Abstract
Background
Patients with metastatic human epidermal growth factor receptor 2-positive breast cancer (HER2+ BC) frequently experience brain metastases (BM). We aimed to define risk factors for the development of BM in patients with HER2+ BC and to report on their outcome.
Methods
This is a retrospective analysis of patients diagnosed with HER2+ BC between January 2000 and December 2014 at Institut Jules Bordet, Belgium. Statistical analyses were conducted with SAS V.9.4 using Kaplan-Meier method and Cox regression analyses.
Results
A total of 483 patients were included of whom 108 (22.4%) developed metastases and 52 (10.8%) BM. Among 96 metastatic patients without BM at diagnosis, 40 (41.7%) developed BM in the course of their disease. In multivariate analysis, risk factors for the development of BM were age ≤40 years (HR 2.10, 95 % CI 1.02 to 4.36), tumour size >2 cm (HR 4.94, 95% CI 1.69 to 14.47), nodal involvement (HR 3.48, 95% CI 1.47 to 8.25), absence or late start (≥6 months after initial diagnosis) of adjuvant anti-HER2 treatment (HR 3.79, 95% CI 1.52 to 9.43 or HR 2.65, 95% CI 1.03 to 6.82) and the development of lung metastases as first site of relapse (HR 6.97, 95% CI 3.41 to 14.24). Twenty-two patients with HER2+ BC and BM sent to our institute for further treatment were included in the outcome analysis. Asymptomatic patients at the time of BM diagnosis showed a better overall survival than symptomatic patients (HR 0.49, 95% CI 0.25 to 0.94).
Conclusion
A considerable number of patients with metastatic HER2+ BC will develop BM. Screening of patients with risk factors for BM might lead to early detection and better outcome. However, randomised controlled trials examining the use of MRI as a screening method for BM in patients with metastatic BC are warranted before such an approach can be recommended.
Keywords: HER2-positive breast cancer, brain metastases, risk factors
Key questions.
What is already known about this subject?
Besides triple-negative breast cancer (BC), HER2-positive (HER2+) BC is the most likely one to metastasize to the brain.
Some risk factors for the development of brain metastases (BM) including young age, pulmonary metastases, negative hormone receptor status and HER2 amplification have been suggested, however, most of the studies included unselected patients with BC, whereas less is known about risk factors for BM for cohorts of only HER2+ BC.
So far, no screening for BM in patients with metastatic HER2+ BC is recommended
What does this study add?
This study found five risk factors for the development of BM in HER2+ BC patients (age ≤40 years, tumour size >2 cm, nodal involvement, absence or late start of adjuvant anti-HER2 treatment and the development of lung metastases as first site of relapse).
Asymptomatic patients at the time of BM diagnosis showed a better overall survival than symptomatic patients.
How might it impact on clinical practice?
Our data suggest that early detection of BM in patients with HER2+ BC might be associated with improved outcome.
However, randomised controlled trials examining the use of MRI as a screening method for BM are warranted.
Introdoction
HER2 overexpression occurs in 15%–25% of all breast cancers (BCs) and is associated—at least in the absence of adequate systemic therapy—with a high recurrence rate, a short disease-free survival, a disposition for brain metastases (BM) and reduced overall survival (OS).1–5 Besides triple-negative BC, HER2-positive (HER2+) BC is the most likely one to metastasize to the brain.6,7 In historical series, median OS for patients with BC and BM treated with whole brain radiotherapy (WBRT) alone was poor and less than 6 months.8 More recent analyses suggest that the prognosis of patients with BC BM and especially with HER2+ BC BM is improving which probably is a result of better systemic treatment options leading to better control of disease outside the central nervous system (CNS).9,10 Furthermore, the implementation of better local treatment options like stereotactic radiosurgery (SRS) has improved the outcome of patients with BM in terms of OS and functional autonomy as compared with WBRT alone.11 As surgical or radiosurgical approaches are limited to patients with less extensive CNS disease, detection of BM at an early stage might improve patients’ outcome and quality of life. Definition of a high-risk group for the development of BC BM that might benefit from imaging screening for BM is needed. Some risk factors for the development of BM including young age, pulmonary metastases, negative hormone receptor status and HER2 amplification have been suggested, however, most of the studies included unselected patients with BC,12–21 whereas less is known about risk factors for BM for cohorts of only HER2+ BC.22–25 In this retrospective, single-institutional analysis, we aimed to define risk factors for the development of BM for early and advanced (metastatic) HER2+ BC as well as to describe the local treatment approaches and outcomes of patients with BM.
