Abstract
Several thalidomide derivatives were synthesized and evaluated for their anti-inflammatory activity. Introduction of the benzyl group to the parent thalidomide is unfavorable in which 2-(1-benzyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (4a) was inactivated. However, the inhibitory activities on TNF-α and IL-6 expression in HaCaT cells were improved by the substitution of a chloro- or methoxy- group at the phenyl position of 4a. The IL-6 inhibitory activity decreased in an order of 5c (69.44%) > 4c (48.73%) > 6c (3.19%) indicating the 3-substituted derivative is more active than the 4-substituted counterpart, which in turn is more active than the 2-substituted counterpart. Among them, 2-[1-(3-chlorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (5c) was found to inhibit TNF-α and IL-6 expression in HaCaT cells with a higher potency than thalidomide and no significant cell cytotoxicity was detected at 10 μM. In psoriasis, Compound 5c reduced IL-6, IL-8, IL-1β and IL-24 in imiquimod-stimulated models. Our results indicated that compound 5c is a potential lead of novel anti-psoriasis agents. Structural optimization of compound 5c and its in vivo assay are ongoing.
Keywords: thalidomide, psoriasis, tumor necrosis factor, anti-inflammatory, anti-proliferative
1. Introduction
Psoriasis is a chronic inflammatory skin disorder that affects 2% of the world population [1]. The symptoms of psoriasis on the skin, such as pain, scaling, and itching, results in decreased quality of life and significant healthcare-related costs [2]. Moreover, patients suffering from moderate-to-severe plaque psoriasis may also suffer from increased risk of depression and cardiovascular disease [3]. The conventional treatments for moderate-to-severe plaque psoriasis, such as methotrexate, cyclosporine, retinoids, hydroxycarbamide, and fumarates, raise awareness of potential significant side effects [4,5]. A new approach to treat psoriasis is to down-regulate the specific cytokines and chemokines. Recently, certain biological treatments targeting specific immune pathways, such as tumor necrosis factor (TNF) (infliximab, adalimumab), T cells (alefacept), and the p40 subunit of interleukin (IL)-12/IL-23 (ustekinumab) were developed with good therapeutic effects [6,7,8]. However, the biologics still present some challenges, such as limited administration route, quality control, and high therapeutic costs. Moreover, some severe side effects have been reported, including reactivation of hepatitis B infection and increased risk of infection for HIV patients, after anti-TNF therapy [9]. Consequently, there is still an urgent need for the discovery of novel compounds that could improve the safety profile with good efficacy.
Thalidomide, a synthetic glutamic acid derivative, was initially developed for epilepsy treatment in the early 1950s. Following a lack of sufficient evidence of anticonvulsant efficacy, it was then marketed as a sedative medication and was also widely used for its antiemetic effect during pregnancy but was soon after withdrawn from the market because of its catastrophic teratogenicity [10,11]. In 1998, thalidomide was approved again for the treatment of erythema nodosum leprosum (ENL) [12,13]. Moreover, it has been rediscovered and marketed as having immunomodulatory and anti-angiogenetic properties [14]. TNF-α, an inflammatory cytokine involved in the pathogenesis of several inflammatory diseases including psoriasis, could be inhibited by thalidomide [15] and its derivatives such as 2-(1-benzyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (4a) [16]. Thalidomide has also been found to inhibit basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), interleukin-6 (IL-6) production, and tumor necrosis factor-induced nuclear factor-κB activation in Jurkat cells [14,17,18,19]. These cytokines, which result in suppression of inflammation, angiogenesis, and the immune response, play a crucial role in psoriasis [20,21,22]. Although it possesses multiple pharmacological applications and is widely used in clinical settings, it still possesses several induced side effects such as sedation, rash, constipation, peripheral neuropathy, dizziness, thromboembolism, and severe teratogenicity [23]. These results have raised great interest not only for clinical use of thalidomide but also the discovery of novel derivatives for better activities and decreased side effects.
