Abstract
Context
Testosterone (T) increases GH secretion in older men with a relative lack of T, in hypogonadal men of all ages, and in patients undergoing sex reassignment. The role of estradiol (E2) in men is less well defined.
Objective
To assess the contribution of aromatization of T to spontaneous nocturnal and stimulated GH secretion.
Participants
Four groups of healthy older men (N = 74, age range 57 to 77 years) were studied. The gonadotropic axis was clamped with the gonadotropin-releasing hormone antagonist degarelix. Three groups received T and one group placebo addback. Two T-replaced groups were treated with anastrozole (an aromatase inhibitor) and either placebo or E2 addback.
Main Outcome Measures
Ten-minute GH concentration profiles were quantified by deconvolution analysis, after overnight (2200 to 0800 hours) sampling, and after combined IV injection of GHRH (0.3 µg/kg) and GHRH-2 (0.3 µg/kg) and withdrawal of a 2-hour somatostatin infusion (1 µg/kg/h).
Results
E2 addback during aromatase inhibition increased basal (P = 0.046), pulsatile (P = 0.020), and total (P = 0.018) GH secretion by 60% to 70%. E2 did not potentiate GH secretory stimuli. Logarithmically transformed pulsatile GH secretion correlated strongly and positively with concurrent E2 concentrations overall (P = 0.028) and under anastrozole treatment (P = 0.005).
Conclusion
E2 administration in older men transdermally stimulates overnight pulsatile GH secretion. The exact site of E2 action cannot be ascertained from these experiments but may include hypothalamic loci involved in GH regulation, especially because GH secretagogue effects on somatotrope pituitary cells were not affected.
Sex steroid concentrations were clamped by aromatase inhibitor in gonadotropin-downregulated men. Transdermal estradiol increased overnight pulsatile GH secretion in a concentration-dependent manner.
Systemic concentrations of testosterone (T), estradiol (E2), GH, IGF-I, and IGF-binding protein 3 decline in healthy older men (1, 2). Relative sex-steroid deprivation in aging accentuates GH and IGF-I depletion, because T stimulates GH and IGF-I production in older men, hypogonadal males of all ages, and patients undergoing (genotypic female-to-male) sex reassignment (1–3). From a clinical vantage, understanding the mechanistic basis of T’s drive of the somatotropic axis is especially relevant in boys with pubertal failure, adults with primary hypogonadism, and men with aging-related hypoandrogenemia. In relation to aging in men, the bioavailabilities of T and E2 decline by 35% to 50% in the eighth compared with third decade of life (1, 4). From a medical perspective, aging is accompanied by progressive osteopenia, sarcopenia, and intra-abdominal obesity (5). Some of these adverse outcomes are remediable by short-term replacement with T or recombinant GH (2), thus linking GH, T, and E2 availability with key body compositional features.
T acts via three major mechanistic pathways: without biotransformation and after conversion to E2 (aromatization) or 5α-DHT (reduction). Estrogenic steroids exert important effects on the GH axis, inasmuch as transgenic silencing of the alpha estrogen receptor (ER) gene in mice lowers systemic IGF-I concentrations, nonaromatizable androgens fail to stimulate GH secretion in the human, and ER antagonists impede, and ER agonists enhance, GH secretion in girls with Turner syndrome, postmenopausal women, male-to-female transsexual patients, and men with prostatic cancer necessitating estrogen therapy (1, 2, 5). Earlier investigations have suggested an important role of aromatization of T to E2 in the stimulatory effect of this androgen on spontaneous and drug-induced GH secretion. Different approaches were used, such as partial ER blocking with tamoxifen and the use of nonaromatizable androgens (6, 7). Another study recorded diminished GH stimulation under aromatase inhibitory treatment (8). However, none of these studies was fully controlled with respect to the endogenous sex steroid milieu, because the foregoing interventions also change T levels. Thus, whether estrogen per se specifically mediates T’s stimulation of GH secretion in men remains unknown. The question is salient, because we found recently that inhibiting the conversion of T to E2 by using anastrozole lowered GH output by 50% in older men but concomitantly elevated T concentrations. Whether exogenous E2 administration could sustain pulsatile GH secretion when T levels do not change in men remains unknown. Therefore, the purpose of this investigation was to assess whether transdermally supplied E2 in subjects with blocked aromatization of T and exogenous T addback under a GnRH receptor antagonist to limit endogenous T changes can rescue spontaneous nocturnal and stimulated GH secretion in older healthy men. This design addresses more explicitly the role of aromatization of T under fixed T availability in GH secretion.
Clinical Protocol
Subjects
Seventy-four healthy, ambulatory, community-dwelling older men (mean age 65 years, range 57 to 77 years) participated in the overnight Clinical Translational Unit (CRU)-based study. Mean body mass index (BMI) was 26.9, range 20 to 36 kg/m2. Volunteers were recruited by newspaper advertisements, local posters, the Clinical Trials Center web page, and community (general and minority) bulletin boards. This was an investigator-initiated double-blind, placebo-controlled prospective study in gonadotropin-downregulated men, approved by the US Food and Drug Administration. Gonadotropin downregulation was accomplished by degarelix (Ferring Pharmaceuticals, Parsippany, NJ) administration. Randomization was IM T vs placebo and transdermal E2vs no treatment in subjects treated with anastrozole (Astra Zeneca Pharmaceuticals, Wilmington, DE) (an aromatase inhibitor). The T/E2 clamp consisted of degarelix 80 mg (given as two subcutaneous injections of 40 mg) once (called day 1), T enanthate or T cypionate (Cardinal Health, Hudson, WI) 100 mg/placebo IM given on day 1 and repeated on days 8 and 15 (range ± 1 day), oral placebo or anastrozole 2 mg once daily for 21 days, and no transdermal patch or an E2 patch calibrated to deliver 0.05 mg/day E2 (Novartis, East Hanover, NJ) beginning on day 1 and changed every 3 days through day 22. Subjects underwent overnight and next-morning sampling on days 20 to 24 to accommodate CRU scheduling.