Methods
We retrospectively analysed patients with HER2+ BC who were diagnosed and/or treated at Institut Jules Bordet (IJB), Brussels, Belgium between January 2000 and December 2014. Eligible patients were identified using the hospital cancer registry, an exhaustive data base of incident tumours for patients consulting at IJB. During the same period of time, 22 additional patients diagnosed with HER2+ BC and BM outside of IJB were referred to our centre for a second opinion and further treatment. These additional patients were included in the outcome but not in the risk factor analysis.
Follow-up data were gathered until December 2016. HER2 status was determined with immunohistochemistry (IHC) and in situ hybridisation at the time of the first biopsy or breast surgery and classified according to the American Society of Clinical Oncology/College of American Pathologists clinical practice guidelines for HER2 testing of 2007 and 2013, respectively, and the Belgian Guidelines for HER2 testing.26,27 Hormone receptor status was determined by IHC using the Allred scoring system.28
Male patients, patients with a history of other malignant tumours or synchronous BCs without HER2 amplification were excluded. Structured data were exported from the hospital cancer registry when available or electronic charts were reviewed for patient and disease characteristics, treatment regimens for primary, metastatic and CNS disease and clinical outcomes. Some doctors opted for screening for BM in case of metastatic, extracranial disease (eg, at the time of metastatic disease diagnosis or regularly in the course of metastatic disease). This screening did not follow any guidelines.
The primary objective was to define risk factors for the development of BM both at the time of initial BC diagnosis and at the time of metastatic disease diagnosis. Secondary objectives included epidemiological aspects of BM and outcome of patients with BM. The Kaplan-Meier method was used to estimate OS and time from initial diagnosis and metastatic disease diagnosis until development of BM. Log-rank tests and Wilcoxon–Mann-Whitney tests have been used for comparisons among groups with the significance probability set at p<0.05. Χ2 tests were used to compare the characteristics of patients with and without BM. When appropriate (>20% of theoretical effectives <5), Fisher’s exact tests were used. Regarding the analysis of risk factors for the development of BM, univariate and multivariate analyses using the Cox proportional hazards model were used. In the multivariate analyses, a ‘backward’ selection method of the covariates was used. The proportional hazards assumption was verified on all the variables using cumulative sums of martingale residuals (assess statement and ‘resample’ option in the SAS procedure ‘Proc Phreg’). If not applicable time-varying covariates were used. The following factors were included in the multivariate analyses of risk factors for BM at the time of initial BC diagnosis: age (at initial BC diagnosis), menopausal status (at initial BC diagnosis), tumour size, nodal status, primary histology, tumour grade, HER2-to-CEP17 ratio, hormone receptor status, type of breast surgery, adjuvant radiotherapy, endocrine treatment, type of chemotherapy and anti-HER2 treatment. For the analysis at the time of metastatic disease diagnosis, the factors included in the multivariate analyses were: age (at metastatic BC (MBC) diagnosis), menopausal status (at MBC diagnosis), primary histology, tumour grade, HER2-to-CEP17 ratio, hormone receptor status, type of treatment for MBC (chemotherapy, endocrine treatment, anti-HER2 treatment), time from initial BC diagnosis to MBC, type (de-novo vs recurrent) and first site of MBC. For the calculation of the risk factors for BM at the time of initial BC diagnosis, patients with de-novo metastatic disease were excluded. For the HER2-to-CEP17 ratio, a cut-off of 5 was used corresponding to the P50. All Statistical analyses were conducted with SAS V.9.4.