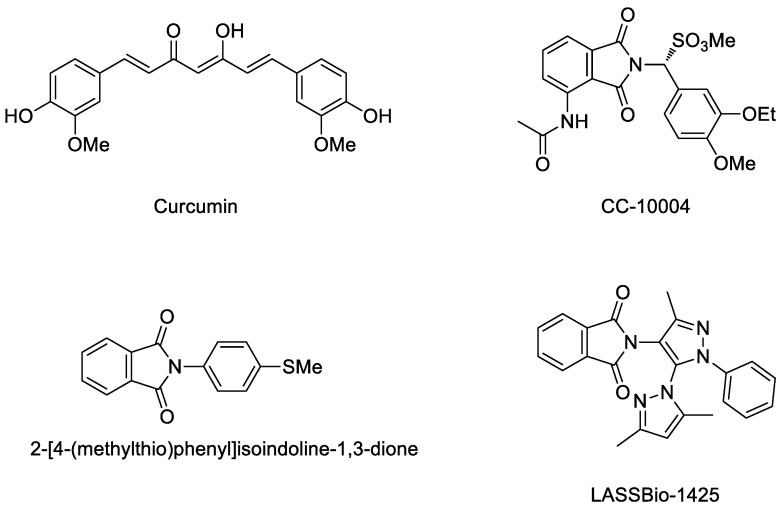
Recently, several new inhibitors (Figure 1) have been in development for such skin diseases. Curcumin has been reported to be an anti-psoriasis drug candidate by interrupting certain cytokines including TNF-α, IL-17, IL6 and INF-γ [24]. CC-10004 was synthesized and developed as an oral treatment for psoriasis [25]. (2-[4-(Methyl-thio)phenyl]isoindoline-1,3-dione) and LASSBio-1425 have been reported as promising anti-inflammatory agents by interrupting TNF-α [26,27]. Strong evidence given by these studies is that the aryl- group and phthalimide are needed for anti-inflammatory activity in psoriasis.
Figure 1.
The chemical structures of curcumin, CC-10004, 2-[4-(methyl-thio)phenyl] isoindoline-1,3-dione, and LASSBio-1425.
We have also synthesized certain polycyclic heterocycles such as furo[3,2:3,4] naphtha[1,2-d]imidazole, benzo[f]indole-4,9-dione and indeno[1,2-c]quinoline derivatives for the evaluation of their anti-inflammatory activities [28,29,30,31,32]. Herein, we describe the discovery of a novel series of thalidomide derivatives that connect thalidomide and selected substituted aryl- groups by different linkers. Our design is aimed at retaining the anti-TNF-α and anti-ILs pharmacological profiles from thalidomide and to enhance the efficacy against specific cytokines related to psoriasis.
2. Results and Discussion
2.1. Chemistry
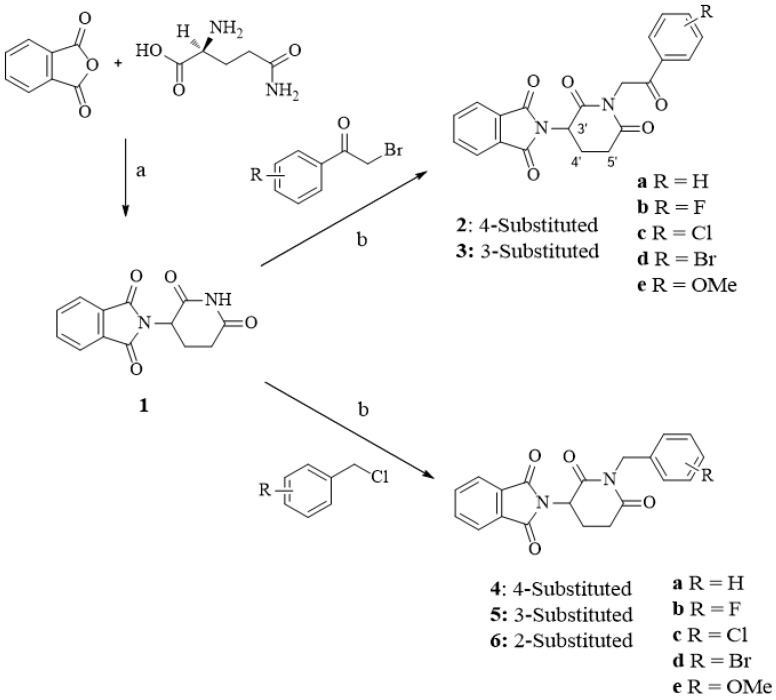
The synthesis of compounds 2a–6e is depicted in Scheme 1. Compound 1 (thalidomide) was first prepared from phthalic anhydride and (l)-glutamine under the condition of acetic anhydride, Et3N and toluene according to the research published by Rao, D.R., et al. 2009 [33]. 2-(1-Benzyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (4a) was reported in the research of Luzzio, F.A., et al. 2003 and synthesized by two-step using phthalimidoglutaric anhydride and benzyl amine as starting material [34]. However, the drawbacks of these synthetic strategies are low-yield, high-cost, and complexity of method. In the present study, the new classes of thalidomide analogues were prepared by the modification of the glutarimide ring using one-step synthetic strategy to add selected benzyl and phenacyl groups. Synthesis of compounds 2a–6e was accomplished by the substitution of thalidomide (1) with selected benzyl chloride and phenacyl bromide under basic conditions of potassium carbonate. The structures of final compounds were confirmed by 1H NMR, 13C NMR spectra (spectra data can be found in Supplementary Materials) and elemental analysis as described in Section 4.
Scheme 1.
Synthesis of thalidomide derivatives 2a–6e.
Reagent and conditions. (a) Acetic anhydride, triethylamine, toluene, reflux, 24 h; (b) potassium carbonate, acetone, stir rigorously, 1–4 days.
2.2. Biology
2.2.1. In Vitro Anti-Psoriasis Activity
Thalidomide Derivatives Attenuated TNF-α and IL-6 Production
