Abstract
Despite being a promising idea that combines several variables related to ventilator-induced lung injury (VILI), the concept of mechanical power (MP) carries a number of limitations, leaves several open questions, lacks proper modelling of positive end-expiratory pressure (PEEP) effects and, more importantly, does not respect the amount of lung tissue subjected to MP. First, the assessment of MP as a measure for development of VILI would have the highest relevance when volume displacement and related pressure changes are measured directly within the lung. Thus, ideally the relationship between MP delivered to the total respiratory system, and that delivered to lung tissue is discerned. Second, MP as defined today relates to the inspiratory phase only, and it is very possible that the expiratory phase will also play a role. Third, the calculation of MP during spontaneous breathing is challenging as airway pressure, flow and esophageal pressure are affected counter-directionally and simultaneously overlapping by the action of the ventilator and the respiratory muscles. Fourth, in its current form, MP is modelled with a positive linear relationship with PEEP, which is based on incorrect mathematical modelling to integrate the role of PEEP into MP. Fifth, the present equation used to calculate MP is qualitatively in disagreement with clinical data on VILI. The reduction of MP to its elastic part, might not only result in a higher association with VILI, but also amplifies an indirect U-shaped relationship with PEEP.
Keywords: Mechanical power (MP), ventilator-induced lung injury (VILI), mechanical ventilation, driving pressure, tidal volume
Introduction
With each breath delivered by the mechanical ventilator a certain amount of energy is transferred to the respiratory system of the patient. This energy is mainly spent to overcome resistance of the airways and to expand the lung parenchyma by increasing the thoracic volume (1-4). A fraction of this energy, however, acts directly on the lung skeleton or extracellular matrix, as such deforming the epithelial and endothelial cells anchored to it (2). Lung tissue ‘conserves’ small amounts of energy with each breath cycle, as the elastic recoil of the lung returns less energy during expiration than that absorbed during inspiration (1-4). Thus, mechanical ventilation is associated with substantial dissipation of energy, probably resulting in ‘heat’ or inflammation, potentially leading to injury of lung tissue, a phenomenon frequently referred to as ventilator-induced lung injury (VILI).
The original concept of VILI took into account only the volume and pressure generated by the ventilator. The likely most effective way to protect the lungs of patients is to avoid volutrauma by using low tidal volumes (5). Pivotal randomized controlled trials (RCTs) performed almost 20 year ago showed that mechanical ventilation with low tidal volumes improves survival of ARDS patients (6,7), a finding that was convincingly confirmed in one meta-analysis (5). Another way to protect the lungs is to prevent atelectrauma by using higher levels of positive end-expiratory pressure (PEEP) (8). Three pivotal RCTs showed no benefit of higher levels of PEEP (9-11), but the results of an individual patient data meta-analysis suggested that in moderate and severe ARDS, mortality is reduced when higher levels of PEEP are used (12). In contrast, a recent RCT showed that in patients with moderate to severe ARDS, the use of lung recruitment maneuvers and titrated higher levels of PEEP were associated with higher mortality, increased risk of barotrauma and longer duration of mechanical ventilation compared to lower levels of PEEP (13).
It has been hypothesized that the extent of VILI depends on the amount of energy transferred (1,2), and tidal volume (VT), plateau pressure (Pplat), respiratory rate (RR) and air flow all relate to the amount of energy generated by the mechanical ventilator (2). The amount of energy per unit of time, expressed in Joules per minute (J/min), is often referred to as the ‘mechanical power’ (MP) (2-4,14,15), which can be calculated through a derived ‘power equation’, increasing its applicability in clinical practice (15). Recently, it was shown that MP is associated with worse outcomes in critically ill patients receiving mechanical ventilation for more than 48 hours (16).
Despite being a promising idea that combines several variables related to VILI, the concept of MP carries a number of limitations, leaves several open questions, lacks proper modelling of effects of PEEP and, more importantly, does not respect the amount of lung tissue subjected to MP.
