Abstract
AIM
To evaluate the long-term efficacy and safety of autologous stem cell transplantation (SCT) for decompensated liver cirrhosis.
METHODS
Consecutive patients with decompensated liver cirrhosis were included and assigned into the SCT group and non-transplantation (non-SCT) group according to whether they received SCT treatment. Patients were followed up for ten years. The long-term survival rate and incidence of hepatocellular carcinoma (HCC) were compared between groups.
RESULTS
A total of 159 patients were enrolled, including 27 cases in the SCT group and 132 cases in the non-SCT group. The baseline characteristics were significantly different between the two groups. Propensity score matching (PSM) was used to match SCT and non-SCT patients. After PSM, 92 subjects were enrolled in the final analysis, including 23 cases in the SCT group and 69 cases in the non-SCT group. The overall mortality was 73.9% and 55.1%, and the median survival period was 48 and 64 mo, respectively. However, no significant difference was found in the long-term survival rate between the two groups (P > 0.05). In addition, the incidence of HCC was higher in the SCT group than in the non-SCT group (47.8% vs 21.7%, P < 0.05). After adjusting for other covariates, SCT (OR = 3.065, 95%CI: 1.378-6.814) and age (OR = 1.061, 95%CI: 1.021-1.102) were independently correlated with the development of HCC in this decompensated liver cirrhosis cohort.
CONCLUSION
Autologous SCT may fail to improve the long-term efficacy and increase the incidence of HCC for decompensated liver cirrhosis. Close monitoring of HCC is strongly recommended in patients undergoing autologous SCT.
Keywords: Decompensated liver cirrhosis, Stem cell transplantation, Hepatocellular carcinoma, Propensity score matching
Core tip: Stem cell therapy has shown short-term efficacy and safety for treatment of liver cirrhosis. However, the tumorigenicity of stem cells requires increased attention.
INTRODUCTION
Liver cirrhosis is a diffuse hepatic process characterized by fibrosis and the conversion of normal liver architecture into structurally abnormal nodules[1]. Patients with decompensated liver cirrhosis usually have symptoms of portal hypertension and hepatic dysfunction, which greatly affects patients’ quality of life and has a high mortality[2]. Currently, there is still a lack of effective treatments for decompensated liver cirrhosis, and symptomatic and supportive therapy and protection of residual hepatocytes remain the predominant strategy for the management of decompensated liver cirrhosis[3].
Orthotopic liver transplantation has been recognized as the best option for the treatment of decompensated liver cirrhosis, which improves both the quality of life and survival[1]. However, this treatment suffers from problems of huge shortage of donor livers, post-surgical complications, immune rejection, high medical expenditure and moral and ethical issues[4,5], which greatly limits its wide application in clinical practices. Development of regenerative treatment strategies for decompensated liver cirrhosis is therefore urgently needed[6,7].
Recently, stem cell-based therapy has become a novel strategy for the treatment of decompensated liver cirrhosis[8], and results from phase I/II clinical trials have shown generalized functional improvements and may be slightly superior to current conventional treatments[9]. It has been demonstrated that stem cells, such as hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and endothelial progenitor cells (EPCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), may be induced to differentiate into hepatocytes under certain conditions, which may promote liver renewal, alleviate hepatic fibrosis and be involved in the repair and reconstruction of the damaged liver. In particular, bone marrow (BM)-MSCs have been prevalently utilized[10]. Results from clinical studies showed that the liver disease patients had alleviation of clinical symptoms following autologous stem cell therapy, suggesting that stem cell therapy has short-term efficacy and safety[11-15]. However, stem cells have multi-lineage differentiation capability[10,16], and stem cell tumorigenicity has been paid increasing attention to the identification of liver cancer stem cells[17-19]. In addition, a limited follow-up period and no controls were assigned in most of the previous clinical studies[11,13], and there is little knowledge on the long-term clinical efficacy and safety of stem cell transplantation (SCT) to date.
In this retrospective cohort study, we aimed to compare the survival rate and incidence of hepatocellular carcinoma (HCC) in decompensated liver cirrhosis patients with and without SCT, so as to evaluate the long-term efficacy and safety of SCT.
MATERIALS AND METHODS
Ethical statement
This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Fujian Medical University, Permission No. 2015[084]. All methods were performed in accordance with the Declaration of Helsinki regarding ethical standards for research involving human subjects.