Production of inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-8 and IL-24 in the skin is increased in various chronic skin diseases including atopic dermatitis and psoriasis [35]. Recently, topical application of imiquimod (IMQ) on mouse skin exacerbated psoriasis at both the local treated areas as well as distant sites, which has led to the development of pre-clinical models of psoriasis using topically applied IMQ cream [36]. Moreover, Schön et al. reported that imiquimod induced upregulation of proinflammatory cytokines including TNF-α, IL-1β, IL-6, and IL-8 in Toll-like receptor (TLR)7- and TLR8-negative cells, such as the keratinocyte-derived cell line HaCaT [37]. Therefore, all the synthesized thalidomide derivatives were evaluated for inhibition of imiquimod (IMQ)-induced TNF-α and IL-6 production in HaCaT cells, and cell viability. The preliminary results of these thalidomide derivatives are summarized in Table 1. Thalidomide (1), which inhibits IL-6 (48.70%) and TNF-α (22.97%) expression in HaCaT cells at 5 μM, was used as a positive control. 2-[2,6-Dioxo-1-(2-oxo-2-phenylethyl)piperidin-3-yl]isoindoline-1,3-dione (2a) was inactivated indicating the introduction of 2-oxo-ethylphenyl side chain in the parent thalidomide is unfavorable. Substitution of bromo atom at the 4-phenyl position of 2a improved IL-6 and TNF-α inhibitory activities in which 2d was weakly active against IL-6 (14.86%). However, its inhibitory activity on TNF-α (30.38%) expression was more potent than thalidomide (22.97%). Compounds 3b, 3c, and 3e were more active than 2b, 2c, and 2e respectively against IL-6 expression, indicating 3-substituted derivatives are superior to their 4-substituted counterparts. Introduction of the benzyl group to the parent thalidomide was also unfavorable in which compound 4a became inactive. However, substitution at 4-phenyl position, such as compounds 4b–e, improved IL-6 inhibitory activity. Among them, compound 4c exhibited comparable IL-6 inhibitory activity to thalidomide (1). The IL-6 inhibitory activity decreased in an order of 5c (69.44%) > 4c (48.73%) > 6c (3.19%) indicating 3-substituted derivative was more active than the 4-substituted counterpart, which in turn was more active than the 2-substituted counterpart. The same trend was observed in which the IL-6 inhibitory activity decreased in an order of 5e (62.12%) > 4e (14.43%) > 6e (2.09%). For 3-substituted derivatives, 3-fluoro derivative 5b was inactive while 3-chloro derivative 5c and 3-methoxy derivative 5e possessed about 1.4-fold stronger IL-6 inhibitory activity than thalidomide. In TNF-α inhibitory activity evaluation, compounds 2d, 2e, 5c, and 6b exhibited more active inhibitory effect than thalidomide. Among them, 2-[1-(3-chlorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (5c) possessed the most potent inhibition of TNF-α, being about 3.4-fold more active than thalidomide and was non-cytotoxic at the concentration of 10 µM. Our results indicated that 5c exhibited more potent inhibitory activity than thalidomide to both IL-6 and TNF-α simultaneously.
