Abstract
Penile cancer (PC) is a relatively rare malignancy in the United States (US) but a greater concern in developing nations. Lymph node imaging remains critical to the staging and treatment of this disease as metastases develop in a predictable, anatomic fashion. Early surgical intervention remains a mainstay in treatment and imaging often aids in decision making. This review highlights the indications for imaging in both low-stage and advanced disease. Furthermore, we discuss the benefits and limitations of currently available imaging for staging of inguinal and pelvic lymph nodes in PC and novel modalities in development.
Keywords: Penile cancer (PC), lymph nodes, MRI, dynamic sentinel node biopsy (DSNB), 18F-FDG-PET scan
Introduction
In the United States (US), penile cancer (PC) is a relatively rare malignancy with an estimated incidence of 2,320 cases in 2018 and nearly 400 deaths (1). Approximately 35–40% of patients with PC will present with ≥ clinical T2 disease (2,3). In developing countries, with large income disparities and those with low rates of circumcision, the incidence of PC is far greater (4). Rural India has three times the incidence of the US, and in Brazil the rate of new PCs may reach 8.3 per 100,000 men (5). When men with PC present for treatment, accurate staging is critical for prognostic and therapeutic information (6). Early identification and surgical removal of inguinal lymph nodes (ILNs) may increase survival in men with metastatic disease (7). On the contrary, men with advanced and potentially unresectable nodal disease may be better suited with neoadjuvant chemotherapy or radiation therapy (8). When considering disease spread to ILNs, the most important prognostic factor is the stage and grade of the penile lesion (9,10). Low-grade and stage (≤ pT1a) tumors have positive ILN rates under 10% while high-grade (≥ pT1b) tumors may metastasize to inguinal nodes in up to 75% of cases (11). Having these risk factors, along with palpable disease in the inguinal region, should heighten the physician’s clinical suspicion for lymphatic spread (9,10). An important caveat is that an examination of the inguinal area is often unreliable, especially in overweight individuals, resulting in many false negatives and false positives (12,13). Because of this, clinicians often rely on imaging modalities to better stage patients prior to treatment (13,14).
Urologists must be aware of the extent of metastatic spread as this is the most important factor predictive of survival (15). Multiple studies have found average 5-year disease-free survival rates of 85–100%, 79–89%, 17–60%, and 0–17% for pN0, pN1, pN2, and pN3, respectively (Table 1) (6,12,16,17). PC has a predictable pattern of metastatic spread: first spreading to the sentinel lymph node (SLN) which is often located within the superficial lymph nodes near the central and superior aspects of the saphenofemoral junction (18,19). Metastases to inferior and deeper inguinal nodes and then pelvic nodes may occur later (18). This review will highlight the indications and available imaging modalities for detecting inguinal and pelvic nodal metastases in the setting of PC.
Table 1. Definitions of pathological staging of inguinal lymph nodes for penile cancer (8).
| Staging | Definition |
|---|---|
| Clinical stage | |
| cNX | Regional lymph nodes cannot be assessed |
| cN0 | No palpable or visibly enlarged inguinal lymph nodes |
| cN1 | Palpable mobile unilateral inguinal lymph node |
| cN2 | Palpable mobile ≥2 unilateral inguinal lymph nodes or bilateral inguinal lymph nodes |
| cN3 | Palpable fixed inguinal nodal mass or pelvic lymphadenopathy unilateral or bilateral |
| Pathologic stage | |
| pNX | Lymph node metastasis cannot be established |
| pN0 | No lymph node metastasis |
| pN1 | ≤2 unilateral inguinal metastases, no ENE |
| pN2 | ≥3 unilateral inguinal metastases or bilateral metastases |
| pN3 | ENE of lymph node metastases or pelvic lymph node metastases |
ENE, extranodal extension.