Results
Patient population
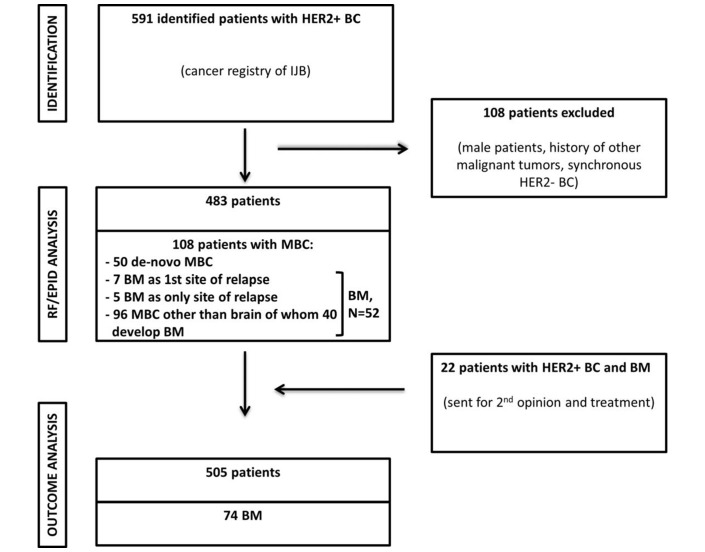
Out of 483 eligible patients identified using the hospital cancer registry, 108 patients (22.4%) were diagnosed with MBC (including BM) and 52 (10.8%) developed BM. Among the 52 BM, 7 patients (13.5%) had BM as first site of metastatic disease and 5 patients (9.6%) developed BM as only site of distant relapse. Among 96 metastatic patients without BM at diagnosis, 40 (41.7%) developed BM in the course of their disease. (figure 1). Table 1 summarises the demographic and disease characteristics of the study population according to the presence or absence of BM. Patients with BM were more likely to be younger, to have no surgery for primary lesion, to have larger tumours and more nodal involvement and less likely to receive anthracyclines+taxanes as (neo)adjuvant chemotherapy and to receive adjuvant endocrine treatment.
Figure 1.
Constitution of the study population. BC, breast cancer; BM, brain metastasis; EPID, epidemiological; HER2-, HER2-negative; HER2+, HER2-positive; IJB, Institut Jules Bordet; MBC, metastatic breast cancer; RF, risk factor.
Table 1.
Patients’ and tumours’ baseline characteristics
| Clinical values | Patients without diagnosis of BM (n=431) No. (%) |
Patients with diagnosis of BM (n=52) No. (%) |
P values |
| Age at dx, median (IQR), years | 53.9 (44.4–62.0) | 49.7 (38.0–60.2) | 0.03 |
| Age at diagnosis | 0.0022 | ||
| ≤40 years | 78 (18.05) | 20 (38.4) | |
| 41–64 years | 282 (65.4) | 24 (46.2) | |
| ≥65 years | 71 (16.55) | 8 (15.4) | |
| Menopausal status at diagnosis | 0.47 | ||
| Premenopausal | 183 (43.9) | 27 (52.9) | |
| Postmenopausal | 225 (53.95) | 23 (45.1) | |
| Perimenopausal | 9 (2.15) | 1 (2.0) | |
| Missing | 14 | 1 | |
| Type of surgery | <0.0001 | ||
| Breast conserving surgery | 135 (31.3) | 5 (9.6) | |
| Radical surgery | 271 (62.9) | 33 (63.5) | |
| No surgery | 25 (5.8) | 14 (26.9) | |
| Histology | 0.06 | ||
| Invasive carcinoma NST | 387 (91.3) | 43 (89.6) | |
| Lobular carcinoma | 18 (4.2) | 3 (6.25) | |
| Mixt | 19 (4.5) | 2 (4.15) | |
| Others/missing | 7 | 4 | |
| Tumour size | <0.0001 | ||
| pT1 | 194 (45.5) | 5 (10.0) | |
| pT2 | 143 (33.6) | 19 (38.0) | |
| pT3–4 | 89 (20.9) | 26 (52.0) | |
| Missing | 5 | 2 | |
| Nodal status | 0.0002 | ||
| pN0 | 243 (56.6) | 14 (27.5) | |
| pN1 | 162 (37.8) | 32 (62.7) | |
| pN2 | 14 (3.3) | 1 (2.0) | |
| pN3 | 10 (2.3) | 4 (7.8) | |
| Missing | 2 | 1 | |
| Tumour grade | 0.46 | ||
| G1 | 10 (2.4) | 0 (0) | |
| G2 | 138 (32.5) | 15 (29.4) | |
| G3 | 276 (65.1) | 36 (70.6) | |
| Missing | 7 | 1 | |
| Hormone receptor status | 0.53 | ||
| ER and/or PR positive | 308 (71.5) | 35 (67.3) | |
| ER and PR negative | 123 (28.5) | 17 (32.7) | |
| HER2-to-CP17 ratio | 0.18 | ||
| <5 | 241 (55.9) | 24 (46.2) | |
| ≥5 | 190 (44.1) | 28 (53.8) | |
| Type of chemotherapy | 0.0085 | ||
| Anthracyclines | 82 (19.0) | 18 (34.6) | |
| Taxanes | 37 (8.6) | 6 (11.5) | |
| Anthracyclines+taxanes | 261 (60.6) | 20 (38.5) | |
| Other | 7 (1.6) | 3 (5.8) | |
| None | 44 (10.2) | 5 (9.6) | |
| Type of anti-HER2 treatment | 0.14 | ||
| Trastuzumab | 277 (64.3) | 27 (51.9) | |
| Trastuzumab+lapatinib | 10 (2.3) | 1 (1.95) | |
| Trastuzumab+pertuzumab | 19 (4.4) | 1 (1.95) | |
| Lapatinib | 6 (1.4) | 0 (0) | |
| None | 119 (27.6) | 23 (44.2) | |
| Adjuvant endocrine therapy in patients with HR+BC | 0.003 | ||
| Administered | 275 (89.3) | 25 (71.4) | |
| Not administered | 33 (10.7) | 10 (28.6) | |
| Adjuvant radiotherapy | 0.65 | ||
| Yes | 146 (33.9) | 16 (30.8) | |
| No | 285 (66.1) | 36 (69.2) |
BM, brain metastases; ER, estrogen receptor; G, tumor grade; HR+BC, hormone receptor-positive breast cancer; N, nodal status; NST, no special type; PR, progesterone receptor; T, tumor size; dx, diagnosis; p, stage given by histopathological examination.