Table 1.
Preliminary anti-psoriasis activity and cytotoxicities of thalidomide derivatives.
| Compounds | % Inhibition at 5 µM | % Viability | ||
|---|---|---|---|---|
| IL-6 | TNF-α | 5 µM | 10 µM | |
| Thalidomide (1) | 48.70 ± 1.10 | 22.97 ± 5.16 | 96.7 ± 3.4 | 86.7 ± 2.9 |
| 2a | 3.85 ± 1.66 | −2.45 ± 2.37 | 94.3 ± 2.5 | 86.0 ± 4.1 |
| 2b | 3.33 ± 0.44 | 5.67 ± 0.13 | 96.3 ± 2.1 | 91.7 ± 3.9 |
| 2c | 3.77 ± 0.56 | 12.93 ± 2.41 | 93.0 ± 4.3 | 94.7 ± 3.3 |
| 2d | 14.86 ± 3.20 | 30.38 ± 8.49 | 90.7 ± 2.1 | 89.7 ± 6.2 |
| 2e | 2.27 ± 1.66 | 39.77 ± 2.29 | 96.7 ± 2.1 | 84.0 ± 2.4 |
| 3b | 17.51 ± 6.41 | 12.69 ± 6.50 | 96.2 ± 3.5 | 89.8 ± 3.8 |
| 3c | 4.56 ± 2.91 | 8.28 ± 6.87 | 100.0 ± 0.3 | 98.1 ± 0.8 |
| 3e | 22.20 ± 0.27 | 11.39 ± 2.20 | 94.0 ± 3.6 | 89.3 ± 5.4 |
| 4a | 2.56 ± 0.52 | −5.21 ± 5.22 | 96.7 ± 2.1 | 93.0 ± 3.7 |
| 4b | 22.49 ± 1.90 | 0.28 ± 0.20 | 94.3 ± 2.9 | 93.3 ± 2.1 |
| 4c | 48.73 ± 2.25 | −1.32 ± 4.14 | 92.0 ± 4.5 | 93.7 ± 3.8 |
| 4d | 14.02 ± 8.97 | 12.59 ± 7.43 | 91.3 ± 3.3 | 91.3 ± 2.7 |
| 4e | 14.43 ± 0.68 | 5.01 ± 4.10 | 93.3 ± 0.5 | 88.0 ± 4.5 |
| 5b | 1.35 ± 0.18 | 2.89 ± 4.07 | 95.3 ± 3.3 | 95.0 ± 2.9 |
| 5c | 69.44 ± 4.39 | 75.01 ± 17.6 | 96.3 ± 2.5 | 90.3 ± 7.0 |
| 5e | 62.12 ± 3.22 | 3.17 ± 0.78 | 95.7 ± 4.0 | 66.3 ± 3.9 |
| 6b | 4.40 ± 1.61 | 34.46 ± 3.85 | 93.7 ± 3.1 | 90.3 ± 2.5 |
| 6c | 3.19 ± 0.80 | 7.35 ± 1.07 | 91.0 ± 2.4 | 58.7 ± 6.1 |
| 6e | 2.09 ± 1.86 | 2.19 ± 6.51 | 98.0 ± 1.6 | 97.0 ± 2.2 |
Results are presented as mean ± standard deviation (n = 3).
