Introduction
It may be hard to argue against the thesis that mechanical ventilation represents one of the most important treatments ever introduced in the care of patients with respiratory failure. Similarly to other supportive measures adopted in intensive care (e.g., renal replacement therapy, extracorporeal supports, etc.), mechanical ventilation—replacing partially or totally the insufficient respiratory muscles—“buys time” until a causal therapy (if available) becomes effective, the lung heals and the patient recuperates respiratory and breathing autonomy. In the 70s, even in the presence of severely impaired lung mechanics, clinicians strived for normal blood O2 and CO2 tension through high-volume and high-pressure ventilation and gross barotrauma (e.g., pneumothorax, pneumomediastinum, gas embolism, subcutaneous emphysema) was as frequent as to consider preemptive positioning of chest drainage in mechanically ventilated patients (1-3). In the last 50 years, a remarkable progress has been made in the understanding of ARDS pathophysiology (4), the complicated relation between the failing lung and mechanical ventilation has become progressively clearer and the complications related to this powerful treatment tool have been grouped under the name “ventilator-induced lung injury” (VILI), introduced in 1993 (5). This generic umbrella-term gathers a wide spectrum of histopathological signs, ranging from mild perivascular edema to overt pneumothorax. Despite decades of dedicated research, though, the exact noxious pathways of mechanical ventilation are still not fully understood and, importantly, the optimal measures to prevent VILI in the most endangered patients [i.e., moderate-severe and severe ARDS patients (6)] have not been determined yet. Herein we will discuss the gist, the potentials and the limits of the youngest concept introduced in the field of mechanical ventilation and VILI prevention: the mechanical power (7).
VILI, ARDS and mechanical ventilation
Actually, we believe that the acronym VILI may express two similar but not identical concepts: ventilation-induced lung injury and ventilator-induced lung injury. The first definition is more generic and accepts the possibility that lung damage occurs even during spontaneous breathing in particular conditions (e.g., ARDS), while the latter stresses the damaging potential of mechanical ventilation and, implicitly, the importance of the ventilatory settings in harm prevention. However, when discussing VILI, we must bear in mind that—although potentially harmful—mechanical ventilation is primarily a lifesaving treatment when spontaneous breathing becomes insufficient or unsustainable. Indeed, as a matter of fact, not establishing mechanical ventilation in moderate-severe and severe ARDS would probably lead to a mortality rate of these patients of approximately 100%. Taking this concept to an extreme, therefore, we could say that severe ARDS would not exist without mechanical ventilation. Differently from some decades ago, the aim of mechanical ventilation in ARDS patients is to provide a gas exchange compatible with life—accepting a certain degree of hypoxemia and/or hypercapnia—through the least damaging ventilation possible. However, despite the most careful level of care, VILI can always occur when ventilating ARDS patients and—differently from the experimental setting, in which VILI can be induced in previously healthy lungs—we are still not able to quantify the mortality directly attributable to mechanical ventilation in the clinical setting, where this treatment is established in already injured lungs. ARDS and VILI are strictly bond together by mechanical ventilation and, once they are established, they merge into a virtually unique, complicated form of lung damage, in which their relative contribution is no longer distinguishable.
As long as we will remain unable to discriminate between ARDS, VILI and their respective attributable mortality, the only reasonable action that we can promote is to minimize the hazard related to mechanical ventilation. In the last decades, many efforts have been made to better define VILI, to understand which component—if any—of the mechanical ventilation prevails in VILI generation and what is the best ventilatory strategy for damage prevention. Unfortunately, we are still searching for the answer to these questions.
Furthermore, we consider the concept of VILI somewhat limited, as it neglects other extremely relevant consequences of mechanical ventilation, such as the hemodynamic effects of high intrathoracic volumes and pressures, which can heavily affect survival in spite of uninjured lungs.
The network of mechanical ventilation
As a matter of fact, mechanical ventilation is a rather complicated network composed by a precise number of elements, set by the physician to ensure an adequate gas exchange with the lowest risk of lung damage: pressures (e.g., end-expiratory, plateau, driving, peak, etc.), volumes (e.g., PEEP volume, tidal volume, etc.), flow, airways and tissue resistances as well as respiratory frequency.
