Abstract
A modified Vip3C protein has been developed that has a spectrum of activity that has the potential to be commercially useful for pest control, and shows good efficacy against Spodoptera frugiperda in insect bioassays and field trials. For the first time Vip3A and Vip3C proteins have been compared to Cry1 and Cry2 proteins in a complete set of experiments from insect bioassays to competition binding assays to field trials, and the results of these complementary experiments are in agreement with each other. Binding assays with radiolabelled toxins and brush border membrane vesicles from S. frugiperda and Helicoverpa armigera show that the modified Vip3C protein shares binding sites with Vip3A, and does not share sites with Cry1F or Cry2A. In agreement with the resulting binding site model, Vip3A-resistant insects were also cross-resistant to the modified Vip3C protein. Furthermore, maize plants expressing the modified Vip3C protein, but not those expressing Cry1F protein, were protected against Cry1F-resistant S. frugiperda in field trials.
Introduction
Bacillus thuringiensis is a gram-positive bacterium that produces a variety of insecticidal protein toxins during its different growth phases. Two major categories of insecticidal proteins produced by B. thuringiensis are delta-endotoxins (Cry and Cyt toxins), that may form crystals within the bacteria during sporulation, and vegetative insecticidal proteins (Vip), which are secreted into the growth medium during vegetative growth. Delta-endotoxins fall into dozens of classes, but all share structural and functional similarities, and all form pores in or otherwise disrupt insect gut brush border membranes1,2. Vip proteins fall into four unrelated classes, consisting of Vip1 plus Vip2, which function as a binary toxin, Vip3, and Vip43. The Vip3 class of proteins appear to function by forming pores in insect gut brush border membranes4. Bacillus thuringiensis pore-forming toxins are believed to interact with receptors in the brush border before forming pores, and the specificity of a given toxin for particular insect species depends largely on the identity of its receptor. The binding of toxins to receptors in the midgut brush border membrane has been shown to play a key role in the mode of action of Cry1 and Cry2 proteins, since many resistant insect strains have been found to have a strong reduction of binding ability5–11. In addition, binding analyses using brush border membrane vesicles (BBMV) derived from insect midguts have proved to be a sound method of predicting or explaining patterns of cross-resistance among B. thuringiensis insecticidal toxins9,12–17. However, only a limited number of studies on a limited number of insect species have been carried out with labeled Cry1Fa, Cry2Ae, Vip3Aa or Vip3C to estimate the affinity of these proteins for membrane binding sites and the concentration of these sites in the brush border. Similarly, very few studies have tested the ability of these particular proteins to compete for each other’s binding sites18–20. The current study adds to the knowledge in this area, helping to establish more firmly the nature of the interactions of these economically important proteins with their target insects.
In the present study we tested and compared the four proteins listed above in a variety of experiments. We evaluated a modified version of Vip3Ca, called ARP150v02, with improved activity against Spodoptera frugiperda, in field efficacy trials, insect bioassays, and brush border membrane binding studies, to explore the extent of cross resistance with commercial Bt proteins. We performed competition binding assays with S. frugiperda and Helicoverpa armigera BBMV, and use these data to propose a binding site model for these insecticidal toxins. Laboratory insect bioassay results and field trial results were consistent with the in vitro binding data, suggesting that binding experiments can be used to help predict the resistance management usefulness of an insecticidal protein used as a trait in crops. The fact that the different types of experiments were in agreement with each other will allow for greater confidence in the use of laboratory-based studies during the development of new commercially relevant toxins such as ARP150v02.
Results
Characteristics of a modified Vip3C protein used in these studies
The amino acid sequence of ARP150v02 differs from Vip3Ca3 at 8 locations near the N-terminus (Fig. 1). The protein was recombinantly expressed and purified from an E. coli vector with an N- terminal 6X His tag, and was tested in bioassays to assess its efficacy and spectrum of activity against agronomically important lepidopteran pests. ARP150v02 showed activity against a number of pests, causing mortality and or stunting in Spodoptera exigua, S. frugiperda, Ostrinia nubilalis, and Agrotis ipsilon (Table 1). Separate bioassays were used to determine the LC50 of ARP150v02 against two insect species. Against S. frugiperda it showed an LC50 of 450 ng/cm2. However, this protein showed low activity against H. armigera, since it was not even able to cause growth inhibition at 6000 ng/cm2 when tested at Universitat de València (Table 2). Only when the protein was tested at a higher dose against H. armigera at CSIRO was activity seen. To test whether ARP150v02 might overcome resistance to Vip3A in insects, the protein was tested against a colony of H. armigera that is resistant to Cry2Ab and Vip3Aa, called Ha-DRES, and against a colony susceptible to those toxins, called Ha-Gr. ARP150v02 killed approximately two-thirds of the susceptible insects at a dose of 10000 ng/cm2 and had an LC50 of 3560 ng/cm2, but had little to no effect on the resistant insects at 10000 ng/cm2 (Table 3), showing that the protein cannot be used to overcome resistance of H. armigera to Vip3Aa.
Figure 1.
ARP150v02 sequence alignment with Vip3Ca3. White indicates non-conservative differences, and grey indicates conservative differences.
Table 1.
Spectrum of activity of ARP150v02 showing semiquantitative activity against several insect species.
| 0.01 µg/m | 40 µg/ml | |||
|---|---|---|---|---|
| Mortality | Stunting | Stunting | ||
| Spodoptera exigua | 0% | − | 75% | ++++ |
| Spodoptera frugiperda | 0% | ++ | 50% | ++++ |
| Ostrinia nubilalis | 0% | + | 0% | +++ |
| Agrotis ipsilon | 0% | − | 0% | +++ |
For stunting, a value of (−) indicates no stunting and a value of (++++) indicates the larvae remained the size of neonates.
Table 2.
Quantitative assay of ARP150v02 insecticidal activity against S. frugiperda and H. armigera.
| LC50 (ng/cm2) | FL95 (ng/cm2) | slope ± SE | |
|---|---|---|---|
| S. frugiperda | 450 | 300–670 | 1.2 ± 0.1 |
| H. armigera | >6000a | — | — |
a No mortality or growth inhibition was observed at this concentration.
