Abstract
Autism is associated with difficulties in predicting and understanding other people’s actions. There is evidence that autistic traits are distributed across a spectrum and that subclinical forms of autistic impairments can also be measured in the typical population. To investigate the association between autistic traits and motor responses to others’ actions, we quantified these traits and measured cortico-spinal excitability modulations in M1 during the observation of actions embedded in congruent, incongruent and ambiguous contexts. In keeping with previous studies, we found that actions observed in congruent contexts elicited an early facilitation of M1 responses, and actions observed in incongruent contexts, resulted in a later inhibition. Correlational analysis revealed no association between autistic traits and the facilitation for congruent contexts. However, we found a significant correlation between motor inhibition and autistic traits, specifically related to social skills and attention to details. Importantly, the influence of these factors was independent from each other, and from the observer’s gender. Thus, results suggest that individuals with higher social deficits and greater detail-processing style are more impaired in suppressing action simulation in M1 when a mismatch between kinematics and context occurs. This points to difficult integration between kinematics and contextual representations in the autistic-like brain.
Introduction
Comprehending the intentions underlying other people’s actions from observing their movements constitutes a crucial ability in humans’ everyday-life. This ability is thought to rely on the mirror neuron system (MNS), a fronto-parietal network that is active during both the observation and the execution of the same or similar action1. Furthermore, there is evidence suggesting that this ability may be anticipatory in nature, enabling for predictive coding of other’s behaviors2–5.
Socio-motor impairments, including difficulties in action prediction and comprehension, are considered to be among the core deficits associated with Autism Spectrum Disorders (ASD)6. It has been argued7 that these impairments could be explained in terms of a global dysfunction in the MNS (i.e., the broken mirror theory of ASD).
Evidence of mirror-like activity in humans comes from single-pulse transcranial magnetic stimulation (spTMS) studies measuring motor facilitation over the primary motor cortex (M1) during action observation. In these studies, the amplitude of TMS-induced motor-evoked potentials (MEPs) are taken as an index of mirror responses in fronto-parietal areas, which enhance the excitability of M1 via cortico-cortical connections8,9. To date, only a few studies have tested the broken mirror theory hypothesis using spTMS, yielding contradictory results. For instance, Theoret, Halligan10 found reduced motor facilitation in individuals with ASD as compared to neurotypical ones while observing meaningless hand movements. Enticott, Kennedy11 showed that, during the observation of transitive hand gestures, ASD individuals presented a significant reduction in M1 responses as compared to controls. However, in a subsequent study in which they used different experimental stimuli (individual vs. interactive hand actions), comparable motor responses were observed in both, ASD and neurotypical individuals12. Furthermore, neuroimaging studies also provide contradictory evidence on whether the MNS is compromised in ASD, with studies either reporting preserved13,14 or abnormal activity15,16 within this network. Thus, overall, a dysfunction in the MNS, by itself, seems to be insufficient in providing a complete account of ASD symptomatology.
To get an insight into the possible reasons for this discrepancy, it is important to note that socio-motor deficits in ASD have been traditionally studied by using non-realistic stimuli (i.e., meaningless movements, snapshots of hands detached from background). These stimuli are not good replicas of the world because they lack the context of everyday-life situations17. Indeed, body movements are not perceived in isolation but with objects, actors, and the relationships among them ‘gluing together’ into a unifying scene. In other words, this integrative aspect, which is inherent to the processing of naturalistic scenes, seems to be missing in most previous studies measuring action comprehension in ASD.
When considered in light of recent models that emphasize the integrative nature of ASD deficits, this aspect become even more critical. For instance, it has been recently suggested18 that the MNS might be modulated by top-down social signals from prefrontal regions and that a failure in this integration could explain ASD socio-motor impairments. Similarly, it has been proposed that ASD individuals could suffer from a deficit in integrating low sensory perceptual aspects and high level intentional representations during action comprehension19. Thus, according to these models, ASD impairments would arise when integrating perceptual evidence with top-down signals. In a similar vein, recent appealing proposals based on the Bayesian framework suggest that autistic observers would fail in optimally combining current sensory evidence with prior expectations and contextual information regarding the likely causes of input20,21.
Interestingly, there is evidence that individuals with subclinical ASD22 exhibit a similar profile (i.e., stronger reliance on the sensory evidence and less sensitivity to context), thus suggesting that autistic-like impairments are also measurable, albeit at lower levels of severity, in neurotypical individuals23,24.
Here, we examined whether individual differences in autistic-like traits relate to the ability of combining perceptual information about observed actions (i.e., perceptual kinematics) with prior expectations derived from naturalistic contexts. We capitalized on previous studies25–27 showing that, during an action prediction task, the observation of kinematics occurring in congruent contexts facilitated M1 excitability at early stages (~300 ms after action onset), while the observation of kinematics in incongruent contexts resulted in a later inhibition of motor facilitation (~500 ms after action onset). These results were interpreted as reflecting the interplay between simulative motor resonance responses triggered by the observation of low-level movement kinematics and top-down signals generated in areas beyond the MNS and conveying information about the intention predicted from the context.
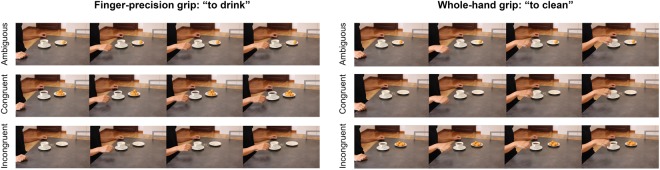
In the current study, we used the same task and similar procedures as in previous studies25–27. More specifically, we recorded MEPs from the FDI and a control muscle in 22 participants while they observed videos of everyday actions embedded in congruent, incongruent or ambiguous contexts. Videos were interrupted before action ending and participants were asked to predict how action will unfold. TMS pulse could be delivered at either 300 ms or 500 ms after action onset. Congruency between action and context was manipulated in terms of compatibility between the pattern of observed movement kinematics and the scenario in which it was embedded. For instance, one of the videos depicted a breakfast scenario (i.e., a mug full of coffee and a plate with some biscuits). If the model grasped the mug by its handle with a precision grip, this condition was coded as congruent. However, if the mug was grasped using a whole-hand grip from the top, this condition was coded as incongruent, given that this type of grasping prevented the model from drinking in a context were the highly expected action was “to drink”. After showing the video, two possible descriptors (i.e., to drink and to clean) were presented on the screen and participants had to select, given contextual and kinematics information, which was the actor’s more likely intention. Importantly, we also included ambiguous contexts (i.e., a mug half full of coffee) in which both types of actions and associated grasping movements were equally plausible (see Fig. 1 and Videos 1–3 for examples of the stimuli used in the study).
