Abstract
Background:
Although there is good evidence that warming of contrast media changes the bolus kinetics and injection pressure of iodinated contrast media, there has been little evidence that it affects clinical adverse event rates in a meaningful way.
Objective:
To determine whether the extrinsic warming of low-osmolality iodinated contrast media to 37°C reduced adverse reactions.
Methods:
Data on adverse reactions were collected from two cohorts, one of which used contrast media at room temperature and the other in which contrast media were warmed to 37°C before administration. Adverse reactions, including allergic-like and physiological reactions, were reviewed. We compared the incidence rates of adverse reactions between the two cohorts by using the χ2 test.
Results:
A total of 70,446 injections in cohort 1 and 203,873 injections in cohort 2 were included. Extrinsic warming reduced the rate of allergic-like reactions to iopromide 370, iopamidol 370, and iohexol 350 (0.32% in cohort 1 versus 0.21% in cohort 2, p = 0.003; 0.14% versus 0.10%, p = 0.046; and 0.32% versus 0.13%, p = .003, respectively). However, the physiological reaction rates could not be reduced (p = 0.057, p = 0.107, and p = 0.962, respectively). The extrinsic warming of iopromide 300 could not reduce adverse reaction rates (allergic-like reaction rates: 0.21% versus 0.16%, p = 0.407; physiological reaction rates: 0.17% versus 0.13%, p = 0.504).
Conclusion:
Extrinsic warming to 37°C before intravenous administration was associated with a reduction in the rate of allergic-like reactions to iopromide 370, iopamidol 370, and iohexol 350.
Keywords: Contrast media, low osmolality, iopromide, iopamidol, iohexol, warming, 37°C, centigrade, reduction, allergic-like reactions, physiological reactions
The extrinsic warming of iodinated contrast medium from room temperature to human body temperature (37°C) reduces its viscosity, particularly for nonionic radiocontrast agents.1 In previous studies, this approach has increased the viscosity-dependent iodine delivery rate with both manual and high-pressure injections when using intravenous (i.v.) catheters.2 The rate of anaphylactoid adverse events due to high-osmolality contrast media (HOCM) decreases when these agents are warmed before i.v. administration.3 Currently, HOCM has been replaced by low-osmolality contrast media (LOCM) at most institutions because of its improved adverse effect profile.4 Therefore, the number of institutions that adopted this practice with expectations to reduce the adverse reactions to LOCM is increasing.
The extrinsic warming of iodine-based contrast media to 37°C before routine clinical i.v. administration was suggested in the American College of Radiology, version 10.15 and the European Society of Urogenital Radiology version 9.06 contrast medium guidelines to minimize complications and improve vascular opacification. Extrinsic warming changes the bolus kinetics and injection pressure of iodinated contrast media. Therefore, iodinated contrast media are primarily warmed to reduce extravasation events. However, until now, only one previous study, with 24,830 injections of iopamidol 370 during a 400-day study period, showed that warming has a significant effect on extravasation rates.7 Analysis of data that are based on a large population study shows that warming positively affects extravasation rates are still limited.7 Thus, we conducted a literature review of multiple data bases and found that no previous studies reported that the extrinsic warming of LOCM before i.v. injection could reduce the rates of acute adverse reactions (i.e., allergic-like and physiological reactions). Therefore, to date, no conclusive data can confirm the effects on adverse reactions of warming LOCM before i.v. administration.
Iodinated contrast media are considered medications. Therefore, the warming of iodinated contrast media is regulated by the Joint Commission, which mandates if contrast media are to be extrinsically warmed.5 It is important to provide conclusive evidence that shows that the warming of contrast medium is practical (e.g., a daily temperature log and regular maintenance for each incubator) and cost effective. Therefore, the present study aimed to determine whether the extrinsic warming of LOCM to human body temperature (37°C) before routine i.v. administration during computed tomography (CT) reduces adverse reaction rates.
METHODS
The ethics review board of Guangdong General Hospital approved this retrospective study and waived the need to obtain informed consent from the patients. B. Zhang, J. Liu, Y. Dong, and B. Guo contributed equally to this work.