Risk factors for the development of BM
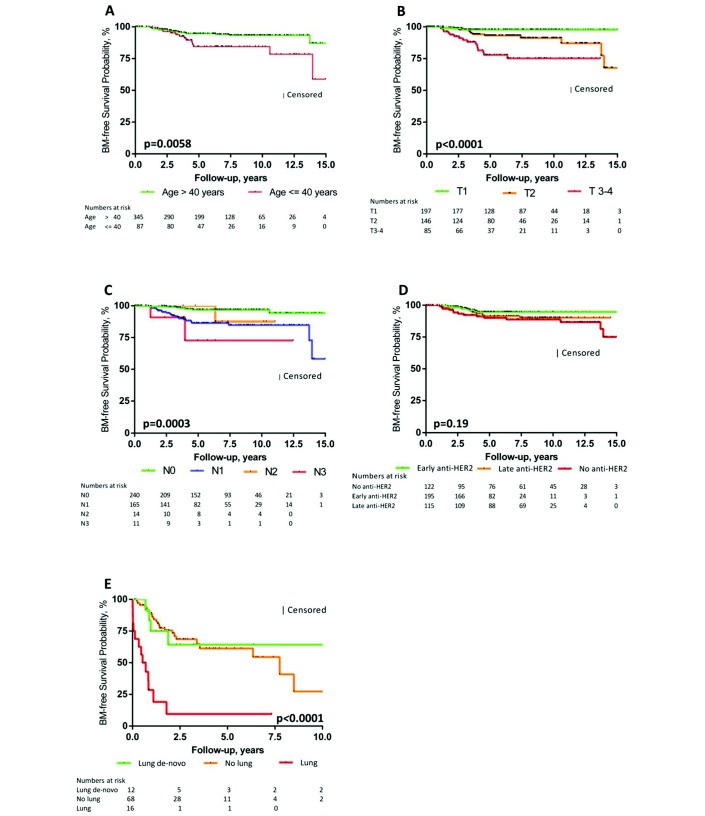
Median follow-up was 64.1 months (IQR 37.3–103.3) for the cohort including 52 patients with BM. Table 2 shows the results of the univariate and multivariate analyses of the risk factors for BM development at the time of initial BC and metastatic disease diagnosis, respectively. In multivariate analysis, risk factors for the development of BM at the time of initial BC diagnosis were age ≤40 years (HR 2.10, 95% CI 1.02 to 4.36), tumour size >2 cm (HR 4.94, 95% CI 1.69 to 14.47), nodal involvement (HR 3.48, 95% CI 1.47 to 8.25) and absence or late start (≥6 months after initial BC diagnosis) of adjuvant anti-HER2 treatment (HR 3.79, 95% CI 1.52 to 9.43 or HR 2.65, 95% CI 1.03 to 6.82). At the time of metastatic disease, the development of lung metastases as first site of relapse but not de-novo metastatic disease involving the lungs was found as an independent risk factor for BM development (HR 6.97, 95% CI 3.41 to 14.24 and HR 0.84, 95% CI 0.29 to 2.46). Figure 2A-E depicts the Kaplan-Meier curves for the variables found to be significant in multivariate analysis. Biological factors such as hormone receptor status, degree of HER2 amplification and tumour grade had no impact on the development of BM both in univariate and multivariate analyses. Furthermore, the type of systemic treatment in the metastatic setting (chemotherapy, anti-HER2 treatment) did not modulate the risk for BM.
