Abstract
Aims
The characteristics of electrocardiographic (ECG) patterns in the general population of adolescents are insufficiently defined. The purpose of this study is to report ECG patterns and their association with anthropometric characteristics.
Methods and results
Twenty-four thousand and sixty-two students of Roman schools, aged 12–19, were screened with ECG and physical examinations. Electrocardiographic abnormalities were classified as either minor/non-clinically relevant or major, and anthropometric measures were evaluated per age class. Obesity prevalence was 20.9%, with a higher rate in younger students (P < 0.008 for all comparisons, except for the pair 16–17 vs. 18–19 years). Stage 1 hypertension was found in 3.14% of adolescents, Stage 2 hypertension in 0.45% of adolescents, and isolated systolic hypertension in 11.7% of adolescents. Heart rate and QT interval corrected for heart rate (QTc) decreased with increasing age. The QTc was longer in females than in males over 14 years. A higher rate of incomplete right bundle branch block (RBBB) was observed in underweight students (21.58% vs. 15.10% in non-underweight students, P < 0.0001). Complete RBBB was the most common major ECG abnormality (1.6%). It was associated with height irrespective of age, sex, and body mass index (odds ratio 17.9; 95% confidence interval 5.0–64.6) and more frequent in students regularly practicing physical activity (1.80% vs. 1.02%, P = 0.0009).
Conclusion
Heart rate and QTc decreased with increasing age. The QTc was longer in females than in males over 14 years. RBBB was the most common major abnormality and was associated with higher stature. The prevalence of some cardiovascular risk factors in adolescents is provided.
Keywords: Adolescent, Electrocardiogram, Antropometry, Cardiovascular risk factors
What’s new?
This study is the largest report on non-selected adolescents which evaluates electrocardiographic (ECG) and anthropometric characteristics divided by age class and the first which attempts to systematically assess the correlations between ECG abnormalities and anthropometric parameters.
The large database supplies relevant findings about the prevalence of some cardiovascular risk factors (smoking, physical activity, blood pressure, and obesity) in adolescents attending schools in a metropolitan city.
The study has demonstrated significant age-related ECG changes which, until now, had never been reported in such a large population of adolescents.
The QT interval corrected for heart rate are longer in females than in males for each age group over 14 years.
The prevalence of ECGs potentially indicative of heart disease is 1.7% with right bundle branch block, the most common major abnormality, found to be associated with higher stature.
Introduction
Most of the current data on the electrocardiographic (ECG) characteristics in young people are based on studies restricted to athletes. Indeed, the vast majority of published reports on ECG screenings in young people include selected populations of adolescents participating in competitive sports and were aimed at screening for conditions that predispose these athletes to sudden cardiac death. However, only a few reports have addressed the prevalence of ECG abnormalities in in a general population of adolescents.1
In an effort to bridge this gap, this report involves a large-scale screening of a non-selected population of adolescents and focuses on describing their ECG and anthropometric characteristics divided by age class. We aimed to assess the prevalence of ECG abnormalities and their association with anthropometric parameters in a young metropolitan population.
We report clinical and ECG data of students attending secondary schools in Rome as collected by the screening programme ‘healthy heart’, which was organized by the non-profit organization ‘Rome’s heart’.
Methods
The ‘healthy heart’ programme is a screening plan designed to evaluate ECG and anthropometric characteristics of young students with a cross-sectional study. The study was conducted in compliance with the Declaration of Helsinki, good clinical practice, and the applicable regulatory requirements. The study population consisted of male and female adolescents aged 12–19 enrolled at 175 schools in Rome, Italy (38% of the total schools in Rome attended by a similar age group), between 2012 and 2014. Schools were included based on their availability to participate during the prespecified screening period and no selection criteria was applied. Before the screening, informed consent was obtained from parents and the results of the screening tests were disclosed to parents and to students. Each student was screened by a health questionnaire and a standard resting 12-lead ECG. The health questionnaire included questions on personal medical history, symptoms of cardiovascular disease, physical activity (defined by regularly practising any physical activity, for at least 1 h, 3 or more times a week), and smoking habits. Experienced nurses performed standard 12-lead ECGs with a 10 mm/mV gain, paper speed of 25 mm/s. ECGs were recorded at a sample rate of 1200 Hz and a bandwidth of 250 Hz, according to recommendations for paediatric ECGs.1–3 Each ECG was performed using Cardiolinear 2100 view system (Cardioline, Trento, Italy; CE certified), with students at rest and in a supine position during quiet respiration. Heart rate, PR interval, QRS axis and duration, and QT interval duration were measured by a computerized system (HES-EKG software, tested by the European project ‘Common Standards for Quantitative Electrocardiography’). The QT interval was corrected for heart rate (QTc) using Bazett’s formula. All ECG tracings were evaluated by one experienced cardiologist skilled in the arrhythmological field and blinded to the medical questionnaire. When an ECG abnormality was detected the trace was reviewed by a committee of three experienced cardiologists. ECG interpretation was done according to current international standards.3–5 ECG abnormalities found were classified as either common and non-clinically relevant (minor abnormalities) or clinically relevant (major abnormalities), as reported in Table 1. Height and weight measurements were performed wearing lightweight clothing and without shoes, and blood pressure measurement in a seated upright position after 5 min of rest. Blood pressure cut-offs to distinguish normal blood pressure, Stage 1 hypertension, Stage 2 hypertension and isolated systolic hypertension, have been established in accordance with 2016 European Society of Hypertension guidelines.6 The body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. In accordance with international standard age- and sex-adjusted cut-offs, subjects were classified into six groups distinguishing between slight, moderate, and severe thinness (I, II, and III degree, respectively), normal weight, and slight or severe obesity (I and II degree, respectively).