Considering the complexity of the system, it is hard to think that, in clinical practice, either of these elements may be sensitive and specific enough as to accurately predict the safety of mechanical ventilation. Conversely, if we could rely on a single measure, comprehensive—and not excluding any—of all the determinants of mechanical ventilation mentioned above, we could gain great advantage when tailoring this treatment on the single patient. However, efforts to identify a “magic number” that could possibly and univocally set a threshold between safe and unsafe ventilation were unsuccessful.
Schematically, we could represent mechanical ventilation as an irregular solid with six components—namely tidal volume, respiratory rate, PEEP, driving pressure, resistances and flow (Figure 1). As shown in this simplified model, each component of the solid has a different area, exemplifying a different relative weight of the components of mechanical ventilation in determining its hazardous potential. Although it is clear that each face of the solid is important in determining its shape and volume, none of them is per se sufficient to describe the solid as a whole. Similarly, although each of the abovementioned respiratory variables contributes to the shape and the impact of mechanical ventilation, none of them is singularly enough to adequately describe it as a whole. Conversely, notwithstanding the role of each component, the combination of all them together would yield the complete picture (i.e., the volume of the solid). Similarly, combining the different components of mechanical ventilation into a comprehensive variable may provide a comprehensive description of its framework. This is, we believe, the role of the mechanical power.
Figure 1.
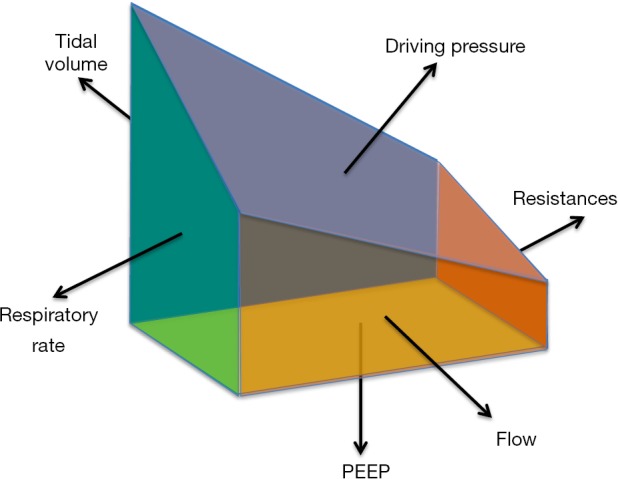
Irregular solid representing the framework of mechanical ventilation.
The mechanical power
When we introduced the concept of mechanical power, we did not invent anything new (7). Indeed, the mechanical power is the mathematical description of the mechanical energy [i.e., (change in volume) × (absolute pressure)] delivered to the respiratory system over time. Accordingly, the mechanical energy equation is obtained by multiplying each component of the equation of motion by the tidal volume. This is the energy delivered to the lung within each respiratory cycle and, multiplied by the respiratory rate, it is equal to the mechanical power applied to the respiratory system (7).
where 0.098 is the conversion factor from L × cmH2O to joule, ∆V is the tidal volume, ELRS is the elastance of the respiratory system, I:E is the inspiratory-to-expiratory time ratio, RAW is the airway resistance and PEEP is the airway pressure at end-expiration.
Among the determinants of the mechanical power, tidal volume (8), driving pressure (9) and PEEP (10) showed a relevant clinical impact in the care of ARDS patients. This evidence, most likely combined with observation-based common sense, has certainly influenced clinical practice leading to the adoption of low-volume and low-pressure mechanical ventilation delivered on a moderate PEEP level (11). The role of the other respiratory variables, however, should not be forgotten. Indeed, in two series of experiments on piglets, Protti, Cressoni and coworkers showed the crucial role of respiratory rate in determining whether a strain of 2 [i.e., tidal volume =2× functional residual capacity (FRC); roughly equivalent to a tidal volume of 3,000 mL in a 70 kg man] was lethal (15 bpm) or not (3–6 bpm) (12,13). Similarly, the same tidal volume contributed differently to VILI depending on the flow (i.e., the rate at which the tidal volume is delivered during inspiration) (14), further underlining the importance of taking the whole package of ventilation-related variables into account when reasoning on VILI.