Table 3.
ARP150v02 insecticidal activity against H. armigera strains, susceptible (Ha-Gr) and resistant to both Cry2Ab and Vip3Aa proteins (Ha-DRES).
| LC50 (ng/cm2) | FL95 (ng/cm2) | slope ± SE | |
|---|---|---|---|
| Ha- Gr | 3560 | 2500–5420 | 1.20 ± 0.09 |
| Ha-DRES | >10000* | — | — |
*Little to no mortality was observed at this concentration.
Binding of 125I-labeled ARP150v02 to BBMV
Total binding and nonspecific binding of ARP150v02 to BBMV from S. frugiperda and H. armigera is shown in Fig. 2a,b. The total binding was very low (around 1.5% at 0.03 mg/ml) with around 50% nonspecific binding in the case of S. frugiperda and almost 80% in the case of H. armigera. Since the binding was low and since the protein shows low toxicity to H. armigera, no further work was done with BBMV from this species. The increase of specific binding reached the asymptotic point at a very low concentration of S. frugiperda BBMV, for which 0.02 mg/ml BBMV were used in the competition assays (less than that made the pellet in the binding reaction too loose so it was easily lost in the washings). Heterologous competition binding assays (competition of a labeled protein by a different unlabeled protein) showed that Vip3Aa, but not Cry1Fa or Cry2Ae, competed for ARP150v02 binding sites (Fig. 3a). The dissociation constant (Kd) and concentration of binding sites (Rt) were estimated from the homologous competition assay (competition of a labeled protein by the same unlabeled protein) (Fig. 3a, solid line). The homologous competition curve fit a one-site model and the estimated Kd value indicated that the binding was of low affinity (Table 4).
Figure 2.
Binding assays at increasing concentrations of BBMV proteins. (a,c,e,g) show results for S. frugiperda BBMV. (b,d,f,h) show results for H. armigera BBMV. (•) total binding, (○) nonspecific binding.
Figure 3.
Competition binding assays with BBMV at increasing concentrations of unlabeled competitors. (a,b,d,f) show results for S. frugiperda BBMV. (c,e,g) show results for H. armigera BBMV. (•) Cry1Fa, (○) Cry2Ae, (■) Vip3Aa, (□) ARP150v02. Each data point represents the mean of at least two replicates and the error bars represent the standard error.
Table 4.
Binding parameters, Kd (dissociation constant) (nM) and Rt (concentration of binding sites) (pmol/mg), for the Vip3 and Cry proteins estimated from the respective homologous competition assays with BBMV from S. frugiperda and H. armigera.
| Protein | Mean ± SE | |||||
|---|---|---|---|---|---|---|
| S. frugiperda | H. armigeraa | |||||
| K d | R t | K d1 | R t1 | K d2 | R t2 | |
| ARP150v02 | 103 ± 55 | 48 ± 39 | — | — | — | — |
| Vip3Aa | 17 ± 5 | 50 ± 11 | 54 ± 10 | 138 ± 23 | — | — |
| Cry1Fa | 0.30 ± 0.02 | 1.30 ± 0.05 | 0.045 ± 0.003 | 0.023 ± 0.014 | 6.0 ± 0.3 | 14.1 ± 0.2 |
| Cry2Ae | 3.4 ± 0.6 | 0.26 ± 0.04 | 1.19 ± 0.03 | 0.40 ± 0.03 | — | — |
aTwo binding sites were obtained for Cry1Fa in this insect species.
Binding of 125I-labeled Vip3Aa to BBMV
Figure 2c,d show the binding of Vip3Aa to BBMV from the two insect species. The total binding was low (5–8% at 0.02 mg/ml of BBMV) with a substantial contribution of nonspecific binding (around 50%). As with labeled ARP150v02, the increase of specific binding reached the asymptotic point at a very low concentration of BBMV in both cases. As a consequence, the competition assays were performed with 0.02 mg/ml. Competition assays showed that ARP150v02, but not Cry1Fa (Cry2Ae was not tested), competed for Vip3Aa binding sites (Fig. 3b,c). The homologous competition curves fit a one-site model and their analysis indicated that the binding was of relatively high affinity in both insect species (Table 4).
Binding of 125I-labeled Cry1Fa to BBMV
Total and nonspecific binding of labeled Cry1Fa to BBMV from S. frugiperda and H. armigera is shown in Fig. 2e,f. Approximately 40% of the labeled protein bound to BBMV (at 0.3 mg/ml of BBMV), with very low nonspecific binding contribution. A concentration of 0.1 mg/ml of BBMV was chosen to carry out the competition assays. The statistical analysis of the homologous competition curves indicated that with S. frugiperda BBMV the curve fit a one-binding site model, whereas with H. armigera BBMV the curve fit a two-binding site model (Fig. 3d,e). Interestingly, the lower affinity binding site in H. armigera was not detected when using preparations of BBMV that had been kept at −80 °C for more than two months (data not shown). In both insect species, the Kd values indicate that binding of Cry1Fa is of high affinity (Table 4).
Heterologous competition binding assays showed that neither ARP150v02, Vip3Aa or Cry2Ae competed with Cry1Fa for binding to the BBMV from both insect species (Fig. 3d,e), indicating that the Cry1Fa binding site (or sites, if there was more than one site with similar Kd values) is not shared with the other three proteins.
Binding of 125I-labeled Cry2Ae to BBMV
Total and nonspecific binding of labeled Cry2Ae to BBMV from the two insect species is shown in Fig. 2g,h. The total binding with S. frugiperda BBMV was relatively low (10% at 0.6 mg/ml of BBMV) and more than 50% was due to nonspecific binding (Fig. 2g). However, with H. armigera BBMV almost 25% total binding was obtained (at 0.6 mg/ml of BBMV) with a very low contribution of nonspecific binding (Fig. 2h). Competition binding assays were carried out with 0.4 mg/ml of BBMV. The results showed that neither ARP150v02, Vip3Aa, or Cry1Fa competed for Cry2Ae binding sites (Fig. 3f,g). The homologous competition curves fit a one-site model and their analysis indicated that the binding was of high affinity in both insect species (Table 4).