Table 1.
Common ECG abnormalities classified as non-clinically relevant (minor abnormalities) and pathological ECG patterns potentially indicative of heart disease (major abnormalities)
| Minor abnormalities | Major abnormalities |
|---|---|
| Sinus bradycardia | II and III degree atrio-ventricular block |
| First-degree atrio-ventricular block | Left anterior fascicle block |
| Incomplete RBBB | Left posterior fascicle block |
| Unspecific repolarization anomalies (early repolarization or juvenile T-wave pattern) | Ventricular pre-excitation |
| Supraventricular and ventricular premature beats | Complete LBBB or RBBB |
| Heterotopic atrial rhythm | Long QTc |
| Junctional rhythm |
ECG, electrocardiographic; LBBB, left bundle branch block; QTc, QT interval corrected for heart rate; RBBB, right bundle branch block.
Statistical analysis
Continuous and binary variables were reported as average ± standard deviation, and as percentage with 95% confidence intervals (CIs), respectively. Missing data were not replaced. Comparisons among age classes as well as association between ECG abnormalities and anthropometric characteristics were performed with linear or logistic mixed model using schools as random intercept. Dunn’s test with Bonferroni’s correction was used for pairwise multiple comparisons. Significance level was set at P = 0.05, after adjusting for multiple comparison when necessary. All statistical analyses were performed with STATA software version 11.1/SE (StataCorp, TX, USA).
Results
Clinical and ECG data of 24 062 students (∼8% of the 298 000 adolescents of the same age group resident in Rome) were collected, representing 80% of the students attending the 175 schools where the screening was performed. The students who were not enrolled were either absent or declined the screening. The mean age was 14.2 ± 2.1 and 12 347 (51%) were male. Over 99% of adolescents screened were Caucasian. The anthropometric characteristics, baseline ECG findings, and main clinical data of the study population divided by age class (12–13 years, 14–15 years, 16–17 years, and 18–19 years) are reported in Table 2.
Table 2.
Anthropometric characteristics, ECG findings, and main clinical data of the study population divided by age class
| Variables | All ages | Age class |
P-values | P-values | |||
|---|---|---|---|---|---|---|---|
| 12–13 | 14–15 | 16–17 | 18–19 | Mixed model analysis | Dunn’s pairwise comparisons | ||
| Only non-significant P-values are reported | |||||||
| N | 24 062 | 11 796 (49%) | 5976 (25%) | 3781 (16%) | 2509 (10%) | ||
| Female, n (%) | 11 715 | 5795 (49) | 2837 (47) | 1945 (51) | 1174 (49) | 0.52 | |
| Height (cm) | 164.4 ± 10.3 | 159.3 ± 8.7 | 167.3 ± 8.8 | 170.4 ± 9.0 | 172.2 ± 1.0 | <0.0001 | |
| Growth rate (cm/years) | 11.7 ± 1.4 | 12.8 ± 0.7 | 11.6 ± 0.7 | 10.3 ± 0.6 | 9.4 ± 0.6 | <0.0001 | |
| Weight (kg) | 56.2 ± 12.6 | 51.3 ± 11.2 | 58.6 ± 11.5 | 62.1 ± 11.5 | 64.5 ± 12.8 | <0.0001 | |
| BMI (kg/m2) | 20.7 ± 5.7 | 20.2 ± 4.1 | 20.9 ± 6.1 | 21.4 ± 6.0 | 21.9 ± 9.3 | <0.0001 | |
| Thinness (%, 95 % CI) | 7.5 (7.1–7.9) | 6.2 (5.7–6.6)* | 6.7 (6.0–7.4)* | 9.9 (9.0–11.0) | 12.1 (10.8–13.5) | <0.0001 | 0.70* |
| I degree | 5.9 (5.6–6.2) | 4.9 (4.5–5.3)* | 5.6 (5.0–6.2)* | 7.0 (6.2–7.9) | 9.3 (8.2–10.6) | <0.0001 | 0.25* |