Measurements to derive MP
The assessment of MP as a measure for development of VILI would have the highest relevance when volume displacement and related pressure changes are measured directly at the lung parenchyma. Thus, ideally the relationship between MP delivered to the total respiratory system, and that delivered to lung tissue is discerned (17). Measurement of pressures in the pleural space, to allow determining the trans-pulmonary pressure, is most often not feasible in daily practice. Esophageal pressure is an accepted surrogate for pleural pressure, allowing lung elastance and lung resistance to be estimated at the bedside. Using esophageal pressure to calculate MP showed that MP higher than 12 J/min resulted in VILI derived in healthy pigs (2), although this study has been criticized for the fact that the extent of VILI was only assessed by computer tomography-based identification of edema (18).
Minimally invasive measurement of esophageal pressure in the clinical environment lacks standardization, is prone to instability and additional perturbations of measured pressure, e.g., caused by activity of esophageal muscles. In addition, esophageal pressure measurement is not regularly performed and may not always be representative in certain scenarios, e.g., during open abdominal or thoracic surgery (19).
Relation between VILI and ventilator settings
In order to use MP as a tool for guidance of ventilation and before the determination of the threshold above which it results in VILI, it is paramount that MP reflects the relationship between VILI and the ventilator settings related to the two mechanisms involved in VILI: volutrauma and atelectrauma. Lung tissue has viscoelastic properties and thus dynamic stress is related to strain and strain rate while during static stress the lung is subject to creep (20). Therefore, stress is mainly determined by PEEP (static stress) as well as VT and RR (dynamic stress) (21).
The relationship between VT and VILI has been studied before (6), and the results of one ARDS Network study, called the ‘ARMA trial’ (7), led to the most influential guideline changes in the care for ARDS patients. A reduction of VT to values below or equal 6 to 8 mL/kg predicted body weight (PBW) reduces mortality by 22% to 50%. Compared to 6 mL/kg PBW, a further reduction of VT to 4 mL/kg PBW improved morphological markers and reduced pulmonary cytokine concentrations in patients with ARDS (22). Thus, it may be assumed that there is a correlation between VT and VILI of linear or even exponential relationship.
In viscoelastic material, stress is associated with static strain and with the strain rate, the change of strain per unit of time. RR, thereto, directly and proportionally defines strain rate and dynamic stress, the important determinant of atelectrauma (3,23). Atelectrauma may be avoided by increasing PEEP to moderate levels, usually levels higher than 5 cmH2O (24). As mentioned above, one meta-analysis that used the data of the three large RCTs of PEEP in patients with ARDS (9-11) suggested survival benefit of high PEEP (12). Recently, however, use of lung recruitment maneuvers and higher, or ‘superhigh’ PEEP, aiming at the best compliance was shown to increase mortality when compared to high PEEP (13). This suggests that an excessive increase of PEEP actually increases the risk of VILI.
Thus, there could be a U-shaped relationship between PEEP and the risk of VILI, and high PEEP aiming at preventing VILI might not be identical to superhigh PEEP aiming at the best lung compliance (23,25).
Calculation of MP
The derivation of the equation to calculate MP from ventilator settings and respiratory mechanics is commenced from the pure physical basics of a uniaxial elastic spring. Mechanical work (MW), or mechanical energy, performed by a spring is defined as the well-known integral of force (F) generated by the spring over the deformation distance x of that spring:
| [1] |
F is generally a function of x, as the spring stiffness changes with deformation length x of the spring. Note that both F(x) and x are directly connected to each other and result from the same mechanical movement. If F can be assumed as constant over x, then, and only in that case, the integral above reduces to pure multiplication of F and x.
Analogously for the three-dimensional case of the respiratory system, the equation for MW in tidal ventilation becomes (26):
| [2] |
where changes of airway pressure (ΔPaw) result from the volume change dV during tidal ventilation. Note that ΔPaw and dV are both again directly related as they have their origin in the same mechanical movement. ΔPaw during tidal ventilation is a function of respiratory system resistance R and elastance E as well as airway flow and volume V (27). Therefore, the equation for MW could be rewritten as follows:
| [3] |
On integration and simplification, assuming constant flow volume–controlled ventilation, inspiratory tidal volume VT and volume independence of R and E the equation for MW based on ventilator settings and respiratory mechanics is derived:
| [4] |
MW is the sum of the dissipated energy in the airways over the resistance (resistive MW) and the energy temporarily stored in the elastic lung tissue (elastic MW). MP is defined as MW divided by the time MW is performed in and, thus, may be derived by multiplication of MW with RR:
| [5] |
MP has the same dependencies as MW on primary ventilator parameters as well as respiratory mechanics and additionally increases with RR (14). Alike MW, MP consists of the two components: resistive MP and elastic MP. The equations for MW and MP derived above differ essentially from the equation widely used (14) by missing the term PEEP·VT. Although this term might be intuitive, especially when visualizing the pressure volume loop (Figure 1), it represents an important flaw in the current state of MP in the literature for the following reasons:
Figure 1.