Indications for imaging
Inguinal imaging is not required for all men with PC. The National Comprehensive Cancer Network (NCCN) guidelines indicate imaging in two clinical situations prior to treatment: in patients with intermediate or high-risk PC (clinical stage ≥ T1b) or in all men with palpable ILNs on physical exam (8). These guidelines suggest obtaining either computed tomography (CT) or magnetic resonance imaging (MRI), with contrast if possible, of the abdomen and pelvis, as well as chest imaging (8). In men who have undergone surgical inguinal lymphadenectomy for pN2-3 disease, surveillance imaging (CT or MRI) is indicated every three months for the first year and then every 6 months thereafter. For patients with unresectable inguinal disease who undergo chemotherapy or radiation therapy as neoadjuvant or primary treatment, repeat CT or MRI of the abdomen and pelvis along with lung imaging is indicated after treatment. In such a clinical scenario, clinicians may consider positron emission tomography (PET)/CT as this modality may have superior diagnostic performance over conventional cross-sectional imaging (8).
Conventional imaging
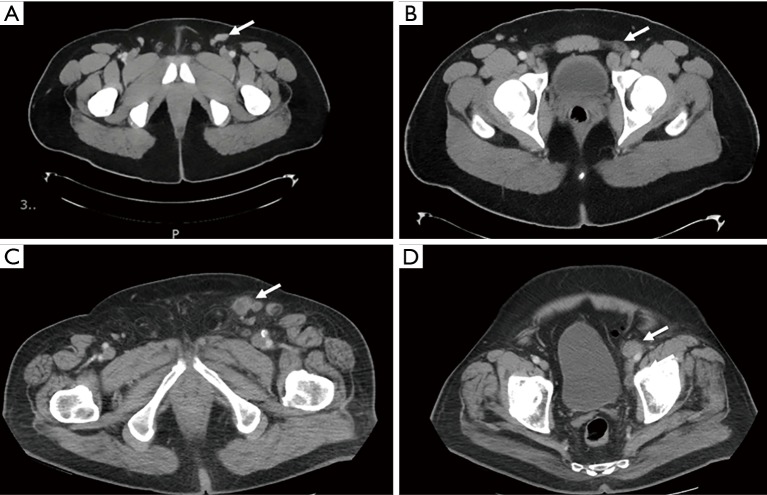
Conventional cross-sectional imaging, such as CT or MRI, often relies on size criteria (e.g., >8–10 mm) to diagnose metastatic spread to lymph nodes. However, based solely on size criteria, a significant number of cancerous nodes may go undiagnosed while benign enlarged nodes may be falsely positive (Figure 1) (20). PC is known to cause inflammatory changes in local nodes explaining a notoriously high rate of false positives findings (21).
Figure 1.
CT scans demonstrating a false-positive and true-positive when staging ILN. A 32-year-old man with HgT2 penile cancer invading into the glans with imaging demonstrating a 2.6 cm left inguinal node (arrow) (A) and 1.9 cm left external iliac node (arrow) (B) all of which were benign after robotic-assisted node dissection. An 82-year-old man with HgT2 penile cancer with 2.2 cm left inguinal node (arrow) (C) with irregular borders and 1.8 cm left external iliac node (arrow) (D). Inguinal lymph node dissection was performed with pathology demonstrating metastatic disease with tumor necrosis and extranodal extension.
Graafland et al. looked at their experience with CT imaging to detect metastatic spread to inguinal and pelvic lymph nodes using different radiologic criteria to identify suspicious nodes. In patients with low risk for inguinal nodal involvement, an 8 mm cut-off in the short axis of the node provided the highest accuracy for predicting a positive node with a sensitivity of 87% and specificity of 81% (22). For patients at high risk of inguinal nodal disease, the criteria with the highest accuracy (88%) was the presence of an irregular nodal border which had an improved specificity of 95% (22). For high-risk patients, a cut off of 8 mm or greater in the short axis yielded a sensitivity of 95% but at the expense of specificity at just 54%. This threshold measurement, 8 mm, also provided high sensitivity (100%) when ruling out ipsilateral pelvic nodal disease but decreased with increasing lymph node diameters (22).