| II degree | 1.2 (1.0–1.3) | 0.8 (0.7–1.0)* | 0.7 (0.5–1.0)* | 2.1 (1.7–2.7)† | 1.8 (1.3–2.5)† | <0.0001 | 0.99* |
| 0.68† | |||||||
| III degree | 0.5 (0.4–0.6) | 0.3 (0.2–0.4)* | 0.3 (0.2–0.5)* | 0.7 (0.5–1.1)† | 0.9 (0.6–1.4)† | <0.0001 | 0.99* |
| 0.67† | |||||||
| Obesity | 20.9 (20.4–21.5) | 27.1 (26.2–27.9) | 17.6 (16.6–18.7) | 13.5 (12.4–14.7)* | 11.8 (10.5–13.1)* | <0.0001 | 0.30* |
| I degree | 17.3 (16.8–17.9) | 22.2 (21.4–23.0) | 14.4 (13.5–15.4) | 11.9 (10.8–13.0)* | 10.0 (8.8–11.2)* | <0.0001 | 0.17* |
| II degree | 3.6 (3.3–3.8) | 4.8 (4.4–5.2) | 3.2 (2.7–3.7) | 1.7 (1.3–2.1)* | 1.8 (1.3–2.4)* | <0.0001 | 0.99* |
| SBP (mmHg) | 119 ± 15 | 117 ± 14 | 121 ± 15 | 122 ± 15* | 122 ± 15* | <0.0001 | 0.99* |
| DBP (mmHg) | 69.8 ± 11.4 | 69.6 ± 11.7 | 70.4 ± 11.4* | 69.5 ± 10.7† | 70.2 ± 10.8*,† | <0.0001 | 0.27* |
| 0.22† | |||||||
| ECG | |||||||
| Heart rate (bpm) | 80.6 ± 16.1 | 83.6 ± 15.8 | 79.6 ± 16.0 | 76.4 ± 15.6 | 74.3 ± 15.1 | <0.0001 | |
| PR interval (ms) | 136 ± 18 | 133 ± 16 | 137 ± 21 | 138 ± 19* | 140 ± 18* | <0.0001 | 0.08* |
| QRS (ms) | 87 ± 8 | 85 ± 7 | 86 ± 9 | 91 ± 9 | 90 ± 8 | 0.09 | |
| QTc (ms) | 413 ± 61 | 417 ± 32 | 412 ± 108 | 408 ± 29 | 404 ± 28 | <0.0001 | |
| Physical activity (%, 95% CI) | 69.7 (69.1–70.3) | 73.1 (72.4–74.0) | 70.2 (69.0–71.3) | 66.4 (64.9–67.9) | 57.6 (55.6–59.5) | <0.0001 | |
| Smoke (%, 95% CI) | 10.3 (9.9–10.7) | 1.9 (1.7–2.1) | 8.1 (7.4–8.8) | 22.8 (21.4–24.2) | 35.9 (34.0–37.8) | <0.0001 | |
Age class was significant in all variables (except for sex, P = 0.52) as a result of analyses performed using generalized linear mixed models with age class as the independent variable and school as random intercept. All post hoc multiple comparisons performed with the Dunn’s test were significant at the required level of P = 0.05 after Bonferroni’s adjustment, except for the * and † marked pairwise P-values reported in the last column. P-values of significant pairwise comparisons were not reported.
BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; QTc, heart rate-adjusted QT interval; SBP, systolic blood pressure.
Anthropometric characteristics and life style
Overall 7.5% of students were underweight and 20.9% obese. The prevalence of obesity was significantly higher in lower age classes (27.1% in age class 12–13 vs. 17.6% in age class 14–15, 13.5% in age class 16–17, and 11.8% in age class 18–19; P < 0.008 for all post hoc comparisons, except for the pair 16–17 vs. 18–19 years). The rate of students exercising decreased with age (from 73.1% to 70.2%, 66.4%, and 57.6%, P < 0.008), with an overall rate of 67%, while the rate of smoking increased with age (from 1.9% to 8.1, 22.8, and 35.9, P < 0.008), with an overall rate of 10.6%.
Arterial blood pressure
Systolic blood pressure was significantly lower in the 12–13 and 14–25 age classes (117 ± 14 mmHg and 121 ± 15 mmHg, respectively) compared with higher age classes (Bonferroni-adjusted P ≤ 0.05). Overall, Stage 1 hypertension was found in 3.14% of adolescents, Stage 2 hypertension in 0.45% of adolescents, and isolated systolic hypertension in 11.7% of adolescents. The Figure 1 shows the distribution of blood pressure values in the different age classes.
Figure 1.