Field Activity of ARP150v02
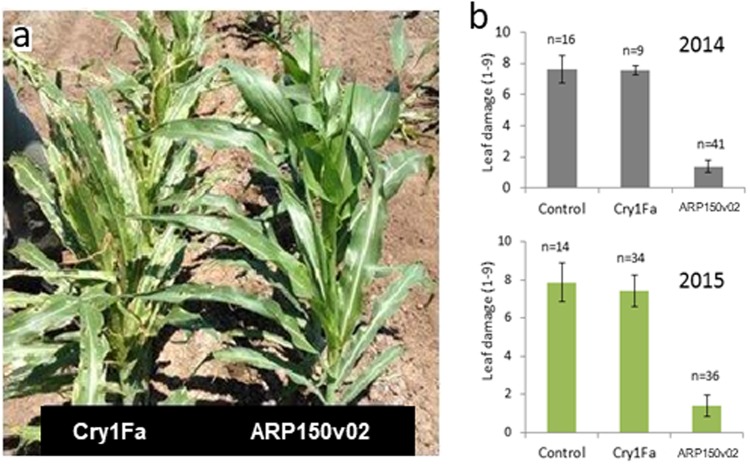
Field trials were conducted in 2014 and 2015 in a region where a natural infestation of Cry1Fa-resistant S. frugiperda is known to persist21 to judge the commercial potential of ARP150v02. To confirm the presence of the Cry1Fa-resistant population, transgenic maize plants expressing Cry1Fa were planted alongside plants expressing ARP150v02 protein. Significant leaf damage was observed in Cry1Fa-expressing plants as well as in negative control plants (not shown in photo), but plants expressing ARP150v02 protein were protected (Fig. 4a). Leaf damage was measured on a 1–9 scale, with 1 representing no visible damage or only pinhole sized lesions, and 9 representing nearly complete destruction of whorl and furl leaves. In both 2014 and 2015, S. frugiperda pressure was high as indicated by the high leaf damage scores of 7.6 and 7.8 respectively in the negative control plants (Fig. 4b). Maize plants expressing Cry1Fa also demonstrated high leaf damage scores of 7.5 and 7.4 (2014 and 2015 respectively) indicating the presence of the Cry1Fa-resistant insect population. However, maize plants expressing the modified Vip3C exhibited excellent control of the resistant S. frugiperda population as seen by the relatively low leaf score of 1.4 in both years (Fig. 4b).
Figure 4.
Field performance of corn plants expressing ARP150v02. (a) Transgenic corn plants expressing ARP150v02 (right) compared to transgenic plants expressing Cry1Fa (left). (b) Average leaf damage scores from field-tested transgenic corn plants, on a scale from 1 to 9, where 1 means no visual damage and 9 means the leaves are almost totally destroyed. The number of transgenic events tested is indicated. Control plants were nontransgenic.
Discussion
Previous studies on Vip3Aa provided LC50 values of 620 ng/cm2 for S. frugiperda22 and 1660 ng/cm2 for H. armigera23, while Vip3Ca was much less toxic to both insect species: a concentration as high as 4000 ng/cm2 of Vip3Ca3 killed 27% of neonate S. frugiperda larvae and 65% of neonate H. armigera larvae after 10 days24. Despite the low toxicity reported for Vip3Ca3 against S. frugiperda, in the current study ARP150v02, which differs from Vip3Ca3 in eight amino acid positions near the N-terminus, showed substantial activity against this insect pest, with an LC50 value of 450 ng/cm2 (Table 2), not significantly different from the value obtained for Vip3Aa. Thus ARP150v02 can potentially be substituted for Vip3Aa for pest control in crops though, like Vip3C, it will not control H. armigera that have developed resistance to Vip3Aa. The amino acid differences between ARP150v02 and Vip3Ca3 led to a reduction in the already low activity that Vip3 proteins exhibit against H. armigera3, suggesting the possibility that modifications that increase effectiveness against some pests may lead to loss of activity against other insect species.
The fact that ARP150v02 does not kill Vip3Aa-resistant H. armigera suggested that it might share binding sites with Vip3Aa, but few competition binding experiments comparing Vip3A and Vip3C proteins have previously been done. This study represents the first time a member of the Vip3C protein family has been radiolabeled, improving the determination of quantitative binding parameters with insect BBMV so that competition between ARP150v02 and Vip3Aa could be directly assessed. The high Kd value for ARP150v02 with S. frugiperda BBMV (103 nM) indicates that binding of this protein is of low affinity. The Kd value for H. armigera BBMV could not be estimated due to the extremely low specific binding and high nonspecific binding obtained. Heterologous competition assays showed that ARP150v02 and Vip3Aa share binding sites in S. frugiperda, whereas the Cry proteins used in this study do not recognize the Vip3 binding sites, consistent with the results of the insect bioassays and field studies.
Vip3Aa binding to S. frugiperda BBMV can be considered to be of high affinity, though the Kd is significantly higher than that of Cry1Fa or Cry2Ae (Table 4). The Kd value obtained is essentially the same as the one reported in a previous study using a different source of protein (Vip3Aa16)25. For H. armigera this is the first time that a Kd value has been obtained for a Vip3 protein. The high Kd value (54 nM) indicates that binding of Vip3Aa to H. armigera BBMV is of low affinity. Although the relationship between binding affinity and insecticidal potency is not always direct26, in our case the lower affinity of Vip3Aa to H. armigera, as compared with S. frugiperda, is correlated with the lower susceptibility of H. armigera to this protein. The same is true for ARP150v02, with its affinity for BBMV from the two insect species correlating with its activity against those species. The results from the heterologous competition assays extend those previously obtained with biotin-labeled Vip3Aa and Vip3Af, in that Cry2Ab (with Heliothis virescens BBMV) and Cry1Fa (with S. frugiperda BBMV) do not compete for Vip3A binding sites19,27. In contrast, ARP150v02 does compete for Vip3Aa binding sites in S. frugiperda and H. armigera (Fig. 3a–c), a result that is consistent with one recently obtained for Vip3 proteins with Mamestra brassicae20.