Subjects
In this retrospective cohort study, patients with decompensated liver cirrhosis admitted to the First Affiliated Hospital of Fujian Medical University (Fuzhou, China) during the period from January 2008 through December 2010 were included. Decompensated liver cirrhosis was diagnosed by previous medical history, blood and imaging examinations or liver biopsy. Those who met the following criteria were excluded from the study: (1) subjects with HCC or cancers in other organs; (2) pregnancy; (3) subjects with severe heart, lung, renal or hematologic diseases; (4) subjects died within a month; (5) subjects with HIV infection, sepsis or other life-threatening infectious diseases; and (6) subjects without any follow-up after discharge from the hospital. All subjects were assigned into the SCT group and non-SCT group according to whether they have received SCT.
Laboratory examinations
Upon admission, all subjects received blood examinations for a prothrombin time (PT) test, a routine blood test, liver and kidney function tests and a blood glucose test, and for determining serum hepatitis B virus (HBV) markers, HBV DNA viral load and serum alpha fetoprotein (AFP) concentration. The liver function was quantified using the Child-Pugh classification and the model for end-stage liver disease (MELD) score. HCC was diagnosed according to the Expert Consensus on Standardization of the Management of HCC in China[20]. Abdominal B ultrasonography, CT or MRI scans were performed to exclude other disorders, including HCC.
SCT
Autologous bone marrow mesenchymal stem cell (BMSC) transplantation or peripheral HSC transplantation was performed according to the patients’ willingness. All subjects signed the informed consent of SCT. For autologous BMSC transplantation, after skin sterilization and local anesthesia, marrow aspiration was performed in bilateral posterior-superior iliac crests. Approximately 100 mL of bone marrow (BM) was mixed evenly with BM storage buffer in a total volume of 180 mL and stored at 4 °C for subsequent experiments. For peripheral HSC transplantation, patients were given 300 μg (1.2 mL) recombinant human granulocyte colony stimulating factor injection for seven successive days to mobilize HSCs before transplantation, which has been demonstrated to be feasible and effective in previous studies[21,22]. HSCs were separated by a stem cell separator, and then 100 mL of HSCs were collected. The number of CD34+ stem cells was counted using flow cytometry, and CD34+ stem cells were obtained at a density of (2.81 ± 1.03) × 106 cells/mL.
Digital subtraction angiography-guided femoral artery puncture and catheterization was performed using the Seldinger technique, and hepatic angiography was conducted to identify the distribution of intrahepatic blood vessels. The catheter was inserted into the hepatic artery, and 100 mL of peripheral HSC (at a speed of 1.5 mL/min) or 180 mL of autologous BMSC (at 3 mL/min) were slowly injected with a microinfusion pump.
Follow-up
The survival and development of HCC was observed through outpatient follow-up visits. The follow up was performed during the period from the time of SCT to December 31, 2017 or death.
Statistical analysis
The patients’ gender, age, cause and Child-Pugh classification were adjusted using propensity score matching (PSM)[23], and the number of cases and controls were matched at a ratio of 1:3 by means of the nearest neighborhood matching and caliper matching, with a caliper width set as 0.2. Non-normally distributed data were described as quartiles and compared using the rank-sum test. Normally distributed data were expressed as mean ± SD and compared using a Student’s t-test. Differences of proportions were tested for statistical significance with a χ2 test. Survival analysis was performed using the Kaplan-Meier method, and the survival rate and incidence of HCC were compared between groups with the log-rank test. The risk factors of HCC were identified using a Cox proportional hazards regression model. All data were analyzed by SPSS 18.0 software (SPSS Inc., Chicago, IL, United States), and a value of P < 0.05 was considered statistically significant.
RESULTS
Comparison of baseline characteristics of overall cases between groups
A total of 218 patients with decompensated liver cirrhosis were admitted to the hospital during the period from January 2008 through December 2010, and 59 patients were excluded; finally, a total of 159 subjects were enrolled, including 27 patients undergoing SCT and 132 patients without transplantation (Figure 1). Of the 27 subjects undergoing SCT, there were 15 cases undergoing autologous bone-marrow SCT and 12 cases of peripheral hematopoietic SCT. There were significant differences in the prevalence of severe liver cirrhosis (Child-Pugh class C), PT, total bilirubin (TBIL) concentration, the prevalence of HBV infection and seropositive rate of HBsAg between the SCT group and the non-SCT group (P < 0.05) before PSM (Table 1). The overall mortality was 47.8% (76/159) in all study subjects, with 77.8% (21/27) mortality in the SCT group and 41.7% (55/132) in the non-SCT group (P < 0.05), and the overall incidence of HCC was 27.0% (43/159), with 40.7% (11/27) incidence in the SCT group and 24.2% (32/132) in the non-SCT group (P < 0.05).