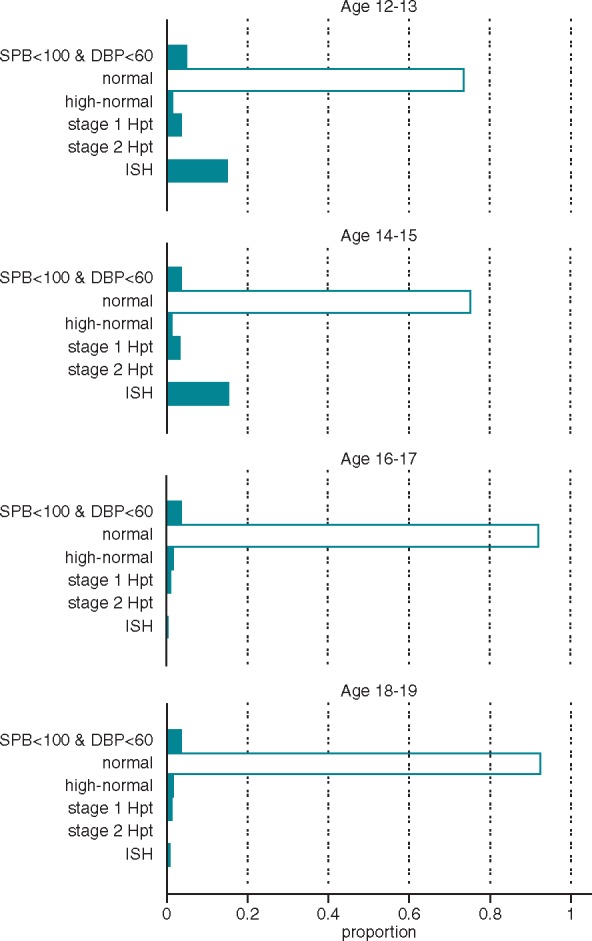
Arterial blood pressure value distribution in the different age classes. Prevalence of low blood pressure values (SBP < 100 mmHg, DBP < 60 mmHg, or both), normal blood pressure (<90th, in students 12–15 years old; <130/85, in students 16 years and older), high normal (>90th to <95th percentile, in students 12–15 years old; 130–139/85–89 mmHg, in students 16 years and older), Stage 1 hypertension (95th–99th percentile and 5 mmHg, in students 12–15 years old; 140–159/90–99 mmHg, in students 16 years and older), Stage 2 hypertension (>99th percentile plus 5mmHg, in students 12–15 years old; 160–179/100–109 mmHg, in students 16 years and older), and isolated systolic hypertension (SBP >95th percentile and DBP <90th percentile, in students 12–15 years old; >140/<90 mmHg in students 16 years and older) observed in the different age classes. Blood pressure values have been adjusted for age, sex, and height. Blood pressure cut-off values of the different categories have been defined in accordance with the classification reported in 2016 guidelines of the European Society of Hypertension.6 DBP, diastolic blood pressure; ISH, isolated systolic hypertension; SBP, systolic blood pressure.
ECG characteristics
The mean heart rate was progressively lower with increasing age, with significant differences among the various age classes (83.6 vs. 79.6, 76.4, and 74.3; P < 0.0001). The duration of PR interval was shorter in lower age classes, however difference between the 16–17 and 18–19 age classes were not significant (P = 0.08 only approached statistical significance after Bonferroni’s correction). The duration of QRS interval showed an upward trend from the class age 12–13 to 16–17 (age effect P = 0.09, random intercept mixed model) and was longer in males than in females in all the age classes (Figure 2). The mean QTc was progressively shorter with increasing age, with significant differences among the various age classes (417 ms vs. 412 ms, 408 ms, and 404 ms; P < 0.0001). Starting at age 14, a longer QTc interval was found in females (Figure 2). Minor abnormalities were present in 4898 (20.3%) students and major abnormalities in 411 (1.7%) students. Both minor and major abnormalities had higher prevalence in males than in females (24.5% vs. 16.6%, P < 0.001 and 2.8% vs. 0.6%, P < 0.001, respectively). The most frequent minor abnormality was an incomplete right bundle branch block (RBBB) present in 15.24% of subjects, whereas the most frequent major abnormality was a complete RBBB, found in 1.57% of subjects. A left bundle branch block (LBBB) was found in 0.01%, a II or III degree atrio-ventricular block in 0.01%, and a long QTc in 0.002% of students. A sinus bradycardia was found in 3.86% of students, with a greater prevalence in adolescents practising regular physical activity (4.8% vs. 1.7%, P < 0.0001). The Table 3 shows the prevalence of major and minor abnormalities in the different age classes.
Figure 2.
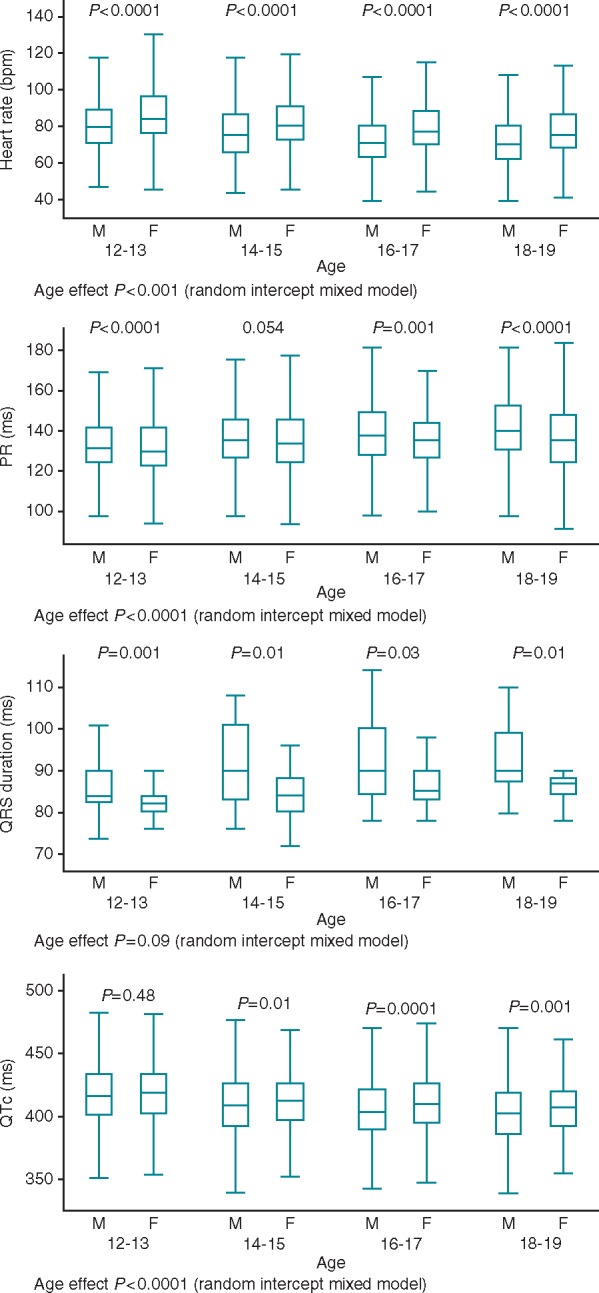
Heart rate, PR, QRS, and QTc interval in males and females in the different age classes. F, females; M, males; QTc, QT interval corrected for heart rate.