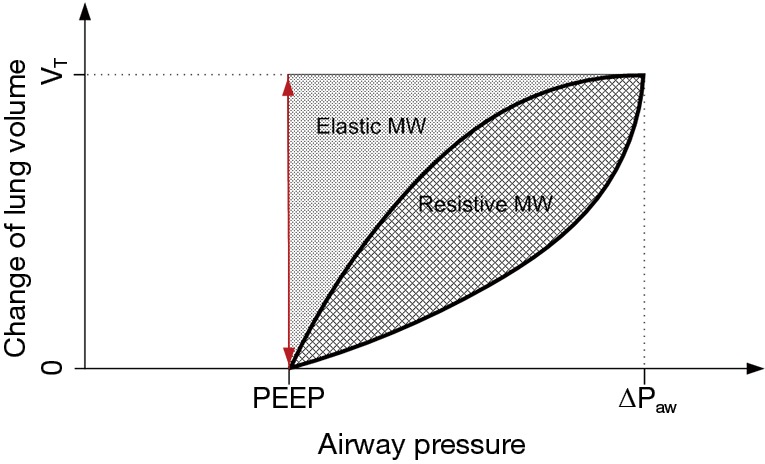
Change of lung volume during tidal ventilation with positive end-expiratory pressure (PEEP), driving pressure (∆Paw) and tidal volume (VT). elastic mechanical work (MW) and resistive mw marked with dotted and crossed pattern areas; airway pressure does not change from zero to PEEP during tidal ventilation and thus no PEEP related mechanical work is present (area is zero, indicated by the red double arrow).
Physically—there is no mechanical movement with volume displacement VT resulting in a pressure variation of PEEP. Therefore, this term does not describe a mechanical energy; one could argue that this term might state an imaginary mechanical energy, which is relevant to VILI after all—in that case, however, both would have to be associated with no other change of pressure or volume. In fact, both the pressure change of PEEP and the volume change VT are each related to other volume and pressure changes in the respiratory system.
Qualitatively—when including the term PEEP·VT into the equations for MW and MP, a linear, positive dependence between MP and PEEP is derived instead of the proposed U-shaped relationship as discussed in the previous section; MP as calculated including the PEEP·VT term, thus, may not be adequately correlate with VILI; theoretically, minimization of PEEP would result in a minimization of MP, though clinical data suggest that minimization of PEEP does not minimize VILI, especially in ARDS patients (12).
Consequently, MP in its present form does not directly include PEEP, one of the most widely discussed settings in critical care in healthy as well as acutely injured lungs.
Is resistive MP relevant for VILI?
As mentioned above, lung injury is induced during ventilation directly on the lung parenchyma by over-distension (volutrauma), by cyclic opening and closing (atelectrauma) or by a combination of both primarily by the generation of alveolar leaks (23). Both, R and E considered in the derivation of MW and MP above, are manifested in two distinct parts of the respiratory system:
The bronchial tree and the conducting airways up until the 12th generation define R and have only limited distensibility—high E (28), and;
The alveoli air flow is minimized and distensibility is maximized due to the parallel structure of the tree and, thus, E is manifested here.
Thus, the question arises, whether MP dissipated in the respiratory system resistance is related to the formation of VILI at all.
In one retrospective study, data from 8,207 critically ill patients was analyzed to elucidate the association between MP in the first 48 hours of mechanical ventilation and VILI. Clinical endpoints included in-hospital and ICU mortality, the number of ventilator-free days at day 28, length of stay in ICU and in the hospital. The results of this study showed that high MP is associated with higher mortality rates, less ventilator-free days and longer stays in the ICU and the hospital. It appeared that RR and driving pressure were the two main components associated with mortality (16).
A reduction of Eq. [5] to elastic MP = 0.5·E·VT2·RR will result in an indirect dependence between MP and PEEP in the U-shaped fashion as elastance E has a sigmoidal relationship with PEEP (29) and a positive linear relationship with RR. Given that an increase of PEEP results in recruitment and in improvement of gas exchange, RR may be reduced to minimize MP and consequently a clinically easily implementable way of managing patients might be based on gas exchange and elastic MP.