In summary, effects of E2 on GH secretion in older men were quantified by the use of anastrozole, a potent and selective aromatase inhibitor, which blocks the conversion of T to E2 (9). Because the resulting decrease in E2 levels would amplify LH secretion, and hence T secretion (10), all subjects were pretreated with degarelix, a potent, selective GnRH antagonist. The resultant low T concentrations were then controlled experimentally via exogenous T or placebo injections. Because E2 levels also fall on degarelix, and even more on anastrozole, we restored E2 concentrations by adding E2 back exogenously, in this case transdermally. This strategy permitted quantification of the influence of a wide range of serum E2 concentrations on GH secretion after an interval of 22 (±2) days.
Subjects arrived in the CRU at or before 1800 hours to permit placement of bilateral forearm IV catheters. A blood sample was obtained at 0800 hours for baseline sex hormone and peptide measurements. Ambulation was allowed to the lavatory. The subject was allowed to sleep during the sampling window. Each subject underwent blood sampling at 10-minute intervals during the 10-hour overnight (from 2200 hours until 0800 hours) stay in the CRU. A single combined injection of GHRH (Bachem Americas, Inc., Torrance, CA) and GH-releasing peptide 2 (GHRP2) (Kaken Pharmaceutical Company, Tokyo, Japan) (both doses 0.3 µg/kg) was given by IV bolus at 0800 hours in 40 subjects to test pituitary effects of the estrogen protocols. Blood sampling at 10-minute intervals was continued for another 2 hours. Thirty-four other subjects received a 2-hour IV somatostatin (Bachem Americas, Inc.) infusion (1 µg/kg/h), after which rebound GH increase was followed for 3 hours by 10-minute blood sampling. At the end of blood sampling, IV catheters were removed and the subjects received a meal before discharge from the CRU. To reduce nutritional confounds, volunteers were given a prescribed meal to ingest on the evening before the sampling session. Subjects received a standardized 10-kcal/kg meal (vegetarian or nonvegetarian) with a macronutrient composition of 20% protein, 50% carbohydrate, and 30% fat. Participants then remained fasting overnight (except for allowable intake of noncaloric and noncaffeinated liquids) until noon. Breakfast was offered before discharge from the CRU.
The protocol was approved by Mayo Institutional Review Board. Witnessed voluntary written consent was obtained before study enrollment. A complete medical history, physical examination, and screening tests of hematological, renal, hepatic, metabolic, and endocrine function were normal. Subjects underwent a single-slice CT of the abdomen, level L3 to L4, as an exploratory measure to evaluate the impact of visceral adiposity on GH secretion.
Criteria for exclusion
Exclusion criteria were recent use of psychotropic or neuroactive drugs; obesity (outside weight range); abnormal laboratory test results; drug or alcohol abuse, psychosis, depression, mania, or severe anxiety; acute or chronic organ system disease; endocrinopathy, other than primary thyroidal failure receiving replacement; nightshift work or recent transmeridian travel (more than three time zones); acute weight change (loss or gain of >2 kg in 6 weeks); allergy to peanut oil (used in injectable T preparation); unwillingness to provide written informed consent; history or suspicion of prostatic disease (elevated prostate-specific antigen, indeterminate nodule or mass, obstructive uropathy); history of carcinoma (excluding localized basal cell carcinoma removed or surgically treated with no recurrence); history of thrombotic arterial disease (stroke, transient ischemic attack, myocardial infarction, angina) or deep vein thrombophlebitis; and history of smoking greater than one pack per day.
Laboratory assays
The 10-minute serum samples were assayed in a single batch in each subject by chemiluminescence GH assay, performed with Beckman robotics Access ultrasensitive human GH assay (Beckman Coulter, Inc., Fullerton, CA). Within-assay precision was 3.8% to 6.5% (range of 100 runs), sensitivity 0.010 μg/L, and specificity 97% 22 kDa GH. The following hormones and peptides were measured in the 0800 hours fasting blood specimen. Estrone, E2, and T were measured by liquid chromatography–tandem mass spectrometry (Agilent Technologies, Inc., Santa Clara, CA); interassay coefficients of variation (CVs) were E2 10.8% at 0.29 pg/mL and 5.1% at 32 pg/mL and T 8.9% at 0.69 ng/dL, 4.0% at 45 ng/dL, and 3.5% at 841 ng/dL. IGF-I and SHBG were quantified by solid-phase chemiluminescent assay on the Siemens Immulite 2000 Automated Immunoassay System (Siemens Healthcare Diagnostics, Deerfield, IL). Interassay CVs for IGF-I were 4.9% at 37 ng/mL and 5% at 225 µg/L; and for SHBG 4.0% at 5.4 nmol/L and 5.9% at 74 nmol/L. Prolactin, FSH, and LH were measured by two-site chemiluminescent sandwich immunoassays on a DXL 800 automated immunoassay system (Beckman Instruments, Chaska, MN). For prolactin the interassay CVs were 3.7%, 2.1%, and 4.8% at 6.1, 16.4, and 34.5 µg/L; for FSH the interassay CVs were 3.6%, 3.2%, and 4.7% at 6.5, 16.7, and 58.0 IU/L, respectively; and for LH, interassay CVs were 9.3%, 6.0%, and 6.0% at 1.4, 15.6, and 48.8 IU/L, respectively.
Analytical methods
Ten-hour overnight and complete GH concentration time series (12 or 15 hours) were subjected to biexponential deconvolution analysis based on independently determined estimates of two-compartment GH disappearance kinetics (11). The extended time series were used to estimate GH secretion after stimulation with GHRH/GHRP2 or somatostatin withdrawal. The primary analytical outcome was the summed mass of GH secreted in pulses over the 10 hours of overnight blood sampling. This outcome measure is relevant, because sex steroid hormones and regulatory peptides uniquely control GH secretory burst mass. Stimulatory effects were also assessed by mean and maximal concentrations. The time at which maximal levels occurred was derived from the raw data.
Statistical assessment
The null hypothesis is that the estrogenic milieu does not determine endogenously driven (spontaneous) pulsatile GH secretion in older men. Here, the statistical endpoint was the summed mass of GH secreted over 10 hours. Analyses included one-way ANOVA across independent groups. Where appropriate, logarithmic transformation of the data were applied. Furthermore, linear regression tools were used. P values of ≤0.05 were considered significant. The study size was based on a 1.8 ± 0.3 (SD)-fold stimulatory effect of estrogen on pulsatile GH secretion observed in women (2). Statistical power to detect a comparable (80%) effect of a wide range of enforced E2 concentrations in a total (combined group) of 60 men is >90% assuming P < 0.05. Calculations were performed with Systat version 13 (Systat Software, Inc., San Jose, CA).