Figure 1.
Examples of stimuli and conditions. Depending on the action, the reach-to-grasp movement kinematics was different in terms of precision vs. whole-hand grips. In addition, the actions could be performed in three different contexts: congruent, incongruent, and ambiguous.
We reasoned that this task suited well for testing whether the amount of autistic-like traits, as measured by the Autistic Quotient28, was associated with different modulation of motor responses according to the congruency between the observed kinematics and the context. Based on the “combinatorial models” suggesting that the integration between perceptual signals and prior or contextual knowledge is compromised in ASD, we hypothesized that during action recognition the individuals who exhibit higher levels of autistic-like traits would show impairments in effectively combining the sensory evidence conveyed by the perceptual kinematics with the overarching action goal representation estimated from the naturalistic context. More specifically, we expected that the high prevalence of autistic traits would be related to a reduction in the facilitatory and inhibitory effects for actions embedded, respectively, in congruent and incongruent contexts.
Results
Behavioral Results
Figure 2 shows participant’s d values for each context and time-point. The RM-ANOVA performed on these values yielded a significant main effect of context (F2, 42 = 78.88, p < 0.0001, = 0.78). Non-significant main effect of time (p = 0.88) or interactions between time and context were observed (p = 0.43). Post-hoc comparisons (MSE = 0.40, df = 42) showed that, across the two time-windows, kinematics embedded in congruent contexts (M = 1.32, SD = 0.32) was better recognized as compared to that observed in ambiguous (M = 0.50, SD = 0.45, p = 0.0001) or incongruent ones (M = −0.37, SD = 0.83, p = 0.0001). In addition, kinematics observed in ambiguous contexts was better recognized than that observed in incongruent ones (p = 0.0001).
Figure 2.
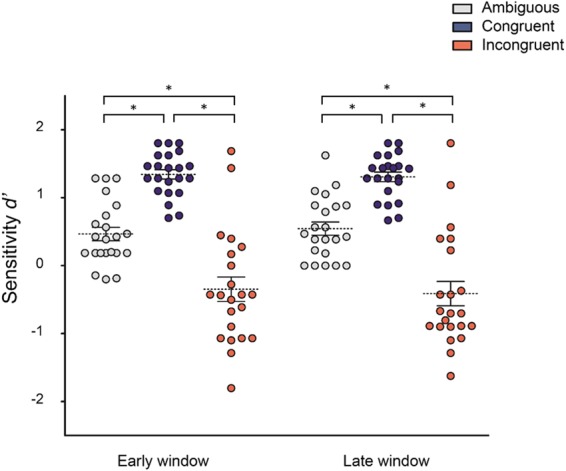
Behavioural results. Participants’ performance in predicting the course of the observed actions (expressed as d’) during the 3 action observation conditions (Congruent, Incongruent, and Ambiguous) at the 2 time-windows (early, late). Data points represent individual participants. Asterisks indicate significant comparison (p < 0.05). Error bars represent SEM and dotted lines MEAN.
MEP Results
The raw mean amplitudes of MEPs recorded from the FDI and the ECR muscles in the different conditions are reported in Table 1.
Table 1.
Mean raw MEP amplitudes for each experimental condition.
| Ambiguous | Congruent | Incongruent | ||||
|---|---|---|---|---|---|---|
| FDI | ECR | FDI | ECR | FDI | ECR | |
| 300 ms | 0.959 ± 0.148 | 0.511 ± 0.054 | 1.028 ± 0.159 | 0.468 ± 0.044 | 0.931 ± 0.141 | 0.496 ± 0.058 |
| 500 ms | 0.839 ± 0.112 | 0.449 ± 0.049 | 0.962 ± 0.158 | 0.472 ± 0.048 | 0.740 ± 0.107 | 0.457 ± 0.048 |
Values corresponding to the raw amplitudes (mean ± SEM) of MEPs recorded from the FDI and the ECR muscles in the 3 observation conditions (Congruent, Incongruent, and Ambiguous) for the time-points of interest (300 ms and 500 ms).
The dependent-sample t-test (two-tailed) performed on the log-transformed amplitudes of MEPs recorded from the FDI and the ECR baseline blocks revealed no main effect of muscle (t21 = 1.68, p = 0.10), suggesting that MEPs recorded from both muscles were comparable. Thus, for each muscle, these baseline values were subtracted from the log-transformed MEPs during the action observation conditions to derive normalized index of motor facilitation.
