publications suggest that the opposite may be true. We recently reported the counterintuitive observation that greater arterial medial fibrosis was associated with greater postoperative AVF blood flow and lower AVF maturation failure (7). Another study of patients with arteriovenous grafts found that the frequency of graft interventions was inversely proportionate to preexisting venous intimal thickness, arterial medial fibrosis, and arterial micro-calcification (13). Finally, our preliminary data suggested an association between a more perpendicular fiber configuration in the preoperative veins with higher post-operative AVF blood flow rate (37). Thus, the contributions of various arterial and venous wall histological features to AVF development require further detailed studies.
The strengths of our study include the quantification of several vascular histologic features from both advanced CKD patients and control subjects, the inclusion of both venous and arterial samples, and the use of a state-of-the-art method (SHG imaging) to quantify the configuration of medial collagen fibers.
One limitation of our study is that all CKD patients were enrolled from a single dialysis center, so the results may not generalize to CKD patients in other centers. Additionally, we had limited clinical information on the control subjects, some of which may have had other co-morbidities (possibly including CKD) associated with vascular abnormalities. We do know, however, that none of the controls were dialysis patients. We also must consider the possibility that postmortem changes or the embalming solution may have contributed to vascular abnormalities in the control subjects. However, this seems less likely given the observation that the controls had greater arterial intimal hyperplasia but less venous intimal hyperplasia than the CKD patients. It seems unlikely that postmortem changes or the embalming solution could have opposite effects on arteries and veins.
Conclusions
There are major abnormalities in histologic features in the vasculature of CKD patients, which may affect its physiologic properties. Future research is needed to investigate whether these abnormalities affect the maturation outcomes of arteriovenous fistulas and other cardiovascular events.
Acknowledgments
This study was supported by the U.S. National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) grants R01DK085027 to M.A. and R01DK100505 to Y.T.S, and the U.S. National Heart, Lung and Blood Institute (NHLBI) grants R00HL122368 to Z.C. We also acknowledge the statistical assistance by the University of Utah Study Design and Biostatistics Center, with funding, in part, from the U.S. National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH grant 8UL1TR000105 (formerly NIH grant UL1RR025764). SHG microscopy imaging was performed at the Cell Imaging Core Facility, a part of the Health Sciences Cores at the University of Utah. Microscopy equipment was obtained using the U.S. National Center for Research Resources Shared Equipment grant 1S10RR024761-01.
Portions of this manuscript were submitted in the abstract form for presentation at the 2017 American Society of Nephrology annual meeting in New Orleans, LA, USA.
M. Allon is a Consultant for CorMedix. A. K. Cheung was a member of the Data and Safety Monitoring Board for a trial on vascular graft co-sponsored by Humacyte, Inc. and the National Heart, Lung, and Blood Institute, as well as a member of the Clinical Events Committee and Data Safety and Monitoring Board for the Novel Endovascular Access Trial sponsored by TVA Medical, Inc.
References
- 1.Stompor T. Coronary artery calcification in chronic kidney disease: An update. World J Cardiol. 2014;6:115–129. doi: 10.4330/wjc.v6.i4.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLoach SS, Mohler ER., 3rd Peripheral arterial disease: a guide for nephrologists. Clin J Am Soc Nephrol. 2007;2:839–846. doi: 10.2215/CJN.04101206. [DOI] [PubMed] [Google Scholar]
- 3.Afsar B, Turkmen K, Covic A, Kanbay M. An update on coronary artery disease and chronic kidney disease. Int J Nephrol. 2014;2014:767424. doi: 10.1155/2014/767424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allon M, Litovsky S, Young CJ, et al. Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis. 2011;58:437–443. doi: 10.1053/j.ajkd.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allon M, Robbin ML, Young CJ, et al. Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol. 2013;8:1750–1755. doi: 10.2215/CJN.02740313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allon M, Robbin ML, Umphrey HR, et al. Preoperative arterial micro-calcification and clinical outcomes of arteriovenous fistulas for hemodialysis. Am J Kidney Dis. 2015;66:84–90. doi: 10.1053/j.ajkd.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiu YT, Litovsky SH, Cheung AK, et al. Preoperative vascular medial fibrosis and arteriovenous fistula development. Clin J Am Soc Nephrol. 2016;11:1615–1623. doi: 10.2215/CJN.00500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabbara M, Duque JC, Martinez L, et al. Pre-existing and Postoperative Intimal Hyperplasia and Arteriovenous Fistula Outcomes. Am J Kidney Dis. 2016;68:455–464. doi: 10.1053/j.ajkd.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez-Padron RI, Allon M. Impact of pre-existing arterial and venous pathology on vascular access outcomes. Clin J Am Soc Nephrol. 2016;11:1495–503. doi: 10.2215/CJN.01860216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YO, Song HC, Yoon SA, et al. Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistula in hemodialysis patients. Am J Kidney Dis. 2003;41:422–428. doi: 10.1053/ajkd.2003.50051. [DOI] [PubMed] [Google Scholar]
