Abstract
Anxiety-related bias in the recognition memory based on trait anxiety has induced some studies. Their results, however, were conflicting. In fact, anxious differences not only differed from personality traits but also from different anxiety mood levels. We explored the emotional memory bias in both trait and state anxiety individuals, the high trait and high state anxiety group, the high trait and low state anxiety group, the low trait and high state anxiety group, and the low trait and low state anxiety group, on classic recognition paradigm using event-related potentials (ERPs). The behavioral results showed high state anxiety levels increased the d’ of negative words, regardless of the trait anxiety of participant is high or low, and a lower d’ of recognition memory for negative words than for neutral and positive words in all participants. Moreover, Electrophysiological results supported the findings of behavior, showing an earlier N400 (250–500 ms) latency elicited for new-negative words in high state level than in low state levels in right parietal region. These results suggested that the memory bias to negative events resides in state anxiety, but not in trait anxiety.
Introduction
Anxiety is anticipation of future threat, it’s more often associated with muscle tension and vigilance in preparation for future danger and cautious or avoidant behaviors, some the level of anxiety is reduced by pervasive avoidance behaviors1,2. There has been a large increase in the incidence rates of anxiety disorders and symptoms over the years, however, it is hard to investigate anxiety independently due to the high comorbidity rate (as much as 50%)3. Trait anxiety, innate predisposition, is a relatively stable personality trait with the reaction of the individual differences4. Studies found that high trait anxiety of human was extremely similar with anxiety disorders about behavioral and cognitive features, so individuals with high trait anxiety from a non-clinical group have a predisposition for an anxiety-related bias in emotional and cognitive processing, high trait anxiety is considered to be a prerequisite for psychiatric disorders5,6. In other words, individuals with high trait anxiety may be a proper sample to uncover the characters of anxiety, understanding the neural basis in non-clinically anxious individuals will provide a broad picture of the neuro-scientific basis of anxiety disorders7.
Cognitive bias, especially the memory processing, were the focus of many researchers, whereas the evidence for anxiety-related recognition memory bias is still scant and inconsistent across studies8–12, some studies showed a bias to recognize threatening items13–15, some got a result contrary16–20. Up to now, whether there has a robust bias in trait anxiety under the negative recognition processing is still unclear. Cognitive-motivational view consider the effect of negative biases in anxiety is not only trait anxiety, but also have situational context, state anxiety, prior learning, biological preparedness21. And model of William et al. representing the cognitive mechanisms underlying biases in initial orienting to treat in anxiety is interaction effect of state and trait anxiety on attentional bias22. Anxiety can be classified as trait anxiety and state anxiety23,24. State anxiety is a common mood in daily life, and it can be a kind of brief emotional experience, accompanied by physiological arousal and subjective feelings (e.g., nervous, fear) as environmental change4. Specifically, the correlation is very high between trait and state anxiety, many studies had found that the effect of state anxiety was stronger than trait anxiety to task performance25–27, furthermore, a behavioral research found that the effect of recognition negative biases was state anxiety alone, not trait anxiety12. The factor, state anxiety levels (e.g. high vs. low), might affect the size and direction of threat bias in recognition tasks21,28. Thus, the present study intends to control the state anxiety level into high and low state anxiety groups by experiment operation. Since in most studies state anxiety has more significant impact on task performance than trait anxiety25–27,29, we predicted state anxiety would have the most obvious infection on memory bias than trait anxiety.
Therefore, in order to explore whether individuals with high trait anxiety and state anxiety have anxiety recognition bias, we employed a classic recognition paradigm, used the neutral, positive and negative words as stimulus. We added experimental operation between learning and testing phase to induce/calm state anxiety, the experimental operation was that participants were asked to read one of two scenarios describing different events and then image that they were experiencing the event29–31, what’s more, studies have showed that this experimental operation was very effective in inducing state anxiety (negative mood states)27,30–32. Thence, participants were divided into high state anxiety group and low state anxiety group by the experiment operation. Meanwhile, participants were also divided into high and low trait group according to their trait anxiety scores. Altogether, four groups (high state with high trait group, high state with low trait group, low state with high trait group and low state with low trait group) were involve in the present study. Given the stronger effect of state anxiety than trait anxiety to task performance25–27, and relatively stable personality of the trait anxiety, high trait individuals might induce higher state anxiety compared to low trait anxiety individuals, we predicted state anxiety is an intervening variable between trait anxiety and emotional valence events. If there existed anxiety recognition bias, the high state with high trait group would obtain the maximized effect, followed by the high state with low anxiety group, low state with high anxiety group, low state with low trait anxiety. Further, event-related potential (ERP) possess excellent temporal resolution and a continuous measure of processing, thus, we collected participants’ electroencephalography (EEG) data synchronously. Many studies claimed that the N400 was related with semantic processing33–35, moreover, was influenced by the effects of emotional valence36–38, and, participants’ emotional state36,39,40. Thence, the index of parietal N400 might be sensitive and effective to emotional words in present study.
Method
Participants
Two hundred participants were selected by the random sampling method from five grades’ undergraduates, who took elective courses in psychology in Xinxiang Medical University. All the participants were tested by the Trait Anxiety Inventory (the second 20 questions of state - trait anxiety questionnaire)41. The T-AI consists of 20 self-report items that measure anxiety-related trait personality, with high internal consistency and test-reliability ranging from 0.73 to 0.86 across multiple samples23,42. The top and down 27% of the all trait anxiety score were selected for the study and the Chinese norm score of trait anxiety are 41.11 ± 7.74 (males) and 41.31 ± 7.54 (females) respectively43. Participants were recruited and divided into two groups with the following standard of evaluation: participants scored higher than or equal to 45 were assigned to the high trait anxiety group, while the ones scored lower than or equal to 40 were assigned to the low trait anxiety group. Seventy-four participants (38females; mean age = 20.45 ± 1.59 years) were recruited depending on the scores. They got the formal experiment in the laboratory of psychology. Altogether, thirty-seven participants (18males, 19females; mean score = 50.32 ± 5.34) were assigned to the high trait anxiety group, and thirty-seven participants (18males, 19females; mean score = 34.51 ± 4.63) were assigned to the low trait anxiety group and then an independent samples t test showed this difference to be significant t (72) = 0.35, p < 0.01. Three participants were excluded from further analysis because of noisy electroencephalography. Thence, the induced state anxiety group consisted of 20 high trait anxiety participants (10 males, 10 females) and 17 low trait anxiety participants (7 males, 10 females); the non-induced state anxiety group consisted of 16 high trait anxiety participants (7 males, 9 females) and 16 low trait anxiety participants (9 males, 7 females). All participants were right-handed, and have normal or corrected-to-normal vision.