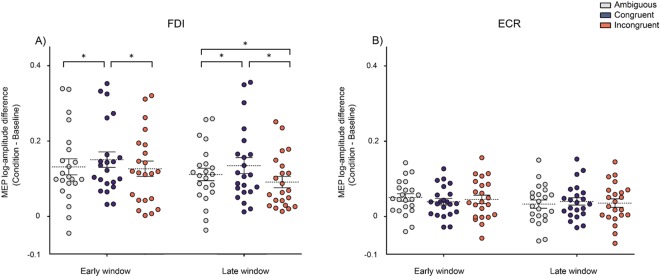
To test how the intensity of motor facilitation of the two muscles was modulated by time and context, we entered normalized MEPs amplitudes into a 2 × 2 × 3 (muscle × time × context) RM-ANOVA. A significant intercept (F1,21 = 65.83, p < 0.0001, = 0.75) was found, ensuring that the overall motor facilitation index was different from 0. The omnibus RM-ANOVA revealed a main effect of muscle (F1, 21 = 12.86, p = 0.001, = 0.37), indicating that, overall, the FDI showed a greater activation as compared to the ECR muscle throughout the experiment. A main effect of context (F2, 42 = 3.79, p = 0.03, = 0.15) was also found, showing that the observation of actions in congruent contexts elicited a greater cortico-spinal excitability (CSE) facilitation as compared to those observed in incongruent ones. Finally, a main effect of time was observed (F1, 21 = 12.73, p = 0.001, = 0.37), indicating that CSE facilitation was greater in the early time window as compared to the later one. Furthermore, we found a significant muscle × time (F1, 21 = 8.05, p = 0.009, = 0.27), and muscle × context (F2, 42 = 10.21, p = 0.0002, = 0.32) interactions, which were further qualified by a significant 3-way interaction of all variables (F2, 42 = 4.07, p = 0.024, = 0.16).
Post-hoc tests (MSE = 0.0005, df = 21) on the muscle × time interaction indicated that, despite the fact that CSE was greater for the FDI as compared to the ECR in both time windows (all ps < 0.001), both muscles showed increasing levels of CSE in the earlier time window and compared to the later one. Crucially, the post-hoc tests (MSE = 0.00053, df = 42) on the muscle × context, indicated that the FDI muscle (all ps < 0.004), but not the ECR muscle (all ps > 0.81), was modulated by context. Finally, post-hoc tests (MSE = 0.00024, df = 42) performed on the 3-way interaction showed that, in the earlier time-window (300 ms), motor facilitation for the FDI was enhanced for actions observed within a congruent context (M = 0.144, SD = 0.1) as compared to those observed in ambiguous (M = 0.131, SD = 0.09, p = 0.006) and incongruent ones (M = 0.126, SD = 0.09, p = 0.001), which in turn did not differ (p = 0.48). No modulations were observed for the ECR (all ps > 0.08).
In the later time-window (500 ms) after action onset, the facilitation effect for the FDI was still present (Congruent > Ambiguous, p = 0.0008), together with an inhibition of motor response, with lower normalized MEP amplitudes for actions observed within an incongruent context (M = 0.087, SD = 0.07) as compared to those observed within the congruent (M = 0.129, SD = 0.1; p = 0.0001) and ambiguous (M = 0.111, SD = 0.07; p = 0.0001) ones. No modulations were again observed for the ECR (all ps > 0.40). Please see Fig. 3.
Figure 3.
MEPs results. Amplitudes of MEPs recorded from the FDI (a) and ECR (b) muscles during the 3 action observation conditions (Congruent, Incongruent, and Ambiguous) at the 2 time-windows (early, late). Data points represent individual participants. Asterisks indicate significant comparison (p < 0.05). Error bars represent SEM and dotted lines MEAN.
Correlation Results
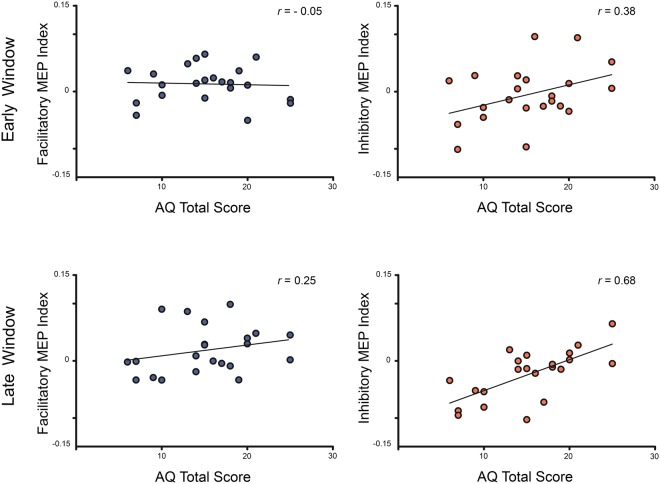
We ran a Pearson’s correlation analysis to examine whether individual differences in ASD traits were associated with the facilitatory and/or inhibitory indexes. Please see Table 2 where the mean, standard deviation, and range for AQ scores are shown. No correlations were observed for the facilitatory index (Early time-window, r = −0.05, p = 0.82; Late time-window, r = 0.25; p = 0.26). However, the inhibitory index in the later time-window (500 ms) positively correlated with the AQ total score (r = 0.679; p = 0.001), showing that the higher the level of autistic traits the higher the level of motor responses for actions observed in incongruent contexts as compared to ambiguous ones. A post hoc power analysis conducted with the G*Power software29 using as input the results of the correlation between the inhibitory MEP index and the AQ in the late time window, showed that we reached a power of 0.95 with 22 participants. These results indicate that individuals exhibiting higher levels of autistic traits showed an impairment in inhibiting their motor responses to the observed kinematics when the context pointed to an incongruent overarching goal. In addition, no correlations were observed in the early time-window (r = 0.389; p = 0.07). See Fig. 4.
Table 2.
Mean (SD) scores and range for the total AQ and AQ subscales.
| Mean | SD | Range | |
|---|---|---|---|
| AQ Total | 15.18 | 5.45 | 6–25 |
| Social skills | 2.09 | 1.50 | 0–7 |
| Attention switching | 4 | 2.02 | 1–8 |
| Attention to detail | 4.77 | 2.22 | 2–10 |
| Communication | 1.77 | 1.71 | 0–7 |
| Imagination | 2.27 | 1.48 | 0–6 |
Figure 4.
Pearson correlation results. Scatter plots illustrating the relationship between Facilitatory (Congruent – Ambiguous) and Inhibitory (Incongruent – Ambiguous) MEP indexes and the AQ total scores at the 2 time-windows (early, late).