Results
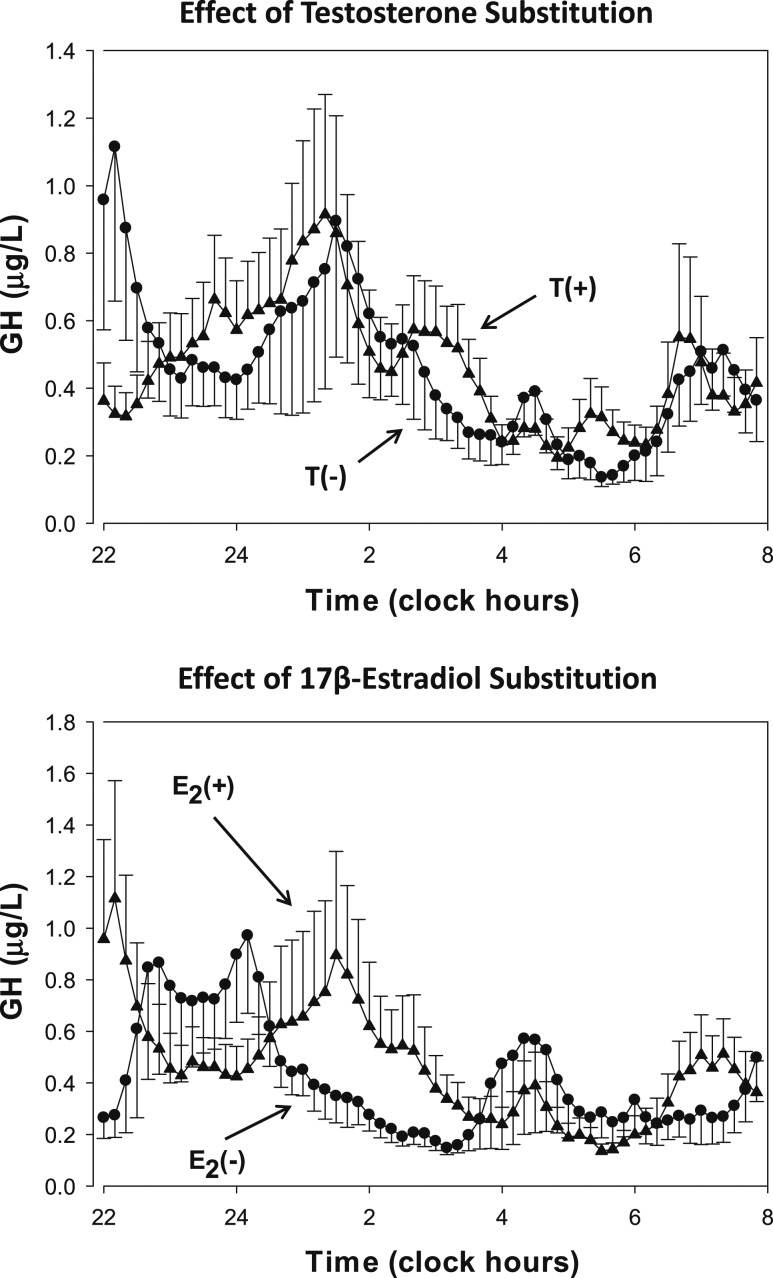
As shown in Table 1, the four groups of subjects were comparable with respect to age, BMI, and screening hormone levels. The GH profiles of the first 10 hours of blood sampling are shown in Fig. 1. The upper panel exhibits mean ± SEM. GH concentrations from 10-minute sampling in the groups treated with degarelix to suppress gonadotropin and T secretion. Note that 18 subjects received T addback and 16 placebo. No obvious visual differences between the profiles are present. Details of the deconvolution analyses are shown in Table 2. Serum E2 and T levels were low in nonsubstituted men with downregulated gonadotropin secretion, but GH secretion was unaffected compared with men who received T addback. In addition, approximate entropy was similar in both groups, indicating unchanged feedback of GH secretion in the T- and E2-depleted men.
Table 1.
Demographic Data
| Variable | (n = 16) D/T(−) | (n = 18) D/T(+) | (n = 20) D/T(+)/A/E2(−) | (n = 20) D/T(+)/A/E2(+) | ANOVA P |
|---|---|---|---|---|---|
| Age, y | 64 ± 0.8 | 66 ± 1.0 | 65 ± 1.0 | 65 ± 1.2 | 0.69 |
| BMI, kg/m2 | 28.1 ± 1.1 | 27.7 ± 0.8 | 25.4 ± 0.5 | 26.9 ± 0.9 | 0.12 |
| TSH, mU/L | 2.33 ± 0.2 | 2.76 ± 0.3 | 2.83 ± 0.3 | 2.90 ± 0.3 | 0.44 |
| Prolactin, µg/L | 9.2 ± 0.9 | 8.4 ± 0.6 | 8.7 ± 0.7 | 8.3 ± 0.6 | 0.81 |
| T, ng/dL | 430 ± 30 | 430 ± 21 | 480 ± 22 | 500 ± 28 | 0.11 |
| E2, pg/mL | 22.6 ± 2.5 | 21.4 ± 1.6 | 27.2 ± 1.3 | 24.7 ± 1.3 | 0.08 |
| SHBG, nmol/L | 45 ± 3.3 | 43 ± 3.2 | 42 ± 2.7 | 50 ± 3.3 | 0.35 |
| LH, U/L | 5.0 ± 0.9 | 4.0 ± 0.4 | 5.5 ± 0.8 | 4.0 ± 0.4 | 0.23 |
| FSH, U/L | 9.1 ± 1.0 | 6.6 ± 0.7 | 9.9 ± 1.3 | 6.8 ± 0.6 | 0.12 |
Groups: D/T(−), degarelix + placebo + placebo + no E2 patch; D/T(+), degarelix + T + placebo + no E2 patch; D/T(+)/A/E2(−), degarelix + T + anastrozole + no E2 patch; D/T(+)/A/E2(+), degarelix + T + anastrozole + E2 patch.