Table 2.
Risk factors for the development of BM
| Variable | HR | 95% CI | P values | |
| Time of initial BC diagnosis (excluding de-novo metastatic patients ) | ||||
| Univariate analysis | ||||
| Age at BC diagnosis | >40 years | 1 | ||
| ≤40 years | 2.61 | 1.29 to 5.28 | 0.0078 | |
| Tumour size | T1 | 1 | ||
| T2–4 | 6.68 | 2.34 to 19.06 | 0.0004 | |
| Nodal status | N0 | 1 | ||
| N1–3 | 5.00 | 2.16 to 11.56 | 0.0002 | |
| Type of surgery | BCS | 1 | ||
| Mastectomy | 7.86 | 1.84 to 33.53 | 0.0054 | |
| No breast surgery | 3.70 | 0.33 to 42.1 | 0.29 | |
| Multivariate analysis | ||||
| Age at BC diagnosis | >40 years | 1 | ||
| ≤40 years | 2.10 | 1.02 to 4.36 | 0.045 | |
| Tumour size | T1 | 1 | ||
| T2–T4 | 4.94 | 1.69 to 14.47 | 0.0036 | |
| Nodal status | N0 | 1 | ||
| N1–3 | 3.48 | 1.47 to 8.25 | 0.0045 | |
| Adjuvant anti-HER2 treatment | Early anti-HER2 treatment* | 1 | ||
| Late anti-HER2 treatment† | 2.65 | 1.03 to 6.82 | 0.043 | |
| No anti-HER2 treatment | 3.79 | 1.52 to 9.43 | 0.0042 | |
| Time of metastatic disease diagnosis (including de-novo metastatic patients) | ||||
| Univariate analysis | ||||
| Age at MBC diagnosis | >40 years | 1 | ||
| ≤40 years | 2.11 | 1.09 to 4.10 | 0.028 | |
| Time from initial diagnosis to metastatic disease | <1 year | 1 | ||
| ≥1 year | 2.18 | 1.15 to 4.14 | 0.017 | |
| Type of metastatic disease | De-novo | 1 | ||
| Recurrent | 1.97 | 1.03 to 3.75 | 0.0040 | |
| First metastatic site | Other than lung | 1 | ||
| Lung de-novo | 0.84 | 0.29 to 2.46 | 0.75 | |
| Lung (not de-novo) | 6.97 | 3.41 to 14.24 | <0.0001 | |
| Multivariate analysis | ||||
| First metastatic site | Other than lung | 1 | ||
| Lung de-novo | 0.84 | 0.29 to 2.46 | 0.84 | |
| Lung (not de-novo) | 6.97 | 3.41 to 14.24 | <0.0001 | |
*Start within the first 6 months after BC diagnosis.
†Start ≥6 months after BC diagnosis.
BC, breast cancer; BCS, breast conserving surgery; BM, brain metastases; MBC, metastatic breast cancer; N, nodal status; T, tumor size.
Figure 2.
Kaplan-Meier plots of BM-free survival. Kaplan-Meier plots of BM-free survival according to (A) age at BC diagnosis, (B) tumour size, (C) nodal involvement, (D) adjuvant anti-HER2 treatment (early: start within the first 6 months after BC diagnosis; late: start ≥6 months after BC diagnosis) and (E) site of first relapse. For (A–D), de-novo metastatic patients were excluded from this analysis. BC, breast cancer; BM, brain metastasis; N, nodal status; T, tumour size.
Clinical presentation and local treatments of patients with HER2+ BM
Median time from metastatic disease diagnosis to BM development was 76.2 months. Thirty-three patients (63.5%) developed subsequent BM or progressed locally. Median time between first and second brain events was 7.7 months (IQR 5.3–13.2 months). At the time of the diagnosis of the first BM, 44 out of 74 patients (59.5%), presented with clinical signs related to the CNS event. The most common symptoms were headaches (50.0%), nausea and vomiting (25.0%), confusion and memory impairment (18.2%), paresis (18.2%), aphasia and dysarthria (6.8%) and seizures (6.8%). Thirty patients (40.5%) did not present any symptoms related to their BM, and diagnosis was made as part of routine screening with brain MRI or CT scan.