Ethics Statement
All participants gave their written informed consent in accordance with the Declaration of Helsinki44. The ethics committee of the Xinxiang Medical University approved this study. The experimental methods were carried out in “accordance” with the approved guidelines.
Materials
Materials
Stimulus were selected from Chinese Affective Words System(CAWS)(Wang & Zhou, 2008). There were 60 negative-arousing words (mean valence = 2.54; mean arousal = 6.63), 60 positive-arousing words (mean valence = 7.35; mean arousal = 6.61), and 60 neutral, non-arousing words (mean valence = 5.40; mean arousal = 4.13). Altogether, one hundred eighty words were included in the experiment. Word frequencies were equated across emotion.
Procedure
The experiment was composed of three phases, one study phase, one imagining phase and one test phase. In the study phase, participants were instructed to memory 120 words (40 neutral, 40 negative, 40 positive). Each of the words was displayed for 1500 ms. Then in imagining phase, the instruction 1 was presented to the induced state anxiety group, or the instruction 2 was presented to the non-induced state anxiety group. Before and after the imagining phase, participants were tested by the State-Trait Anxiety Inventory (the first 20 questions of state-trait anxiety questionnaire), it was used to measure participants’ state anxiety level. Immediately following the presentation of the study list, participants made recognition judgments for 120 words, which 60 studied words that had been presented in the study phase, and 60 new words had never been presented before, the words were presented one at a time for 1000 ms each, then the response interface was presented for 2000 ms to made participants delay to respond. All stimuli were presented in white letters against black backgrounds. For items judged to be “old” pressing “F” key, lures judged to be “new” by pressing “J” key. Study and test sequences were randomly ordered for each subject. The instruction 1 and instruction 2 were depicted as follows:
Instruction 1: Hello, welcome to participate in this study. This is a test to inspect the ability of imagination about details.
Now please image that: you got a call from you tutor suddenly, the tutor asked you to go to his office at once, it seems that there will be a very serious matter. Please image all the details and your psychological physiology reaction between the period you got the call and you got to the office.
Please press the space bar to start the test when you understand the introduction.
Instruction 2: Hello, welcome to participate in this study. This is a test to inspect the ability of imagination about details. Now please recall all the details that you got out of the bed and washed in this morning.
Please press the space bar to start the test when you understand the introduction.
ERP data acquisition
Brain electrical activity was recorded from 64 Ag-AgCl scalp sites according to the international 10–20 system in an elastic cap (NeuroScan Prouct). During recording, all electrodes were referenced to Cz and re-referenced off-line to linked mastoids. Channels for horizontal and vertical EOG were computed offline from electrodes recorded from the outer canthi of the eyes and from above and below the right eye, respectively. Electrode impedance was kept below 5 kΩ.
EEG was sampled on-line with a frequency of 500 Hz DC-amplifiers with a band-pass filter of 0.1–100 Hz. Data was filtered off-line by a band-pass filter of 0.1–25 Hz and runned an independent component analysis(ICA) for eye movement correction45.
Data analysis
Behavioral data analysis
A standard way of measuring accuracy is to calculate β and d’, the distance between the means of the memory strength distributions of studied words and unstudied words. The percentages of correct answers on the memory test were subjected to three-way repetitive measure analysis of variance (ANOVA). We applied the theory of detection to the memory test.
According to the signal detection theory (SDT), we defined four possible outcomes for each trial depending on a word of object chosen from the study phase or not (old/new judgement) and participants’ response: hits, misses, false alarms (FA), and correct rejections (CR). Specifically, a hit is an accurate judgement that word has been shown in the study phase (old word); a miss is a failure detection for the old word; a correct rejection is an accurate judgement that the word was not chosen in the study phase; and a false alarm is a failure detection for the new word. Then the P (H) and the P (FA) in the items and the lures were analyzed by the following formula:
The P(H) and the P(FA) in the items and the lures were translated to O(H), O(FA) Z(H) and Z(FA) using PZO translation. Then, the likelihood ratio (β) and discriminability index (d’) in the items and the lures were analyzed by the following formula:
Higher β values (the likelihood ratio or decision criteria, the more the β is, the more strict the criteria is) indicated worse memory performance in this study. Higher d’ values (discriminability index or sensibility, the more the d’ is, the more sensibility is) indicates best memory performance in this study. The β and d’ values in the two conditions were subjected to three-way ANOVA. All p-values were corrected using the Bonferroni adjustment.
ERP data analysis
ERPs were calculated time-locked to the onset of the search display, with segments extending from 100 ms before stimulus onset until 1000 ms afterwards. In analyses, the 100 ms interval preceding target onset served as baseline. Artifacts produced by blinks or eye movements were corrected by subtracting means of ICAs implemented in the EEGLab software45. Trials with incorrect responses were excluded from analysis.
Significance
Studies found that anxiety-related recognition memory bias was still inconsistent. Here, we asked different trait anxiety level individuals under different state anxiety levels to perform classic learn-test task. Our results showed that there existed the memory bias to negative events in state anxiety, but not in trait anxiety.
Results
Manipulation check
The pretest and posttest’s scores of STAI-S of the induced state anxiety groups were calculated separately (pretest mean = 38.85 ± 9.16; posttest mean = 41.28 ± 11.85), and then a paired sample t test showed this difference was significant t (38) = −2.10, p < 0.05. Similarly, the twice scores of STAI-S of the calm state anxiety groups were calculated separately (pretest mean = 36.19 ± 6.99; posttest mean = 36.63 ± 7.61), and the result of paired sample t test showed there was no difference t (31) = −0.54, p > 0.1. Therefore, the experiment manipulation is effective.