Patients
All LOCM-enhanced CTs were performed in all the patients (including children and adults) in cohort 1 (n = 70,446) and cohort 2 (n = 203,873) from January 1, 2007, to December 31, 2015. The two cohorts were derived from two separate hospitals: cohort 1 was from a large tertiary hospital, and cohort 2 was from a different large tertiary hospital. The use of contrast material warmers for all iodinated contrast media continued in cohort 2 since 2007. The decision was not associated with the present study. Cohort 1 did not use contrast material warmers during the study period due to the uncertain effect of warming on adverse reactions. The patients in the present study did not receive any premedication before enhanced CT because the clinical evidence on the effectiveness of premedication is limited and premedication may not prevent anaphylaxis.
All the patients in the two cohorts were thoroughly screened before they underwent enhanced CT. Usually, patients with relative contraindications, such as renal insufficiency, hyperthyroidism, unstable asthma, and/or previous moderate or severe acute adverse reactions, were not injected with iodine-based contrast media unless necessary. No patient was permitted to leave the CT waiting room within 30 minutes after injection, and patients with adverse reactions were followed up for ≥24 hours. Outpatients were followed up via telephone, and inpatients were followed up by their clinical nurses who provided feedback to the nurses in the radiology department. All CT operation rooms were equipped with sufficient rescue drugs and devices. Once an adverse reaction occurs, the emergency department physicians, radiologists, and nurses could use the devices and drugs to preliminarily treat the patients until they are stable. These patients would then be sent to the emergency department for further treatment and observation if necessary.
Contrast Media and Their Administration
The following LOCM were used in cohorts 1 and 2 for i.v. contrast-enhanced CT examinations during the study period5: iopromide 370 (trade name Ultravist, 370 mg I/mL; viscosity of 20.1 cP at 20°C and 9.5 cP at 37°C [Bayer Health Care, Guangzhou, CN]), iopromide 300 (trade name Ultravist, 300 mg I/mL, viscosity of 9.2 cP at 20°C and 4.9 cP at 37°C [Bayer Health Care, Guangzhou, CN]), iopamidol 370 (trade name Isovue, 370 mg I/mL; viscosity of 20.9 cP at 20°C and 9.4 cP at 37°C [Bracco Imaging, Shanghai, CN]), and iohexol 350 (trade name Omnipaque, 350 mg I/mL, viscosity of 23.3 cP at 20°C and 10.6 cP at 37°C [GE Health Care, Guangzhou, CN]). For details, see Online Supplemental Material.
Adverse Reactions
In the two cohorts, adverse reactions (i.e., allergic-like and physiological reactions) were tracked by using the picture archiving and communication system and the self-designed case report form by the nurses in the radiology department from January 1, 2007, to December 31, 2015. The case report form includes age, sex, history of adverse reaction to iodinated contrast medium, history of asthma, additional allergies (e.g., penicillin anaphylaxis), signs and symptoms of the adverse reaction, treatment, clinical outcome, type and dose of contrast medium, and injection rate. Mild adverse reactions included nausea, mild vomiting, urticaria, and itching, and moderate adverse reactions included severe vomiting, marked urticaria, bronchospasm, facial and/or laryngeal edema, and vasovagal attack. Meanwhile, severe adverse reactions included hypotensive shock, respiratory arrest, cardiac arrest, and convulsion. All adverse manifestations were recorded for each adverse reaction to the iodinated contrast material. Allergy-like symptoms were caused by direct toxicity from the contrast media instead of type 1 immunoglobulin E (IgE) mediated reactions. The contrast material–related adverse reactions were classified by the American College of Radiology criteria (version 10.1).5
Statistical Analysis
The χ2 test or the Fisher-Yates test was applied to compare adverse reactions between cohorts 1 and 2. A two-tailed p < 0.05 was considered statistically significant. The Student's t-test was used to compare the mean age and injection rate between the patients with acute adverse reactions in cohorts 1 and 2. Data were analyzed by using the Statistical Package for Social Sciences software version 23.0 (SPSS Inc., Chicago, IL). A power analysis was not conducted because the present study had a large sample size.