Table 3 outlines the local treatment approaches for the first BM in our patient cohort. While 40% of the patients diagnosed without symptoms were treated with SRS, only 15.9% of the symptomatic patients received this type of treatment (p=0.02). Compared with asymptomatic patients, symptomatic patients were treated more often with WBRT alone (63.65% vs 36.7%, p=0.023).
Table 3.
Local treatment approaches for the first BM
| Treatment modality | Asymptomatic patients (n=30) No. (%) |
Symptomatic patients (n=44) No. (%) |
P values |
| Radiotherapy (RT)-based | |||
| WBRT only | 11 (36.7) | 28 (63.65) | 0.023 |
| SRS only | 12 (40) | 7 (15.9) | 0.02 |
| WBRT+SRS | 4 (13.3) | 3 (6.8) | 0.35 |
| No RT | 3 (10) | 6 (13.65) | 0.8 |
| Surgery-based | |||
| Surgery alone | 0 | 1 (2.3) | 1 |
| Surgery+RT | 2 (6.7) | 8 (18.2) | 0.19 |
BM, brain metastases; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Outcome of patients with HER2+ BC and BM
Median follow-up was 64.8 months (IQR 39.6–105.3 months) for the whole study cohort including 72 patients with BM, 62.9 months (IQR 42.1–96.7 months) for patients who received adjuvant anti-HER2 treatment and 76.2 months (IQR 32.1–145.2 months) for patients without such treatment. Median OS for metastatic patients without CNS events was 46.7 months (IQR 23.0–145.6 months) and for patients with BM 20.8 months (IQR 5.36-not reached). Patients without CNS symptoms at the time of the BM diagnosis had a better OS than patients with clinical signs related to their BM (HR 0.49, 95% CI 0.25 to 0.94).
Anti-HER2 treatment after diagnosis of BM had no impact on the development of a second CNS event or on OS.
Discussion
In our analysis, 10.8% of patients with early HER2+ BC and 41.7% of metastatic patients developed BM. These results are in line with the study of Kennecke et al that reported incidence rates of BM of 14.3% and 7.9% after diagnosis of early BC and of 28.7% and 15.4% after diagnosis of metastatic disease for patients treated between 1986 and 1992 for HER2-enriched and luminal/HER2+ BC, respectively.7
Trastuzumab in combination with chemotherapy significantly improves OS and disease-free survival in early HER2+ BC.29,30 However, two meta-analyses reported an increased incidence of BM associated with adjuvant trastuzumab.29,30 Normally, CNS recurrence is preceded by metastases to other organs like lung, liver or bone. Therefore, a better control of extracranial disease with trastuzumab, coupled with the inability of trastuzumab to cross the intact blood–brain barrier, might extend the period of survival to such a degree as to display an increased propensity for BM.10,22,31 In our study, adjuvant anti-HER2 treatment was associated with a reduced risk for the development of BM. However, trastuzumab was approved by the European Medicines Agency only in 2006, and thus the median follow-up for patients who received adjuvant trastuzumab is slightly shorter than for the non-treated patient cohort in our study—a factor that should be taken into consideration when interpreting the results. Furthermore, the chemotherapy backbone as well as the time of trastuzumab start in the adjuvant setting have changed over the years.