Figure 1.
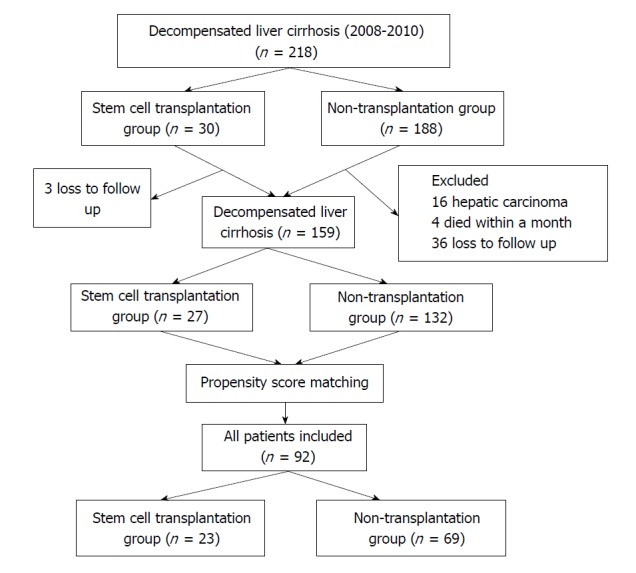
Flowchart of the study subject enrollment.
Table 1.
Comparison of the baseline demographic and clinical characteristics between the stem cell transplantation group and the non-transplantation group
| Characteristic |
Before propensity score matching |
After propensity score matching |
||||
| Stem cell transplantation group (n = 27) | Non-transplantation group (n = 132) | P-value | Stem cell transplantation group (n =23) | Non-transplantation group (n = 69) | P-value | |
| No. of male (%) | 18 (66.7%) | 100 (75.8%) | 0.325 | 15 (65.2%) | 45 (65.2%) | 1 |
| Age (yr) | 53.7 ± 9.7 | 53.3 ± 10.8 | 0.84 | 53.0 ± 9.7 | 55.3 ± 9.8 | 0.351 |
| History of smoking (%) | 6 (22.2) | 33 (25.0) | 0.76 | 4 (17.4) | 15 (21.7) | 0.656 |
| History of alcohol drinking, n (%) | 8 (29.6) | 33 (25.0) | 0.616 | 6 (26.1) | 20 (29) | 0.789 |
| Diabetes, n (%) | 6 (22.2) | 23 (17.4) | 0.556 | 6 (26.1) | 16 (23.2) | 0.778 |
| Family history of liver cancer, n (%) | 3 (11.1) | 10 (7.6) | 0.541 | 3 (13) | 3 (4.3) | 0.144 |
| Child-Pugh B to C ratio | 4:23 | 67:65 | 0.001 | 4:19 | 17:52 | 0.473 |
| MELD score | 14.36 (9.33-18.69) | 10.43 (7.22-15.91) | 0.051 | 15.55 ± 7.66 | 14.21 ± 8.32 | 0.498 |
| HBsAg positive, n (%) | 20 (74.1) | 127 (96.2) | 0 | 20 (87) | 66 (95.7) | 0.144 |
| PT (s) | 21.5 (18.2-26.7) | 18.2 (16.0-20.7) | 0.003 | 22.0 (18.1-27.8) | 19.3 (16.8-22.6) | 0.143 |
| TBIL (µmol/L) | 88.7 (46.6-141.1) | 38.4 (23.1-111.6) | 0.012 | 73.2 (46.6-137.9) | 46.4 (26.6-155.6) | 0.21 |
| ALB (g/L) | 26.89 ± 4.36 | 28.16 ± 5.63 | 0.268 | 27.08 ± 4.59 | 26.27 ± 6.02 | 0.56 |
| ALT (U/L) | 45 (30-60) | 54 (35-113) | 0.055 | 47 (36-63) | 48 (30-93) | 0.701 |
| PLT (× 109/L) | 79.11 ± 40.39 | 92.66 ± 58.39 | 0.252 | 81.78 ± 41.33 | 90.49 ± 65.35 | 0.551 |
| AFP (ng/mL) | 7.62 (3.68-20.80) | 6.90(2.73-33.87) | 0.889 | 7.93 (3.9-29.37) | 6.3 (2.47-23.88) | 0.564 |
| HBeAg titer (s/copies) | 0.45 (0.34-11.26) | 0.5 (0.34-24.68) | 0.808 | 0.45 (0.34-11.26) | 0.48 (0.34-22.13) | 0.906 |
| LogHBV DNA (IU/mL) | 4.45 ± 1.21 | 4.47 ± 1.44 | 0.966 | 4.45 ± 1.21 | 4.65 ± 1.48 | 0.097 |
| Cause, n (%) | ||||||
| HBV infection | 20 (74.1) | 127 (96.2) | 0 | 20 (87) | 66 (95.7) | 0.154 |
| Alcohol drinking | 4 (14.8) | 1 (0.8) | 1 (4.3) | 0 | ||
| Others | 3 (11.1) | 4 (3) | 2 (8.7) | 3 (4.3) | ||