MP during expiration
MP as defined today relates to the inspiratory phase only, and it is very possible that the expiratory phase will also play a role (17). Indeed, all of the energy accumulated at end inspiration must have dissipated both into the lung structures and into the atmosphere when exhalation is complete. It is interesting and potentially important to know whether controlling expiratory flow (which decreases the fraction of energy expended into the lung) thereby helps to reduce VILI. In fact, such a phenomenon has been reported in two studies (30,31).
MP during spontaneous breathing
MP can be calculated during spontaneous breathing during CPAP, straightforwardly. However, in all assisted modes of ventilation, e.g., BIPAP, PSV or APRV, the derivation of MW and MP is challenging using established methods as discussed above as airway pressure, flow and esophageal pressure are affected counter-directionally and simultaneously overlapping by the action of the ventilator and the respiratory muscles.
Even if appropriately adjusted for resistance, flow, and chest wall elastance, any estimate of lung-delivered MP, using airway pressure or esophageal pressure during spontaneous efforts would reflect both the ventilator’s contribution and respiratory muscle activity counter-directionally and thus would not represent the total energy imparted during inflation (32). In addition, the distribution of MP throughout the lung parenchyma must be determined. It is not known whether it follows the same mal-distribution of stress and strain dictated by lung inhomogeneity (33).
Conclusions and perspective
In its current form, MP is modelled with a positive linear relationship with PEEP, which is based on incorrect mathematical modelling to integrate the role of PEEP into MP. Furthermore, the present equation used to calculate MP is qualitatively in disagreement with clinical data on VILI. The reduction of MP to its elastic part, might not only results in a higher association with VILI, but also amplifies an indirect U-shaped relationship with PEEP. It remains an open issue whether direct modelling of PEEP into the equation for MP is relevant and how the effect of PEEP can be represented in MP.
For the same MP, VILI will be worse if the amount of aerated lung is lower and pulmonary heterogeneity is higher. Therefore, the intensity, i.e., MP delivered per unit of ventilated lung tissue, is probably a more important concept in determining VILI (34). In the present form, the concept of MP does not consider the properties of the lung, and to define a safety threshold of MP for ventilation it must be first normalized either for a standard lung volume or for the amount of aerated lung tissue (17).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Serpa Neto A, Amato MBP, Schultz MJ. Dissipated Energy is a Key Mediator of VILI: Rationale for Using Low Driving Pressures. In: Vincent JL. editor. Annual Update in Intensive Care and Emergency Medicine 2016. Cham: Springer International Publishing, 2016:311-21. [Google Scholar]
- 2.Cressoni M, Gotti M, Chiurazzi C, et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016;124:1100-8. 10.1097/ALN.0000000000001056 [DOI] [PubMed] [Google Scholar]
- 3.Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 2013;41:1046-55. 10.1097/CCM.0b013e31827417a6 [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L, Carlesso E, Cadringher P, et al. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl 2003;47:15s-25s. 10.1183/09031936.03.00021303 [DOI] [PubMed] [Google Scholar]
- 5.Putensen C, Theuerkauf N, Zinserling J, et al. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 2009;151:566-76. 10.7326/0003-4819-151-8-200910200-00011 [DOI] [PubMed] [Google Scholar]
- 6.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. 10.1056/NEJM199802053380602 [DOI] [PubMed] [Google Scholar]
- 7.Acute Respiratory Distress Syndrome Network , Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 8.Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 1988;137:1159-64. 10.1164/ajrccm/137.5.1159 [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. 10.1056/NEJMoa032193 [DOI] [PubMed] [Google Scholar]
- 10.Mercat A, Richard JCM, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. 10.1001/jama.299.6.646 [DOI] [PubMed] [Google Scholar]