Figure 1.
Serum GH concentration (y-axis) vs time (x-axis) series in healthy older men sampled overnight every 10 min for 10 h. Data are the mean ± SEM. All subjects were treated with degarelix to suppress the gonadal axis. Three groups received T addback, and of these two groups also received anastrozole, an aromatase inhibitor. One-half of these subjects received transdermal E2 addback. Addback is indicated in the panels by the + sign.
Table 2.
Effects of T or E2 Administration in Subjects Treated With Degarelix
| Replacement | T(−) (n = 16) | T(+) (n = 17) | P |
|---|---|---|---|
| IGF-I, µg/L | 108 ± 8.4 | 111 ± 7.4 | 0.40 |
| E2, pg/mL | 9.4 ± 1.9 | 31.3 ± 3.5 | <0.0001 |
| T, ng/dL | 163 ± 35 | 760 ± 61 | <0.0001 |
| Basal GH secretion, µg/L/10 h | 3.7 ± 0.6 | 3.7 ± 0.7 | 0.33 |
| Pulsatile GH secretion, µg/L/10 h | 11.6 ± 2.9 | 14.1 ± 2.1 | 0.34 |
| Total GH secretion, µg/L/10 h | 18.3 ± 3.3 | 17.8 ± 2.3 | 0.39 |
| Mean GH pulse mass, µg/L | 2.5 ± 0.4 | 3.1 ± 0.6 | 0.28 |
| Approximate entropy, dimensionless | 0.737 ± 0.100 | 0.859 ± 0.071 | 0.18 |
| Replacement | E2(−) (n = 20) | E2(+) (n = 19) | P |
|---|---|---|---|
| IGF-I, µg/L | 106 ± 6.5 | 114 ± 6.1 | 0.18 |
| E2, pg/mL | 1.20 ± 0.24 | 82 ± 18 | <0.0001 |
| T, ng/dL | 748 ± 71 | 844 ± 66 | 0.33 |
| Basal GH secretion, µg/L/10 h | 2.6 ± 0.3 | 4.2 ± 0.8 | 0.046 |
| Pulsatile GH secretion, µg/L/10 h | 14.1 ± 3.2 | 21.3 ± 3.7 | 0.020 |
| Total GH secretion, µg/L/10 h | 16.8 ± 3.5 | 25.5 ± 4.3 | 0.018 |
| Mean GH pulse mass, µg/L | 2.4 ± 0.5 | 4.1 ± 0.9 | 0.018 |
| Approximate entropy, dimensionless | 0.785 ± 0.045 | 0.728 ± 0.046 | 0.19 |
Data are shown as the mean ± SEM. GH secretion outcomes were compared after logarithmic transformation and corrected for visceral fat mass. Boldface denotes P < 0.05 significance.
The lower panel of Fig. 1 shows the serum GH profiles of the men treated with the aromatase inhibitor, anastrozole, in addition to T addback (all subjects). Half of the subjects received transdermal E2 as well as T replacement. Sex hormone concentrations and results of the deconvolution analyses are presented in Table 2. E2 levels were much higher in the subjects treated with transdermal substitution, whereas T levels were similar and normal in both groups. GH secretion was higher in men treated with E2, especially the pulsatile component. The latter was also reflected in increased secretory burst mass. The GH increase within physiological boundaries did not result in changed regularity of secretion, as indicated by unchanged approximate entropy or in increased serum IGF-I concentration.
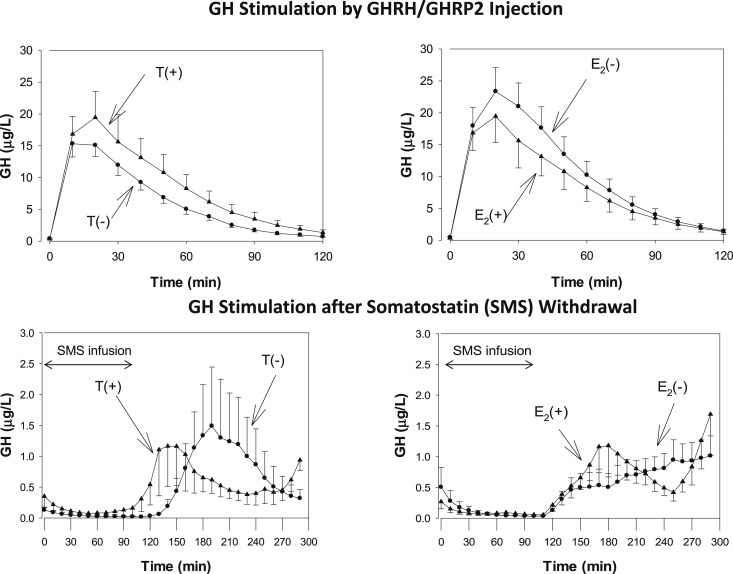
After the overnight blood sampling, approximately one-half of the subjects (N = 38) received an IV injection of GHRH and GHRP2, and the other one-half (N = 34) received somatostatin. The latter tool was applied to study the endogenous stimulation of GH after withdrawal of somatostatin administration after 2 hours. The plots of the concentration profiles for these two stimulation protocols are shown in Fig. 2, and the statistical analyses are displayed in Table 3. After the termination of the somatostatin infusion, the peak GH concentration occurred 40 minutes earlier in men receiving T compared with T-depleted men. An analog but nonsignificant shift in peak GH time was noted for men receiving E2 compared with those not treated with E2. GHRH/GHRP2 injection occurred slightly but not significantly later (about 5 minutes) in subjects treated with anastrozole. GH secretion was assessed by different methods (i.e., mean GH concentration, maximal GH concentration, and pulsatile GH secretion; the latter was calculated with deconvolution of the time series). The three approaches did not detect differences between the four groups. Thus, neither T nor E2 potentiated somatostatin or GHRH/GHRP2-stimulated GH secretion.
Figure 2.