MELD: Model for end-stage liver disease; HBsAg: Hepatitis B surface antigen; PT: Prothrombin time; TBIL: Total bilirubin; ALB: Albumin; ALT: Alanine aminotransferase; PLT: Platelet; AFP: Alpha fetoprotein; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus.
Baseline characteristics of patients after PSM
Since the Child-Pugh classification, HBV infection, gender and age were reported to correlate with the prognosis of liver cirrhosis[24,25], the subjects’ gender, age, cause and Child-Pugh classification were adjusted using PSM at a caliper width of 0.2 and a ratio of 1:3, and finally 92 subjects were enrolled in the final analysis (Figure 1). The subjects in the SCT group after PSM (n = 23) had a mean age of 53.0 ± 9.7 years and included 12 cases undergoing autologous bone-marrow stem-cell transplantation and 11 cases of peripheral hematopoietic stem-cell transplantation, and the subjects in the non-SCT group (n = 69) had a mean age of 55.3 ± 9.8 years. The subjects in both groups had a median follow-up period of 42 mo (range: 1-118 mo). Following PSM, no significant differences were detected in the demographic and clinical features between the two groups (P > 0.05) (Table 1).
Impact of SCT on survival
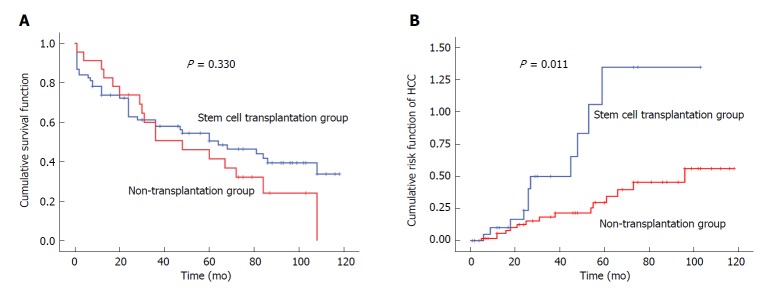
Of the 92 patients with decompensated liver cirrhosis, there were 55 deaths during the study period, with an overall mortality rate of 59.8%. There were 17 deaths in the SCT group (73.9% mortality), including five cases dying of gastrointestinal bleeding, seven cases dying of end-stage HCC, three cases dying of hepatic failure and two cases dying of cerebrovascular accidents. There were 38 deaths in the non-SCT group (55.1% mortality), including 13 cases dying of gastrointestinal bleeding, 13 cases dying of hepatic failure, five cases dying of HCC, and seven cases dying of other causes (lung cancer, laryngeal cancer, arrhythmia, electrolyte disorders and infection). The median survival period was 48 mo in the SCT group and 64 mo in the non-SCT group (Figure 2). No significant difference was found in the survival rate between the two groups (P > 0.05) (Table 2 and Figure 2A).
Figure 2.
Long-term outcomes of the study subjects. A: Survival curve of the study subjects; B: Risk curve for hepatocellular carcinoma in the study subjects. HCC: Hepatocellular carcinoma.
Table 2.