RESULTS
A total of 70,446 injections in cohort 1 and 203,873 injections in cohort 2 were included in this hospital-based study. Adverse reactions after the i.v. administration of iopromide 370, iopromide 300, iopamidol 370, and iohexol 350 occurred in 223 patients (112 women, with a mean age of 45 years [range, 10–78 years]; and 111 men, with a mean age of 47 years [range, 4–77 years]) in cohort 1. Adverse reactions after the i.v. administration of iopromide 370, iopromide 300, iopamidol 370, and iohexol 350 occurred in 428 patients (215 women, with a mean age of 53 years [range, 4–88 years]; and 213 men, with a mean age of 51 years [range, 4–91 years]) in cohort 2.
Effects of the Extrinsic Warming of LOCM on Adverse Reactions
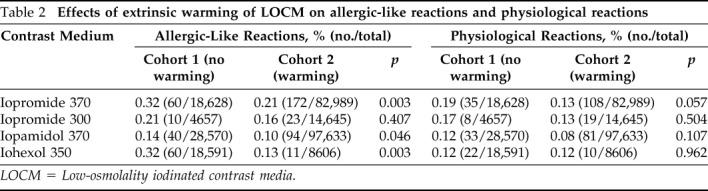
The number of contrast-enhanced CTs and adverse reactions to each contrast medium (iopromide 370, iopromide 300, iopamidol 370, and iohexol 350) in cohorts 1 and 2 during the study period are depicted in Table 1. The rates of adverse reactions and allergic-like reactions to these LOCM in cohort 1 were significantly higher than those in cohort 2 (adverse reaction rates: 0.32% [223/70,446] in cohort 1 versus 0.21% [428/203,873] in cohort 2, p < 0.001; allergic-like reaction rates: 0.22% [158/70,446] versus 0.14% [291/203,873], p < 0.001). However, the physiological reaction rates (0.12% [85/70,446] versus 0.10% [208/203,873], p = 0.192) were not reduced by extrinsic warming.
Table 1.
Comparison of acute adverse reaction rates in cohorts 1 and 2
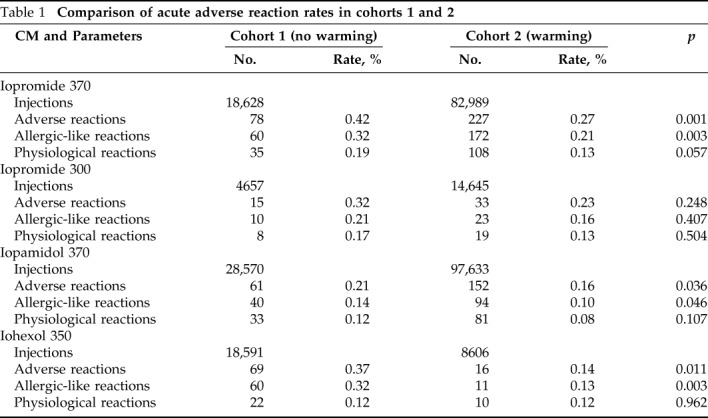
As shown in Table 2, the extrinsic warming of iopromide 370, iopamidol 370, and iohexol 350 was associated with a reduction in adverse reactions (p = 0.001, p = 0.036, and p = 0.011, respectively) and allergic-like reactions (p = 0.003, p = 0.046, and p = 0.003, respectively) but not physiological reactions (p = 0.057, p = 0.107, and p = 0.962, respectively). The extrinsic warming of iopromide 300 did not reduce the allergic-like reactions (p = 0.407) and physiological reactions (p = 0.504).
Table 2.
Effects of extrinsic warming of LOCM on allergic-like reactions and physiological reactions
LOCM = Low-osmolality iodinated contrast media.
Summary of Adverse Reactions in Cohorts 1 and 2
The patient characteristics, injection characteristics, adverse reaction manifestations, severity of adverse reaction, treatment, and clinical outcome of the adverse reactions from the i.v. injection of LOCM in the two cohorts during the study period are shown in Tables 3 and 4. All adverse reactions in the two cohorts eventually resolved after proper treatment, with the patients returning to their usual state of health.