Behavioral data
d’. A 2 (state anxiety: high vs. low) × 2 (trait anxiety: high vs. low) × 3 (emotional valence: negative vs positive vs neutral) repeated-measure ANOVA was performed. The interaction between valence and state anxiety was significant, F (2, 66) = 6.048, p < 0.01, η2 = 0.083. Subsequent simple effect analyses showed that significant differences between high state anxiety group and calm group about the d’ were found of negative words (F (1, 67) = 20.16, p < 0.01), but not in neutral and positive words (ps > 0.1), showing that the memory recognition processing was different to negative words and the other two groups’ words. In addition, state anxiety influenced memory retrieval phase on recognition task, but trait anxiety did not. Moreover, only the d’ of negative words of high state anxiety group was larger than calm group. The results showed that there was significant main effect of emotional valence, F (2, 66) = 55.66, p < 0.001, η2 = 0.454, and state anxiety, F (1, 67) = 7.34, p < 0.01, η2 = 0.10. There was no significant difference between positive words and neutral words, but the d’ of negative words was smaller than positive words and neutral words. No other statistical differences were found (all ps > 0.1). Table 1 Mean accuracy (%) of trait, state anxiety and emotional valence for all conditions above. Table 2. Mean d’ of trait, state anxiety and emotional valence for all conditions above.
β. B 2 (state anxiety: high vs. low) × 2 (trait anxiety: high vs. low) × 3 (emotional valence: negative vs positive vs neutral) repeated-measure ANOVA was performed. We have not found any differences about β. Table 2. Mean β of trait, state anxiety and emotional valence for all conditions above.
Table 1.
Mean accuracy (%) of trait, state anxiety and emotional valence for all conditions above.
| Negative | Neutral | Positive | |
|---|---|---|---|
| induced state and high trait anxiety | 0.62 ± 0.09 | 0.66 ± 0.08 | 0.68 ± 0.10 |
| induced state and low trait anxiety | 0.61 ± 0.09 | 0.70 ± 0.07 | 0.69 ± 0.07 |
| non-induced state and high trait anxiety | 0.62 ± 0.07 | 0.67 ± 0.06 | 0.70 ± 0.07 |
| non-induced state and low trait anxiety | 0.61 ± 0.11 | 0.66 ± 0.07 | 0.66 ± 0.08 |
Table 2.
Mean β and d’ of trait, state anxiety and emotional valence for all conditions above.
| Negative | Neutral | Positive | ||||
|---|---|---|---|---|---|---|
| β | d’ | β | d’ | β | d’ | |
| induced state and high trait anxiety | 1.11 ± 0.30 | 0.63 ± 0.52 | 1.12 ± 0.68 | 1.21 ± 0.72 | 1.15 ± 0.72 | 1.00 ± 0.51 |
| induced state and low trait anxiety | 1.04 ± 0.21 | 0.53 ± 0.59 | 1.36 ± 0.76 | 1.09 ± 0.71 | 1.26 ± 0.44 | 1.23 ± 0.45 |
| non-induced state and high trait anxiety | 1.26 ± 0.76 | −0.03 ± 0.62 | 1.36 ± 0.71 | 1.21 ± 0.50 | 1.25 ± 0.57 | 1.03 ± 0.27 |
| non-induced state and low trait anxiety | 1.14 ± 0.44 | −0.01 ± 0.45 | 1.29 ± 0.75 | 0.96 ± 0.63 | 1.08 ± 0.49 | 1.09 ± 0.53 |
ERP data
N400 (250–500 ms timing window)
According to previous researches, N400 was related to memory46,47 and semantic processing33–35, and even to the emotional valence36–38 and participants’ emotional state36,39,40. Here, we used the parietal N400 as an index on recognition memory. The latency and peak of the N400 were measured from 250 to 500 ms, after the stimulus onset, as defined in previous researches48–51, centroparietal (CPz, CP1/2, CP3/4, CP5/6) and parietal (Pz, P1/2, P3/4, P5/6), latencies and peaks of typically maximum amplitude regions for N400 were analyzed.
For the latencies of N400, a 2 (trait anxiety: high/low) × 2 (state anxiety: high/low) × 3 (emotional valence: negative/neutral/positive) × 2 (responses: old/new) repeated measures ANOVA were analyze. The interactions of valence, response and state anxiety were significant over right parietal (i.e., P4) and centroparietal (i.e., CP1/CPZ/CP2/CP4/CP6). There are significances about the interaction of response, trait and state anxiety over right parietal (i.e., P1/P4) and centroparietal (i.e., CP1/CPZ/CP2/CP4/CP6). The other main effects and interactions were not significant or just significant in few sites, so no further analysis were carried out on them. The specific data were affixed in Table 3.
Table 3.
The latencies of N400 were analyzed with a 2 (trait anxiety: high/low) × 2 (state anxiety: high/low) × 3 (emotional valence: negative/neutral/positive) × 2 (responses: old/new) repeated measures ANOVA.