Table 3.
Prevalence of minor and major ECG abnormalities per age class, % (95% confidence interval)
| Age class |
||||
|---|---|---|---|---|
| 12–13 | 14–15 | 16–17 | 18–19 | |
| Major abnormalities | ||||
| Complete RBBB | 1.60 (1.37–1.83) | 1.77 (1.44–2.10) | 1.59 (1.19–1.98) | 0.92 (0.54–1.30) |
| Left anterior fascicular block | 0.18 (0.10–0.25) | 0.10 (0.02–0.18) | 0.16 (0.03–0.28) | 0.12 (0.01–0.25) |
| Ventricular pre-excitation | 0.07 (0.02–0.11) | 0.08 (0.01–0.16) | 0.03 (0.02–0.08) | 0.28 (0.007–0.48) |
| II/III AV block | 0.02 (0.01–0.04) | 0.02 (0.01–0.04) | – | – |
| Complete LBBB | 0.01 (0.01–0.02) | 0.02 (0.02–0.05) | – | – |
| Minor abnormalities | ||||
| Incomplete RBBB | 14.5 (13.9–15.1) | 16.3 (15.3–17.2) | 15.8 (14.6–16.9) | 15.4 (14.0–16.8) |
| Sinus bradycardia | 2.40 (2.13–2.68) | 4.40 (3.88–4.92) | 5.53 (4.80–6.26) | 6.90 (5.90–7.89) |
| Unspecific repolarization anomalies | 1.25 (1.10–1.45) | 0.99 (0.74–1.24) | 0.66 (0.40–0.92) | 0.56 (0.27–0.85) |
| Heterotopic atrial rhythm | 0.51 (0.39–0.65) | 0.60 (0.41–0.80) | 0.58 (0.34–0.82) | 0.56 (0.27–0.85) |
| Supraventricular premature beats | 0.11 (0.05–0.17) | 0.30 (0.16–0.44) | 0.16 (0.03–0.28) | 0.24 (0.05–0.43) |
| Ventricular premature beats | 0.22 (0.13–0.30) | 0.15 (0.05–0.25) | 0.13 (0.02–0.25) | 0.08 (0.03–0.19) |
| I degree AV block | 0.08 (0.03–0.13) | 0.13 (0.04–0.23) | 0.13 (0.02–0.25) | 0.12 (0.01–0.25) |
| Junctional rhythm | – | 0.02 (0.02–0.05) | 0.10 (0.00–0.20) | – |
AV, atrio-ventricular; LBBB, left bundle branch block; RBBB, right bundle branch block.
ECG abnormality correlations with anthropometric characteristics
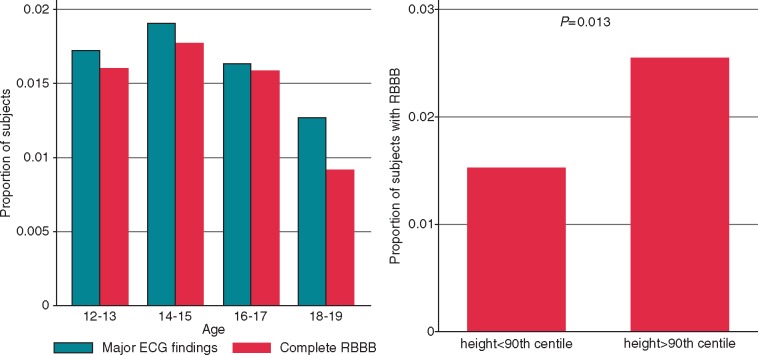
The prevalence of minor abnormalities was significantly higher in normal or taller students (height >10° percentiles) compared with shorter students (height <10° percentiles): 20.7% vs. 16.7%, respectively (P < 0.001). The rate of ECG minor abnormalities has been observed to increase significantly with the severity of thinness [20.6% in subjects with normal BMI vs. 26.3% in I degree thinness (P < 0.001), 27.3% in II degree thinness (P = 0.06), and 31.1% in III degree thinness (P = 0.03)]. Inversely, minor ECG abnormalities rates were found to decrease with the degree of obesity (22.2% in normal BMI vs. 14.9% in I degree obesity; P < 0.0001, and 11.9% in II degree obesity; P < 0.0001; see Supplementary material online, Table S1). The higher prevalence of minor abnormalities in underweight students and the lower prevalence in obese subjects is due to the higher rate of incomplete RBBB observed in underweight students (21.58% in underweight students vs. 15.10% in non-underweight students, P < 0.0001) and the lower rate of incomplete RBBB found in obese students (10.23% vs. 17% in non-obese students, P < 0.0001). Major ECG abnormalities have been found with higher prevalence in taller subjects (height >90°percentiles) compared with normal or short subjects (height <90°percentiles): 1.7% vs. 2.7%, respectively (P = 0.021). This finding is due to the higher prevalence of RBBB observed in taller subjects (3.4% in height >90°percentile vs. 1.3% in height <90°percentile, P < 0.0001). Table 4 shows 90° percentiles of height in each age class along with the relative RBBB prevalence: height ≥1.70 m in age class 12–13 or ≥1.79 in age class 14–15 was associated with a 77% increased odds of RBBB (P < 0.0001) after adjusting for sex; similarly, but with weaker evidence, students over 1.82 m and 1.85 m in height had almost two-fold increased odds of RBBB in age classes 16–17 and 18–19, respectively. Overall, we observed an inverse association of RBBB prevalence with increasing age (Figure 3). Uni- and multivariate analyses of association of RBBB to some demographic and anthropometric variables, showed that RBBB prevalence was significantly associated with height even after adjusting for age, sex, and BMI (odds ratio 17.9; 95% CI 5.0–64.6) (Table 5).