Table 3.
Summary of adverse reactions after intravenous administration of LOCM in cohort 1 and cohort 2
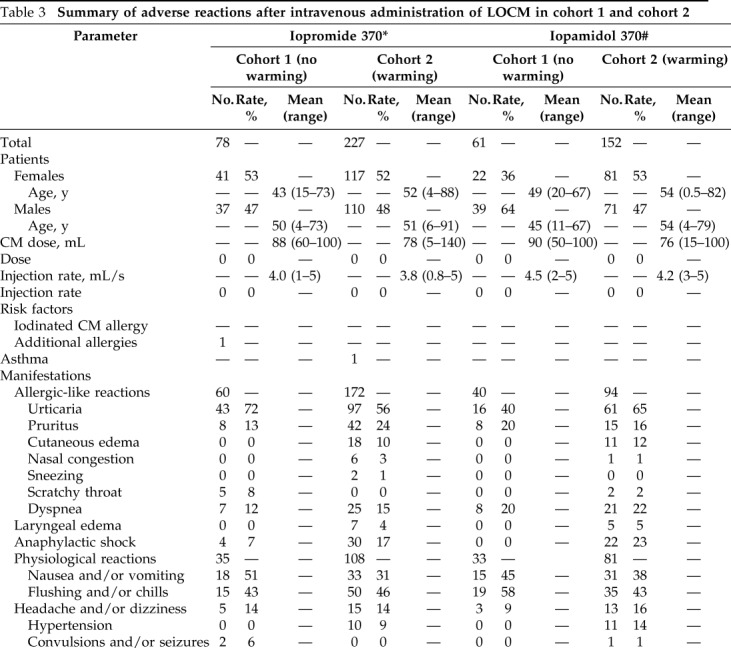
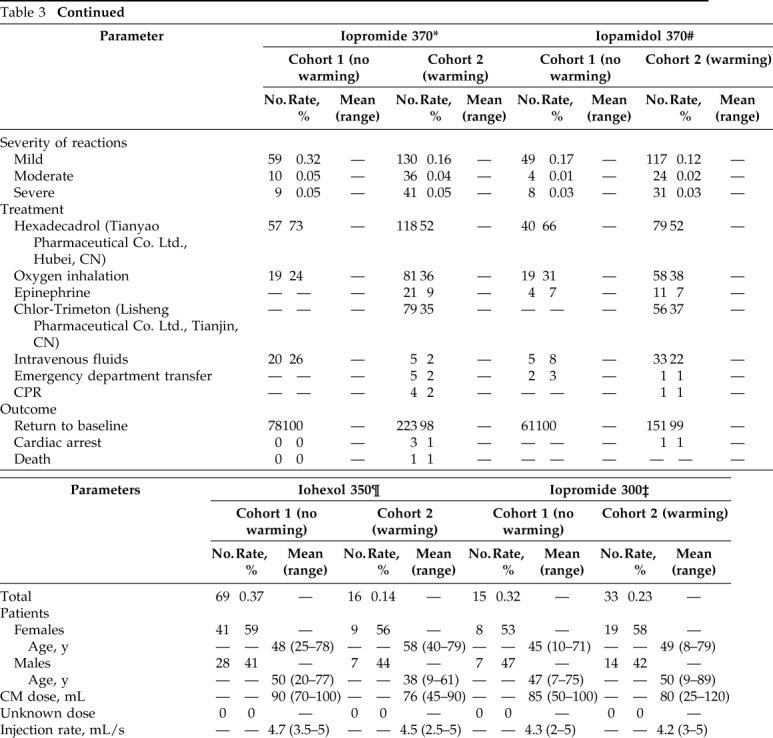
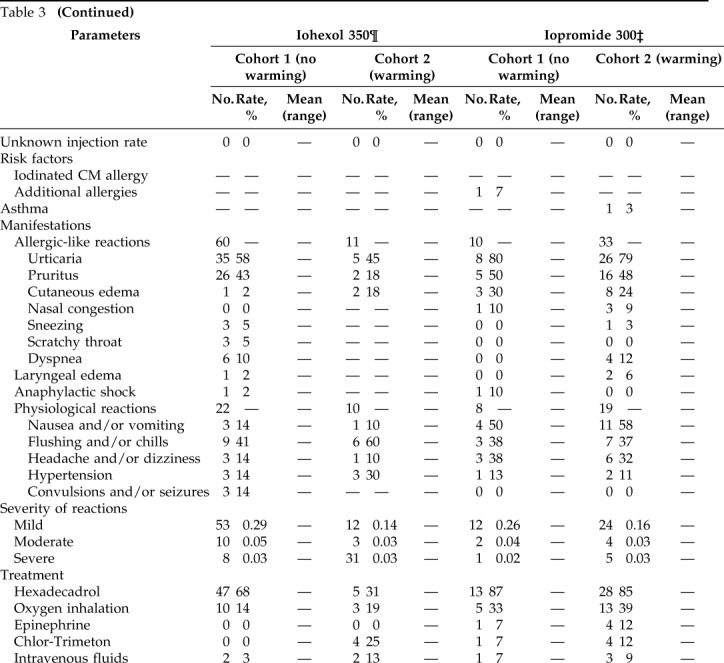
LOCM = Low-osmolality iodinated contrast media; CM = contrast media; CPR = cardiopulmonary resuscitation.
*There were 18,628 Iopromide 370 injections in cohort 1 and 82,989 injections in cohort 2.
#There was 28,570 Iopamidol 370 injections in cohort and 97,633 injections in cohort 2.
§Patients had more than one symptom after a single injection of CM.
¶There were 18,591 iohexol 350 injections in cohort 1 and 8606 injections in cohort 2.
‡There were 4657 iopromide 300 injections in cohort 1 and 14,645 injections in cohort 2.
Discussion
Based on the two cohorts with a large population, we further analyzed the effects of the extrinsic warming of LOCM to 37°C on adverse reaction rates. For the first time, we found that the extrinsic warming of iopromide 370, iopamidol 370, and iohexol 350 resulted in a significant reduction in allergic-like reactions but not in physiological reactions. In 1996, Vergara and Seguel3 performed a nonrandomized prospective study in which each group of patients was injected via i.v. with a specific contrast medium and temperature combination without overlap. Analysis of the results showed a minimal but significant reduction in adverse events (except for extravasations) after the extrinsic warming of HOCM to 37°C. The warmed HOCM had a reduced adverse reaction rate of 10% compared with the rate of 12% for the same nonwarmed (22°C) HOCM (p < 0.05).
The results of the present study were challenged by earlier double-blind research,8 which showed no significant difference in the rate of adverse events caused by warmed HOCM and HOCM at room temperature. However, this finding might be underpowered due to the absence of extravasation events.8 Currently, the use of HOCM has been replaced by LOCM at most institutions because of its improved adverse effect profile. Based on the limited studies about HOCM warming and the package inserts for iodinated contrast media, several institutions warm their contrast media to 37°C before i.v. administration. However, when considering that the contrast media are considered to be medications, their warming is subjected to the regulation of the Joint Commission, with a daily temperature log and regular maintenance required for each warming device. As a result, some institutions began to reconsider the use of warming devices and to reevaluate whether the extrinsic warming of LOCM is significantly beneficial, particularly low-rate (<5 mL/s) applications.