The homologous competition experiments with Cry1Fa indicate that it binds with very high affinity to BBMV from both insect species (Table 4), in agreement with previous studies28,29. The lower Kd values for S. frugiperda obtained in the present study compared to earlier ones17,28,29, and the finding of two binding sites in H. armigera, may be due to the use of more highly purified protein and fresh BBMV. The results from the heterologous competition experiments are in agreement with previous studies that showed that Vip3A and Cry2A proteins do not bind to Cry1Fa binding sites in S. frugiperda19,28 and Helicoverpa zea18.
Cry2Ae also bound with high affinity to BBMV from the two species (Table 4). This is the first time that the Kd value of this protein to S. frugiperda BBMV has been reported. For H. armigera, the Kd value obtained (1.19 nM) is in good agreement with the ones previously reported for Cry2Ae and for Cry2Ab in H. armigera and other heliothine species (Kd values between 2.3 and 6.5 nM)11,15,18. The results from the heterologous competition experiments extend those obtained with another heliothine species, H. zea, in that the Cry2Ae binding site is not shared by Cry1Fa or Vip3Aa18.
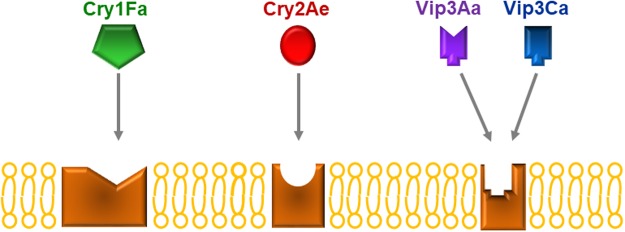
Taking all the heterologous competition results together we can propose a binding site model for the four B. thuringiensis proteins and the two insect species in this study (Fig. 5), in which Cry1Fa and Cry2Ae each bind to independent binding sites, not shared by the Vip3 proteins, and that Vip3Aa and ARP150v02 bind to a common binding site not shared by the Cry proteins. According to this model, the choice of one of these Vip3 proteins to be expressed in a Bt crop should be made on the basis of differences in their spectrum of activity, but not as a solution for resistance management.
Figure 5.
Binding site model for the Cry and Vip3 proteins in this study for S. frugiperda and H. armigera.
The competition binding results fit together with the laboratory bioassays using insects resistant to both Cry2 and Vip3A. ARP150v02 was active against susceptible H. armigera, but was inactive against a colony that was resistant to Vip3A proteins. We did not determine if the cross-resistance was due to loss of binding, or due to a change in a subsequent step in the toxicity process. Binding experiments with BBMV isolated from resistant insects would be one way to address this question. In addition to being in agreement with the laboratory insect bioassays, the binding results are also in agreement with field trials using maize expressing Cry1Fa or ARP150v02. Spodoptera frugiperda that were resistant to Cry1F did extensive damage to plants expressing Cry1F, but did minimal damage to plants expressing ARP150v02. This is the first time a full set of experiments has been used to connect competition binding experiments, insect bioassays, and field trials in one study. This agreement between laboratory in vitro binding experiments, laboratory insect bioassays, and field trials confirms the utility of the laboratory experiments in predicting the performance of transgenic crops in the field, at least for the case of ARP150v02, a fact that could be useful in the future development of commercial insect control traits. Performing binding experiments is typically much quicker and easier than obtaining resistant insect colonies, so being able to use binding experiments to predict the resistance management potential of a trait could be of great value.
The differences in amino acid sequence between ARP150v02 and other Vip3Ca proteins are located near the N-terminus of the protein. These differences increased activity against S. frugiperda and decreased activity against H. armigera, showing that the N-terminal region of the protein plays a role in the spectrum of activity. However, ARP150v02 still competes with Vip3Aa for the same binding sites in S. frugiperda BBMV, indicating that the changes in activity are not due to changes in the site of action of the protein. The differences in activity may be due to other factors, such as the stability of the protein in the insect gut, binding affinity, or steps in the intoxication process that come after receptor binding.
Materials and Methods
Expression and purification of B. thuringiensis proteins
Cry2Ae (accession number AAQ52362), Vip3Aa1 (accession number AAC37036) and ARP150v02 were produced from Escherichia coli BL21 and Cry1Fa (accession number AAA22348) from E. coli WK6. Escherichia coli cells were grown in TB-medium for three to four hours at 37 °C with shaking until reaching an OD600 between 0.6 and 0.9. Then, the culture was made 1 mM IPTG and incubated for 18 h to induce the expression of the recombinant proteins. The culture medium was centrifuged at 9000 g for 30 min at 4 °C to proceed with the lysis.
For Cry1Fa purification, the pelleted cells were resuspended in lysis buffer (50 mM Tris-HCl, 5 mM EDTA, 100 mM NaCl, pH 8) with vigorous vortexing and then lysed by 2 min sonication (10 sec ON, 10 sec OFF). After centrifugation, the insoluble material (containing the Cry1Fa inclusion bodies) was washed three times with lysis buffer and then solubilized in carbonate buffer (50 mM sodium carbonate, 100 mM NaCl, 10 mM DTT, pH 10.5) for 2 h at 37 °C. The solubilized protein was activated with 10% trypsin for 1 h at 37 °C and then dialyzed at 4 °C against 20 mM Tris/HCl (pH 8.6). The activated Cry1Fa was further purified on a HiTrap Q HP column (5 ml bed volume) equilibrated in the same dialysis buffer, using an Äkta explorer 100 system (GE Healthcare, UK). Cry1Fa was eluted with a linear gradient of 1 M NaCl (0–80% in 100 ml). The fractions containing Cry1F were pooled and DTT was added at a final concentration of 10 mM. After 5 min incubation at room temperature, the mixture was loaded onto a gel filtration column (Superdex 200 10/300 GL, GE Healthcare, UK) pre-equilibrated with 20 mM Tris, 150 mM NaCl, 10 mM DTT, pH 8.6. The most concentrated peak fractions containing Cry1F (Fig. 6) were pooled and the DTT was eliminated by overnight dialysis (with one buffer change) at 4 °C against 20 mM Tris, 150 mM NaCl, pH 8.6.