Table 4.
Complete right bundle branch block prevalence by age class
| Age class | 90th centile height (m) | RBBB prevalence (%) | RBBB prevalence (%) | OR (95% CI)a | P-value |
|---|---|---|---|---|---|
| <90th centile height | ≥90th centile height | ||||
| 12–13 | 1.70 | 1.28 | 3.60 | 1.77 (1.32–2.30) | <0.0001 |
| 14–15 | 1.79 | 1.59 | 3.01 | ||
| 16–17 | 1.82 | 1.04 | 4.76 | 1.89 (1.11–3.21) | 0.018 |
| 18–19 | 1.85 | 0.91 | 1.09 | ||
| All | 1.78 | 1.29 | 3.41 |
CI, confidence interval; OR, odds ratio; RBBB, right branch bundle block.
Sex-adjusted OR as results of logistic random intercept (school level) mixed model.
Figure 3.
Prevalence of major ECG abnormalities and complete RBBB by age class and of complete RBBB by stature subgroup. Left panel: prevalence of major ECG anomalies and complete RBBB in each age class. Right panel: prevalence of complete RBBB in subgroups of height < and >90th centile. ECG, electrocardiographic; RBBB, right bundle branch block.
Table 5.
Right bundle branch block association with demographic and physical characteristics
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Age (years) | 0.96 (0.89–1.03) | 0.28 | 0.91 (0.84–0.99) | 0.048 |
| Sex (m = 0; f = 1) | 0.15 (0.11–0.21) | <0.001 | 0.17 (0.12–0.24) | <0.001 |
| Height (m) | 61.1 (18.5–201.6) | <0.001 | 14.0 (3.7–54.8) | <0.001 |
| BMI (kg/m2) | 0.91 (0.88–0.94) | <0.001 | 0.88 (0.85–0.92) | <0.0001 |
Uni- and multivariate analyses of association of right bundle branch block to some demographic and anthropometric variables reported as OR. Results obtained with logistic mixed model having the variable of interest as covariate and schools as random intercept.
BMI, body mass index; CI, confidence interval; f, female; m, male; OR, odds ratio.
Furthermore, a significant correlation between physical activity and RBBB was found. 1.80% of students regularly exercising had a complete RBBB vs. 1.02% of students who referred not to practice regular physical activity (P = 0.0009 as a result of logistic mixed model with random intercept at school level and adjusting for age and height).
Discussion
Very few data are available about the association between anthropometric characteristics and ECG patterns. Data which are available were largely obtained from adult populations and when adolescents were evaluated, major ECG abnormalities were not systematically assessed.7 Furthermore, published ECG findings in large young populations were mainly focused on athletes as report of pre-participation evaluation aiming at sudden cardiac death prevention,8 not systematically dealing with anthropometric parameters8–11 and in some cases focused on young males in military conscription older than our population. To the best of our knowledge this is the largest report on non-selected adolescents which evaluates ECG and anthropometric characteristics and the first which attempts to assess the correlations between ECG abnormalities and anthropometric parameters. Furthermore, this large database supplies relevant findings about the prevalence of some cardiovascular risk factors (smoking, physical activity, blood pressure, and obesity) in adolescents attending schools in a metropolitan city.