| df | CP1 | CPZ | CP2 | CP4 | CP6 | P1 | P4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | η2 | F | η2 | F | η2 | F | η2 | F | η2 | F | η2 | F | η2 | ||
| responses | 1, 67 | 1.72 | 0.03 | 3.25 | 0.05 | 1.02 | 0.02 | 0.03 | 0.01 | 0.70 | 0.01 | 0.19 | 0.01 | 0.14 | 0.01 |
| responses*trait | 1, 67 | 1.41 | 0.02 | 0.02 | 0.01 | 2.14 | 0.03 | 2.65 | 0.04 | 2.06 | 0.03 | 0.01 | 0.01 | 0.70 | 0.01 |
| responses*state | 1, 67 | 2.09 | 0.03 | 0.76 | 0.01 | 2.14 | 0.03 | 0.26 | 0.01 | 0.03 | 0.01 | 0.06 | 0.01 | 1.17 | 0.02 |
| responses*trait*state | 1, 67 | 7.78** | 0.10 | 6.61* | 0.09 | 6.10* | 0.08 | 10.70** | 0.14 | 4.07* | 0.06 | 9.18** | 0.12 | 6.53* | 0.09 |
| emotion | 2, 66 | 1.70 | 0.25 | 2.22 | 0.03 | 4.14* | 0.06 | 1.46 | 0.02 | 0.93 | 0.01 | 6.59** | 0.09 | 0.93 | 0.01 |
| emotion*trait | 2, 66 | 0.21 | 0.01 | 0.60 | 0.01 | 0.79 | 0.01 | 0.52 | 0.01 | 0.21 | 0.01 | 0.82 | 0.01 | 0.24 | 0.21 |
| emotion*state | 2, 66 | 0.50 | 0.01 | 2.14 | 0.03 | 0.66 | 0.01 | 0.25 | 0.01 | 1.53 | 0.02 | 0.17 | 0.01 | 0.60 | 0.01 |
| emotion*trait*state | 2, 66 | 1.19 | 0.02 | 1.44 | 0.02 | 1.13 | 0.02 | 0.87 | 0.01 | 2.46 | 0.04 | 0.78 | 0.01 | 0.37 | 0.01 |
| responses*emotion | 2, 66 | 0.20 | 0.03 | 0.69 | 0.01 | 0.45 | 0.01 | 2.06 | 0.03 | 1.90 | 0.03 | 0.58 | 0.01 | 1.68 | 0.02 |
| responses*emotion*trait | 2, 66 | 2.55 | 0.04 | 1.15 | 0.02 | 0.20 | 0.01 | 0.46 | 0.01 | 0.31 | 0.01 | 1.10 | 0.02 | 1.03 | 0.02 |
| responses*emotion*state | 2, 66 | 4.80* | 0.07 | 6.27** | 0.09 | 7.18** | 0.10 | 4.55* | 0.06 | 4.50* | 0.06 | 2.33 | 0.34 | 4.85* | 0.07 |
| response*emotion*trait*state | 2, 66 | 0.61 | 0.01 | 0.23 | 0.01 | 0.14 | 0.01 | 1.00 | 0.02 | 0.39 | 0.01 | 0.56 | 0.01 | 0.15 | 0.02 |
| trait | 1, 67 | 0.01 | 0.01 | 0.03 | 0.01 | 0.00 | 0.00 | 0.32 | 0.01 | 0.01 | 0.01 | 0.08 | 0.01 | 0.22 | 0.01 |
| state | 1, 67 | 0.25 | 0.01 | 0.23 | 0.01 | 0.10 | 0.01 | 0.10 | 0.01 | 1.06 | 0.02 | 1.41 | 0.02 | 1.45 | 0.02 |
| trait*state | 1, 67 | 1.16 | 0.02 | 0.13 | 0.01 | 0.34 | 0.01 | 1.45 | 0.02 | 0.71 | 0.01 | 1.85 | 0.03 | 2.09 | 0.03 |
Notes: *<0.05, **<0.01.
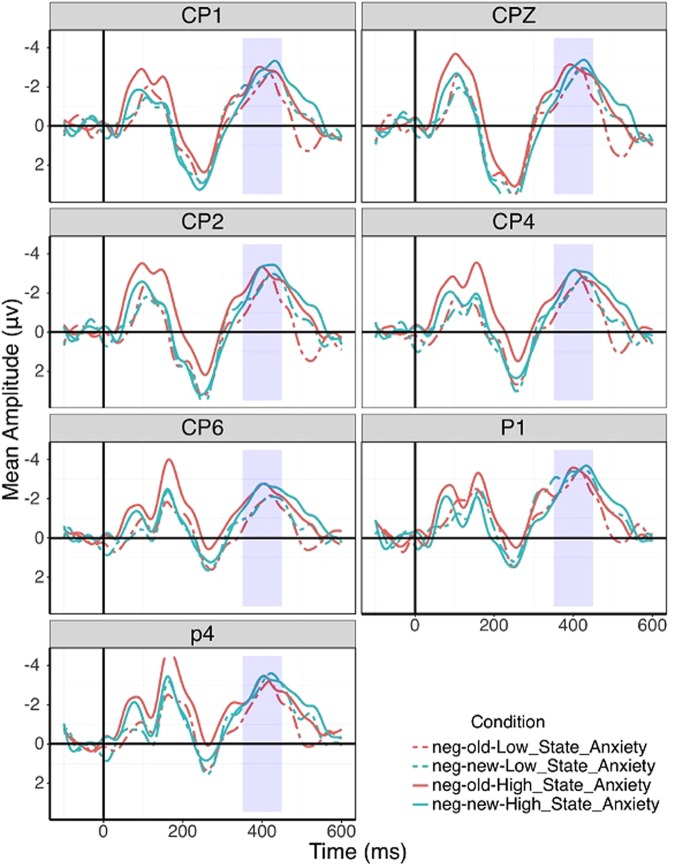
For the interaction of valence, response and state anxiety, we reanalyzed it with a 2 (state anxiety: high/low) × 3 (emotional valence: negative/neutral/positive) × 2 (responses: old/new) repeated measures ANOVA. There were significances about the interaction of valence, response and state anxiety over right parietal (CP1: F(2, 68) = 4.72, p < 0.05, η2 = 0.06; CPZ: F(2, 68) = 6.37, p < 0.01, η2 = 0.08; CP2: F(2, 68) = 7.40, p < 0.01, η2 = 0.10; CP4: F(2, 68) = 4.60, p < 0.05, η2 = 0.06; CP6: F(2, 68) = 4.64, p < 0.05, η2 = 0.06; P4: F(2, 68) = 4.94, p < 0.05, η2 = 0.07). No other statistical differences were found (all ps > 0.1). Subsequent simple main effect tests showed that the latency induced by negative words was shorter than calm group under the new words condition in anxious state group (CP1: F(1, 69) = 5.86, p < 0.05, η2 = 0.08; CPZ: F(1, 69) = 10.20, p < 0.01, η2 = 0.13; CP2: F(1, 69) = 6.77, p < 0.05, η2 = 0.09; CP4: F(1, 69) = 3.55, p < 0.1, η2 = 0.05; CP6: F(1, 69) = 7.29, p < 0.01, η2 = 0.10; P4: F(1, 69) = 5.46, p < 0.05, η2 = 0.07), there was no same differences among other parts. For the interaction of response, trait and state anxiety, we reanalyzed it with a 2 (state anxiety: high/low) × 3 (trait anxiety: high/low) × 2 (responses: old/new) repeated measures ANOVA. There were significances about the interaction of response, trait and state anxiety over parietal (CP1: F(1, 67) = 7.78, p < 0.01, η2 = 0.10; CPZ: F(1, 67) = 6.61, p < 0.05, η2 = 0.09; CP2: F(1, 67) = 6.10, p < 0.05, η2 = 0.08; CP4: F(1, 67) = 10.69, p < 0.01, η2 = 0.14; CP6: F(1, 67) = 4.07, p < 0.05, η2 = 0.06; P1: F(1, 67) = 9.18, p < 0.05, η2 = 0.12; P4: F(1, 67) = 5.11, p < 0.05, η2 = 0.07). No other statistical differences were found (all ps > 0.1). Subsequent simple main effect tests showed that the latency induced by new words was shorter than calm group in high trait anxiety group(CP1: F(1, 67) = 5.17, p < 0.05, η2 = 0.07; CP2: F(1, 67) = 3.99, p < 0.1, η2 = 0.06; CP4: F(1, 67) = 4.84, p < 0.05, η2 = 0.07; CP6: F(1, 67) = 4.00, p < 0.1, η2 = 0.07; P1: F(1, 67) = 5.96, p < 0.05, η2 = 0.08; P4: F(1, 67) = 6.05, p < 0.05, η2 = 0.08), there was no same differences among other parts. Figure 1. Mean N400s of above 7 electrode sites for all four conditions to negative words.