Comparison of the survival rate and incidence of liver cancer between the stem cell transplantation group and the non-transplantation group
| Time | Stem cell transplantation group | Non-transplantation group | χ2 value | P-value | |
| (n = 23) | (n = 69) | ||||
| Survival rate | 3-mo | 95.70% | 84.10% | 0.951 | 0.33 |
| 6-mo | 91.30% | 82.60% | |||
| 1-yr | 87.00% | 73.80% | |||
| 2-yr | 73.90% | 62.90% | |||
| 3-yr | 50.80% | 58.10% | |||
| 4-yr | 46.20% | 54.50% | |||
| 5-yr | 41.60% | 50.70% | |||
| 6-yr | 32.30% | 44.20% | |||
| 7-yr | 24.30% | 39.50% | |||
| Incidence of liver cancer | 3-mo | 0 | 0 | 6.3 | 0.011 |
| 6-mo | 4.80% | 1.70% | |||
| 1-yr | 9.50% | 5.50% | |||
| 2-yr | 20.80% | 14.10% | |||
| 3-yr | 41.40% | 19.30% | |||
| 4-yr | 49.80% | 22.40% | |||
| 5-yr | 66.50% | 29.00% | |||
| 6-yr | 66.50% | 36.30% | |||
| 7-yr | 83.30% | 42.70% |
Impact of SCT on the incidence of HCC
Of the 92 patients with decompensated liver cirrhosis, 26 patients developed HCC during the study period, with an incidence rate of 28.3%. There were 11 and 15 cases that developed HCC in the SCT group and non-SCT group, with 47.8% and 21.7% incidence, respectively, and a significant difference was observed between the two groups (P < 0.05). In addition, the 1-, 3-, 5- and 7-year incidence of HCC were all significantly higher in the SCT group than in the non-SCT group (P < 0.05) (Table 2 and Figure 2B).
Risk factors of HCC
In the univariate Cox regression analysis, SCT and age were found to correlate with the development of HCC (P < 0.05), while the medical history of diabetes, history of smoking, history of alcohol drinking, HBV infection, sex, Child-Pugh classification and family history of HCC in the first-degree relatives were not associated with the development of HCC (P > 0.05) (Table 3).
Table 3.
Cox regression analysis of risk factors of hepatocellular carcinoma
| Variable |
Univariate Cox regression analysis |
Multivariate Cox regression analysis |
||||
| HR | 95%CI | P | HR | 95%CI | P | |
| Stem cell transplantation | 2.664 | 1.211-5.859 | 0.015 | 3.065 | 1.378-6.814 | 0.006 |
| Age | 1.055 | 1.016-1.096 | 0.006 | 1.055 | 1.016-1.096 | 0.006 |
| Sex | 1.588 | 0.728-3.467 | 0.246 | |||
| History of diabetes | 1.098 | 0.439-2.741 | 0.842 | |||
| History of smoking | 1.475 | 0.675-3.223 | 0.330 | |||
| History of alcohol consumption | 1.546 | 0.698-3.423 | 0.283 | |||
| HBsAg positivity | 0.664 | 0.086-5.117 | 0.694 | |||
| Child-Pugh classification | 1.301 | 0.522-3.246 | 0.573 | |||
| Family history of liver cancer | 1.283 | 0.303-5.444 | 0.735 | |||
HBsAg: Hepatitis B surface antigen.
In the multivariate Cox regression, SCT (OR = 3.065, 95%CI: 1.378-6.814) and age (OR = 1.061, 95%CI: 1.021-1.102) were independently correlated with the development of HCC in this decompensated cirrhotic cohort (Table 3).
DISCUSSION
Recently, SCT has achieved great successes in the treatment of liver diseases[8]. However, there are still a large number of unsolved problems to date, such as the long-term efficacy and safety of SCT, which remain to be investigated[26].
Results from the clinical studies have shown that SCT achieves a satisfactory short-term efficacy for the treatment of decompensated liver cirrhosis[11,12]; however, the transplantation does not seem to increase the long-term efficacy[12,27,28]. In 53 liver failure patients caused by hepatitis B, a single transplantation with autologous BMSCs did not result in significant differences in liver function or MELD score between the transplantation group and controls three years after transplantation, and the 192-wk follow-up revealed no significant difference in the survival rate between the two groups, suggesting no marked improvements in long-term efficacy[12]. A recent meta-analysis to examine the clinical outcomes of the transplantation of stem cells from various human tissue sources in cirrhotic patients showed no significant difference in the mortality between the treatment and control groups, and concluded that SCT could improve liver function but appeared to not be significant in increasing the survival in cirrhotic patients[29]. In the current study, a 10-year follow-up revealed 73.9% (17/23) deaths in the decompensated liver cirrhosis cases undergoing SCT. We did not find a significant difference in the survival rate between the two groups, which was similar to previous reports[12,29]. The plausible explanation is that SCT can minimally reverse portal hypertension and the development of cancer in decompensated cirrhosis, as most causes of death were due to gastrointestinal bleeding and HCC in patients undergoing SCT in this cohort.