Anthropometric characteristics, life style, and arterial blood pressure
The prevalence of adolescent smokers found in this study (10.3%) was similar to that reported among adolescents in the United States (US) (8% in 2014 and 10.6% in 2012).12 The prevalence of adolescents that do not exercise was found to increase with age as reported in a U.S. nationwide survey.13 The overall prevalence of obesity observed in our adolescent population (20.9%) is similar to the 20.5% rate reported among subjects with the same age range by the National Health and Nutrition Examination Survey conducted in the US in 2011–12.14 The relevance of this data is due to the evidence that the obesity in adolescence is a risk factor for coronary heart disease in young adulthood. On the other hand, the prevalence of thinness among adolescents is also non-negligible (7.5%). Considering that the screening has involved a population of students living in a big metropolitan of a developed country, the non-negligible prevalence of underweight adolescents warrants further analysis from a nutritional and psychological point of view. The prevalence of high blood pressure values found in this report (Stage 1 hypertension found in 3.14% of adolescents, Stage 2 hypertension in 0.45% of adolescents, and isolated systolic hypertension in 11.7% of adolescents 11.1%) is in the range of the values observed in other studies conducted in Europe (2.2–13%).6
ECG findings and their correlations with anthropometric characteristics
Although recently there has been a great effort by international experts to establish standard recommendations for ECG interpretation in young athletes,5 there is still a lack of general consensus on the criteria for defining an abnormal ECG in the general populations of adolescents.1
This report shows age related changes in the ECGs of adolescents, such as a progressive reduction in heart rate associated with a decrease in the QTc interval up until 18–19 years old. Previous observations regarding ECG parameters which were carried out mainly in paediatric subjects up to 16 years old15,16 were typically much smaller and showed a similar trend in the reduction of heart rate and QTc. The strength of our data is that it was obtained from a large sample including the general adolescent population and the systematic analysis was conducted by dividing the population into small age classes of only 2 years. We found QTc intervals greater in females than in males for each age group over 14 years. This observation confirms with more robust data the results of a previous study which focused on the heart rate and QTc interval of 781 children aged 10–18 years.17 Overall ECG abnormalities were found more often in males than in females, consistent with other reports,7–10 with a total prevalence of ECG abnormalities of 22%, close to that found in another Italian study which involved a smaller sample of students.11 The higher prevalence of an incomplete RBBB reported in underweight subjects and lower rate in obese subjects is consistent with data observed in adults, where the incomplete RBBB was found to be associated with a lower BMI.18 It may be speculated that this is due to the systematic differences in precordial lead placement and the different cardiac location within the chest in subject with very low or very high BMI. Previous reports of ECG screening in youths found that around 2% of ECG major anomalies suggested heart disease,9 similar to our report. The complete RBBB, the most common major abnormality detected in this study, was found to have a higher prevalence than in another report conducted in the US which included subjects ranging in age from 14–19 years.9 A higher frequency of RBBB in taller subjects was observed, and the RBBB was found to have a significant association with stature irrespective of age, sex, and BMI. Furthermore, we attempt to establish a cut-off height value over which the subjects were at higher risk for RBBB. The observed relationship between RBBB and height may suggest that taller stature is associated with an anomalous conduction in the right bundle branch. Of note, a recent study has found a similar relationship between the height of adults and an altered cardiac conduction system.19 Moreover, conduction disturbances are not rare in acromegaly, an endocrine disease characterised by anomalous growth, where RBBB has been found in up to 10% of patients.20 With this study, we also found a higher prevalence of RBBB among subjects regularly practising physical activity. This finding has been previously reported in literature and may be the consequence of an excess wall stress that causes structural and electrical remodelling.
Left bundle branch block, despite its infrequency, was found to have a higher rate than the US study which reported no cases of LBBB.9 Second and third degree atrio-ventricular block were found with a similar rate to the U.S. study9 whereas no specific data are available from any of the Italian reports. Two students had a third degree atrio-ventricular block, both were obese and after pacemaker implantation noticed the disappearance of some symptoms previously not associated with the bradycardia, such as postprandial headache and exertion dyspnoea. The rate of a long QTc was lower than previous U.S. report,9 probably because, according to international recommendations1 and unlike the previous U.S. study, all ECG measurements in this study were carried out by a computerized system. The ventricular pre-excitation was rare and the prevalence was found to be consistent with the previous Italian study.11 The absence of students who presented short QTc or ECG features of hypertrophic cardiomiopathy, arrhythmogenic right ventricular cardiomyopathy, and Brugada 1 pattern may be explained by the very low prevalence of these conditions in the general population with, for some of these, an even lower prevalence rate in young people.
Limitations
It is worthy to address some study limitations. The first ECG interpretation was done by a single cardiologist who was skilled in electrocardiography and in arrhythmology, and only ECGs with an abnormality were re-evaluated by a committee of three cardiologists. A second limitation pertains to the classification of minor and major abnormalities, which was mainly done based on the international standards for ECG interpretation in athletes,5 since a consensus about the normal findings of adolescent ECGs is still lacking. The classification of the ECG abnormalities found was done on the basis of the interpretation of a single ECG, therefore, variable behaviours over time of some ECG abnormalities could not be detected. In addition, cut-off values employed to define ECG abnormalities, although based on current international recommendations, may actually represent continuous variables with age related changes and this aspect should be considered in interpreting study results. Furthermore, the study has involved a population of adolescents attending secondary schools of Rome, therefore, all students enrolled lived in a big metropolitan and their habits are unavoidably conditioned by this specific social background. In addition, since the pubertal stage affects the QT interval in females, a further issue is the lack of information regarding the onset of periods. Finally, we do not have complete data about the results of diagnostic exams performed on students with major ECG abnormalities. In particular, echocardiographic data would have permitted an examination of the right ventricle and the detection of possible causes of the relatively frequent RBBB. Because of the cross-sectional design of this study not even the outcomes are available. Therefore, the prognostic relevance of the ECG findings reported cannot be established.