Figure 1.
The separate N400s of above 7 electrode sites for all four conditions to negative words.
The peaks of N400 were analyzed with a 2 (trait anxiety: high/low) × 2 (state anxiety: high/low) × 3 (emotional valence: negative/neutral/positive) × 2 (responses: old/new) repeated measures ANOVA. The results showed that there was significant main effect of response, the amplitude of new word was stronger than old word (CP5: F(1, 67) = 4.06, p < 0.05, η2 = 0.06; CP3: F(1, 67) = 3.84, p < 0.1, η2 = 0.04; CP1: F(1, 67) = 5.39, p < 0.05, η2 = 0.07; CPz: F(1, 67) = 5.03, p < 0.05, η2 = 0.07; CP2: F(1, 67) = 6.25, p < 0.05, η2 = 0.09; CP4: F(1, 67) = 6.75, p < 0.05, η2 = 0.09; CP6: F(1, 67) = 3.39, p < 0.1, η2 = 0.05; P5: F(1, 67) = 6.49, p < 0.05, η2 = 0.08; P3: F(1, 67) = 3.66, p < 0.1, η2 = 0.05; P1: F(1, 67) = 4.20, p < 0.05, η2 = 0.06; Pz: F(1, 67) = 2.84, p < 0.1, η2 = 0.04; P2: F(1, 67) = 4.82, p < 0.05, η2 = 0.07; P4: F(1, 67) = 4.19, p < 0.05, η2 = 0.06; P6: F (1, 67) = 3.57, p < 0.1, η2 = 0.05), there was no same differences among other parts. The result, ERPs of words correctly judged as ‘new’ have been observed to be more negative-going than ERPs elicited by words correctly judged as “old”, named “old/new effect”, accorded with previous research results in recognition memory task52–55.
Discussion
The aim of the present study was to investigate whether anxious individuals have memory bias to such negative events. Participants’ electroencephalography brain activities and responses were recorded while they were performing a classic recognition task. The results showed high state anxiety levels increased the d’ of negative words, moreover, an earlier N400 (250–500 ms) latency was elicited for new-negative words in high state levels than in low state levels in right parietal region, which suggested there existed the recognition memory bias to negative events in state anxiety (mood factor). Trait anxiety (personality factor), however, did not influence recognition memory.
Notably, the d’ of negative words of high state anxiety individuals was larger than that of low state anxiety individuals, whether it’s high or low trait anxiety individuals in the present study. A higher d’ indicates that the signal can be more readily detected, d’ depends on the intensity (i.e., the physical properties of the stimulus materials) and the sensitivity of the individual, and the sensitivity of the individual is affected by his/her pre se ability and physiological state56,57. Considering that the increasing state anxiety level might induce the vulnerable of negative information processing, which reflected in the change of d’, high state anxiety individuals may have a stronger vigilance of threatening information than low state anxiety individuals. Furthermore, the result also showed there did not exist significant difference in β, which suggested that subjects’ subjective motivation was similar for the three types of words, and this result support to no negative response bias existed. Moreover, the d’ of recognition memory was lower for negative words than both neutral and positive words in all participants. The data indicated a significant emotional valence effect, many studies reported that the false recognition of negative stimuli was higher than of the neutral stimuli58–60, which consistent to our results that the participants’ ability of distinguishing new and old negative words was poorer than that of neutral and positive words, healthy individuals would enhance negative affective processing, meanwhile, they also have the capacity away from negative processing, finally, there still existed greater d’ on positive and neutral words relative to negative words. State anxiety increasing may induce the negative memory bias in the present study.
Moreover, we found that the evidence in ERP data supported the finding of behavior. It is interesting to note that the enhancement was found in the new negative words, that the latency of N400 of new negative words in high state levels was shorter than that in low state levels in right parietal region. It suggested that state anxiety influenced the new negative words but not old negative words. Many studies have claimed that the latency of N400 was related with semantic processing61–64, moreover, many studies had found that N400 was influenced by the effects of emotional valence36–38, and the effect of participants’ emotional state36,39,40. So, the index of parietal N400 was sensitive and effective to emotional words in different state anxiety levels in present study. Here, the latency of N400 was the index that indicated memory recollection of semantics of words, and the result suggested the latency of N400 was influenced by the effect of emotional valence of words and state anxiety levels. As for we found the difference of latency only in right parietal region, we considered that it is due to the lateralization of emotion process, convincing evidence had indicated that the left hemisphere is dominant for positive emotions and the right hemisphere is dominant for negative emotions65–69. Hence, our results that the difference of ERP data of the negative words in right parietal region were consistent with previous studies.