Finally, no correlations were observed between the AQ total score and the d prime indexes (all ps > 0.11).
Multiple Linear Regression Model
To identify the relative importance of factors contributing to the observed association between the autistic traits and the contextual modulation of motor responses, we used a multiple linear regression model with the 5 AQ subscales scores and gender as predictors and the inhibitory index as the dependent variable. The model explained a significant proportion of motor inhibition variability (adjusted R2 = 61%; F = 6.48; p = 0.002). The factors Social skill (β = 0.63, p = 0.002), Gender (β = 0.51, p = 0.008) and Attention to detail (β = 0.40, p = 0.01) showed significant regression coefficients. All other factors remained non-significant (all ps > 0.11). See Table 3 for further details. Overall, these results suggest that individuals with higher social skill deficits and greater detail-focused processing style are more impaired in inhibiting their motor responses when actions are observed in incongruent contexts. Furthermore, this impairment is greater in men as compared to women.
Table 3.
Multiple regression analysis results.
| Coefficients | B | Std. Error | Beta | t | p | Collinearity Statistics | |
|---|---|---|---|---|---|---|---|
| Tolerance | VIF | ||||||
| Intercept | −0.137 | 0.022 | −6.207 | <0.001* | |||
| Gender | 0.045 | 0.015 | 0.519 | 3.046 | 0.008 * | 0.640 | 1.564 |
| Social skills | 0.018 | 0.005 | 0.633 | 3.764 | 0.002 * | 0.656 | 1.524 |
| Attention switching | −0.006 | 0.004 | −0.298 | −1.681 | 0.113 | 0.589 | 1.697 |
| Attention to detail | 0.008 | 0.003 | 0.401 | 2.801 | 0.013 * | 0.907 | 1.103 |
| Communication | 0.003 | 0.004 | 0.118 | 0.731 | 0.476 | 0.711 | 1.406 |
| Imagination | −0.002 | 0.005 | −0.083 | −0.517 | 0.612 | 0.717 | 1.395 |
Discussion
In the present study, we aimed to investigate the potential relationship between the autistic traits in a neurotypical sample and the motor resonance responses evoked by the observation of others’ actions embedded in naturalistic contexts.
At the neurophysiological level, we replicated previous findings25,26 by showing that the observation of movement kinematics embedded in congruent contexts led to an early facilitation of CSE, while the observation of the same movements in incongruent contexts resulted in a later inhibition of motor responses. Similar modulations were obtained at the behavioral level, with increased or decreased performance in kinematics recognition when actions were observed in congruent or incongruent contexts, respectively, as compared to ambiguous ones.
When correlating the AQ total score with the facilitatory and inhibitory MEP indexes, we found a specific and strong positive association between the level of autistic traits and the ability to inhibit M1 simulative responses if these where incompatible with the overarching intention cued by the context. Specifically, we found that individuals higher in autistic traits showed increased motor facilitation for actions embedded in incongruent as compared to ambiguous contexts, pointing to a failure in down-regulating M1 activity when a mismatch occurred.
Many studies have put the social skills impairments in ASD in relation to altered representation of other’s kinematics10,11. However, the origin of these deficits remains poorly understood, with studies either reporting preserved13,14,30 or abnormal MNS activity in ASD10,11,15,16,31. Here, in line with those studies showing no alteration of motor resonance in ASD, we found no correlation between autistic traits and motor facilitation during observation and prediction of actions embedded in ambiguous contexts (control condition). Furthermore, no correlations were observed between the AQ scores and performance in kinematics recognition, thus suggesting, in line with Bayesian accounts on ASD, that autistic-like observers are not impaired in coding perceptual kinematics per se but rather in combining it with context-based expectations21.
Importantly, however, autistic traits predicted an altered response to observed actions when these were embedded in incongruent contexts. This points to a specific alteration of the integration between perceptual kinematics and expectations of others’ intentions derived from the context.
Interestingly, our data show that autistic traits predicted the extent of inhibition of motor responses to observation of kinematics embedded in incongruent contexts, but not the facilitation in response to congruent ones. Thus, our data add to previous views on ASD by showing that autistic traits are specifically associated to the sensitivity of the motor system in mapping kinematics when they are incongruent with the observers’ expectations, rather than to a general deficit in coding these two information cues. In fact, facilitation for congruent contexts and inhibition for incongruent ones may reflect different mechanisms. In our previous studies, we found different time-courses in the motor responses associated to these effects, suggesting that while the early facilitation might reflect representation of the contextual expectations within the MNS, the later inhibition likely reflects modulation from higher cortical areas, beyond the MNS, which are call into play to solve the conflict25,26. Accordingly, during this later time-window, the ability to “mute” or silence the incompatible action representation was reduced in those individuals with higher autistic traits. Thus, our evidence points to alterations of later processes in the autistic-like brain, which fails to down-regulate M1 activity when the predicted action kinematics mismatches contextual expectations about others’ behaviors.
A core hypothesis derived from current Bayesian approaches to ASD is that sensory signals are weighted more highly when integrated with prior or contextual information (for a recent Review, see32). Our findings are in line with this suggestion but further specify that the greater reliance on sensory information mainly occurs in individuals with high levels of autistic traits when there is a mismatch between contextual information and sensory data. Indeed, if autistic traits were generally associated to greater weighting of sensory input we should have also observed a negative correlation between AQ-scores and the facilitatory MEP index, since individuals with higher levels of autistic traits should be less facilitated by congruent contexts, mostly relying on perceptual kinematics. Thus, according to our findings, the altered use of context-based expectations in ASD might be dependent on the degree of informativeness of the environment and/or on a failure in updating expectations when a discrepancy between what was expected and what was actually perceived (i.e., prediction errors) takes place.