MRI is another cross-sectional imaging technique to stage ILNs. Lucchesi et al. found 13 of 15 (86.7%) cases of ILN involvement using MRI compared to physical exam which identified just 7 of the 15 (46.7%) nodes (23). MRI may not provide significant additional information over CT scans for ILN imaging but MRI may provide additional information when staging the primary tumor (Figure 2) (24). MR images of the primary penile tumor are optimally obtained after intracorporeal injection (ICI) of 10 µg of prostaglandin E1 with axial, sagittal and coronal images then obtained (25). Gadolinium contrast may help to identify lymph nodes but only T1- and T2-weighted images are necessary to evaluate penile anatomy and tumor extension (25). MRI of the penis shows the muscular wall of the urethra, tunica albuginea, and Buck’s fascia to all be hypointense on both T1 and T2 weighted images while the corpora cavernosa and corpus spongiosum have intermediate signal intensity on T1-weighted imaging and high signal intensity on T2-weighted imaging (25). Hanchanale et al. studied 100 patients with clinical T1–T3 PC using MRI after prostaglandin E1 ICI and found MRI to have a sensitivity and specificity for tunica albuginea invasion of 82.1% and 73.6%, respectively (26). The sensitivity and specificity of urethral invasion was 62.5% and 82.1% in this study (26).
Figure 2.
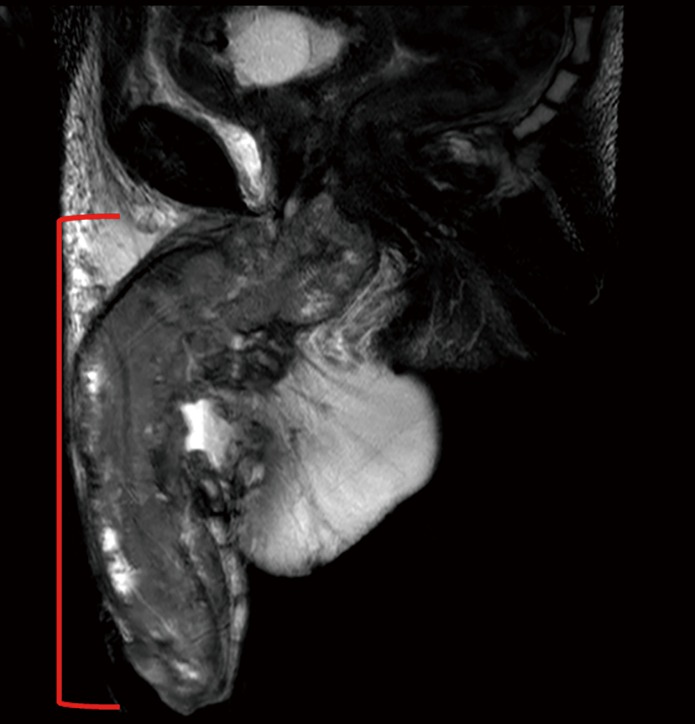
A 47-year-old male with penile cancer. Sagittal T2W MR image shows a large heterogenous mass involving almost the entire penis (red bracket).
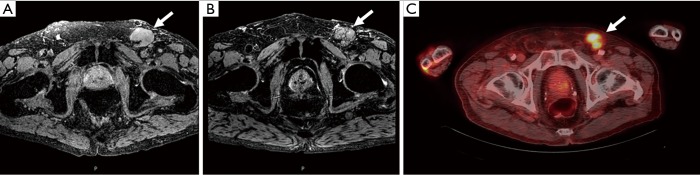
A unique approach to MR imaging utilizes a novel contrast agent, ultra-small superparamagnetic iron oxide particles (USPIO) such as ferumoxtran-10 or commercially available ferumoxytol. The former has a half-life of 25 hours while the latter has shorter half-life of 14–15 hours and is less prone to allergic reactions (20,27). Ferumoxtran-10 nanoparticles are taken up within the penile lymphatics, phagocytosed by resident macrophages, and results in nodal accumulation of contrast (20). Metastatic nodes are often lacking in macrophages and therefore accumulate less of this nanoparticle (20). Nanoparticles within a benign lymph node result in a decreased T2* (susceptibility weighted) images along with bright T2 images. In contrast, malignant nodes will have bright T2 and T2*-weighted images (Figure 3) (20). This technology has been utilized for multiple cancers and has been proven effective for PC as well (28,29). Tabatabaei et al. studied ferumoxtran-10 in seven men with stage T1b–T2 PC and found a sensitivity of 100%, specificity of 97%, positive predictive value of 81.2%, and negative predictive value of 100% (29).