Serum GH concentration vs time series after combined IV injection of GHRH/GHRP2 (upper panels) and after withdrawal of IV somatostatin infusion (lower panels). The left panels show the responses in men treated with T [T(+)] compared with men treated with placebo [T(−)]. The right panels show effects of E2 and placebo treatment [E2(+) and E2(−), respectively]. Sex steroid treatment evoked earlier (but not higher) peak GH concentrations.
Table 3.
GH Secretion After Somatostatin Withdrawal and Injection of GHRH and GHRP2
| Group | D/T(−) | D/T(+) | D/T(+)/A/E2(−) | D/T(+)/A/E2(+) | ANOVA |
|---|---|---|---|---|---|
| Somatostatin withdrawal | |||||
| No. of subjects | 7 | 9 | 9 | 9 | |
| Mean GH, µg/L | 0.74 ± 0.41 | 0.67 ± 0.24 | 0.67 ± 0.17 | 0.79 ± 0.18 | 0.64 |
| Maximal GH concentration, µg/L | 1.9 ± 0.9 | 2.1 ± 0.8 | 1.7 ± 0.3 | 2.5 ± 0.6 | 0.65 |
| *Time of maximum, min | 90 ± 12 | 46 ± 7a | 96 ± 13 | 56 ± 10b | 0.004 |
| Pulsatile GH secretion, µg/L/2 h | 8.4 ± 5.1 | 5.8 ± 2.3 | 6.2 ± 2.1 | 6.8 ± 1.4 | 0.92 |
| GHRH/GHRP2 injection | |||||
| No. of subjects | 9 | 9 | 11 | 11 | |
| Mean GH, µg/L | 5.8 ± 0.8 | 8.0 ± 1.9 | 9.7 ± 2.2 | 9.9 ± 1.7 | 0.36 |
| Maximal GH concentration, µg/L | 16.1 ± 1.9 | 19.9 ± 4.1 | 21.1 ± 3.9 | 23.6 ± 3.7 | 0.53 |
| Time of maximum, min | 14.4 ± 1.8 | 14.4 ± 1.8 | 19.1 ± 1.6 | 18.2 ± 1.2c | 0.08 |
| Pulsatile GH secretion µg/L/2 h | 33.3 ± 4.1 | 51.9 ± 11.9 | 65.1 ± 15.6 | 62.5 ± 11.5 | 0.27 |
Data are shown as the mean ± SEM. Boldface denotes P < 0.05.
Abbreviations: A, anastrozole; D, degarelix; E2(+), 17β−Ε2 addback; E2(−), no 17β− E2 addback; T(+), testosterone addback; T(−), no testosterone addback.
T(−) vs T(+) post hoc contrast after ANOVA, P = 0.009.
E2(−) vs E2(+) post hoc contrast after ANOVA, P = 0.011.
Anastrozole vs no anastrozole post hoc contrast after ANOVA, P = 0.012.
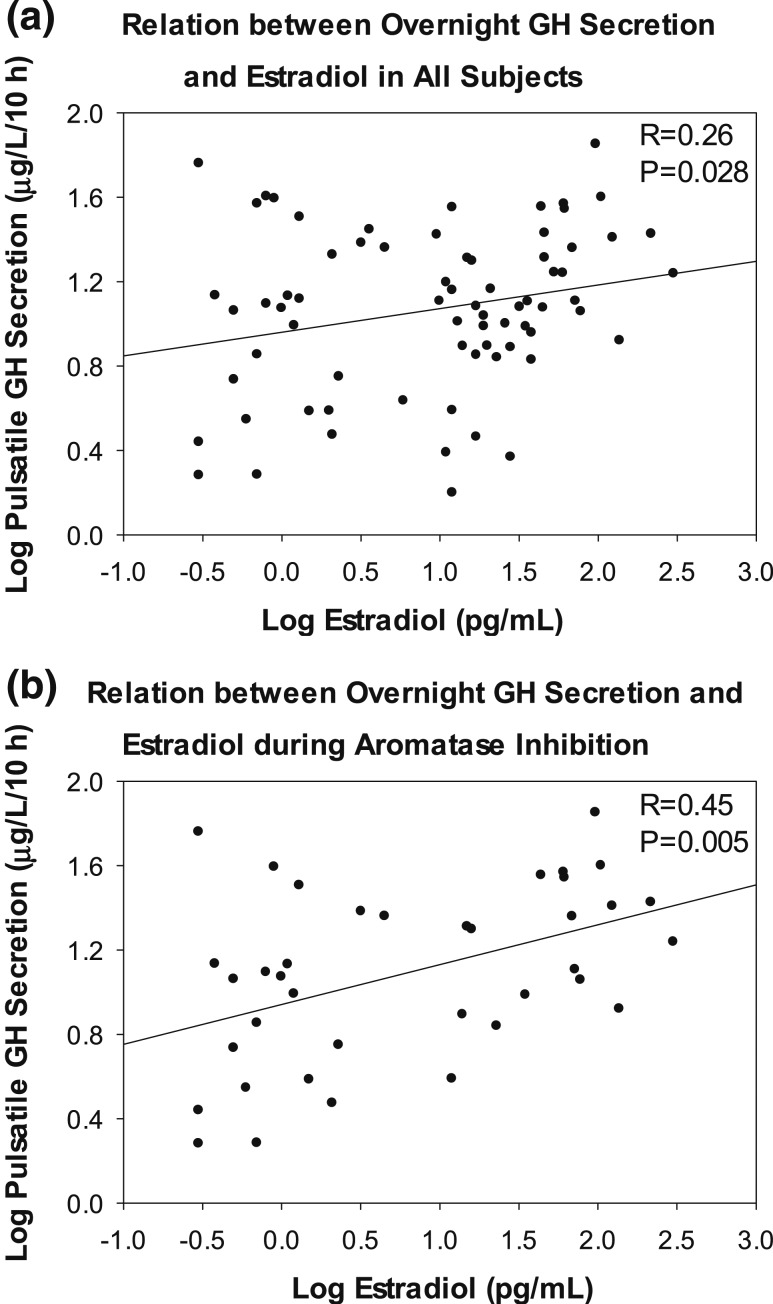
Pulsatile GH secretion correlated with serum E2 concentrations (Fig. 3). The correlation was strongest when both the dependent variable (GH secretion) and the independent variable (serum E2 concentration) were logarithmically transformed (R = 0.26, P = 0.028, β = 0.002 ± 0.001). Restricting the analysis to the subjects treated with the aromatase inhibitor, anastrozole, with and without E2 addback strengthened the correlation (R = 0.45, P = 0.005). Comparable positive regressions existed for log total GH secretion and log mean GH secretory burst mass regressed on log E2 concentration (R = 0.44, P = 0.006 and R = 0.45, P = 0.005, respectively). As expected, significant negative relations were present between overnight GH secretion (dependent variable) and visceral fat mass (independent variable), determined by single-slice CT (pulsatile secretion R = 0.38, P = 0.001; total secretion R = 0.36, P = 0.002; mean pulse mass R = 0.31, P = 0.009.