Unsolved issues of the mechanical power
Normalization
The appropriateness of whatever ventilatory setting can be judged only taking into account the object of mechanical ventilation: the respiratory system within each individual patient. Indeed, ideally, the clinician setting the ventilator follows the individualized evaluation of the specific characteristics of each patient’s respiratory system and general status. This line of thinking should be applied whenever an extensive property (e.g., volume, energy) is of concern or whenever an intensive property (e.g., pressure) is discussed in relation to a specific system. Indeed, dimensions matter as, for example, the mechanical energy required to ventilate a healthy elephant is certainly much bigger than the one necessary to ventilate a mouse with ARDS (for the same mechanical power, the lung volume determines the intensity: the power transferred per unit of area). We are still struggling to understand which is the best way to normalize the mechanical power. Regarding subjects with healthy lungs, it might be sufficient to normalize the mechanical power to the lung volume obtained with different formulas based on the dimension of the body. Unfortunately, although this approximation can be accepted in healthy subjects, it does not at all seem adequate in the setting of ARDS, where the ventilatable lung varies according to the severity of the disease (15). In this case, therefore, a more accurate measurement of the FRC and the normalization of the mechanical power by FRC appear to be the most reasonable approach to the problem of relating the energy administered to the respiratory system to the final subject receiving the possibly noxious energy [i.e., the baby lung (16)].
Relative weight of its components
Each determinant of the mechanical power contributes with a different “specific weight” to the computation of the mechanical power. Indeed, mathematically, the mechanical power will increase with the exponent of 2 of the tidal volume, the exponent of 1.4 of the respiratory frequency and the exponent of 1 of PEEP (7). Even if this allows speculating on the relative importance of the different components in VILI formation, the real effect in terms of lung damage and mortality is yet to be determined. Unfortunately, the pathway leading to injury is probably very far from being as straightforward as to depend on the mere increase in mechanical power or its single contributing variables. Most likely, we can figure a scenario in which each variable is not, per se, good or evil, but can range from too-little to too-high, depending not only on its absolute value normalized for the patient’s lung, but also on the other ventilatory variables used. This applies also to the mechanical power itself.
Looking for a threshold
The mechanical power is a continuous variable that describes the energy given to the respiratory system and the lung over time. Similarly, VILI is a continuous entity characterized by a wide range of manifestations, in which identifying a starting point is impossible in the clinical setting. Although higher levels of mechanical power correlate with more severe lung damage, we still do not know under which conditions the injury starts. It would most likely be of high clinical relevance to find a value of mechanical power below which the mechanical ventilation could be considered safe (safety threshold) or above which the mechanical ventilation setting should be considered hazardous (hazard threshold). In recent experiments, we showed that a lung mechanical power threshold of about 12–13 J/min and respiratory mechanical power of 25 J/min yielded to more significant (and potentially lethal) lung damage than a lower mechanical power (13). The most relevant problems in defining a threshold are related to the duration and the intensity of the mechanical power.
The mechanical power in the respiratory cycle
So far, the mechanical power has been expressed as an average value of energy delivered to the respiratory system in one minute. However, although this concept allows summarizing the different variables in a unifying concept, to understand the pathway through which the mechanical power may hurt the lung parenchyma requires the understanding of its distribution throughout the respiratory cycle. For what the inspiratory phase is concerned, there is no doubt that the intensity of power delivered at the beginning of inspiration is far greater in pressure-controlled that in volume-controlled ventilation. In the first case, in a very short time, all the pressure and volume are delivered, while they are more evenly distributed through the inspiratory phase in volume-controlled ventilation. Possibly more relevant, however, is the understanding of the mechanical power impact during the expiratory phase. Indeed, at the end of expiration, the amount of the potential energy accumulated is equal to the total energy delivered during the inspiratory phase minus the fraction of energy, which has been spent to overcome the surface forces and the airway and tissue resistances. The potential energy accumulated into the lung must be released during expiration. Only two pathways do exist: the airways and the lung parenchyma and the atmosphere. A faster and more uncontrolled expiration induces a greater energy fraction dissipated into the lung. A more controlled expiration, leads to a greater fraction of energy dissipated into the atmosphere than into the lung (17,18). Therefore, there is a wide field of research to exactly define the relationship between mechanical power distribution and VILI. In this area, it is very well possible that the expiratory phase is more relevant for VILI formation than previously thought.