Another important finding from our study came from the regression analysis, which showed that the model including the AQ-subscales and gender explained a significant proportion of motor inhibition variability, with social skill, attention to detail and gender being the regressors giving the greatest contributions. Thus, we found that the differential inhibition of M1 was related to individual differences in ASD-like traits associated to both social (i.e., social skill) and non-social (i.e., attention to detail) aspects. Furthermore, these associations were independent from the observer’s gender, which showed an independent effect with greater impairments in men than women. This later finding is in line with previous studies using the AQ and showing consistent gender differences, such that neurotypical men obtain significantly higher AQ scores than neurotypical women33,34.
While the correlations with social impairments and gender were expected, the finding of an independent influence on motor inhibition from the attention to detail subscale deserves further discussion. This subscale measures non-social aspects related to perceptual atypicalities. Indeed, a perceptual phenomenon that has been repeatedly associated to ASD is the presence of a local processing bias, which seems to be independent of social functioning35 and theory of mind abilities36. In other words, individuals with ASD perceive the world differently, exhibiting a detail-focused style when processing visual information. Interestingly, evidence from a recent meta-analysis37 suggests that the altered perceptual organization in ASD may be related to a shift towards slower global processing. In this view, perceptual atypicalities would be mainly related to timing alterations, with ASD individuals being slower in global processing as compared to neurotypical controls. Furthermore, it has been shown that differences in the temporal interplay between local and global processing particularly affect the construction of dynamic visual representations38. For instance, when the time to integrate a motion signal is short, ASD perceptual deficits become more pronounced, suggesting that global motion processing in ASD evolves more slowly over time39. Within this context, our finding of an independent contribution of the attention to detail subscale could be related to a delayed integration of the dynamic relationships between the contextual and the kinematics cues. This temporal delay might explain the absence of down-regulation of M1 activity in the late time window and opens the possibility that inhibition for incongruent contexts could actually occur but in a later time-window. However a limitation of our experimental design is that CSE was measured up to 500 ms after action onset, not allowing us to test this hypothesis.
Another major limitation is the small sample size for a correlational design (n = 22), which requires caution when generalizing and interpreting current results. However, the post hoc analysis showed a more than acceptable power for our study40. It is worth noting that previous studies41,42 measuring MEPs in neurotypical individuals and correlating motor activity with AQ scores have used smaller samples. Nevertheless, further research is clearly required to extend current findings to larger population.
Conclusions
Collectively, our findings suggest that autistic traits might be related to differences in the extent to which individuals are able to integrate context-based expectations and current sensory evidence. More specifically, our finding of an association between autistic traits with inhibition for incongruent contexts -but not with facilitation for congruent ones-, points to a specific difficulty in integrating perceptual information and expectations derived from the context when a mismatch occurs. Overall, these findings are in line with current proposals on ASD speaking in favor of an abnormal interplay between simulative motor mapping of low-level movement kinematics in the MNS and top-down signals from areas beyond this network during action prediction and recognition.
Methods
Participants
Twenty-two individuals recruited at the University of Udine took part in the study (13 women, M = 22.9, SD = 3.06). Participants were all right-handed according to the Standard Handedness Inventory43, had normal acuity in both eyes and were free from any contraindication to TMS44. They gave their written informed consent before the beginning of the experiment and received course credits for participating in the study. The experimental procedures were approved by the local Ethics Committee (Comitato Etico Regionale Unico, Friuli Venezia Giulia, Italy) and were carried out in accordance with the ethical standards of the Declaration of Helsinki. None of the participants reported history of neurological, psychiatric, or other major medical problems. No discomfort or adverse effects during TMS were reported or noticed in any of the participants.
After the experiment, participants completed the Italian version45 of the Autism Spectrum Quotient (AQ)28. The AQ is a self-report questionnaire which quantifies the degree to which individuals with normal intelligence have the traits associated to the autistic spectrum (social abnormalities, communication difficulties, and stereotyped and rigid behaviour), via assessing five different factors: Social skills, Attention switching, Attention to detail, Communication and Imagination. Each factor is assesed by 10 items and higher scores are obtained by individuals endorsing more autistic-like behaviour.
Stimuli and Task
Videos were taken from Amoruso and Urgesi (2016). They were recorded in color with a Canon EOS 550D camera at 30 frames per second and were further edited with Adobe Premiere Pro CS3 3.0. Length was matched across videos so that they had an equivalent duration (15 frames for a total of 500 ms). All videos depicted a female model performing reach-to-grasp movements of different objects with her right hand. Depending on the kinematics (precision vs whole-hand grips), each object could be grasped to perform either one of two possible actions. For instance, in the case of the object “mug”, the actions were a) to drink and b) to clean, each of them performed with their correspondent kinematics: reaching-to-grasp the mug by its handle using a precision grip, and reaching-to-grasp the mug from the top using a whole-hand grip, respectively46.
Each action was recorded in 3 different contexts: congruent, incongruent, and ambiguous. In the congruent condition, the action suggested by the context was compatible with the action suggested by the kinematics. Conversely, in the incongruent condition, the context interfered with the kinematics by cueing to the opposite action. Finally, in the ambiguous condition, contextual cues were not informative about which action was likely to unfold (see Fig. 1 and Videos 1–3 for examples of each condition). For a complete description of objects, action labels, grips, contexts, and their possible combinations, please refer to Amoruso and Urgesi27.
Stimuli were validated in a previous study27, which confirmed the appropriate manipulation of the plausibility of each action when embedded in congruent, ambiguous, and incongruent contexts (with parametrically decreasing levels of plausibility, respectively). Moreover, to ensure that the observed motor modulations were actually due to the manipulation of the contextual information and not to differences in the movement kinematic profiles of the same action across scenes, we performed a frame-by-frame kinematic analysis of the videos. This analysis showed that kinematics were comparable across contexts25.
In a two-alternative forced choice (2AFC) task, participants were requested to watch the videos and predict action unfolding. A temporal occlusion paradigm was used, with the videos being stopped before the model made contact with the object. Note that, by doing this, participants were able to observe the pre-shaping of the hand configuration during the reaching-to-grasp phase of the movement but not the grasping movement itself. To build-up their predictions, participants were instructed to pay attention to both aspects of the scene: the kinematic information conveyed by the model’s hand and the contextual information in terms of objects configurations. The inclusion of ambiguous trials, in which the context was not informative, prevented participants from focusing attention only on the contextual cues when giving their responses.