Figure 3.
Linear regressions between log E2 concentrations and log overnight pulsatile GH secretion in (a) all subjects and (b) men treated with anastrozole.
The effect of T on spontaneous 10-hour GH secretion was investigated in the groups that did not receive the aromatase inhibitor. There was a borderline significant correlation between T and pulsatile GH secretion (R = 0.35, P = 0.04) but not for total GH secretion or GH secretory burst mass. When all subjects were included, there was no correlation between serum T and pulsatile GH secretion (R = −0.12, P = 0.30). E2 concentrations varied with visceral fat mass (R = 0.32, β = 0.060 ± 0.025, P = 0.02), excluding subjects receiving the E2 dermal patch. Finally, leptin strongly correlated with visceral fat mass (R = 0.72, P < 0.0001, β = 0.060 ± 0.025).
E2 did not increase GHRH/GHRP2-stimulated GH secretion in all groups combined or in anastrozole-treated subjects (R = 0.27, P = 0.10; R = 0.28, P = 0.22, respectively). Log-transformed rebound GH secretion after somatostatin infusion was nonsignificantly related to E2 in all subjects (R = 0.30, P = 0.08) and in subjects treated with anastrozole (R = 0.43, P = 0.07).
Discussion
Basal (nonpulsatile), pulsatile, and total GH secretion, and especially GH secretory pulse mass, were increased in E2-depleted men treated with anastrozole plus or minus transdermal E2 addback. In addition, E2 concentrations were strongly positively correlated with pulsatile GH secretion in all subjects combined (R = +0.2, P = 0.28) and more strongly in the subjects who received the aromatase inhibitor (R = +0.45, P = 0.005). The influence of E2 on the GH axis more generally has been known for four decades. Synthetic estrogens, such as diethylstilbestrol and ethinyl E2, but also conjugated estrogens, increase GH concentrations in women and decrease serum somatomedin-C (IGF) concentrations (12). Furthermore, GH concentrations rise in the late follicular phase compared with the early follicular phase of the menstrual cycle, in parallel with increasing E2 levels in normal-cycling women (13, 14). However, GH levels in the middle of the luteal phase tend to be comparable to early follicular phase levels, suggesting that progesterone or other factors may have an attenuating effect on GH secretion (13–15). Moreover, GH secretion declines after menopause but can be stimulated by E2 administration (16, 17). Comparable data on E2 administration have not been available in men. Indeed, in male-to-female transsexual patients administered estrogen suppressed GH, possibly because of concurrent cyproterone use (18). However, because of the high levels of E2, this study does necessarily indicate the exact magnitude of the contribution of E2 to GH secretion under physiological conditions in older adults.
GH is secreted in discrete bursts under the regulation of GHRH, synthesized in arcuate nuclei; GHRP (of which ghrelin is the endogenous 28–amino acid prototype, synthesized in the hypothalamus, pituitary gland, and stomach); and somatostatin, synthesized in paraventricular nuclei. Negative feedback on GH secretion is mediated by IGF-I and by GH itself (1), which may further alter GH secretion in aging, adiposity, and selected sex steroid milieus (17, 19, 20). The current study examined the effect of E2 on spontaneous nocturnal and exogenous stimulated GH secretion in older men in a sex steroid–clamped milieu. Under the conditions of short-term hypogonadism with or without sex steroid addback, transdermal E2 augmented basal, pulsatile, and total GH secretion by 50% to 60%. The main effect was mediated via an increase of 70% in GH secretory burst mass. These findings are consistent with other less direct studies. Patients with inactivating mutations of the aromatase cytochrome P450 gene, in either exon IV or IX of the CYP19A1 gene, are rare genetic models for the role of E2 in men. Male patients with this mutation exhibit low IGF-I concentrations and show severely blunted GH secretion after an arginine-GHRH challenge (21, 22). However, long-term treatment (months) with transdermal 17β-E2 in four such patients did not normalize the blunted GH response or restore IGF-I levels. Detailed studies in female CYP19A1 knockout mice (intact or ovariectomized) revealed diminished expression of pituitary mRNA Pit1 and GH and of GHRH and GHRP receptors with increased expression of somatostatin subtype receptors. High-dose E2 administration for 21 days in the murine model rescued GH synthesis and secretion and restored normal GHRH, GHRP, and Pit1 signaling pathways and the expression of somatostatin receptor mRNAs (23, 24). The apparent disparity of the effect of E2 on the GH axis between the single human study and those in mice might be species dependent or E2 dose and time related. This question warrants further studies, as are now feasible in small animals (25, 26). Moreover, the E2 concentration-dependent GH data suggested here provide key dose-response estimates in healthy older men.
The correlation between T and pulsatile GH secretion was weak in this 3-week study. Six months of T supplementation in older men elevates T and E2 concentrations and amplifies pulsatile GH secretion by 70% (27). Moreover, in older men with an intact gonadotropin system, pulsatile GH secretion is related to serum E2, T, and DHT concentrations and restrained by anastrozole inhibition (8). Possible explanations for this discrepancy are that the duration of degarelix-induced hypogonadism was too brief for downregulation of the GH axis, that degarelix itself unexpectedly suppressed the T effect, or that T concentrations achieved by addback here were too low. The latter notion is suggested by the capability of higher doses of T to stimulate GH secretion in older men, whereas lower doses are ineffective (3).