Is the mechanical power the final word on VILI?
Otherwise stated: is the mechanical power really better? This, we believe, may be a misleading question, as the mechanical energy delivered to the lung is nothing but the product of the determinants of the mechanical ventilation: volume and pressure. Therefore, the rationale behind both low tidal volume (8) and low driving pressure (9) ventilation and the reasons for the open lung approach (i.e., very high PEEP combined with high pressure recruitments maneuvers) failure (10,19) are synthetically (and “physically”) combined in the mechanical energy. Considering the absolute pressure instead of the delta pressure, the energy has the advantage of including PEEP—i.e., the pressure the ventilator must deliver at each breath before starting inflating the respiratory system—into the equation (20,21) (Figure 2). Eventually, the transformation of energy into power introduces the importance of the frequency at which a given energy is applied (i.e., the respiratory rate). The unifying nature of the mechanical power, therefore, frustrates any attempt of comparison: it would be roughly equivalent to comparing a book to one of its chapters. Notwithstanding the importance of each chapter, the book will always provide a more complete view of the story.
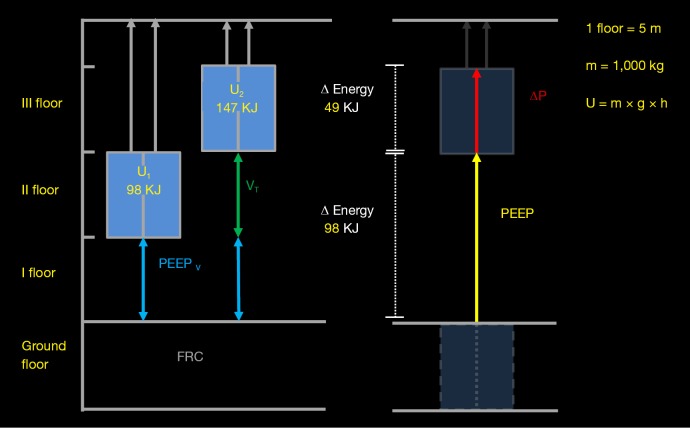
Figure 2.
This diagram explains why PEEP is part of the absolute pressure (and energy) delivered to the lung at each tidal cycle. In the example, we simplistically consider a lift (blue box) moving from the second to the third floor of a building, symbolizing the respiratory system expanding from a PEEP volume to an end-inspiratory volume (PEEP volume + tidal volume). The ground floor represents the FRC. Assuming a lift mass (m) of 1,000 kg, a gravitational acceleration (g) of 9.8 m/s2, and a height (h) of 5 m for each floor, when the lift is at the second floor, its potential energy (U = m × g × h) is equal to 98 KJ. This level of energy corresponds to the energy due to PEEP (PEEPE = PEEP × PEEP volume). In order to reach the third floor, the cords hanging the lift must apply an even greater energy (147 KJ). The delta energy (49 KJ) between the second and the third floor represents the additional energy that must be given in order to elevate the lift of one floor. Similarly, to achieve an end-inspiratory volume greater than PEEP volume from PEEP, a pressure equal to PEEP + driving pressure has to be added. If, for any reason, a lower energy is given to the lift or a lower pressure is given to the lung, the first one will go down, while the second will deflate instead of inflating. PEEP, positive end-expiratory pressure; PEEPV, PEEP volume; VT, tidal volume; ΔP, driving pressure.