Electromyography (EMG) Recording and spTMS
Single-pulse TMS was applied to the left M1 using a Magstim 200 stimulator (maximum output = 2T at coil surface, pulse duration = 250 μsec, rise time = 60 μsec, The Magstim Company, Carmarthenshire, Wales, UK) connected to a 70-mm figure-of-eight coil (Magstim polyurethane-coated coil). MEPs were recorded simultaneously from the First Dorsal Interosseous (FDI) and from the Extensor Carpi Radialis (ECR, control muscle) of the right hand during task performance. Muscle selection was based on previous findings using the current paradigm25–27 and evidence from spTMS studies showing that while both muscles are synergistically activated during the observation of reach-to-grasp movements47, only the FDI corticospinal representation is differently modulated by the observation of precision vs. whole-hand grips48,49. Thus, we expected a greater involvement of the FDI muscle as compared to the ECR. Surface Ag/AgCl disposable electrodes (1 cm diameter) were placed in a belly-tendon montage for each muscle. The EMG signal was amplified, filtered (band-pass 5 Hz to 20 kHz) and recorded with the Biopac MP-36 system (BIOPAC Systems, Inc., Goleta, CA) at a sampling rate of 50 kHz.
The coil was positioned tangentially on the scalp, with the handle pointing backward and approximately 45° lateral from the midline, perpendicular to the line of the central sulcus50. This orientation was chosen based on the finding that the lowest motor threshold is achieved when the induced electric current in the brain is flowing perpendicular to the central sulcus51,52. The optimal scalp position (OSP) for inducing MEPs in both muscles was detected by moving the coil in 1-cm steps over the left M1 and by delivering TMS pulses at constant intensity until the largest MEPs for the FDI were found. Then, the OSP was marked with a pen on a tight-fitting bathing cap worn by participants. The coil was held on the scalp by a holder with an articulated arm, and its position with respect to the mark was continuously checked to compensate for small movements of the participants’ head.
The intensity of magnetic pulses was set at 120% of the individual resting motor threshold (rMT), defined as the minimum intensity of the stimulator output able to produce MEPs with amplitudes of at least 50 μV with 50% probability in higher threshold muscle (Rossini et al. 1994) in 5 out of 10 consecutive pulses. The rMT ranged from 38% to 59% (M = 46%, SD = 5.4%). To ensure that there was no unwanted background EMG activity before the magnetic pulse, the signal from both muscles was continuously verified online by visually monitoring the EMG signal. MEPs’ peak-to-peak amplitudes (in mV) were collected and stored in a computer for off-line analysis.
Trial Structure
Stimuli were presented using E-prime V2 software (Psychology Software Tools) on a 21 inch CRT monitor (resolution, 1024 × 768 pixels; refresh frequency, 60 Hz). Participants sat in a comfortable armchair in a dimly lit room ~1 m away from the monitor with prone hands resting on a pillow. They were instructed to pay attention to the displayed stimuli and to avoid moving their right hand. Videos appeared at the center of the screen on a neutral background (subtending ~ 15.96° × 11.97° of visual angle). Trials started with a visual warning cue lasting for 5 s (the Italian word “attendi”, in English “attention”), followed by the video presentation lasting 500 ms. TMS pulses were delivered online at either 300 ms or 500 ms after action onset. After the video, a frame was presented with the verbal descriptors of the two possible goals (e.g., “versare” and “spostare”, in English “to pour” and “to place” respectively; one located up and the other located down) written in black on a white background. This frame remained on the screen until a response was recorded. Participants verbalize their responses (by saying “su” or “giù”, in English “up” or “down”, respectively) and the experimenter recorded the answer. The location of the descriptors was counterbalanced, ensuring that in half of the trials one of the descriptors was presented up and, in the other half, it was presented down. This procedure enabled us to prevent participants from planning their response on the basis of the descriptors’ spatial location. Verbal responses were used to prevent that peripheral muscular contraction artifacts resulting from button press contaminated MEPs. Importantly, verbal responses were required after the TMS pulse was delivered53. A total of 42 videos (14 actions embedded in three different contexts) were used. Each video was presented twice, resulting in 84 stimuli randomly presented in two blocks of 42 trials each. Prior to video presentation, baseline corticospinal excitability was assessed by acquiring 10 MEPs while participants passively watched a fixation cross.
Data Analysis
Behavioral data
Individual performance expressed as d prime values (d’), a bias-corrected measure of sensitivity in discriminating between 2 categories54, was calculated for each condition. In the d’ analysis, ‘precision grips’ identified as ‘precision grips’ were considered as “hits” and ‘whole-hand grips’ identified as ‘precision grips’ were considered as “false alarms”. More specifically, correct responses were defined by the kinematics, not by the context. The d’ values were calculated by transforming the response proportion to z-scores, and then subtracting the z-score that corresponds to the false-alarm rate from the z-score that corresponds to the hit rate55. The d’ values were subjected to a repeated-measures analysis of variance (RM-ANOVA) with Time (early, late) and Context (congruent, incongruent, and ambiguous) as within-subject variables.
MEP data
Individual mean peak-to-peak (in mV) amplitudes of MEPs recorded from the FDI and ECR muscles were calculated separately for each condition. Since background EMG is known to modulate MEP amplitude, it was assessed in each participant by calculating the mean rectified EMG signal across a 100 ms interval prior to TMS. MEPs with preceding background EMG deviating from the mean by >2 SD were removed from further analysis. The total percentage of excluded MEPs (FDI, 300 ms: congruent, 5.84%; incongruent, 6.16%; ambiguous, 7.79%; FDI, 500 ms: congruent, 8.44%; incongruent, 8.76%; ambiguous, 6.16%; ECR, 300 ms: congruent, 6.49%; incongruent, 7.46%; ambiguous, 9.09%; ECR, 500 ms: congruent, 6.81%; incongruent, 8.76%; ambiguous, 8.11%) did not differ between conditions and muscles (all Fs < 1.33; all Ps > 0.27). Furthermore, after removing contaminated trials, at least 10 MEP trials (>70%) remained in each experimental condition for all participants.