Previous studies have demonstrated the short-term safety of SCT[11,13,29,30]; however, its long-term safety has not been fully demonstrated. Results from previous clinical studies have demonstrated that SCT does not increase the risk of developing HCC[11-13,29,31]; however, the follow-up periods (no more than two years) in those studies were not long enough to observe the development of cancer. In this study, the 10-year follow-up showed a gradual increase in the incidence of HCC in the SCT group with the extension of the follow-up period. This significant difference between two groups suggested a possible tumorigenicity of SCT in patients with decompensated liver cirrhosis. Stem cells have a strong self-renewal capability and multi-lineage differentiation potential[16], and tumorigenicity of BM stem cell has been observed in animal experiments[32,33], which provides theoretical evidence for the findings from the present study. As reported by a recent review, the 5-year cumulative incidence of all second malignancies after autologous SCT for hematological disorders is 4.3%, and the 15-year cumulative incidence is 8%-15.3%[17], indicating a gradually increased risk for malignancy in patients with SCT therapy. The risk of HCC in cirrhosis patients might be associated with the activation of hepatic stellate cells and secretion of multiple growth factors and cytokines[34,35]. This may produce a microenvironment for developing HCC, thereby promoting the development and progression of HCC[35]. Follow-up of the fate of administered stem cells using combined imaging methods has been proposed as a method to discriminate tumorigenic transformation. In the future, this technology can be used to monitor liver cancer after SCT[36,37].
Approximately 80% of HCC develops from liver cirrhosis[1]. Multiple factors have been identified as the risk factors of HCC in liver fibrotic patients[31,38,39]. In the current study, only two factors, SCT and age, were included in the multivariate Cox regression model. Multiple risk factors of HCC were excluded during PSM, such as HBV infection, resulting in no statistical significance of conventional risk factors during the univariate Cox regression analysis. However, this did not deny the significance of these variables. In addition, Cox hazard regression analysis identified age as the risk factor of HCC in patients with liver fibrosis, which may be attributed to the longer duration of liver fibrosis in older patients.
The current study has some limitations: (1) Considering the likelihood of tumorigenicity of stem cells, cancers may occur in both the liver and other organs[17,18]; however, we only found four cancers in organs other than the liver, which cannot be analyzed; and (2) This is a single-center retrospective cohort study, although we tried to match patients with and without SCT by PSM. However the selection bias and confounding bias cannot be completely excluded. Further randomized, prospective clinical trials with larger sample sizes and extension of follow-up period are required to evaluate the long-term efficacy and safety of stem cell therapy for decompensated liver cirrhosis.
In summary, the results of the present study demonstrate that SCT fails to increase the long-term survival rate and increase the incidence of HCC in patients with decompensated liver cirrhosis, indicating an unsatisfactory long-term efficacy and safety. It is suggested that close monitoring of HCC is required in patients with decompensated liver cirrhosis undergoing SCT.
ARTICLE HIGHLIGHTS
Research background
Decompensated liver cirrhosis greatly affects patients’ life quality and expectancy. However, the tumorigenicity of stem cells impedes them as a basis for regenerative medicine treatment.
Research motivation
This study evaluates the long-term efficacy and safety of autologous stem cell transplantation (SCT) for decompensated liver cirrhosis based on ten years of follow-up.
Research objectives
We aimed to compare the survival rate and incidence of hepatocellular carcinoma (HCC) in decompensated liver cirrhosis patients with and without SCT, so as to evaluate the long-term efficacy and safety of SCT.
Research methods
Consecutive patients with decompensated liver cirrhosis were included and assigned into the SCT group and non-transplantation (non-SCT) group according to whether they received SCT treatment. Patients were followed up for ten years.
Research results
The incidence of HCC was higher in the SCT group than in the non-SCT group. After adjusting for other covariates, SCT and age were independently correlated with the development of HCC in this decompensated liver cirrhosis cohort.