Meanwhile, the result also showed that in high trait anxiety participants, the latency of new words in induced state anxiety group was shorter than calm group, but there was no significance in emotional valence. Thus, it might become another evidence that there did not existed negative recognition biases in trait anxiety individuals. The difference had not been found in low trait anxiety group, there might exist stronger state anxiety level in high trait anxiety group than low trait anxiety group, there might really exist individual difference between high and low trait anxiety individuals, but none in recognition memory. It found more vigilance in high state anxiety than low state anxiety level in high trait anxiety individuals. In addition, in the present study, the peak of N400s were found the “old/new” effect, ERPs of words correctly reject have been observed to be more negative-going than ERPs elicited by words hit, was consistent with previous research results in recognition memory task52–55, nevertheless, there was no difference in trait and state anxiety levels, which may imply there was no difference for the degree of semantic processing both trait and state anxiety, the finding need to be further examined
On the whole, the behavioral outcome reflected state anxiety affected only negative words on memory retrieval phase, further, the physiological results suggested that state anxiety affected only the new words instead of old words about negative words, the effect was accorded with previous studies, which found the difference was in new words and not old words52,70,71. According to vigilance-avoidance hypothesis72–77, vigilance for negative events could enhance succeeding memory trace of the negative event for anxious individuals. Anxious individuals always more preferentially attend to negative events, encode them more deeply and retrieve memories more easily and correctly, as it could make anxious individuals considering the current situation more negative than it actually was. However, it has been shown that anxious individuals could avoid a prolonged or deep processing of negative information78, which lessened the number of rehearsals during the coding stage or limited retrieval of negative information. Therefore, the effect of vigilance could be reduced or even overturned by the effect of avoidance. In this respect, for the memory of a negative events of anxious individuals, hyper-vigilance and avoidance may conflict with each other. In the present study, the results showed the effect of avoidance might be stronger than the effect of hyper-vigilance, but the vigilance to negative information of high state anxiety individuals was stronger than of which low state anxiety individuals. State anxiety still leaded to recognition memory bias, but trait anxiety did not. Further, from an adaptive perspective, such anxiety enhances negative processing, thus increases vigilance level against danger in a threatening environment79,80, notably, increases vigilance towards novels stimuli81. In low state anxiety levels, healthy individuals may tend to avoid, sometimes can act in “blissful ignorance”82. For example, it may be that inhibiting the processing of new negative words processing (and, hence, making more errors in response to negative information) is adaptive. In fact, to process all stressors in a constantly changing but remains safety environment would be cognitively unnecessary and wasteful. As such, “blissful ignorance,” or an avoidance to fearful stimuli, may increase adaptive fitness under low state anxiety levels conditions83. However, in high state anxiety levels, this situation reversed, such that it becomes adaptive to focus on all aversive information in order is to avoid harm, it also means that enhance vigilance for negative stimulus to avoid harm. As predicting, the effect of avoidance was still stronger than the effect of hyper-vigilance, there was no real threat to participants in experiment in inducing state anxiety condition. But, relative to the condition of low state anxiety levels, subjects in high state anxiety levels could serve the purpose of increasing vigilance to new negative words, could better distinguish to the new or old judge of negative words. State anxiety would still lead to recognition memory bias.
Conclusion
Individuals with high trait anxiety form a non-clinical group with a predisposition for an emotional and cognitive processing bias that is considered to be a pre-existing condition for psychiatric disorders. Our results showed high trait anxiety individuals had no recognition memory bias, but, individuals with high state anxiety existed recognition memory bias to negative events. The finding recognition memory was influenced by state anxiety, rather than trait anxiety. There still existed limitation that whether encoding stage was influenced by state and trait anxiety levels. There is an adaptive perspective that, in low state anxiety levels, individuals inhibit negative processing. But in high state anxiety levels, individuals could increase vigilance to new-negative words to avoid potential threat, enhancing distinguish the new/old judgement of negative words.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (NSFC31400870); the National Natural Science Foundation of China (31600927), the humanities and social science research Project of Henan Colleges and Universities (2017-ZZJH-422); the support project for the Disciplinary group of Psychology and Neuroscience, Xinxiang Medical University (2016PN-KFKT-28).
Author Contributions
G.Z., M.Z. and Q.Y. designed this experiment, Q.Y., B.W., Q.Z. and X.L. performed the experiment, Q.Y., X.L. and Q.Z. analyzed the data, G.Z., M.Z., B.W., Q.Z. and Q.Y. wrote the paper, and all authors reviewed the paper.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guang Zhao, Email: zhaoguang721@163.com.
Meng Zhang, Email: mengzhang.1985@163.com.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V. (American Psychiatric Association, 2013).
- 2.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137. doi: 10.1016/S0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 3.Craske MG, Stein MB. Anxiety. Lancet. 2016;388:3048. doi: 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- 4.Spielberger CD. Anxiety: current trends in theory and research. Anxiety Current Trends in Theory & Research. 1972;1:23–49. [Google Scholar]
- 5.Mcnally R. Simulating buying center decision processes: propositions and methodology. Journal of Business & Industrial Marketing. 2002;17:167–180. doi: 10.1108/08858620210419790. [DOI] [Google Scholar]
- 6.Quidé Y, Witteveen AB, El-Hage W, Veltman DJ, Olff M. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: A systematic review. Neurosci Biobehav Rev. 2012;36:626. doi: 10.1016/j.neubiorev.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Eysenck MW. Applied cognitive psychology: Implications of cognitive psychology for clinical psychology and psychotherapy. J Clin Psy. 2004;60:393. doi: 10.1002/jclp.10252. [DOI] [PubMed] [Google Scholar]
- 8.Amir N, Foa EB, Coles ME. Implicit memory bias for threat-relevant information in individuals with generalized social phobia. J Abnorm Psychol. 2000;109:713–720. doi: 10.1037/0021-843X.109.4.713. [DOI] [PubMed] [Google Scholar]
- 9.Saunders J. Selective memory bias for self-threatening memories in trait anxiety. Cognition & Emotion. 2013;27:21. doi: 10.1080/02699931.2012.683851. [DOI] [PubMed] [Google Scholar]
- 10.Herrera, S., Montorio, I., Cabrera, I. & Botella, J. Memory bias for threatening information related to anxiety: an updated meta-analytic review. J Cogn Psychol (2017).
- 11.Mitte K. Memory bias for threatening information in anxiety and anxiety disorders: A meta-analytic review. Psychol Bull. 2008;134:886. doi: 10.1037/a0013343. [DOI] [PubMed] [Google Scholar]
- 12.Attwood AS, et al. State anxiety and emotional face recognition in healthy volunteers. Roy Soc Open Sci. 2017;4:160855. doi: 10.1098/rsos.160855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui, L. et al. Electrophysiological Correlates of Emotional Source Memory in High-Trait-Anxiety Individuals. Front Psychol7 (2016). [DOI] [PMC free article] [PubMed]
- 14.Eden AS, et al. Brief learning induces a memory bias for arousing-negative words: an fMRI study in high and low trait anxious persons. Front Psychol. 2015;6:1226. doi: 10.3389/fpsyg.2015.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toffalini E, Mirandola C, Coli T, Cornoldi C. High trait anxiety increases inferential false memories for negative (but not positive) emotional events. Pers Individ Dif. 2015;75:201–204. doi: 10.1016/j.paid.2014.11.029. [DOI] [Google Scholar]
- 16.Cooper RM, Rowe AC, Penton-Voak IS. The role of trait anxiety in the recognition of emotional facial expressions. J Anxiety Disord. 2008;22:1120–1127. doi: 10.1016/j.janxdis.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Inaba M, Ohira H. Reduced recollective memory about negative items in high trait anxiety individuals: An ERP study ☆. Int J Psychophysiol. 2009;74:106–113. doi: 10.1016/j.ijpsycho.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Surcinelli P, Codispoti M, Montebarocci O, Rossi N, Baldaro B. Facial emotion recognition in trait anxiety. J Anxiety Disord. 2006;20:110–117. doi: 10.1016/j.janxdis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Toffalini E, Mirandola C, Drabik MJ, Melinder A, Cornoldi C. Emotional negative events do not protect against false memories in young adults with depressive–anxious personality traits. Pers Individ Dif. 2014;66:14–18. doi: 10.1016/j.paid.2014.02.042. [DOI] [Google Scholar]
- 20.White CN, Ratcliff R, Vasey MW, Mckoon G. Using diffusion models to understand clinical disorders. J Math Psychol. 2010;54:39–52. doi: 10.1016/j.jmp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36:809–848. doi: 10.1016/S0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 22.Williams, J. M. G. Cognitive psychology and emotional disorders. (John Wiley & Sons, 1997).