Although we are not sure that the mechanical power will be the “final word” on VILI, we strongly believe that it represents a convenient and more global viewpoint over the extremely complicated interaction between the lung and the mechanical ventilator.
Conclusions
The mechanical power is a physiologically sound, comprehensive concept that greatly simplifies the way we look at mechanical ventilation. It has the advantage of considering the “big picture”, frequently lost when assessing individual respiratory variables such as driving pressure, tidal volume or PEEP. We believe that the mechanical power could become a valuable ally for tailoring mechanical ventilation—particularly in ARDS patients—especially when addressing crucial decisions, such as the use of extracorporeal support.
Bearing yet in mind the relevant still unsolved obstacles to the use of the mechanical power, we believe that this young concept will provide significant benefit to clinical practice, which, unlike RCTs, does not take place in predefined conditions.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kumar A, Pontoppidan H, Falke KJ, et al. Pulmonary barotrauma during mechanical ventilation. Crit Care Med 1973;1:181-6. 10.1097/00003246-197307000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman JE, Dunbar BS, Klingenmaier CH. Management of subcutaneous emphysema, pneumomediastinum, and pneumothorax during respirator therapy. Crit Care Med 1975;3:69-73. 10.1097/00003246-197503000-00004 [DOI] [PubMed] [Google Scholar]
- 3.Hayes DF, Lucas CE. Bilateral tube thoracostomy to preclude fatal tension pneumothorax in patients with acute respiratory insufficiency. Am Surg 1976;42:330-1. [PubMed] [Google Scholar]
- 4.Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med 2000;342:1360-1. 10.1056/NEJM200005043421808 [DOI] [PubMed] [Google Scholar]
- 5.Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med 1993;21:131-43. 10.1097/00003246-199301000-00024 [DOI] [PubMed] [Google Scholar]
- 6.Maiolo G, Collino F, Vasques F, et al. Reclassifying Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2018;197:1586-95. 10.1164/rccm.201709-1804OC [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. 10.1007/s00134-016-4505-2 [DOI] [PubMed] [Google Scholar]
- 8.ARDS Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 9.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- 10.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I , Cavalcanti AB, Suzumura EA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 12.Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 2011;183:1354-62. 10.1164/rccm.201010-1757OC [DOI] [PubMed] [Google Scholar]
- 13.Cressoni M, Gotti M, Chiurazzi C, et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016;124:1100-8. 10.1097/ALN.0000000000001056 [DOI] [PubMed] [Google Scholar]
- 14.Protti A, Maraffi T, Milesi M, et al. Role of Strain Rate in the Pathogenesis of Ventilator-Induced Lung Edema. Crit Care Med 2016;44:e838-45. 10.1097/CCM.0000000000001718 [DOI] [PubMed] [Google Scholar]
- 15.Cressoni M, Chiumello D, Carlesso E, et al. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology 2014;121:572-81. 10.1097/ALN.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Pesenti A. The concept of "baby lung". Intensive Care Med 2005;31:776-84. 10.1007/s00134-005-2627-z [DOI] [PubMed] [Google Scholar]
- 17.Schumann S, Goebel U, Haberstroh J, et al. Determination of respiratory system mechanics during inspiration and expiration by FLow-controlled EXpiration (FLEX): a pilot study in anesthetized pigs. Minerva Anestesiol 2014;80:19-28. [PubMed] [Google Scholar]
- 18.Gattinoni L, Marini JJ, Collino F, et al. The future of mechanical ventilation: lessons from the present and the past. Crit Care 2017;21:183. 10.1186/s13054-017-1750-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipulli F, Vasques F, Duscio E, et al. Atelectrauma or volutrauma: the dilemma. J Thorac Dis 2018;10:1258-64. 10.21037/jtd.2018.02.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonetti T, Vasques F, Rapetti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017;5:286. 10.21037/atm.2017.07.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, Collino F, Maiolo G, et al. Positive end-expiratory pressure: how to set it at the individual level. Ann Transl Med 2017;5:288. 10.21037/atm.2017.06.64 [DOI] [PMC free article] [PubMed] [Google Scholar]