Raw MEP values were log-transformed to address non-normality resulting from positive skew56. MEP amplitudes recorded during action observation conditions were expressed as difference values from MEPs recorded during baseline. These normalized action-observation MEPs were analyzed by means of a 3-way RM-ANOVA with Muscle (FDI, ECR), Time (early, late) and Context (congruent, incongruent and ambiguous) as within-subjects’ variables. Estimates of the effect size were obtained using the partial eta-squared. Post-hoc analysis was carried out using the Newman-Keuls test. The analyses were implemented in Statistica software v.10 (Statsoft, Tulsa, OK) and α value was set at 0.05.
Correlation Analysis
First, we applied the Shapiro-Wilk test to determine the distribution of the variables included in the correlational analysis. Importantly, all the variables showed a normal distribution (all ps > 0.10). Thus, we calculated Pearson correlation coefficients between the AQ-scores and the mean MEP values obtained in the ambiguous condition (control condition). Since no correlations were found (Early time-window, r = −0.145, p = 0.51; Late time-window, r = −0.208; p = 0.35), we computed two MEP indexes obtained by subtracting, for each subject, the mean normalized MEPs value for the congruent minus the ambiguous condition (facilitatory MEP index) and the incongruent minus the ambiguous condition (inhibitory MEP index). Briefly, these indexes reflect that the higher the facilitatory MEP index, the higher the facilitation for congruent contexts and the higher the inhibitory index, the lower the inhibition for incongruent contexts, respectively. We then calculated Pearson correlations between the individual AQ scores and the facilitatory and inhibitory MEP indexes in the early and late time windows. A Bonferroni correction prcedure was applied to control for multiple comparisons (α = 0.012; 4 comparisons).
An analogue procedure was performed for the d prime values. Since no correlations were found for the ambiguous conditions and the AQ scores at the behavioral level (Early time-window, r = −0.167, p = 0.45; Late time-window, r = −0.326; p = 0.13), the two indexes for the early and late time windows were computed and correlated separately.
Multiple Linear Regression Analysis
Finally, we conducted a multiple linear regression analysis to identify factors that may contribute to the MEP effects. We followed standard procedures for assessing multicollinearity and confirmed that tolerance (all values < 0.9) and variance inflation factors (all VIF < 1.69) were low, overall indicating that multicollinearity did not affect our statistical analysis.
The five AQ subscales were entered as predictors together with gender, since it has been shown that gender differences in autistic traits are present in the nonclinical population, with men scoring significantly higher than women24.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the European Commission (MCSA-H2020-NBUCA, grant N. 656881), the Ministero Istruzione Universita‘ e Ricerca (Futuro In Ricerca, FIR 2012, Prot. N. RBFR12F0BD; to C.U.), and from Istituto di Ricovero e Cura a Carattere Scientifico ‘E. Medea’ (Ricerca Corrente 2016, Ministero Italiano della Salute; to C.U.).
Author Contributions
L.A. and C.U. designed the study. L.A. and A.F. performed the experiment. L.A. and C.U. analysed the data and wrote the paper. All authors reviewed the manuscript.
Data Availability
The datasets used in the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33827-8.
References
- 1.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 2.Aglioti SM, et al. Action anticipation and motor resonance in elite basketball players. Nat Neurosci. 2008;11(9):1109–16. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- 3.Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cogn Process. 2007;8(3):159–66. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maranesi M, et al. Mirror neuron activation prior to action observation in a predictable context. J Neurosci. 2014;34(45):14827–32. doi: 10.1523/JNEUROSCI.2705-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urgesi C, et al. Simulating the future of actions in the human corticospinal system. Cereb Cortex. 2010;20(11):2511–21. doi: 10.1093/cercor/bhp292. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, et al. Autism as a disorder of prediction. Proc Natl Acad Sci USA. 2014;111(42):15220–5. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 8.Avenanti A, Candidi M, Urgesi C. Vicarious motor activation during action perception: beyond correlational evidence. Front Hum Neurosci. 2013;7:185. doi: 10.3389/fnhum.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finisguerra A, et al. Generalization of motor resonance during the observation of hand, mouth, and eye movements. J Neurophysiol. 2015;114(4):2295–304. doi: 10.1152/jn.00433.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theoret H, et al. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol. 2005;15(3):R84–5. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Enticott PG, et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry. 2012;71(5):427–33. doi: 10.1016/j.biopsych.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Enticott PG, et al. Interpersonal motor resonance in autism spectrum disorder: evidence against a global “mirror system” deficit. Front Hum Neurosci. 2013;7:218. doi: 10.3389/fnhum.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinstein I, et al. Normal movement selectivity in autism. Neuron. 2010;66(3):461–9. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pokorny JJ, et al. The Action Observation System when Observing Hand Actions in Autism and Typical Development. Autism Res. 2015;8(3):284–96. doi: 10.1002/aur.1445. [DOI] [PubMed] [Google Scholar]