Figure 6.
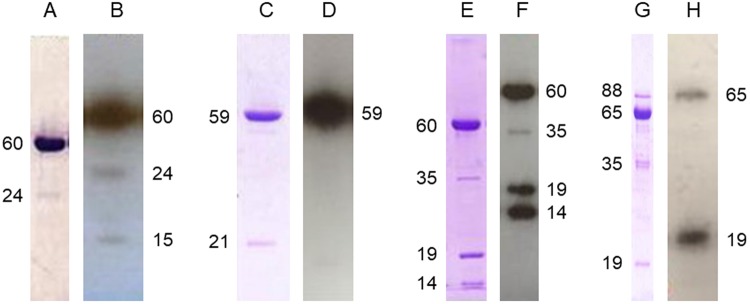
SDS-PAGE analysis of the purity of the protein preparations used for labeling as revealed by Coomassie blue (A,C,E,G) or autoradiography (B,D,F,H). (A,B) Cry1Fa; (C,D) Cry2Ae; (E,F) Vip3Aa; (G,H) ARP150v02. Numbers on the side refer to the approximate molecular weight of the main bands. The figure shows full length lanes of SDS-PAGE analyses performed separately.
For Cry2Ae (which carried a his-MBP tag at the N-terminus), the pelleted cells were resuspended in lysis buffer (50 mM sodium carbonate, 200 mM NaCl, 1 mM DTT, pH 8.9) and 800 µg of lysozyme and 0.2 mg of DNase was added per gram of pellet and incubated at 37 °C. Lysis was completed by 2 min sonication (10 sec on, 10 sec off). The supernatant (after centrifugation) was loaded onto a 5 ml MBP affinity column (MBPTrap HP from GE Healthcare Life Sciences) pre-equilibrated in lysis buffer and then eluted in the same buffer with 10 mM maltose. Fractions containing Cry2Ae were pooled and activated with 4% trypsin overnight at 4 °C without agitation (this step released the his-MBP tag). The fractions containing Cry2Ae were pooled and DTT was added at a final concentration of 10 mM. After 5 min incubation at room temperature, the mixture was loaded onto a gel filtration column (Superdex 200 10/300 GL, GE Healthcare, UK) equilibrated in 50 mM carbonate buffer, 50 mM NaCl, 10 mM DTT, pH 8.9. The most concentrated peak fractions containing Cry2Ae were pooled and reloaded onto a HiTrap Q HP column equilibrated in 50 mM carbonate buffer, 50 mM NaCl, pH 8.9, in an Äkta Explorer 100 chromatography system. The activated Cry2Ae was recovered in the flow through (free from the his-MBP tag), concentrated using Centricon centrifugal filters (Millipore) and dialyzed overnight against 20 mM Tris pH 8.6, 150 mM NaCl, to eliminate the remaining DTT. The purified Cry2Ae protein was flash frozen and stored at −20 °C until used for radiolabeling (Fig. 6). The Cry2Ae protein used as unlabeled competitor in the binding assays was prepared as indicated before, except for the DTT treatment and the gel filtration chromatography step.
For Vip3Aa, the pelleted cells were resuspended in lysis buffer (20 mM phosphate buffer, 200 mM NaCl, pH 7.4) and lysed by 2 min sonication (10 sec on, 10 sec off). After centrifugation at 27000 g for 30 min and supernatant filtration through 0.20 µm filters, the soluble Vip3Aa protein in the cell lysate was purified by isoelectric point precipitation (Ipp). The pH 6.0 was empirically selected as the best pH for the Ipp because it yielded the purest Vip3Aa protein from E. coli BL21 lysate. The pH of the Vip3Aa-containing cell lysate was lowered by the dropwise addition of acetic acid until the pH was 6.0. After centrifugation, the precipitated protein was resuspended in 20 mM Tris, pH 9, and dialyzed against the same buffer. The dialyzed protein was divided into two aliquots. The aliquot which was meant to be used for labeling was further purified on a HiTrap Q HP column as described above. The fractions containing the purified protein were pooled and subjected to trypsin treatment with 1% trypsin (w/w) for 2 h at 37 °C (Fig. 6). The aliquot to be used as competitor in the binding assays was activated by trypsin, as above, without any further purification. Both protein samples were stored at −20 °C until used.
For ARP150v02 (which carried a his-tag at the N-terminus), the pelleted cells were resuspended in lysis buffer (PBS, 300 mM NaCl, pH 7.4) and lysed by 2 min sonication (10 sec on, 10 sec off). After centrifugation at 27000 g for 30 min and supernatant filtration through 0.20 µm filters, the soluble ARP150v02 protein was purified by means of a Hi-Trap chelating HP column (GE Healthcare) charged with Ni2+. The ARP150v02 sample was loaded onto a column equilibrated with 50 mM phosphate buffer, pH 8.0, containing 10 mM imidazole. After washing with 50 mM phosphate buffer, pH 8.0, with 40 mM imidazole, the bound proteins were eluted by the same buffer containing 200 mM imidazole. Fractions (1 ml) were collected in tubes containing EDTA to give a final concentration of 5 mM after collecting the eluate. Fractions containing the ARP150v02 protein were pooled, diluted to 1 mg/ml and dialyzed against 20 mM Tris, 300 mM NaCl, pH 9. The purified ARP150v02 protein was kept at −20 °C and used for bioassays at the University of València. For binding experiments, ARP150v02 was further activated with 24% of trypsin (w/w) and let stand for 3 days at 30 °C. Finally, the activated protein, without any further purification (Fig. 6), was kept at −20 °C until use.