Figure 3.
An 84-year-old male with penile cancer. Axial T2*-weighted MR image shows an enlarged lymph node in the left inguinal region (arrow) (A). Axial T2*-weighted MR image obtained 24 hours after intravenous injection of ferumoxytol shows persistently high signal intensity within the left inguinal adenopathy (arrow) (B) which suggests metastatic involvement of penile cancer. Axial 18F-FDG-PET/CT image shows tracer uptake within the left inguinal adenopathy which confirms presence of metastasis (arrow) (C).
SLN biopsy
The original lymph drainage of the penis was described in 1977 by Ramon Cabanas who performed 100 lymphangiograms (LAGs) in men with both benign and malignant penile lesions (19). In such LAGs, the SLN was mapped to the antero-medial border of the epigastric-saphenous junction which was therefore recommended to be resected based on anatomical landmarks. Furthermore, in 31 cases where SLN biopsy was negative, there was no disease recurrences within 3- and 5-year overall survival rate was 90% (19). Despite this promising data, additional studies showed that the use of Cabanas’ anatomic landmarks alone may yield false-negative findings due to altered lymphatic drainage (30,31).
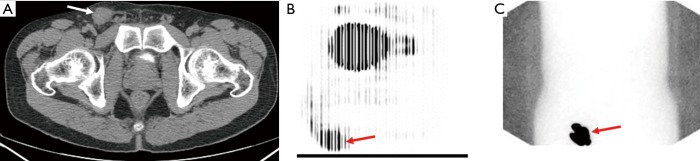
Due to the risk of false-negative results in SLN biopsies, physicians turned to other, real-time methods to identify lymphatic drainage combining dynamic sentinel node biopsy (DSNB) techniques used to diagnose other malignancies (32). Isosulfan blue dye travels through the lymphatic channels allowing surgeons to visualize channels intraoperatively and had previously been used in malignant melanoma to visualize the SLN (32). Furthermore, Krag et al. developed a technique of using a gamma-ray detector after injection of a radiolabeled technetium (99mTc)-nanocolloid for localization of the SLN in breast cancer (33). Horenblas et al. first combined these procedures and performed LSG on 55 patients with T2–T3 PC disease using both blue dye and a radiolabeled 99mTc-nanocolloid (34). One day prior to surgery, 99mTc-nanocolloid is injected near the tumor site and immediate dynamic imaging was performed, followed by static images at 30 minutes and 2 hours post injection (Figure 4). These locations were marked on the patient’s skin to plan for surgery the following day. Blue dye was then injected in a similar manner immediately prior to surgery to aid in intraoperative identification. In the initial series, only one patient had tumor recurrence after negative DSNB. A subsequent study of 250 more surgically explored groins found that the false-negative rate decreased from 19.2% to 4.8% and the complication rate decreased from 10.2% to 5.7% as these practitioners gained experience (35).
Figure 4.
A 38-year-old man with HgT1 penile cancer who underwent wide local excision of primary lesion. Axial CT imaging (A) demonstrates 2.8 cm right inguinal lymph node (arrow). 18F-FDG PET (B) image shows tracer uptake within the right inguinal adenopathy (arrow) which confirms presence of metastasis. Lymphoscintigraphy was performed (C) showing sentinel node corresponding with nodal mass (arrow). Inguinal lymph node dissection was performed with final pathology revealing 3.5 cm nodal metastasis and 9 benign lymph nodes.