In order to establish a screening for BM for patients with BC, two factors should be fulfilled: First, a considerable number of patients develop BM and second, early detection and treatment of BM is associated with an improved outcome. In our analysis, only 2.5% of the patients developed BM as first and/or only site of distant relapse; however, 41.7% of the metastatic patients developed BM in the course of their disease. So far, no study examined the role of regular imaging screening for BC BM, and thus there is no evidence that early detection and treatment of BM is associated with an improved outcome. Miller et al assessed the prevalence and impact on OS of occult CNS metastases in patients with metastatic BC that were screened for participation into one of four clinical trials evaluating novel antiangiogenic agents.20 Among 155 patients, 23 (14.8%) had occult CNS metastases. The study found similar OS for patients with CNS involvement, whether diagnosed clinically (symptomatic) or on screening (occult). However, several limitations exist to draw clear conclusions on the role of BM screening: the screening period was from 1998 to 2001; the management of patients with BM at that time differed from the one applied today. Twenty-one patients (91.3%) with occult BM were treated with WBRT, but no patient received surgery or SRS. Furthermore, that study did not address neurological outcomes even though it is mentioned that patients with occult CNS disease did not develop neurological symptoms like focal deficits, seizures or need for chronic corticosteroids. The current management of patients with BM depends on the performance status, the number, size and localisation of the metastases and the status of the disease outside the brain.32,33 In general, only patients with a limited number of CNS lesions that are smaller than 3 cm and without extensive symptomatic oedema are candidates for SRS.33 The phase III trial of Andrews et al showed an improvement in OS and functional autonomy for patients treated with SRS after WBRT (compared with WBRT alone) in case only one BM was present. However, this study included all solid tumour types and was dominated by non-small cell lung cancer, and patients with BC made up only 10% of the study population. Nevertheless, early diagnosis of BM might be favourable even in patients with BC but remains to be proven. No imaging screening for BM is recommended after primary BC treatment,34 and given the low incidence of BM as first site of distant relapse, we do not consider it appropriate to screen all patients with early HER2 +BC. Some centres suggest regular MRI screening for patienst with metastatic BC with high risk features. However, no clear definition of risk factors exists. Some studies evaluated possible risk factors for BM among patients with HER2+ metastatic BC. Suggested risk factors in this setting are the presence of visceral metastases and a premenopausal status,23 a negative hormone receptor status,22 and time from initial diagnosis to distant relapse shorter than 2 years.24 In our analysis, besides the absence of adjuvant anti-HER2 treatment, young age and locally advanced disease at the time of initial diagnosis and the development of lung metastases as first site of relapse in the advanced setting were independently associated with an increased risk for the development of BM. In our study, about 40% of the patients with BM did not present any CNS symptoms. As compared with symptomatic patients with BM, asymptomatic patients were treated more often with SRS and showed an improved OS. However, our study has several limitations that need to be taken into account. The use of retrospective collection of data is easy to perform and cost effective, however, this approach is not perfect. Our analysis conveys a time period of 14 years. During these years, the management of patients with HER2+ BC and BM has changed. The follow-up of our patients was not standardised and changed over the years. Thus, our study population is quite heterogeneous, and the potential for bias in our results cannot be ignored. Furthermore, the decision for screening for BM in case of metastatic, extracranial disease was at the treating physician’s discretion and did not follow any guidelines which could result in selection bias. Therefore, randomised controlled trials examining the use of MRI as a screening method for BM in patients with metastatic BC are warranted before such an approach can be recommended.
Footnotes
Contributors: CM, AA: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. CM, LT, CD, AA: study conception or design. CM, LT, CD, EdA, MM, MP, J-MN, MJP, AA: acquisition, analysis or interpretation of data. CM, LT, CD, EdA, MM, MP, J-MN, MJP, AA: critical revision of the manuscript for important intellectual content. MM, MP: statistical analysis.
CM, LT, CD, EdA, MM, MP, J-MN, MJP, AA: administrative, technical or material support.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The contents of this article are solely the responsibility of the authors.
Competing interests: MJP declares the following competing of interest: she has served as a consultant for Roche outside the submitted work. EdA declares the following competing of interests: he has received research grants and travel grants from Roche and served on an advisory board of Roche outside the submitted work. CM declares that he has received travel grants form Mundipharma and Amgen outside the submitted work. All other authors declare no competing interests.
Patient consent: Not required.