- 11.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. 10.1001/jama.299.6.637 [DOI] [PubMed] [Google Scholar]
- 12.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 13.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators , Cavalcanti AB, Suzumura ÉA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. 10.1007/s00134-016-4505-2 [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L, Tonetti T, Quintel M. Intensive care medicine in 2050: ventilator-induced lung injury. Intensive Care Med 2018;44:76-8. 10.1007/s00134-017-4770-8 [DOI] [PubMed] [Google Scholar]
- 16.Serpa Neto A, Deliberato R, Johnson AE, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Medicine 2018. (In Press). 10.1007/s00134-018-5375-6 [DOI] [PubMed] [Google Scholar]
- 17.Gattinoni L, Marini JJ, Collino F, et al. The future of mechanical ventilation: lessons from the present and the past. Crit Care 2017;21:183. 10.1186/s13054-017-1750-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spieth PM, Knels L, Kasper M, et al. Effects of vaporized perfluorohexane and partial liquid ventilation on regional distribution of alveolar damage in experimental lung injury. Intensive Care Med 2007;33:308-14. 10.1007/s00134-006-0428-7 [DOI] [PubMed] [Google Scholar]
- 19.Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. 10.1164/rccm.201312-2193CI [DOI] [PubMed] [Google Scholar]
- 20.Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol 2011;1:1317-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protti A, Votta E, Gattinoni L. Which is the most important strain in the pathogenesis of ventilator-induced lung injury: dynamic or static? Curr Opin Crit Care 2014;20:33-8. 10.1097/MCC.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 22.Terragni PP, Del Sorbo L, Mascia L, et al. Tidal Volume Lower than 6 ml/kg Enhances LungProtection Role of Extracorporeal Carbon Dioxide Removal. Anesthesiology 2009;111:826-35. 10.1097/ALN.0b013e3181b764d2 [DOI] [PubMed] [Google Scholar]
- 23.Hamlington KL, Bates JHT, Roy GS, et al. Alveolar leak develops by a rich-get-richer process in ventilator-induced lung injury. PLoS One 2018;13:e0193934. 10.1371/journal.pone.0193934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L, Pesenti A, Bombino M, et al. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology 1988;69:824-32. 10.1097/00000542-198812000-00005 [DOI] [PubMed] [Google Scholar]
- 25.Güldner A, Braune A, Ball L, et al. Comparative Effects of Volutrauma and Atelectrauma on Lung Inflammation in Experimental Acute Respiratory Distress Syndrome: Crit Care Med 2016;44:e854-65. 10.1097/CCM.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 1950;2:592-607. 10.1152/jappl.1950.2.11.592 [DOI] [PubMed] [Google Scholar]
- 27.Pedley TJ, Schroter RC, Sudlow MF. The prediction of pressure drop and variation of resistance within the human bronchial airways. Respir Physiol 1970;9:387-405. 10.1016/0034-5687(70)90094-0 [DOI] [PubMed] [Google Scholar]
- 28.Venegas JG, Harris RS, Simon BA. A comprehensive equation for the pulmonary pressure-volume curve. J Appl Physiol 1998;84:389-95. 10.1152/jappl.1998.84.1.389 [DOI] [PubMed] [Google Scholar]
- 29.Goebel U, Haberstroh J, Foerster K, et al. Flow-controlled expiration: a novel ventilation mode to attenuate experimental porcine lung injury. Br J Anaesth 2014;113:474-83. 10.1093/bja/aeu058 [DOI] [PubMed] [Google Scholar]
- 30.Schumann S, Goebel U, Haberstroh J, et al. Determination of respiratory system mechanics during inspiration and expiration by FLow-controlled EXpiration (FLEX): a pilot study in anesthetized pigs. Minerva Anestesiol 2014;80:19-28. [PubMed] [Google Scholar]
- 31.Protti A, Maraffi T, Milesi M, et al. Role of Strain Rate in the Pathogenesis of Ventilator-Induced Lung Edema. Crit Care Med 2016;44:e838-45. 10.1097/CCM.0000000000001718 [DOI] [PubMed] [Google Scholar]
- 32.Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2014;189:149-58. [DOI] [PubMed] [Google Scholar]
- 33.Marini JJ, Jaber S. Dynamic predictors of VILI risk: beyond the driving pressure. Intensive Care Med 2016;42:1597-600. 10.1007/s00134-016-4534-x [DOI] [PubMed] [Google Scholar]
- 34.Neto AS, Hemmes SN, Pelosi P, et al. Role of shear stress in ventilator-induced lung injury - Authors’ reply. Lancet Respir Med 2016;4:e43. 10.1016/S2213-2600(16)30161-8 [DOI] [PubMed] [Google Scholar]