DSNB is indicated in men with PC who are at intermediate to high risk of having metastases to the ILNs without palpable disease (6). This procedure should be done bilaterally with the goal of preventing the morbidity of bilateral ILN dissections (ILND). However, if the DSNB is positive, a complete ILND should be performed. DSNB should only be performed at experienced centers to minimize the chance of a false-negative node result.
The principle behind LSG was then merged with CT technology to perform single-photon emission computed tomography-CT (SPECT-CT). Leijte et al. performed both standard DSNB while at the same time performing SPECT-CT at 2 hours post-injection of 99mTc-nanocolloid (18). Combining these techniques gave both the functional information of the lymphatic drainage and the spatial resolution of CT. The technique was performed in 86 clinically negative groins and with the combination of both techniques, lymphatic drainage was demonstrated in 95.3%. SNL was not detected in the zones inferior to the saphenofemoral junction nor was there drainage directly into the pelvis (18). Another study of this combined technology showed SPECT/CT to have a high sensitivity and specificity of 88.8% and 86.7%, respectively (36).
Another novel method of identifying the SLN and patterns of drainage requires a fluorescent dye, indocyanine green (ICG), which is injected near the primary lesion site prior to inguinal lymphadenectomy (37,38). Markuszewski et al. compared ICG to 99mTc-nanocolloid radiotracer which was injected with ICG prior to surgery and then used a gamma-ray detector and near infrared fluorescence (NIRF) camera to detect these compounds, respectively (38). Both methods of SLN biopsy were able to detect SLNs intraoperatively, including those with metastases (38). Robotic-assisted inguinal lymphadenectomy with a NIRF camera may become a more standardized approach in performing this procedure with comparable node yields and lower morbidity. ICG may become a more commonly utilized method of intraoperative identification of lymph nodes during such procedures (37,39). Furthermore, ICG and 99mTc-nanocolloid can be combined into one compound for hybrid approaches to first guide the surgeon via radiotracer mapping and then further intraoperative visualization with ICG (40).
Ultrasound (US) has also been used to assess inguinal nodes during the time of surgery. Kroon et al. performed fine-needle aspiration cytology (FNAC) prior to DSNB or inguinal node dissection in 83 clinically negative groins and found US with FNAC to have a sensitivity and specificity of 39% and 100%, respectively (41). Intraoperative US has also been applied to palpable nodal disease during DSNB to identify more metastatic nodes that were missed during DSNB possibly due to lymphatic channel blockage (42). Contrast-enhanced ultrasonography (CEUS) is another novel technique that has been used in other malignancies for sentinel node mapping but has yet to see use with PC. For example, breast cancer often utilizes a SLN biopsy and this technique was studied in 54 patients and found to have a sensitivity of 89% with all five cancerous nodes detected (43). Another example supports this concept when applied to ILNs. Lahtinen et al. found the sensitivity of the procedure to be 81.2% in identifying SLN from vulvar cancer, enabling complete resection intraoperatively (44).
Another modality to evaluate metastatic lymph nodes in PC is PET imaging utilizing 18F-fluorodeoxyglucose (18F-FDG) which is taken up by malignant cells more rapidly (45). This functional imaging is then combined with the anatomic information obtained from CT (Figure 4). Souillac et al. compared the accuracy of 18F-FDG-PET/CT in both patients with clinically positive and clinically negative inguinal node exams (46). 18F-FDG-PET/CT was found to have a sensitivity of 75% and specificity of 87.5% in clinically negative patients while the sensitivity was 37.5% and 97.2% in patients with clinically positive nodes (46). This low sensitivity highlights the importance to incorporate clinical suspicion, based on physical exam and tumor stage, into decision making. A meta-analysis of seven total studies showed an overall sensitivity of 80.9% and specificity of 92.4% for detection of lymph nodes. However, sensitivity varied based on clinical exam with an increase in sensitivity to 96.4% in clinically positive exams, decreasing to 56.5% in patients with negative exams (47).