The present data indicate that E2 and T did not affect GH secretion driven by the combined infusion of submaximal doses of GHRH and GHRP2. This result agrees with the observation in young men that short-term severe hypogonadism does did not modify the GH response to maximally effective GHRH and GHRP2 (28). In older men, T administration for 3 weeks, either alone or with dutasteride, a 5α-reductase inhibitor, also did not affect GH secretion after GHRH and GHRP2 stimulation, despite a tenfold decrease in serum DHT concentrations. However, coadministration of anastrozole diminished the GH response to these same stimuli (8), thus emphasizing E2 as the principal determinant of GH secretion in men as well as women. We also observed no effect of T and E2 here on GH secretion induced by somatostatin withdrawal (29). However, postsomatostatin peak GH secretion occurred earlier in T-supplemented subjects and in subjects treated with E2 during aromatization inhibition, suggesting that a sex steroid effect on GH pulse timing may also be primarily E2 mediated.
Direct studies of the human pituitary gland and specific cell types are not possible in the human in vivo. Therefore, most of our knowledge is based on animal experiments in vivo or in vitro and on human autopsy material. ERs, including classic α and β nuclear receptors and G protein–coupled E2 receptors, are widely distributed in mammals, including the anterior pituitary gland (30, 31). In addition, human, rat, and mouse pituitary glands also produce aromatase mRNA (32–34). Further evidence for a direct effect of E2 on GH synthesis was obtained in two different somatotroph cell lines (GH3 and MtT/S lines), as mediated by the α and β ERs (35). The latter authors found that the somatotroph-specific ERα knockout mouse model had diminished mRNA expression of GH, PRL, and POU1F1 and that in this ER knockout animal E2 administration had no effect on GH mRNA. Accordingly, estrogens regulate not only GHRH, GHRP, and somatostatin neurons and receptors but also the pituitary gland to finely tune GH secretion. The degree to which local intrapituitary vis-à-vis systemic conversion of T to E2 could further regulate GH synthesis and secretion is less clear. However, pituitary tissue incubated in vitro or implanted under the kidney capsule in vivo responds to E2 with increased GH synthesis in animals, supporting the notion of direct pituitary effects (36). The present indirect data of nonpotentiated GH release under GHRH/GHRP2 and somatostatin seem to disfavor direct effects.
In summary, short-term (transdermal) E2 administration in gonadotropin- and aromatase-inhibited healthy older men with experimentally controlled T addback stimulates pulsatile GH secretion. The E2 effect is strongly E2 concentration dependent. The exact site of E2 action cannot be established from these experiments alone but might include hypothalamic GHRH, ghrelin, and somatostatin-regulating neurons or pituitary somatotroph cells directly.
Acknowledgments
We thank Jill Smith for support of manuscript preparation, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo Clinic research nursing staff for implementing the protocol. Matlab versions of the approximate entropy and deconvolution methods are available from veldhuis.johannes@mayo.edu.
Financial Support: Supported in part by grants R01 AG019695, R01 AG029362, R01 AG031763, and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). The project described was supported by grants UL1 TR000135 from the National Center for Advancing Translational Sciences and 60NANB10D005Z from the National Institute of Standards and Technology. Contents are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- BMI
body mass index
- CRU
Clinical Translational Unit
- CV
coefficient of variation
- E2
estradiol
- ER
estrogen receptor
- GHRP2
GH-releasing peptide 2
- T
testosterone
References
- 1. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19(6):717–797. [DOI] [PubMed] [Google Scholar]
- 2. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27(2):101–140. [DOI] [PubMed] [Google Scholar]
- 3. Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87(2):825–834. [DOI] [PubMed] [Google Scholar]
- 4. Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak-detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev. 1988;9(1):3–37. [DOI] [PubMed] [Google Scholar]
- 5. Veldhuis JD, Erickson D, Iranmanesh A, Miles JM, Bowers CY. Sex-steroid control of the aging somatotropic axis. Endocrinol Metab Clin North Am. 2005;34(4):877–893, viii. [DOI] [PubMed] [Google Scholar]
- 6. Stocco DM, Sodeman TC. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem. 1991;266(29):19731–19738. [PubMed] [Google Scholar]
- 7. Veldhuis JD, Metzger DL, Martha PM Jr, Mauras N, Kerrigan JR, Keenan B, Rogol AD, Pincus SM. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)–insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab. 1997;82(10):3414–3420. [DOI] [PubMed] [Google Scholar]
- 8. Veldhuis JD, Mielke KL, Cosma M, Soares-Welch C, Paulo R, Miles JM, Bowers CY. Aromatase and 5alpha-reductase inhibition during an exogenous testosterone clamp unveils selective sex steroid modulation of somatostatin and growth hormone secretagogue actions in healthy older men. J Clin Endocrinol Metab. 2009;94(3):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plourde PV, Dyroff M, Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30(1):103–111. [DOI] [PubMed] [Google Scholar]
- 10. Veldhuis JD, Iranmanesh A. Short-term aromatase-enzyme blockade unmasks impaired feedback adaptations in luteinizing hormone and testosterone secretion in older men. J Clin Endocrinol Metab. 2005;90(1):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiedemann E, Schwartz E, Frantz AG. Acute and chronic estrogen effects upon serum somatomedin activity, growth hormone, and prolactin in man. J Clin Endocrinol Metab. 1976;42(5):942–952. [DOI] [PubMed] [Google Scholar]