One study on the effect of extrinsic warming of LOCM to 37°C on adverse events was published in 2012.7 In this retrospective study of 24,830 injections (<6 mL/s), the investigators compared the rates of extravasations and allergic-like reactions to iopamidol 370 and iopamidol 300 at 200 days before (period 1) and 200 days after (period 2) the cessation of extrinsic warming at an institution.7 The cessation of extrinsic warming did not affect the rate of adverse reactions to i.v. injections of iopamidol 300; however, it nearly tripled the adverse event rates (0.43% [8/1851] versus 1.25% [26/2074]; p = 0.02) for iopamidol 370.7 The effect of warming may be significant for iopamidol 370 but not for iopamidol 300; however, warming of iopamidol 370 did not reduce allergic-like reaction rates (0.16% [3/1851] versus 0.39% [8/2074]; p = 0.42), which may be due to few allergic-like reaction events and small sample sizes during the two periods; therefore, a statistically significant difference was difficult to obtain.7
In our study, all iodinated contrast media in cohort 2 were extrinsically warmed to 37°C by using incubators before i.v. administration since 2007. Thus, our study aimed to reveal the significance of this unvalidated practice. As expected, the extrinsic warming of iopromide 370, iopamidol 370, and iohexol 350 to 37°C was associated with a significant reduction in adverse reaction rates, which was likely associated with the relatively high dynamic viscosity of these contrast media at 20°C (20.1 cP, 20.9 cP, and 23.3 cP, respectively). When these contrast media are warmed to human body temperature (37°C), their viscosities could be reduced by ≥50%.9 It is accepted that contrast media increase blood viscosity at a high shear rate and reduce erythrocyte velocity, platelet aggregation, capillary perfusion, and oxygen supply.10 The decrease in contrast medium viscosity may improve these adverse effects. In addition, compared with the warmed media, media at room temperature are cold irritants to the body, which elevate heart rate and blood pressure, and increase the release of mast cell mediators.11,12 Therefore, the warming of contrast media reduces these adverse events. However, this was not true for iopromide 300 because its extrinsic warming did not reduce the adverse reaction rates, which may be due to its already relatively low viscosity even at room temperature (9.2 cP at 20°C). Another reason for its insignificance could be the small amount of iopromide 300 that was used in the two cohorts.
Interestingly, after separating allergic-like reactions and physiological reactions, only allergic-like reactions could be reduced by warming the contrast media. This finding was not reported in previous studies, and this may be explained by some underlying reasons. First, the assessment of physiological reactions is somewhat subjective compared with that of allergic-like reactions, and it may not be associated with iodinated contrast media. Second, physiological reactions to iodinated contrast media differ in terms of mechanism from allergic-like reactions, and they are caused by direct toxicity from the use of contrast media. Allergic-like reactions are often idiosyncratic, and they may differ immunologically from true allergies mediated by IgE despite their similar clinical presentations.13 In allergic-like reactions, mast cells and basophils degranulate owing to direct stimulation rather than the release of IgE by the immune system.13,14 However, the exact pathogenesis of allergic-like reactions remains unclear.15,16
Some investigators found that allergic-like reactions are likely associated with the route of injection and injection rate.17 However, Jacobs et al.18 reported that the injection rate was not correlated with the allergic-like reaction rate. In our study, all iodinated contrast media were injected by i.v. under high pressure via 20-gauge catheters, and no significant difference was observed in the mean injection rate. Therefore, we could not discuss the influence of the injection rate and route of injection on adverse reaction rates.
The present study had some limitations. First, the dose of iopromide 300 that was used in the present study (n = 4657 in cohort 1 and n = 14,645 in cohort 2) was relatively small, which accounted for only 10–15% of the total iopromide consumption. Second, we did not include a control group of subjects who were free of any of the adverse reactions after i.v. LOCM. The presence of a control group would have allowed the identification of additional risk factors that are not associated with extrinsic warming and those that contribute to the incidence of adverse reactions. However, this was not the purpose of our study. Our primary concern was whether the extrinsic warming of LOCM reduced adverse reaction rates. Also, the two cohorts had different staffs and locations. Different personnel assessed the adverse reactions, and this could have influenced the results and led to bias.
CONCLUSION
Extrinsic warming to 37°C reduced allergic-like reaction rates to iopromide 370, iopamidol 370, and iohexol 350, which are LOCM. The results of the present study were clinically significant and were in accordance to the latest contrast media guidelines. Moreover, contrast media guideline promote a practical way of administering medications to help alleviate patient burden.