Conclusions
The systematic analysis of ECG characteristics divided per age class has demonstrated significant age-related ECG changes which, until now, had never been reported in such a large population of adolescents. In this homogenous population of adolescents, the prevalence of ECGs potentially indicative of heart disease was 1.7%, and the RBBB was the most common major abnormality. The reported correlation between ECG findings and anthropometric characteristics is a real novelty. Particularly we have found that taller adolescents were more likely to have complete RBBB. Furthermore, the evaluation of anthropometric parameters together with blood pressure measurements and unhealthy habits supplies relevant findings about the prevalence of some cardiovascular risk factors in adolescents.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We thank Gioia Cassoni for technical assistance.
Funding
This work was funded by the Peretti Foundation (Rome, Italy) and the Rome Foundation (Rome, Italy).
Conflict of interest: A.G. is an employee of Biotronik-Italia, and participated in the study and contributed to statistical analyses independently and without remuneration. Biotronik was not involved in this research study and did not provide grants, funding, or any form of financial support. The remaining authors have no relationship with industry or other entities to disclose.
References
- 1. Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR. et al. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol 2014;64:1479–514. [DOI] [PubMed] [Google Scholar]
- 2. Dickinson DF. The normal ECG in childhood and adolescence. Heart 2005;91:1626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gulizia MM, Casolo G, Zuin G, Morichelli L, Calcagnini G, Ventimiglia V. et al. ANMCO/AIIC/SIT Consensus Information Document: definition, precision, and suitability of electrocardiographic signals of electrocardiographs, ergometry, Holter electrocardiogram, telemetry, and bedside monitoring systems. Eur Heart J Suppl 2017;19:D190–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A. et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:976–81. [DOI] [PubMed] [Google Scholar]
- 5. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM. et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol 2017;69:1057–75. [DOI] [PubMed] [Google Scholar]
- 6. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A. et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 2016;34:1887–920. [DOI] [PubMed] [Google Scholar]
- 7. Sun GZ, Li Y, Zhou XH, Zhang XG, Zheng LQ, Li Y. et al. Association between obesity and ECG variables in children and adolescents: a cross-sectional study. Exp Ther Med 2013;6:1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelliccia A, Culasso F, Di Paolo FM, Accettura D, Cantore R, Castagna W. et al. Prevalence of abnormal electrocardiograms in a large, unselected population undergoing pre-participation cardiovascular screening. Eur Heart J 2007;28:2006–10. [DOI] [PubMed] [Google Scholar]
- 9. Marek J, Bufalino V, Davis J, Marek K, Gami A, Stephan W. et al. Feasibility and findings of large-scale electrocardiographic screening in young adults: data from 32,561 subjects. Heart Rhythm 2011;8:1555–9. [DOI] [PubMed] [Google Scholar]
- 10. Chandra N, Bastiaenen R, Papadakis M, Panoulas VF, Ghani S, Duschl J. et al. Prevalence of electrocardiographic anomalies in young individuals: relevance to a nationwide cardiac screening program. J Am Coll Cardiol 2014;63:2028–34. [DOI] [PubMed] [Google Scholar]
- 11. De Lazzari C, Genuini I, Gatto MC, Cinque A, Mancone M, D'Ambrosi A. et al. Screening high school students in Italy for sudden cardiac death prevention by using a telecardiology device: a retrospective observational study. Cardiol Young 2017;27:74–81. [DOI] [PubMed] [Google Scholar]
- 12. Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE.. Monitoring the Future National Survey Results on Drug Use, 1975-2015: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, University of Michigan; 2016. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf (1 March 2018, date last accessed). [Google Scholar]
- 13. Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J. et al. Youth risk behavior surveillance—United States, 2015. MMWR Surveill Summ 2016;65:1–174. [DOI] [PubMed] [Google Scholar]
- 14. Ogden CL, Carroll MD, Kit BK, Flegal KM.. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rijnbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA.. New normal limits for the paediatric electrocardiogram. Eur Heart J 2001;22:702–11. [DOI] [PubMed] [Google Scholar]
- 16. Davignon A, Rautaharju P, Boisselle E, Soumis F, Mégélas M, Choquette A.. Normal ECG standards for infants and children. Pediatr Cardiol 1980;1:123–31. [Google Scholar]
- 17. Pearl W. Effects of gender, age, and heart rate on QT intervals in children. Pediatr Cardiol 1996;17:135–6. [DOI] [PubMed] [Google Scholar]
- 18. Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E.. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J 2013;34:138–46. [DOI] [PubMed] [Google Scholar]
- 19. Kofler T, Thériault S, Bossard M, Aeschbacher S, Bernet S, Krisai P. et al. Relationships of measured and genetically determined height with the cardiac conduction system in healthy adults. Circ Arrhythm Electrophysiol 2017;10:e004735.. [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues EA, Caruana MP, Lahiri A, Nabarro JD, Jacobs HS, Raftery EB.. Subclinical cardiac dysfunction in acromegaly: evidence for a specific disease of heart muscle. Heart 1990;64:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.