- 13. Faria ACS, Bekenstein LW, Booth RA Jr, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf). 1992;36(6):591–596. [DOI] [PubMed] [Google Scholar]
- 14. Ovesen P, Vahl N, Fisker S, Veldhuis JD, Christiansen JS, Jørgensen JO. Increased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the periovulatory interval in normal women. J Clin Endocrinol Metab. 1998;83(5):1662–1667. [DOI] [PubMed] [Google Scholar]
- 15. Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, Demott-Friberg R, Barkan AL. Growth hormone secretory dynamics over the menstrual cycle. Endocr J. 2000;47(5):549–556. [DOI] [PubMed] [Google Scholar]
- 16. Veldhuis JD, Anderson SM, Kok P, Iranmanesh A, Frystyk J, Ørskov H, Keenan DM. Estradiol supplementation modulates growth hormone (GH) secretory-burst waveform and recombinant human insulin-like growth factor-I–enforced suppression of endogenously driven GH release in postmenopausal women. J Clin Endocrinol Metab. 2004;89(3):1312–1318. [DOI] [PubMed] [Google Scholar]
- 17. Roelfsema F, Veldhuis JD. Growth hormone dynamics in healthy adults are related to age and sex and strongly dependent on body mass index. Neuroendocrinology. 2016;103(3-4):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Kesteren P, Lips P, Deville W, Popp-Snijders C, Asscheman H, Megens J, Gooren L. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab. 1996;81(6):2227–2232. [DOI] [PubMed] [Google Scholar]
- 19. Weltman A, Weltman JY, Roy CP, Wideman L, Patrie J, Evans WS, Veldhuis JD. Growth hormone response to graded exercise intensities is attenuated and the gender difference abolished in older adults. J Appl Physiol (1985). 2006;100(5):1623–1629. [DOI] [PubMed] [Google Scholar]
- 20. Veldhuis JD, Erickson D, Wigham J, Weist S, Miles JM, Bowers CY. Gender, sex-steroid, and secretagogue-selective recovery from growth hormone-induced feedback in older women and men. J Clin Endocrinol Metab. 2011;96(8):2540–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rochira V, Zirilli L, Maffei L, Premrou V, Aranda C, Baldi M, Ghigo E, Aimaretti G, Carani C, Lanfranco F. Tall stature without growth hormone: four male patients with aromatase deficiency. J Clin Endocrinol Metab. 2010;95(4):1626–1633. [DOI] [PubMed] [Google Scholar]
- 22. Rochira V, Balestrieri A, Faustini-Fustini M, Borgato S, Beck-Peccoz P, Carani C. Pituitary function in a man with congenital aromatase deficiency: effect of different doses of transdermal E2 on basal and stimulated pituitary hormones. J Clin Endocrinol Metab. 2002;87(6):2857–2862. [DOI] [PubMed] [Google Scholar]
- 23. Yan M, Jones ME, Hernandez M, Liu D, Simpson ER, Chen C. Functional modification of pituitary somatotropes in the aromatase knockout mouse and the effect of estrogen replacement. Endocrinology. 2004;145(2):604–612. [DOI] [PubMed] [Google Scholar]
- 24. Yan M, Jones ME, Hernandez M, Liu D, Simpson ER, Chen C. Oestrogen replacement in vivo rescues the dysfunction of pituitary somatotropes in ovariectomised aromatase knockout mice. Neuroendocrinology. 2005;81(3):158–166. [DOI] [PubMed] [Google Scholar]
- 25. Xie TY, Ngo ST, Veldhuis JD, Jeffery PL, Chopin LK, Tschöp M, Waters MJ, Tolle V, Epelbaum J, Chen C, Steyn FJ. Effect of deletion of ghrelin-o-acyltransferase on the pulsatile release of growth hormone in mice. J Neuroendocrinol. 2015;27(12):872–886. [DOI] [PubMed] [Google Scholar]
- 26. Huang L, Steyn FJ, Tan HY, Xie TY, Veldhuis JD, Ngo ST, Chen C. The decline in pulsatile GH secretion throughout early adulthood in mice is exacerbated by dietary-induced weight gain. Endocrinology. 2012;153(9):4380–4388. [DOI] [PubMed] [Google Scholar]
- 27. Muniyappa R, Sorkin JD, Veldhuis JD, Harman SM, Münzer T, Bhasin S, Blackman MR. Long-term testosterone supplementation augments overnight growth hormone secretion in healthy older men. Am J Physiol Endocrinol Metab. 2007;293(3):E769–E775. [DOI] [PubMed] [Google Scholar]
- 28. Veldhuis JD, Keenan DM, Bailey JN, Miles JM, Bowers CY. Preservation of GHRH and GH-releasing peptide-2 efficacy in young men with experimentally induced hypogonadism. Eur J Endocrinol. 2009;161(2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. degli Uberti EC, Ambrosio MR, Cella SG, Margutti AR, Trasforini G, Rigamonti AE, Petrone E, Müller EE. Defective hypothalamic growth hormone (GH)–releasing hormone activity may contribute to declining GH secretion with age in man. J Clin Endocrinol Metab. 1997;82(9):2885–2888. [DOI] [PubMed] [Google Scholar]
- 30. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zárate S, Seilicovich A. Estrogen receptors and signaling pathways in lactotropes and somatotropes. Neuroendocrinology. 2010;92(4):215–223. [DOI] [PubMed] [Google Scholar]
- 32. García-Barrado MJ, Blanco EJ, Catalano-Iniesta L, Sanchez-Robledo V, Iglesias-Osma MC, Carretero-Hernández M, Rodríguez-Cobos J, Burks DJ, Carretero J. Relevance of pituitary aromatase and estradiol on the maintenance of the population of prolactin-positive cells in male mice. Steroids. 2016;111:121–126. [DOI] [PubMed] [Google Scholar]
- 33. Galmiche G, Corvaisier S, Kottler ML. Aromatase gene expression and regulation in the female rat pituitary. Ann N Y Acad Sci. 2006;1070(1):286–292. [DOI] [PubMed] [Google Scholar]
- 34. Kadioglu P, Oral G, Sayitoglu M, Erensoy N, Senel B, Gazioglu N, Sav A, Cetin G, Ozbek U. Aromatase cytochrome P450 enzyme expression in human pituitary. Pituitary. 2008;11(1):29–35. [DOI] [PubMed] [Google Scholar]
- 35. Avtanski D, Novaira HJ, Wu S, Romero CJ, Kineman R, Luque RM, Wondisford F, Radovick S. Both estrogen receptor α and β stimulate pituitary GH gene expression. Mol Endocrinol. 2014;28(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. García-Rudaz MC, Ropelato MG, Escobar ME, Veldhuis JD, Barontini M. Augmented frequency and mass of LH discharged per burst are accompanied by marked disorderliness of LH secretion in adolescents with polycystic ovary syndrome. Eur J Endocrinol. 1998;139(6):621–630. [DOI] [PubMed] [Google Scholar]