- 23.Spielberger, C. D. State-Trait Anxiety Inventory for Adults: Sampler Set: Manual, Test, Scoring Key. (1983).
- 24.Spielberger, C. D., Gorsuch, R. L. & Lushene, R. E. State Trait Anxiety Inventory. (1970).
- 25.Hardy L, Beattie S, Woodman T. Anxiety‐induced performance catastrophes: Investigating effort required as an asymmetry factor. Brit J Psy. 2007;98:15–31. doi: 10.1348/000712606X103428. [DOI] [PubMed] [Google Scholar]
- 26.Rutherford E, MacLeod C, Campbell L. BRIEF REPORTNegative selectivity effects and emotional selectivity effects in anxiety: Differential attentional correlates of state and trait variables. Cognition & Emotion. 2004;18:711–720. doi: 10.1080/02699930341000121. [DOI] [Google Scholar]
- 27.Waechter S, Stolz JA. Trait Anxiety, State Anxiety, and Attentional Bias to Threat: Assessing the Psychometric Properties of Response Time Measures. Cognit Ther Res. 2015;39:441–458. doi: 10.1007/s10608-015-9670-z. [DOI] [Google Scholar]
- 28.White, C. N., Ratcliff, R. & Vasey, M. W. Anxiety-related threat bias in recognition memory: the moderating effect of list composition and semantic-similarity effects. Cognition & Emotion, 1 (2015). [DOI] [PubMed]
- 29.Nelson AL, Purdon C, Quigley L, Carriere J, Smilek D. Distinguishing the roles of trait and state anxiety on the nature of anxiety-related attentional biases to threat using a free viewing eye movement paradigm. Cognition & Emotion. 2015;29:504–526. doi: 10.1080/02699931.2014.922460. [DOI] [PubMed] [Google Scholar]
- 30.Okawa K, Ichinohe T, Kaneko Y. Anxiety may enhance pain during dental treatment. Bulletin of Tokyo Dental College. 2005;46:51. doi: 10.2209/tdcpublication.46.51. [DOI] [PubMed] [Google Scholar]
- 31.Raghunathan R, Pham MT. All Negative Moods Are Not Equal: Motivational Influences of Anxiety and Sadness on Decision Making. Organ Behav Hum Decis Process. 1999;79:56. doi: 10.1006/obhd.1999.2838. [DOI] [PubMed] [Google Scholar]
- 32.Carriere J. The effects of trait and state anxiety on attention to emotional images: An eye-tracking study. Cognition & Emotion. 2012;26:1390–1411. doi: 10.1080/02699931.2012.662892. [DOI] [PubMed] [Google Scholar]
- 33.Chwilla DJ, Kolk HH, Vissers CT. Immediate integration of novel meanings: N400 support for an embodied view of language comprehension. Brain Res. 2007;1183:109. doi: 10.1016/j.brainres.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4:463. doi: 10.1016/S1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- 35.Chwilla DJ, Kolk HH. Accessing world knowledge: evidence from N400 and reaction time priming. Cogn Brain Res. 2005;25:589. doi: 10.1016/j.cogbrainres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Chwilla DJ, Virgillito D, Vissers CT. The relationship of language and emotion: N400 support for an embodied view of language comprehension. J Cogn Neurosci. 2011;23:2400–2414. doi: 10.1162/jocn.2010.21578. [DOI] [PubMed] [Google Scholar]
- 37.Herbert C, Junghofer M, Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45:487. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiefer M, Schuch S, Schenck W, Fiedler K. Mood States Modulate Activity in Semantic Brain Areas during Emotional Word Encoding. Cereb Cortex. 2007;17:1516–1530. doi: 10.1093/cercor/bhl062. [DOI] [PubMed] [Google Scholar]
- 39.KD F, Kirson DA, Moreno EM, Kutas M. Effects of transient, mild mood states on semantic memory organization and use: An event-related potential investigation in humans. Neurosci Lett. 2001;305:149–152. doi: 10.1016/S0304-3940(01)01843-2. [DOI] [PubMed] [Google Scholar]
- 40.Pratt NL, Kelly SD. Emotional states influence the neural processing of affective language. Soc Neurosci. 2008;3:434. doi: 10.1080/17470910802188339. [DOI] [PubMed] [Google Scholar]
- 41.Spielberger, C. STAI manual for the State-trait anxiety inventory. Self-Evaluation Questionnaireiv, 1–24 (1970).
- 42.Spielberger, C. D. & Sydeman, S. J. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. (John Wiley & Sons, Inc., 2010).
- 43.Zheng, X. et al. In Chinese Mental Health Journal 13–15 (1993).