For bioassays carried out at CSIRO with H. armigera susceptible or resistant to Cry2Ab and Vip3A, the Cry1Ac protein was produced from Bt strain HD73 as described in Akhurst et al.30. Cry2Ab toxin was produced from a clone of the cry2Ab gene of B. thuringiensis variety kurstaki HD-1 in B. thuringiensis. The original clone was provided by L. Masson (National Research Council, Montreal, QC, Canada). The concentration of toxin was estimated by scanning the gel and analyzing the density of the toxin band relative to a bovine serum albumin (BSA) standard using Scion Image 1.62 software (Scion Corporation, Frederick, MD). A Vip3Aa clone in E. coli was used as a source of toxin. Production and calibration of the Vip3Aa toxin was described in Mahon et al.31.
Insect strains and bioassays
Laboratory colonies of S. frugiperda and H. armigera were reared on artificial diet and maintained at 25 ± 3 °C with 70 ± 5% RH and at 16/8 h (light/dark) photoperiod. The insecticidal activity of ARP150v02 was measured by a surface contamination assay using seven different concentrations of the protein (protoxin form). For each concentration and control, 16 neonate larvae of S. frugiperda and H. armigera were used. Serial dilutions of ARP150v02 protein were prepared either in PBS pH 7.4 (three replicates) or in 50 mM sodium carbonate, pH 10.5 (three replicates). Mortality was scored after 7 days. Since the mortality values were not significantly different in either buffer, the six replicates were combined and analyzed by probit analysis (POLO-PC, LeOra Software, 1987) to obtain the LC50 value (concentration that kills 50% of the insects).
In addition to the bioassays carried out with a H. armigera laboratory strain of insects originally collected from the field in Spain, the susceptibility of a Vip3Aa-susceptible laboratory strain and a double resistant strain of H. armigera from Australia was compared. The general laboratory strain designated GR is susceptible to Cry and Vip3A toxins. It was created in 2010 by bulk mating 5-6 individuals from 40 iso-female families that scored negative for Cry1Ac, Cry2Ab and Vip3A resistance in F2 screens. Details of the susceptible colony are provided in Mahon et al.32. The double resistant H. armigera colony (resistant to Cry2Ab and Vip3Aa) is designated DRES. It was created from individual resistance alleles isolated from the field as described in Walsh et al.33. The rearing methods were modified from those of Teakle and Jensen34, as described in Mahon et al.35 and Mahon et al.36. Bioassays with toxins were conducted using the surface contamination method as described for Cry2Ab assays35. Briefly, approximately 300 µl of standard diet was added diet to each well of plastic trays shaped with 96 wells sized so that the when containing diet, the upper surface accessible by larvae was approximately 0.57 cm2 in area. Once the diet had cooled, 0.2 ml of a solution containing an appropriate concentration of toxin was added and allowed to air dry. One neonate was placed in each well before it was sealed with a perforated heat-sensitive lid. Trays were incubated at 25 °C for 7 days by which time control larvae normally reach 3rd or 4th instar. Dead and alive larvae (capable of coordinated movement when prodded) were counted and the larval instars of surviving larvae were scored.
Radiolabeling of Cry and Vip3 proteins
Iodination was performed using trypsin-activated proteins (25 μg) by the chloramine-T method15,37. Cry1Fa, Cry2Ae, and Vip3Aa were mixed with 0.5 mCi of 125I and 1/3 (vol/vol) of 18 mM chloramine-T. For ARP150v02, the amount of 125I was reduced to 0.125 mCi to prevent inactivation of the protein. The excess of free 125I was separated from the labeled protein using a Bio-Gel P-30 desalting column (165 × 1.7 mm inner diameter) (Bio-Rad) equilibrated in 20 mM Tris pH 8.6, 150 mM NaCl, 0.1% BSA. The purity of the 125I-labeled proteins was checked by analyzing the elution fractions by SDS-PAGE with further exposure of the dry gel to an X-ray film (Fig. 6). The specific activity of the labeled proteins was calculated based on the input toxin, the radioactivity eluting in the protein peak, and the percentage of radioactivity in the toxin band vs. that in minor bands as reveled by the autoradiography. The estimated specific activity of the labeled proteins was 31 mCi/mg for Cry1Fa, 38 mCi/mg for Cry2Ae, 2 mCi/mg for Vip3Aa, and 0.54 mCi/mg for ARP150v02.
BBMV preparation
Last-instar larvae of S. frugiperda and H. armigera were dissected on ice-cold MET buffer (0.3 M mannitol, 5 mM EGTA, 17 mM Tris–HCl, pH 7.5). The midguts were carefully pulled out of the body carcass, stored in ice-cold MET buffer for no longer than 15 min, blotted on filter paper and then flash frozen in liquid nitrogen and stored at −80 °C. BBMV were prepared by the differential magnesium precipitation method38 and stored at −80 °C. The protein concentration in the BBMV preparations was determined by the Bradford method (1976) using BSA as standard.
Binding assays
Binding assays were performed by incubating BBMV with radiolabeled protein (0.045 nM for Cry proteins and 1.2 nM for Vip3 proteins) at room temperature in a 0.1 ml final volume for different times and buffers depending on whether the labeled protein was Cry or Vip3. For labeled Cry proteins the incubation was carried out for 60 min in binding buffer A (8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl, pH 7.4, 0.1% BSA)5,15, whereas for labeled Vip3 proteins the conditions were 90 min and buffer B (20 mM Tris-HCl, 150 mM NaCl, 1 mM MnCl2, pH 7.4, 0.1% BSA)25. Prior to use, the buffer in which the BBMV were stored was exchanged to the appropriate binding buffer by centrifuging the BBMV sample for 10 min at 16000 g (in a refrigerated centrifuge) and resuspending the pellet in the binding buffer. The incubation was stopped by centrifuging the tubes at 16000 g for 10 min at 4 °C and the pellet was washed once with 500 µl of cold buffer. The radioactivity retained in the pellet was measured in a model 2480 WIZARD2 gamma counter. An excess of unlabeled protein (2000 ng) was used to estimate the non-specific binding. Specific binding was calculated by subtracting the non-specific binding from the total binding.