Research conclusions
Autologous SCT may fail to improve the long-term efficacy and increase the incidence of HCC for decompensated liver cirrhosis.
Research perspectives
Close monitoring of HCC is strongly recommended in patients undergoing autologous SCT.
Footnotes
Institutional review board statement: This study was reviewed and approved by the institutional board of the First Affiliated Hospital, Fujian Medical University.
Informed consent statement: Our patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after the patient agreed to treatment by verbal consent. Individuals cannot be identified by the data presented.
Conflict-of-interest statement: All authors declare no potential conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: June 25, 2018
First decision: July 19, 2018
Article in press: August 26, 2018
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Labusca L, Liu DW, He XH S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
Contributor Information
Ming-Fang Wang, Liver Center, the First Affiliated Hospital, Fujian Medical University, Fuzhou 350005, Fujian Province, China.
You-Bing Li, Liver Center, the First Affiliated Hospital, Fujian Medical University, Fuzhou 350005, Fujian Province, China.
Xiao-Juan Gao, Fujian Provincial Governmental Hospital, Fuzhou 350001, Fujian Province, China.
Hao-Yang Zhang, School of Biological Sciences, the University of Hong Kong, Hong Kong, China.
Su Lin, Liver Center, the First Affiliated Hospital, Fujian Medical University, Fuzhou 350005, Fujian Province, China.
Yue-Yong Zhu, Liver Center, the First Affiliated Hospital, Fujian Medical University, Fuzhou 350005, Fujian Province, China. zhuyueyong@fjmu.edu.cn.
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 2.Poordad FF. Presentation and complications associated with cirrhosis of the liver. Curr Med Res Opin. 2015;31:925–937. doi: 10.1185/03007995.2015.1021905. [DOI] [PubMed] [Google Scholar]
- 3.Lerschmacher O, Koch A, Streetz K, Trautwein C, Tacke F. [Management of decompensated liver cirrhosis in the intensive care unit] Med Klin Intensivmed Notfmed. 2013;108:646–656. doi: 10.1007/s00063-013-0259-6. [DOI] [PubMed] [Google Scholar]
- 4.El-Masry M, Puig CA, Saab S. Recurrence of non-viral liver disease after orthotopic liver transplantation. Liver Int. 2011;31:291–302. doi: 10.1111/j.1478-3231.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 5.Abdeldayem HM, Allam NA, Salah E, Mostafa Aziz A, Kashkoush S, Adawy NM, Gad H, Helmy A. Moral and ethical issues in living-donor liver transplant in Egypt. Exp Clin Transplant. 2009;7:18–24. [PubMed] [Google Scholar]
- 6.Giri S, Bader A. Personalized and Regenerative Medicine for Liver Diseases. Curr Stem Cell Res Ther. 2016;11:692–705. doi: 10.2174/1574888x10666151026115128. [DOI] [PubMed] [Google Scholar]
- 7.Kholodenko IV, Yarygin KN. Cellular Mechanisms of Liver Regeneration and Cell-Based Therapies of Liver Diseases. Biomed Res Int. 2017;2017:8910821. doi: 10.1155/2017/8910821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiota G, Itaba N. Progress in stem cell-based therapy for liver disease. Hepatol Res. 2017;47:127–141. doi: 10.1111/hepr.12747. [DOI] [PubMed] [Google Scholar]
- 9.Kwak KA, Cho HJ, Yang JY, Park YS. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can J Gastroenterol Hepatol. 2018;2018:4197857. doi: 10.1155/2018/4197857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsolaki E, Yannaki E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J Gastroenterol. 2015;21:12334–12350. doi: 10.3748/wjg.v21.i43.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 12.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 13.Spahr L, Chalandon Y, Terraz S, Kindler V, Rubbia-Brandt L, Frossard JL, Breguet R, Lanthier N, Farina A, Passweg J, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8:e53719. doi: 10.1371/journal.pone.0053719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37:16. doi: 10.1186/s41232-017-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936–941. doi: 10.1097/MEG.0b013e3283488b00. [DOI] [PubMed] [Google Scholar]
- 16.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Danylesko I, Shimoni A. Second Malignancies after Hematopoietic Stem Cell Transplantation. Curr Treat Options Oncol. 2018;19:9. doi: 10.1007/s11864-018-0528-y. [DOI] [PubMed] [Google Scholar]