18F-FDG-PET/CT has additional value in predicting the nodal status outside of the groin. Graafland et al. evaluated 18 patients with metastatic spread to inguinal nodes and then underwent evaluation of their pelvic nodes and found a sensitivity of 91% and specificity of 100% (48). Another study of patients with advanced disease, all of whom had inguinal disease and 21/48 (43.8%) who presented with distant metastases found 18F-FDG-PET/CT to have a sensitivity and specificity of 82% and 93%, and overall was able to detect 33% more metastatic lesions than cross-sectional imaging alone (49).
Conclusions
Accurate staging of ILNs is important to administer the multiple modalities required in the treatment of PC and in the proper sequence. Conventional cross-sectional imaging such as CT and MRI rely mainly on size criteria to determine benign vs. malignant lymph nodes and may yield to false-negatives and positives. MRI may contribute an additional role in staging of the primary tumor. DSNB performed with radiotracers and dyes incorporates functional information and may yield false-negative rates as low as 5%. 18F-FDG-PET imaging is indicated in advanced disease and has high specificity and sensitivity which increases with stage. Many imaging and surgical methods are available to identify suspicious nodes but physicians’ clinical suspicion, based on the primary tumor grade and stage, should weigh heavily on management decisions.
Acknowledgements
This research was supported by the Intramural Research Program of the National Cancer Institute, NIH.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Pham MN, Deal AM, Ferguson JE, 3rd, et al. Contemporary survival trends in penile cancer: Results from the National Cancer Database. Urol Oncol 2017;35:674.e1-9. 10.1016/j.urolonc.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 3.Burt LM, Shrieve DC, Tward JD. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys 2014;88:94-100. 10.1016/j.ijrobp.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Douglawi A, Masterson TA. Updates on the epidemiology and risk factors for penile cancer. Transl Androl Urol 2017;6:785-90. 10.21037/tau.2017.05.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol 2004;5:240-7. 10.1016/S1470-2045(04)01427-5 [DOI] [PubMed] [Google Scholar]
- 6.Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. 10.1016/j.eururo.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 7.Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol 2005;173:816-9. 10.1097/01.ju.0000154565.37397.4d [DOI] [PubMed] [Google Scholar]
- 8.NCCN Guidelines Version 2.2018 Staging Penile Cancer, 2018.
- 9.Alkatout I, Naumann CM, Hedderich J, et al. Squamous cell carcinoma of the penis: predicting nodal metastases by histologic grade, pattern of invasion and clinical examination. Urol Oncol 2011;29:774-81. 10.1016/j.urolonc.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 10.Ficarra V, Zattoni F, Artibani W, et al. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J Urol 2006;175:1700-4; discussion 1704-5. [DOI] [PubMed]
- 11.Hungerhuber E, Schlenker B, Karl A, et al. Risk stratification in penile carcinoma: 25-year experience with surgical inguinal lymph node staging. Urology 2006;68:621-5. 10.1016/j.urology.2006.03.052 [DOI] [PubMed] [Google Scholar]
- 12.Leone A, Diorio GJ, Pettaway C, et al. Contemporary management of patients with penile cancer and lymph node metastasis. Nat Rev Urol 2017;14:335-47. 10.1038/nrurol.2017.47 [DOI] [PubMed] [Google Scholar]
- 13.Derakhshani P, Neubauer S, Braun M, et al. Results and 10-year follow-up in patients with squamous cell carcinoma of the penis. Urol Int 1999;62:238-44. 10.1159/000030405 [DOI] [PubMed] [Google Scholar]