To determine the appropriate concentration of BBMV to use in competition assays, the labeled proteins were incubated with increasing concentrations of BBMV in the binding buffer. Competition assays consisted on the incubation of the labeled proteins with the appropriate concentration of BBMV (0.1 mg/ml for Cry1Fa, 0.4 mg/ml for Cry2Ae, 0.02 mg/ml for Vip3Aa and ARP150v02) (the same BBMV concentration was used for both insect species) at increasing concentrations of unlabeled competitor. Dissociation constant (Kd) and concentration of binding sites (Rt) were estimated using the LIGAND software39.
Field trials
Field trials were conducted by DM Crop Research Group, Inc. at the Illinois Crop Improvement Farm in Juana Diaz, Puerto Rico. Each experimental unit consisted of a one-row plot of maize measuring approximately 12.5 feet. Plots were arranged using a randomized complete block design with four replications. Efficacy was determined by evaluating all positive plants in each plot. Traditional, non-GMO inbred B110 and a commercial hybrid were used as negative controls. A soil applied insecticide was applied at planting. In addition, foliar insecticides were applied up to the V4 leaf stage to control thrips and other early plant stage insect pests. Approximately twenty days after the last insecticide application, each plot was assigned a fall armyworm leaf damage rating (1–9; where 1 = no visual damage and 9 = leaves almost totally destroyed40. The damage was typical of S. frugiperda, with very large lesions. Egg masses and moths present at the time of leaf evaluation were all identified as S. frugiperda. No Helicoverpa zea or H. armigera larvae were observed feeding on leaves. A number of plants were dissected to verify that only S. frugiperda larvae were present.
Acknowledgements
We thank Rosa María González-Martínez for her help in rearing the insect colonies at the University of Valencia. We thank Dr. Dan Moellenbeck of DM Crop Research Group, Inc. for designing and carrying out the field trials. The resistant alleles in H. armigera were originally isolated by CSIRO (Sharon Downes) with funding from the CRDC (Cotton Research and Development Corporation).
Author Contributions
T.W.K. interpreted the results and helped write the paper. M.C. purified the toxins and performed and analyzed the data of binding assays and bioassays at the University of Valencia. J.W. coordinated the experiments between the three collaborating institutions. T.W. and B.J. performed the bioassays on the resistant insect populations and helped write the paper. J.M. conceived of the experiments and established the three-way collaboration. J.F. designed the binding experiments, analyzed the binding data, and helped write the paper.
Competing Interests
T.W.K., J.W. and J.M. were employees of Bayer U.S., Crop Science Division, at the time of submission of this manuscript, and state that they adhere to the Scientific Reports editorial and publishing policies. The other authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Theodore W. Kahn and Maissa Chakroun contributed equally.
References
- 1.Palma L, Muñoz D, Berry C, Murillo J, Caballero P. Toxins (Basel) 2014. Bacillus thuringiensis Toxins: An overview of their biocidal activity; pp. 3296–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vachon V, Laprade R, Schwartz JL. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J. Invertebr. Pathol. 2012;111:1–12. doi: 10.1016/j.jip.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Chakroun M, Banyuls N, Bel Y, Escriche B, Ferré J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol. Mol Biol. Rev. 2016;80:329–350. doi: 10.1128/MMBR.00060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MK, Walters FS, Hart H, Palekar N, Chen JS. The mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3A differs from that of Cry1Ab d-endotoxin. Appl. Environ. Microbiol. 2003;69:4648–4657. doi: 10.1128/AEM.69.8.4648-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Rie J, McGaughey WH, Johnson DE, Barnett BD, Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990;247:72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- 6.Ferré J, Real MD, Van Rie J, Jansens S, Peferoen M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc. Natl. Acad. Sci. USA. 1991;88:5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MK, Rajamohan F, Gould F, Dean DH. Resistance to Bacillus thuringiensis CryIA δ-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl. Environ. Microbiol. 1995;61:3836–3842. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballester V, Granero F, Tabashnik BE, Malvar T, Ferré J. Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella) Appl. Environ. Microbiol. 1999;65:1413–1419. doi: 10.1128/aem.65.4.1413-1419.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-Cabrera J, Escriche B, Tabashnik BE, Ferré J. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectinophora gossypiella) Insect Biochem. Mol. Biol. 2003;33:929–935. doi: 10.1016/S0965-1748(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 10.Gahan LJ, Pauchet Y, Vogel H, Heckel DG. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010;6:e1001248. doi: 10.1371/journal.pgen.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caccia S, et al. Binding site alteration is responsible for field-isolated resistance to Bacillus thuringiensis Cry2A insecticidal proteins in two Helicoverpa species. PLoS One. 2010;5:e9975. doi: 10.1371/journal.pone.0009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferré J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 13.Hernández CS, Ferré J. Common receptor for Bacillus thuringiensis toxins Cry1Ac, Cry1Fa, and Cry1Ja in Helicoverpa armigera, Helicoverpa zea, and Spodoptera exigua. Appl. Environ. Microbiol. 2005;71:5627–5629. doi: 10.1128/AEM.71.9.5627-5629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Cabrera J, et al. Toxicity and mode of action of Bacillus thuringiensis Cry proteins in the Mediterranean corn borer, Sesamia nonagrioides (Lefebvre) Appl. Environ. Microbiol. 2006;59:1828–1837. doi: 10.1128/AEM.72.4.2594-2600.