Ethics approval: Institut Jules Bordet Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Slamon DJ, Clark GM, Wong SG, et al. . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 2. Slamon DJ, Godolphin W, Jones LA, et al. . Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–12. 10.1126/science.2470152 [DOI] [PubMed] [Google Scholar]
- 3. Gusterson BA, Gelber RD, Goldhirsch A, et al. . Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) breast cancer study group. J Clin Oncol 1992;10:1049–56. 10.1200/JCO.1992.10.7.1049 [DOI] [PubMed] [Google Scholar]
- 4. Chia S, Norris B, Speers C, et al. . Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 2008;26:5697–704. 10.1200/JCO.2007.15.8659 [DOI] [PubMed] [Google Scholar]
- 5. Tandon AK, Clark GM, Chamness GC, et al. . HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 1989;7:1120–8. 10.1200/JCO.1989.7.8.1120 [DOI] [PubMed] [Google Scholar]
- 6. Arvold ND, Oh KS, Niemierko A, et al. . Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat 2012;136:153–60. 10.1007/s10549-012-2243-x [DOI] [PubMed] [Google Scholar]
- 7. Kennecke H, Yerushalmi R, Woods R, et al. . Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–7. 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 8. Mahmoud-Ahmed AS, Suh JH, Lee SY, et al. . Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys 2002;54:810–7. 10.1016/S0360-3016(02)02967-X [DOI] [PubMed] [Google Scholar]
- 9. Park YH, Park MJ, Ji SH, et al. . Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 2009;100:894–900. 10.1038/sj.bjc.6604941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawood S, Broglio K, Esteva FJ, et al. . Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 2008;19:1242–8. 10.1093/annonc/mdn036 [DOI] [PubMed] [Google Scholar]
- 11. Andrews DW, Scott CB, Sperduto PW, et al. . Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. The Lancet 2004;363:1665–72. 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 12. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. . Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865–72. 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- 13. Evans AJ, James JJ, Cornford EJ, et al. . Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol 2004;16:345–9. 10.1016/j.clon.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 14. Slimane K, Andre F, Delaloge S, et al. . Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol 2004;15:1640–4. 10.1093/annonc/mdh432 [DOI] [PubMed] [Google Scholar]
- 15. Pestalozzi BC, Zahrieh D, Price KN, et al. . Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 2006;17:935–44. 10.1093/annonc/mdl064 [DOI] [PubMed] [Google Scholar]
- 16. Nam BH, Kim SY, Han HS, et al. . Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 2008;10:R20 10.1186/bcr1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tham YL, Sexton K, Kramer R, et al. . Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 2006;107:696–704. 10.1002/cncr.22041 [DOI] [PubMed] [Google Scholar]
- 18. Rudat V, El-Sweilmeen H, Brune-Erber I, et al. . Identification of breast cancer patients with a high risk of developing brain metastases: a single-institutional retrospective analysis. BMC Cancer 2014;14:289 10.1186/1471-2407-14-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heitz F, Rochon J, Harter P, et al. . Cerebral metastases in metastatic breast cancer: disease-specific risk factors and survival. Ann Oncol 2011;22:1571–81. 10.1093/annonc/mdq625 [DOI] [PubMed] [Google Scholar]
- 20. Miller KD, Weathers T, Haney LG, et al. . Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol 2003;14:1072–7. 10.1093/annonc/mdg300 [DOI] [PubMed] [Google Scholar]
- 21. Graesslin O, Abdulkarim BS, Coutant C, et al. . Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 2010;28:2032–7. 10.1200/JCO.2009.24.6314 [DOI] [PubMed] [Google Scholar]
- 22. Stemmler HJ, Kahlert S, Siekiera W, et al. . Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast 2006;15:219–25. 10.1016/j.breast.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 23. Gori S, Rimondini S, De Angelis V, et al. . Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist 2007;12:766–73. 10.1634/theoncologist.12-7-766 [DOI] [PubMed] [Google Scholar]
- 24. Duchnowska R, Dziadziuszko R, Czartoryska-Arłukowicz B, et al. . Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat 2009;117:297–303. 10.1007/s10549-008-0275-z [DOI] [PubMed] [Google Scholar]
- 25. Tonyali O, Coskun U, Yuksel S, et al. . Risk factors for brain metastasis as a first site of disease recurrence in patients with HER2 positive early stage breast cancer treated with adjuvant trastuzumab. Breast 2016;25:22–6. 10.1016/j.breast.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 26. Wolff AC, Hammond ME, Hicks DG, et al. . Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 27. Wolff AC, Hammond ME, Schwartz JN, et al. . American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–45. 10.1200/JCO.2006.09.2775 [DOI] [PubMed] [Google Scholar]
- 28. Harvey JM, Clark GM, Osborne CK, et al. . Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999;17:1474–81. 10.1200/JCO.1999.17.5.1474 [DOI] [PubMed] [Google Scholar]
- 29. Moja L, Tagliabue L, Balduzzi S, et al. . Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012:CD006243 10.1002/14651858.CD006243.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin W, Jiang Y, Shen Z, et al. . Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS One 2011;6:e21030 10.1371/journal.pone.0021030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res 2007;13:1648–55. 10.1158/1078-0432.CCR-06-2478 [DOI] [PubMed] [Google Scholar]
- 32. Tsao MN, Rades D, Wirth A, et al. . Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210–25. 10.1016/j.prro.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramakrishna N, Temin S, Chandarlapaty S, et al. . Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2100–8. 10.1200/JCO.2013.54.0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khatcheressian JL, Hurley P, Bantug E, et al. . Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:961–5. 10.1200/JCO.2012.45.9859 [DOI] [PubMed] [Google Scholar]