- 11.Alpers CE, Imrey PB, Hudkins KL, et al. Histopathology of veins obtained at hemodialysis arteriovenous fistula creation surgery. J Am Soc Nephrol. 2017;28:3076–3088. doi: 10.1681/ASN.2016050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wali MA, Eid RA, Dewan M, Al-Homrany MA. Pre-existing histopathologic changes in the cephalic vein of renal failure patients before arteriovenous fistula (AVF) construction. Ann Thorac Cardiovasc Surg. 2006;12:341–348. [PubMed] [Google Scholar]
- 13.Allon M, Litovsky S, Young CJ, et al. Correlation of pre-existing vascular pathology correlation with arteriovenous graft outcomes in hemodialysis patients. Am J Kidney Dis. 2013;62:1122–1129. doi: 10.1053/j.ajkd.2013.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sander EA, Barocas VH. Comparison of 2D fiber network orientation measurement methods. J Biomed Mater Res A. 2009;88:322–331. doi: 10.1002/jbm.a.31847. [DOI] [PubMed] [Google Scholar]
- 15.Rezakhaniha R, Agianniotis A, Schrauwen JT, et al. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol. 2012;11:461–473. doi: 10.1007/s10237-011-0325-z. [DOI] [PubMed] [Google Scholar]
- 16.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32:659–668. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E, Najjar SS, Cornish TC, Halushka MK. A comprehensive histopathological evaluation of vascular medial fibrosis: insights into the pathophysiology of arterial stiffening. Atherosclerosis. 2010;208:69–74. doi: 10.1016/j.atherosclerosis.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Disthabanchong S. Vascular calcification in chronic kidney disease: Pathogenesis and clinical implication. World J Nephrol. 2012;1:43–53. doi: 10.5527/wjn.v1.i2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drueke TB, Massy ZA. Chronic kidney disease: Medial or intimal calcification in CKD-does it matter? Nat Rev Nephrol. 2011;7:250–251. doi: 10.1038/nrneph.2011.41. [DOI] [PubMed] [Google Scholar]
- 20.Tesauro M, Mauriello A, Rovella V, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281:471–482. doi: 10.1111/joim.12605. [DOI] [PubMed] [Google Scholar]
- 21.Liu MW, Roubin GS, King SB., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 22.Lee T, Somarathna M, Hura A, et al. Natural history of venous morphologic changes in dialysis access stenosis. J Vasc Access. 2014;15:298–305. doi: 10.5301/jva.5000212. [DOI] [PubMed] [Google Scholar]
- 23.Roy-Chaudhury P, Arend L, Zhang J, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Kang L, Grande JP, Hillestad ML, et al. A new model of an arteriovenous fistula in chronic kidney disease in the mouse: beneficial effects of upregulated heme oxygenase-1. Am J Physiol Renal Physiol. 2016;310:F466–476. doi: 10.1152/ajprenal.00288.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer S, Kokozidou M, Heiss C, et al. Chronic kidney disease aggravates arteriovenous fistula damage in rats. Kidney Int. 2010;78:1312–1321. doi: 10.1038/ki.2010.353. [DOI] [PubMed] [Google Scholar]
- 26.Liang A, Wang Y, Han G, Truong L, Cheng J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol. 2013;304:F1413–1420. doi: 10.1152/ajprenal.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Liang A, Luo J, et al. Blocking Notch in endothelial cells prevents arteriovenous fistula failure despite CKD. J Am Soc Nephrol. 2014;25:773–783. doi: 10.1681/ASN.2013050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung AK, Imrey PB, Alpers CE, et al. Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol. 2017;28:3005–3013. doi: 10.1681/ASN.2016121355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vink A, Schoneveld A, Poppen M, DPdK, Borst C, Pasterkamp G. Morphometric and immunohistochemical characterization of the intimal layer throughout the arterial system of elderly humans. Anat. 2002;200:97–103. doi: 10.1046/j.0021-8782.2001.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osika W, Dangardt F, Gronros J, et al. Increasing peripheral artery intima thickness from childhood to seniority. Thromb Vasc Biol. 2007;27:671–676. doi: 10.1161/01.ATV.0000256468.95403.6f. [DOI] [PubMed] [Google Scholar]
- 31.Holzapfel GA. Collagen in arterial walls: biomechanical aspects. In: Fratzl P, editor. Collagen: structure and mechanics. Springer US; 2008. [Google Scholar]
- 32.Lindeman JH, Ashcroft BA, Beenakker JW, et al. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc Natl Acad Sci U S A. 2010;107:862–865. doi: 10.1073/pnas.0910312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010;21:1637–1640. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- 34.Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Heart. 2010;96:817–823. doi: 10.1136/hrt.2009.184879. [DOI] [PubMed] [Google Scholar]
- 35.Tomiyama H, Tanaka H, Hashimoto H, et al. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis. 2010;212:345–350. doi: 10.1016/j.atherosclerosis.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Wang MC, Wu AB, Cheng MF, Chen JY, Ho CS, Tsai WC. Association of arterial stiffness indexes, determined from digital volume pulse measurement and cardiovascular risk factors in chronic kidney disease. Am J Hypertens. 2011;24:544–549. doi: 10.1038/ajh.2010.266. [DOI] [PubMed] [Google Scholar]
- 37.Shiu YT, Litovsky SH, Cheung AK, et al. Association between preoperative venous medial collagen fiber configuration and arteriovenous fistula development. American Society of Nephrology Kidney Week; 2016; Chicago, IL. 2016. [Google Scholar]