- 15.Dapretto M, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martineau J, et al. Atypical activation of the mirror neuron system during perception of hand motion in autism. Brain Res. 2010;1320:168–75. doi: 10.1016/j.brainres.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. 2012;78(17):1354–62. doi: 10.1212/WNL.0b013e3182518375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton AF. Reflecting on the mirror neuron system in autism: a systematic review of current theories. Dev Cogn Neurosci. 2013;3:91–105. doi: 10.1016/j.dcn.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ondobaka S, et al. Action recognition depends on observer’s level of action control and social personality traits. PLoS One. 2013;8(11):e81392. doi: 10.1371/journal.pone.0081392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellicano E, Burr D. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16(10):504–10. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Palmer C. J., Paton B., Kirkovski M., Enticott P. G., Hohwy J. Context sensitivity in action decreases along the autism spectrum: a predictive processing perspective. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1802):20141557–20141557. doi: 10.1098/rspb.2014.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barttfeld P, et al. Organization of brain networks governed by long-range connections index autistic traits in the general population. J Neurodev Disord. 2013;5(1):16. doi: 10.1186/1866-1955-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruzich E, et al. Measuring autistic traits in the general population: a systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Mol Autism. 2015;6:2. doi: 10.1186/2040-2392-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amoruso L, Finisguerra A, Urgesi C. Tracking the Time Course of Top-Down Contextual Effects on Motor Responses during Action Comprehension. J Neurosci. 2016;36(46):11590–11600. doi: 10.1523/JNEUROSCI.4340-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amoruso L, Finisguerra A, Urgesi C. Contextualizing action observation in the predictive brain: Causal contributions of prefrontal and middle temporal areas. Neuroimage. 2018;177:68–78. doi: 10.1016/j.neuroimage.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Amoruso L, Urgesi C. Contextual modulation of motor resonance during the observation of everyday actions. Neuroimage. 2016;134:74–84. doi: 10.1016/j.neuroimage.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 28.Baron-Cohen S, et al. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17. doi: 10.1023/A:1005653411471. [DOI] [PubMed] [Google Scholar]
- 29.Faul F, et al. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 30.Cusack JP, Williams JH, Neri P. Action perception is intact in autism spectrum disorder. J Neurosci. 2015;35(5):1849–57. doi: 10.1523/JNEUROSCI.4133-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattaneo L, et al. Impairment of actions chains in autism and its possible role in intention understanding. Proc Natl Acad Sci USA. 2007;104(45):17825–30. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer CJ, Lawson RP, Hohwy J. Bayesian approaches to autism: Towards volatility, action, and behavior. Psychol Bull. 2017;143(5):521–542. doi: 10.1037/bul0000097. [DOI] [PubMed] [Google Scholar]
- 33.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6(6):248–254. doi: 10.1016/S1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 34.Baron-Cohen S, et al. Attenuation of typical sex differences in 800 adults with autism vs. 3,900 controls. PLoS One. 2014;9(7):e102251. doi: 10.1371/journal.pone.0102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behrmann M, Thomas C, Humphreys K. Seeing it differently: visual processing in autism. Trends Cogn Sci. 2006;10(6):258–64. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 37.Van der Hallen R, et al. Global processing takes time: A meta-analysis on local-global visual processing in ASD. Psychol Bull. 2015;141(3):549–73. doi: 10.1037/bul0000004. [DOI] [PubMed] [Google Scholar]
- 38.Robertson CE, Baron-Cohen S. Sensory perception in autism. Nat Rev Neurosci. 2017;18(11):671–684. doi: 10.1038/nrn.2017.112. [DOI] [PubMed] [Google Scholar]
- 39.Robertson CE, et al. Atypical integration of motion signals in Autism Spectrum Conditions. PLoS One. 2012;7(11):e48173. doi: 10.1371/journal.pone.0048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen, J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates (1988).
- 41.Lepage JF, Tremblay S, Theoret H. Early non-specific modulation of corticospinal excitability during action observation. Eur J Neurosci. 2010;31(5):931–7. doi: 10.1111/j.1460-9568.2010.07121.x. [DOI] [PubMed] [Google Scholar]
- 42.Puzzo I, et al. Reduced cortico-motor facilitation in a normal sample with high traits of autism. Neurosci Lett. 2009;467(2):173–7. doi: 10.1016/j.neulet.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 43.Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11(3):230–8. doi: 10.1016/S0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- 44.Rossi S, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruta L, et al. The Autism-Spectrum Quotient–Italian version: a cross-cultural confirmation of the broader autism phenotype. J Autism Dev Disord. 2012;42(4):625–33. doi: 10.1007/s10803-011-1290-1. [DOI] [PubMed] [Google Scholar]
- 46.Iacoboni M, et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005;3(3):e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finisguerra A, et al. Dissociated Representations of Deceptive Intentions and Kinematic Adaptations in the Observer’s Motor System. Cereb Cortex. 2018;28(1):33–47. doi: 10.1093/cercor/bhw346. [DOI] [PubMed] [Google Scholar]
- 48.Urgesi C, et al. Motor facilitation during action observation: topographic mapping of the target muscle and influence of the onlooker’s posture. Eur J Neurosci. 2006;23(9):2522–30. doi: 10.1111/j.1460-9568.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 49.Urgesi C, et al. Mapping implied body actions in the human motor system. J Neurosci. 2006;26(30):7942–9. doi: 10.1523/JNEUROSCI.1289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Lazzaro V, et al. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998;109(5):397–401. doi: 10.1016/S0924-980X(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 51.Brasil-Neto JP, et al. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9(1):132–6. doi: 10.1097/00004691-199201000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85(1):17–21. doi: 10.1016/0168-5597(92)90096-T. [DOI] [PubMed] [Google Scholar]
- 53.Gentilucci M, et al. Repetitive transcranial magnetic stimulation of Broca’s area affects verbal responses to gesture observation. J Cogn Neurosci. 2006;18(7):1059–74. doi: 10.1162/jocn.2006.18.7.1059. [DOI] [PubMed] [Google Scholar]
- 54.Macmillan NA, Kaplan HL. Detection theory analysis of group data: estimating sensitivity from average hit and false-alarm rates. Psychol Bull. 1985;98(1):185–99. doi: 10.1037/0033-2909.98.1.185. [DOI] [PubMed] [Google Scholar]
- 55.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31(1):137–49. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- 56.Osborne, J. Notes on the use of data transformations. Pract. Assess. Res. Eval. 8(6) (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.