- 18.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 19.Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–2805. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye SL. [Expert consensus on standardization of the management of primary liver cancer] Zhonghua Ganzangbing Zazhi. 2009;17:403–410. [PubMed] [Google Scholar]
- 21.Sharma M, Rao PN, Sasikala M, Kuncharam MR, Reddy C, Gokak V, Raju B, Singh JR, Nag P, Nageshwar Reddy D. Autologous mobilized peripheral blood CD34(+) cell infusion in non-viral decompensated liver cirrhosis. World J Gastroenterol. 2015;21:7264–7271. doi: 10.3748/wjg.v21.i23.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salama H, Zekri AR, Bahnassy AA, Medhat E, Halim HA, Ahmed OS, Mohamed G, Al Alim SA, Sherif GM. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol. 2010;16:5297–5305. doi: 10.3748/wjg.v16.i42.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pingault JB, Côté SM, Petitclerc A, Vitaro F, Tremblay RE. Assessing the independent contribution of maternal educational expectations to children’s educational attainment in early adulthood: a propensity score matching analysis. PLoS One. 2015;10:e0119638. doi: 10.1371/journal.pone.0119638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piekarska A, Zboinska J, Szymczak W, Kuydowicz J. Independent prognostic factors in patients with liver cirrhosis. Hepatogastroenterology. 2008;55:1034–1040. [PubMed] [Google Scholar]
- 25.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Di Nardo P, Singla D, Li RK. The challenges of stem cell therapy. Can J Physiol Pharmacol. 2012;90:273–274. doi: 10.1139/y2012-016. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Li J, Li P, Bai M, Guo Y, Han M, Zhang F, Ahmed R, Jin S. Stem cell transplantation for the treatment of liver diseases: A systematic review and meta-analysis. Turk J Gastroenterol. 2016;27:499–508. doi: 10.5152/tjg.2016.16398. [DOI] [PubMed] [Google Scholar]
- 28.Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 29.Qi X, Guo X, Su C. Clinical outcomes of the transplantation of stem cells from various human tissue sources in the management of liver cirrhosis: a systematic review and meta-analysis. Curr Stem Cell Res Ther. 2015;10:166–180. doi: 10.2174/1574888x09666141112114011. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Torimura T, Iwamoto H, Kurogi J, Inoue H, Hori Y, Sumie S, Fukushima N, Sakata M, Koga H, et al. CD34(+) cell therapy is safe and effective in slowing the decline of hepatic reserve function in patients with decompensated liver cirrhosis. J Gastroenterol Hepatol. 2014;29:1830–1838. doi: 10.1111/jgh.12622. [DOI] [PubMed] [Google Scholar]
- 31.Mohamadnejad M, Ashrafi M, Alimoghaddam K, Vosough M, Mardpour S, Azimian V, Aghdami N, Bagheri M, Abdollahzadeh L, Bashtar M, et al. Surveillance for hepatocellular carcinoma after autologous stem cell transplantation in cirrhosis. Middle East J Dig Dis. 2012;4:145–149. [PMC free article] [PubMed] [Google Scholar]
- 32.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 33.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 34.Thompson AI, Conroy KP, Henderson NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015;15:63. doi: 10.1186/s12876-015-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014;34:834–843. doi: 10.1111/liv.12465. [DOI] [PubMed] [Google Scholar]
- 36.Labusca LS, Herea DD, Radu E, Danceanu C, Chiriac H, Lupu N. Human Adipose Derived Stem Cells and Osteoblasts Interaction with Fe-Cr-Nb-B Magnetic Nanoparticles. J Nanosci Nanotechnol. 2018;18:5143–5153. doi: 10.1166/jnn.2018.15330. [DOI] [PubMed] [Google Scholar]
- 37.Labusca L, Herea DD, Mashayekhi K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J Stem Cells. 2018;10:43–56. doi: 10.4252/wjsc.v10.i5.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takami T, Terai S, Sakaida I. Novel findings for the development of drug therapy for various liver diseases: Current state and future prospects for our liver regeneration therapy using autologous bone marrow cells for decompensated liver cirrhosis patients. J Pharmacol Sci. 2011;115:274–278. doi: 10.1254/jphs.10r13fm. [DOI] [PubMed] [Google Scholar]
- 39.Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: A prospective cohort study in Korea. Cancer. 2018;124:2748–2757. doi: 10.1002/cncr.31406. [DOI] [PubMed] [Google Scholar]