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández-Rodríguez CS, Van Vliet A, Bautsoens N, Van Rie J, Ferré J. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl. Environ. Microbiol. 2008;74:7654–7659. doi: 10.1128/AEM.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández-Martínez P, et al. Lack of Cry1Fa binding to the midgut brush border membrane in a resistant colony of Plutella xylostella moths with a mutation in the ABCC2 locus. Appl. Environ. Microbiol. 2012;78:6759–61. doi: 10.1128/AEM.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakka SRK, et al. Field-Evolved Mode 1 Resistance of the fall armyworm to transgenic Cry1Fa-expressing corn associated with reduced Cry1Fa toxin binding and midgut alkaline phosphatase expression. Appl. Environ. Microbiol. 2016;82:1023–1034. doi: 10.1128/AEM.02871-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouffon C., Van Vliet A., Van Rie J., Jansens S., Jurat-Fuentes J. L. Binding Sites for Bacillus thuringiensis Cry2Ae Toxin on Heliothine Brush Border Membrane Vesicles Are Not Shared with Cry1A, Cry1F, or Vip3A Toxin. Applied and Environmental Microbiology. 2011;77(10):3182–3188. doi: 10.1128/AEM.02791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sena JA, Hernández-Rodríguez CS, Ferré J. Interaction of Bacillus thuringiensis Cry1 and Vip3A proteins with Spodoptera frugiperda midgut binding sites. Appl. Environ. Microbiol. 2009;75:2236–2237. doi: 10.1128/AEM.02342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomis-Cebolla J, et al. Insecticidal spectrum and mode of action of the Bacillus thuringiensis Vip3Ca insecticidal protein. J. Invertebr. Pathol. 2017;142:60–67. doi: 10.1016/j.jip.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Storer NP, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010;103:1031–1038. doi: 10.1603/EC10040. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Martínez P, Hernández-Rodríguez C, Rie JV, Escriche B, Ferré J. Insecticidal activity of Vip3Aa, Vip3Ad, Vip3Ae, and Vip3Af from Bacillus thuringiensis against lepidopteran corn pests. J. Invertebr. Pathol. 2013;113:78–81. doi: 10.1016/j.jip.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz de Escudero I, et al. A screening of five Bacillus thuringiensis Vip3A proteins for their activity against lepidopteran pests. J. Invertebr. Pathol. 2014;117:51–55. doi: 10.1016/j.jip.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Palma L, et al. Vip3C, a novel class of vegetative insecticidal proteins from Bacillus thuringiensis. Appl. Environ. Microbiol. 2012;78:7163–7165. doi: 10.1128/AEM.01360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakroun M, Ferré J. In vivo and in vitro binding of Vip3Aa to Spodoptera frugiperda midgut and characterization of binding sites by 125I radiolabeling. Appl. Environ. Microbiol. 2014;80:6258–6265. doi: 10.1128/AEM.01521-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfersberger MG. The toxicity of two Bacillus thuringiensis delta-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membranes for the toxins. Experientia. 1990;15:475–477. doi: 10.1007/BF01954236. [DOI] [PubMed] [Google Scholar]
- 27.Lee MK, Miles P, Chen JS. Brush border membrane binding properties of Bacillus thuringiensis Vip3A toxin to Heliothis virescens and Helicoverpa zea midguts. Biochem. Biophys. Res. Commun. 2006;339:1043–1047. doi: 10.1016/j.bbrc.2005.11.112. [DOI] [PubMed] [Google Scholar]
- 28.Hernández-Rodríguez CS, Hernández-Martínez P, Van Rie J, Escriche B, Ferré J. Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS One. 2013;8:e68164. doi: 10.1371/journal.pone.0068164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández-Rodríguez CS, Hernández-Martínez P, Van Rie J, Escriche B, Ferré J. Specific binding of radiolabeled Cry1Fa insecticidal protein from Bacillus thuringiensis to midgut sites in lepidopteran species. Appl. Environ. Microbiol. 2012;78:4048–4050. doi: 10.1128/AEM.07591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhurst RJ, James W, Bird LJ, Beard C. Resistance to the Cry1Ac delta-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) J. Econ. Entomol. 2003;96:1290–1299. doi: 10.1093/jee/96.4.1290. [DOI] [PubMed] [Google Scholar]
- 31.Mahon RJ, Downes SJ, James B. Vip3A resistance alleles exist at high levels in Australian targets before release of cotton expressing this toxin. PLoS One. 2012;7:e39192. doi: 10.1371/journal.pone.0039192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahon RJ, Olsen KM, Garsia KA, Young SR. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. J. Econ. Entomol. 2007;100:894–902. doi: 10.1093/jee/100.3.894. [DOI] [PubMed] [Google Scholar]
- 33.Walsh TK, et al. Dual Cry2Ab and Vip3A Resistant Strains of Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae); Testing linkage between loci and monitoring of allele frequencies. J. Econ. Entomol. 2014;107:1610–1617. doi: 10.1603/EC13558. [DOI] [PubMed] [Google Scholar]
- 34.Teakle, R. E. & Jensen, J. M. Heliothis armigera in Handbook of insect rearing (ed. Singh, P. & Moore, R. F.) 313–322 (Elsevier, 1985).
- 35.Mahon RJ, Olsen KM, Downes S, Addison S. Frequency of alleles conferring resistance to the Bt toxins Cry1Ac and Cry2Ab in Australian populations of Helicoverpa armigera (Lepidoptera: Noctuidae) J. Econ. Entomol. 2007;100:1844–1853. doi: 10.1093/jee/100.6.1844. [DOI] [PubMed] [Google Scholar]
- 36.Mahon RJ, Young S. Selection experiments to assess fitness costs associated with Cry2Ab resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) J. Econ. Entomol. 2010;103:835–842. doi: 10.1603/EC09330. [DOI] [PubMed] [Google Scholar]
- 37.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur. J. Biochem. 1989;186:239–47. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 38.Wolfersberger M, et al. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp. Biochem. Physiol. 1987;86A:301–308. doi: 10.1016/0300-9629(87)90334-3. [DOI] [PubMed] [Google Scholar]
- 39.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 40.Davis FM, Ng SS, Williams WP. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Miss. Agric. Forestry Exp. Stn. Tech. Bull. 1992;186:1–9. [Google Scholar]