- 14.Heyns CF, Fleshner N, Sangar V, et al. Management of the lymph nodes in penile cancer. Urology 2010;76:S43-57. 10.1016/j.urology.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Srinivas V, Morse MJ, Herr HW, et al. Penile cancer: relation of extent of nodal metastasis to survival. J Urol 1987;137:880-2. 10.1016/S0022-5347(17)44281-9 [DOI] [PubMed] [Google Scholar]
- 16.Marconnet L, Rigaud J, Bouchot O. Long-term followup of penile carcinoma with high risk for lymph node invasion treated with inguinal lymphadenectomy. J Urol 2010;183:2227-32. 10.1016/j.juro.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 17.Ficarra V, Akduman B, Bouchot O, et al. Prognostic factors in penile cancer. Urology 2010;76:S66-73. 10.1016/j.urology.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 18.Leijte JA, Valdes Olmos RA, Nieweg OE, et al. Anatomical mapping of lymphatic drainage in penile carcinoma with SPECT-CT: implications for the extent of inguinal lymph node dissection. Eur Urol 2008;54:885-90. 10.1016/j.eururo.2008.04.094 [DOI] [PubMed] [Google Scholar]
- 19.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [DOI] [PubMed] [Google Scholar]
- 20.Eisner BH, Feldman AS. Nanoparticle imaging for genitourinary cancers. Cancer Biomark 2009;5:75-9. 10.3233/CBM-2009-0553 [DOI] [PubMed] [Google Scholar]
- 21.Hughes B, Leijte J, Shabbir M, et al. Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World J Urol 2009;27:197-203. 10.1007/s00345-008-0288-6 [DOI] [PubMed] [Google Scholar]
- 22.Graafland NM, Teertstra HJ, Besnard AP, et al. Identification of high risk pathological node positive penile carcinoma: value of preoperative computerized tomography imaging. J Urol 2011;185:881-7. 10.1016/j.juro.2010.10.058 [DOI] [PubMed] [Google Scholar]
- 23.Lucchesi FR, Reis RB, Faria EF, et al. Incremental value of MRI for preoperative penile cancer staging. J Magn Reson Imaging 2017;45:118-24. 10.1002/jmri.25339 [DOI] [PubMed] [Google Scholar]
- 24.Heyns CF, Mendoza-Valdes A, Pompeo AC. Diagnosis and staging of penile cancer. Urology 2010;76:S15-23. 10.1016/j.urology.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Rajesh A. Magnetic resonance imaging of penile cancer. Magn Reson Imaging Clin N Am 2014;22:191-9, vi. 10.1016/j.mric.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Hanchanale V, Yeo L, Subedi N, et al. The accuracy of magnetic resonance imaging (MRI) in predicting the invasion of the tunica albuginea and the urethra during the primary staging of penile cancer. BJU Int 2016;117:439-43. 10.1111/bju.13041 [DOI] [PubMed] [Google Scholar]
- 27.Bashir MR, Bhatti L, Marin D, et al. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging 2015;41:884-98. 10.1002/jmri.24691 [DOI] [PubMed] [Google Scholar]
- 28.Harisinghani MG, Saksena MA, Hahn PF, et al. Ferumoxtran-10-enhanced MR lymphangiography: does contrast-enhanced imaging alone suffice for accurate lymph node characterization? AJR Am J Roentgenol 2006;186:144-8. 10.2214/AJR.04.1287 [DOI] [PubMed] [Google Scholar]
- 29.Tabatabaei S, Harisinghani M, McDougal WS. Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J Urol 2005;174:923-7; discussion 927. 10.1097/01.ju.0000170234.14519.19 [DOI] [PubMed] [Google Scholar]
- 30.Wespes E, Simon J, Schulman CC. Cabanas approach: is sentinel node biopsy reliable for staging penile carcinoma? Urology 1986;28:278-9. 10.1016/0090-4295(86)90004-X [DOI] [PubMed] [Google Scholar]
- 31.Perinetti E, Crane DB, Catalona WJ. Unreliability of sentinel lymph node biopsy for staging penile carcinoma. J Urol 1980;124:734-5. 10.1016/S0022-5347(17)55633-5 [DOI] [PubMed] [Google Scholar]
- 32.Yeung LL, Brandes SB. Dynamic sentinel lymph node biopsy as the new paradigm for the management of penile cancer. Urol Oncol 2013;31:693-6. 10.1016/j.urolonc.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 33.Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 1993;2:335-9; discussion 340. 10.1016/0960-7404(93)90064-6 [DOI] [PubMed] [Google Scholar]