Footnotes
Funded with support from the following organizations: the National Scientific Foundation of China (81571664), the Science and Technology Planning Project of Guangdong Province (2014A020212244, 2016A020216020), the Scientific Research General Project of Guangzhou Science Technology and Innovation Commission (201605110912158), and the China Postdoctoral Science Foundation (2016M600145)
The authors have no conflicts of interest to declare pertaining to this article
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1. Rees CR, Merchun G, Becker GJ, Klatte EC, Mail JT, Mulry C. In vitro study of high-pressure catheters and various contrast agents. Radiology. 1988; 166:53–56. [DOI] [PubMed] [Google Scholar]
- 2. Kok M, Mihl C, Mingels AA, et al. Influence of contrast media viscosity and temperature on injection pressure in computed tomographic angiography: a phantom study. Invest Radiol. 2014; 49:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Vergara M, Seguel S. Adverse reactions to contrast media in CT: effects of temperature and ionic property. Radiology. 1996; 199:363–366. [DOI] [PubMed] [Google Scholar]
- 4. Valls C, Andía E, Sánchez A, Moreno V. Selective use of low-osmolality contrast media in computed tomography. Eur Radiol. 2003; 13:2000–2005. [DOI] [PubMed] [Google Scholar]
- 5. American College of Radiology. Manual on contrast media. Version 10.1, 2015 [cited 2016 Apr 2]. Available from: www.acr.org.
- 6. European Society of Urogenital Radiology Guidelines on contrast media. Version 9.0, 2014 [cited 2016 Apr 2]. Available from: www.esur.org.
- 7. Davenport MS, Wang CL, Bashir MR, Neville AM, Paulson EK. Rate of contrast material extravasations and allergic-like reactions: effect of extrinsic warming of low-osmolality iodinated CT contrast material to 37 degrees C. Radiology. 2012; 262:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner E, Kentor P, Melamed JL, Rao G, Zeitz HJ. Frequency of anaphylactoid reactions during intravenous urography with radiographic contrast media at two different temperatures. Radiology. 1982; 143:327–329. [DOI] [PubMed] [Google Scholar]
- 9. Cademartiri F, Mollet NR, van der Lugt A, et al. Intravenous contrast material administration at helical 16-detector row CT coronary angiography: effect of iodine concentration on vascular attenuation. Radiology. 2005; 236:661–665. [DOI] [PubMed] [Google Scholar]
- 10. Pugh ND. Haemodynamic and rheological effects of contrast media: the role of viscosity and osmolality. Eur Radiol. 1996; 6(Suppl. 2):S13–S15. [DOI] [PubMed] [Google Scholar]
- 11. Zhu YY, Mao YZ, Wu WL. Comparison of warm and cold contrast media for hysterosalpingography: a prospective, randomized study. Fertil Steril. 2012; 97:1405–1409. [DOI] [PubMed] [Google Scholar]
- 12. Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998; 114:640–648. [DOI] [PubMed] [Google Scholar]
- 13. Schabelman E, Witting M. The relationship of radiocontrast, iodine, and seafood allergies: a medical myth exposed. J Emerg Med. 2010; 39:701–707. [DOI] [PubMed] [Google Scholar]
- 14. Lenhard DC, May E, Morgenroth C, Jost G, Haider W, Pietsch H. Adverse skin reactions to iodinated x-ray contrast agents in healthy rats. Invest Radiol. 2014; 49:779–787. [DOI] [PubMed] [Google Scholar]
- 15. Rose TA, Jr, Choi JW. Intravenous Imaging Contrast Media Complications: The Basics That Every Clinician Needs to Know. Am J Med. 2015; 128:943–949. [DOI] [PubMed] [Google Scholar]
- 16. Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large urban children's hospital–retrospective analysis of data in 12,494 patients. Radiology. 2009; 250:674–681. [DOI] [PubMed] [Google Scholar]
- 17. Federle MP, Chang PJ, Confer S, Ozgun B. Frequency and effects of extravasation of ionic and nonionic CT contrast media during rapid bolus injection. Radiology. 1998; 206:637–640. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs JE, Birnbaum BA, Langlotz CP. Contrast media reactions and extravasation: relationship to intravenous injection rates. Radiology. 1998; 209:411–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.