- 44.Association WM. Declaration of Helsinki. Law Med & Health C. 1991;19:264. doi: 10.1111/j.1748-720X.1991.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 45.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Rugg MD, Nagy ME. Event-related potentials and recognition memory for words. Electroencephalography & Clinical Neurophysiology. 2007;11:251. doi: 10.1016/0013-4694(89)90045-x. [DOI] [PubMed] [Google Scholar]
- 47.Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguado L, Dieguezrisco T, Méndezbértolo C, Pozo MA, Hinojosa JA. Priming effects on the N400 in the affective priming paradigm with facial expressions of emotion. Cogn Affect Behav Neurosci. 2013;13:284–296. doi: 10.3758/s13415-012-0137-3. [DOI] [PubMed] [Google Scholar]
- 49.Dorjee D, Lally N, Darrall-Rew J, Thierry G. Dispositional mindfulness and semantic integration of emotional words: Evidence from event-related brain potentials. Neurosci Res. 2015;97:45–51. doi: 10.1016/j.neures.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Ponz A, et al. Emotion processing in words: a test of the neural re-use hypothesis using surface and intracranial EEG. Soc Cogn Affect Neurosci. 2014;9:619–627. doi: 10.1093/scan/nst034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toivonen M, Rämä P. N400 during recognition of voice identity and vocal affect. Neuroreport. 2009;20:1245–1249. doi: 10.1097/WNR.0b013e32832ff26f. [DOI] [PubMed] [Google Scholar]
- 52.Inaba M, Nomura M, Ohira H. Neural evidence of effects of emotional valence on word recognition. International Journal of Psychophysiology Official Journal of the International Organization of Psychophysiology. 2005;57:165. doi: 10.1016/j.ijpsycho.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Johnson, R. In Handbook of Neuropsychology 135–163.
- 54.Rugg MD, Doyle MC. Event-related potentials and recognition memory for low- and high-frequency words. J Cogn Neurosci. 1992;4:69–79. doi: 10.1162/jocn.1992.4.1.69. [DOI] [PubMed] [Google Scholar]
- 55.Rugg MD, et al. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- 56.Elmes, D. G., Kantowitz, B. H. & Roediger, H. L. Research methods in psychology. (Brooks/Cole Publishing C, 2007).
- 57.Kantowitz, B. H. & Roediger, H. L. Experimental psychology: understanding psychological research. (Rand McNally College Pub. Co., 1978).
- 58.Brainerd CJ, Holliday RE, Reyna VF, Yang Y, Toglia MP. Developmental reversals in false memory: Effects of emotional valence and arousal. J Exp Child Psychol. 2010;107:137–154. doi: 10.1016/j.jecp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howe ML, Candel I, Otgaar H, Malone C, Wimmer MC. Valence and the development of immediate and long-term false memory illusions. Memory. 2010;18:58–75. doi: 10.1080/09658210903476514. [DOI] [PubMed] [Google Scholar]
- 60.Knott LM, Thorley C. Mood-congruent false memories persist over time. Cognition & Emotion. 2014;28:903–912. doi: 10.1080/02699931.2013.860016. [DOI] [PubMed] [Google Scholar]
- 61.Bortoloti R, Pimentel N, De Rose JC. Electrophysiological investigation of the functional overlap between semantic and equivalence relations. Psychol Neurosci. 2014;7:183–191015. doi: 10.3922/j.psns.2014.015. [DOI] [Google Scholar]
- 62.Deacon D, Mehta A, Tinsley C, Nousak JM. Variation in the latencies and amplitudes of N400 and NA as a function of semantic priming. Psychophysiology. 2010;32:560–570. doi: 10.1111/j.1469-8986.1995.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 63.Nigam A, Hoffman JE, Simons RF. N400 to semantically anomalous pictures and words. J Cogn Neurosci. 1992;4:15–22. doi: 10.1162/jocn.1992.4.1.15. [DOI] [PubMed] [Google Scholar]
- 64.Renoult L, Wang X, Calcagno V, Prévost M, Debruille JB. From N400 to N300: Variations in the timing of semantic processing with repetition. Neuroimage. 2012;61:206–215. doi: 10.1016/j.neuroimage.2012.02.069. [DOI] [PubMed] [Google Scholar]
- 65.Avram J, Balteş FR, Miclea M, Miu AC. Frontal EEG activation asymmetry reflects cognitive biases in anxiety: evidence from an emotional face Stroop task. Appl Psychophysiol & Biofeedback. 2010;35:285. doi: 10.1007/s10484-010-9138-6. [DOI] [PubMed] [Google Scholar]
- 66.Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 2004;67:7. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Cunningham WA, Espinet SD, Deyoung CG, Zelazo PD. Attitudes to the right- and left: frontal ERP asymmetries associated with stimulus valence and processing goals. Neuroimage. 2005;28:827–834. doi: 10.1016/j.neuroimage.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 68.Davidson RJ. Emotion and Affective Style: Hemispheric Substrates. Psychol Sci. 1992;3:39–43. doi: 10.1111/j.1467-9280.1992.tb00254.x. [DOI] [Google Scholar]
- 69.Harmonjones E, Lueck L, Fearn M, Harmonjones C. The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychol Sci. 2006;17:434–440. doi: 10.1111/j.1467-9280.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 70.Maratos EJ, Allan K, Rugg MD. Recognition memory for emotionally negative and neutral words: an ERP study. Neuropsychologia. 2000;38:1452. doi: 10.1016/S0028-3932(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 71.Ortony A, Turner TJ, Antos SJ. A puzzle about affect and recognition memory. J Exp Psychol Learn. 1983;9:725–729. doi: 10.1037/0278-7393.9.4.725. [DOI] [Google Scholar]
- 72.Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin Psychol Rev. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Derakshan N, Eysenck MW, Myers LB. Emotional information processing in repressors: The vigilance-avoidance theory. Cognition & Emotion. 2007;21:1585–1614. doi: 10.1080/02699930701499857. [DOI] [Google Scholar]
- 74.Hock M, Egloff B. [Interindividual differences in priming and memory effects of threatening stimuli: effect of cognitive avoidance and vigilant anxiety coping] Z Exp Psychol. 1998;45:149–166. [PubMed] [Google Scholar]
- 75.Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition & Emotion. 2004;18:689–700. doi: 10.1080/02699930341000158. [DOI] [Google Scholar]
- 76.Wieser MJ, Pauli P, Weyers P, Alpers GW, Mühlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: an eye-tracking study. J Neural Transm. 2009;116:717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
- 77.Zhai J, Chen X, Ma J, Yang Q, Liu Y. The vigilance-avoidance model of avoidant recognition: An ERP study under threat priming. Psychiatry Res. 2016;246:379–386. doi: 10.1016/j.psychres.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 78.Koster EH, Crombez G, Verschuere B, Van DS, Wiersema JR. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behav Res Thera. 2006;44:1757. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn Affect Behav Ne. 2011;11:217–227. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cornwell BR, et al. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alloy LB, Abramson LY. Judgment of contingency in depressed and nondepressed students: sadder but wiser? J Exp Psychol Gen. 1979;108:441–485. doi: 10.1037/0096-3445.108.4.441. [DOI] [PubMed] [Google Scholar]
- 83.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.