- 34.Horenblas S, Jansen L, Meinhardt W, et al. Detection of occult metastasis in squamous cell carcinoma of the penis using a dynamic sentinel node procedure. J Urol 2000;163:100-4. 10.1016/S0022-5347(05)67982-7 [DOI] [PubMed] [Google Scholar]
- 35.Leijte JA, Kroon BK, Valdes Olmos RA, et al. Reliability and safety of current dynamic sentinel node biopsy for penile carcinoma. Eur Urol 2007;52:170-7. 10.1016/j.eururo.2007.01.107 [DOI] [PubMed] [Google Scholar]
- 36.Lutzen U, Naumann CM, Marx M, et al. A study on the value of computer-assisted assessment for SPECT/CT-scans in sentinel lymph node diagnostics of penile cancer as well as clinical reliability and morbidity of this procedure. Cancer Imaging 2016;16:29. 10.1186/s40644-016-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjurlin MA, Zhao LC, Kenigsberg AP, et al. Novel Use of Fluorescence Lymphangiography During Robotic Groin Dissection for Penile Cancer. Urology 2017;107:267. 10.1016/j.urology.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 38.Markuszewski M, Polom W, Cytawa W, et al. Comparison of Real-Time Fluorescent Indocyanine Green and (99m)Tc-Nanocolloid Radiotracer Navigation in Sentinel Lymph Node Biopsy of Penile Cancer. Clin Genitourin Cancer 2015;13:574-80. 10.1016/j.clgc.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Jaipuria J, Goel A, et al. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J Urol 2018;199:1518-25. 10.1016/j.juro.2017.12.061 [DOI] [PubMed] [Google Scholar]
- 40.KleinJan GH , van Werkhoven E, van den Berg NS, et al. The best of both worlds: a hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur J Nucl Med Mol Imaging 2018;45:1915-25. 10.1007/s00259-018-4028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroon BK, Horenblas S, Deurloo EE, et al. Ultrasonography-guided fine-needle aspiration cytology before sentinel node biopsy in patients with penile carcinoma. BJU Int 2005;95:517-21. 10.1111/j.1464-410X.2005.05330.x [DOI] [PubMed] [Google Scholar]
- 42.Naumann CM, van der Horst S, van der Horst C, et al. Reliability of dynamic sentinel node biopsy combined with ultrasound-guided removal of sonographically suspicious lymph nodes as a diagnostic approach in patients with penile cancer with palpable inguinal lymph nodes. Urol Oncol 2015;33:389 e9-14. [DOI] [PubMed]
- 43.Sever A, Jones S, Cox K, et al. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 2009;96:1295-9. 10.1002/bjs.6725 [DOI] [PubMed] [Google Scholar]
- 44.Lahtinen O, Eloranta M, Anttila M, et al. Preoperative sentinel lymph node localization in vulvar cancer: preliminary experience with inguinal intradermal contrast-enhanced ultrasound. Eur Radiol 2018;28:2089-95. 10.1007/s00330-017-5155-7 [DOI] [PubMed] [Google Scholar]
- 45.Ottenhof SR, Vegt E. The role of PET/CT imaging in penile cancer. Transl Androl Urol 2017;6:833-8. 10.21037/tau.2017.04.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souillac I, Rigaud J, Ansquer C, et al. Prospective evaluation of (18)F-fluorodeoxyglucose positron emission tomography-computerized tomography to assess inguinal lymph node status in invasive squamous cell carcinoma of the penis. J Urol 2012;187:493-7. 10.1016/j.juro.2011.10.033 [DOI] [PubMed] [Google Scholar]
- 47.Sadeghi R, Gholami H, Zakavi SR, et al. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin Nucl Med 2012;37:436-41. 10.1097/RLU.0b013e318238f6ea [DOI] [PubMed] [Google Scholar]
- 48.Graafland NM, Leijte JA, Valdes Olmos RA, et al. Scanning with 18F-FDG-PET/CT for detection of pelvic nodal involvement in inguinal node-positive penile carcinoma. Eur Urol 2009;56:339-45. 10.1016/j.eururo.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, Li W, Liang F. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in penile cancer. Oncotarget 2016;7:48600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]