Effect of Compound 5c on Cell Viability
To determine the cytotoxic effect of 5c on HaCaT cells, cell viability was assessed by performing neutral red staining assay. Neutral red staining is widely used for measuring the cell viability or cytotoxicity. The morphological characteristics of the HaCaT cells were illustrated using a phase-contrast microscope. The viable HaCaT cells incorporate neutral red in lysosomes whereas, dead or damaged cells do not take up the dye (Figure 2A). HaCaT cells were treated with various concentrations of 5c ranging from 1 to 500 μM. As shown in Figure 2B, compound 5c showed over 80% of cell viability in 100 μM and over 90% of cell viability in 50 μM, which indicated that 5c possessed excellent inhibitory activity of proinflammatory cytokines with low cytotoxicity.
Figure 2.
HaCaT cells were treated with 1–500 μM of 5c respectively and cell viability was assessed by Neutral Red assay. Data are presented as mean ± SD (n = 3).
Compound 5c Attenuated IL-1β, IL-6, IL-8 and IL-24 Production
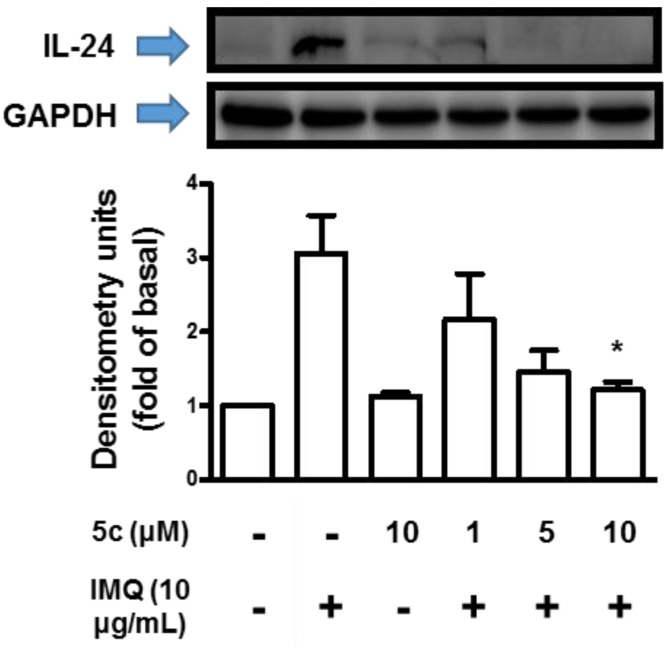
To evaluate the effect of interleukin family related to psoriasis treated with compound 5c, HaCaT cells was stimulated with IMQ (10 μg/mL) and assessed by ELISA or western blot. As shown in Figure 3A, the amount of IL-1β decreased over 80% in the presence of 10 μM compound 5c and the dose-dependent effect was revealed in IMQ-stimulated assay. In Figure 3B, IL-6 secreted by cells stimulated with IMQ showed apparent decrease with 5 and 10 μM compounds 5c. The dose-dependent effect was observed by the IL-6 secretion of the cells treated with IMQ. The results of IL-8 are shown in Figure 3C. Compound 5c caused limited effect on IL-8 in IMQ-treated cells but the attenuation of IL-8 and the slightly dose-dependent effect could still be observed. In Figure 4, secretion of IL-24 was almost inhibited with 10 μM compound 5c in IMQ-stimulated western blot assay and the dose-dependent effect could be observed in IMQ-stimulated assay.
Figure 3.
Effect of 5c on inhibition of inflammatory cytokines in differentiated HaCaT cells was done by ELISA (A) IL-1β, (B) IL-6 and (C) IL-8. The cells were incubated with IMQ alone, IMQ with different concentrations of 5c in media containing 2% fetal bovine serum (FBS) for 24 h at 37 °C. Data are presented as mean ± SD (n = 3).
Figure 4.
Effect of 5c on inhibition of IL-24 in differentiated HaCaT cells was done by Western Blot. The cells were incubated with IMQ alone, IMQ with different concentrations of 5c in media containing 2% FBS for 24 h at 37 °C. Data are presented as mean ± SD (n = 3).
Compound 5c Inhibited MAPKs Signaling Transduction Pathway
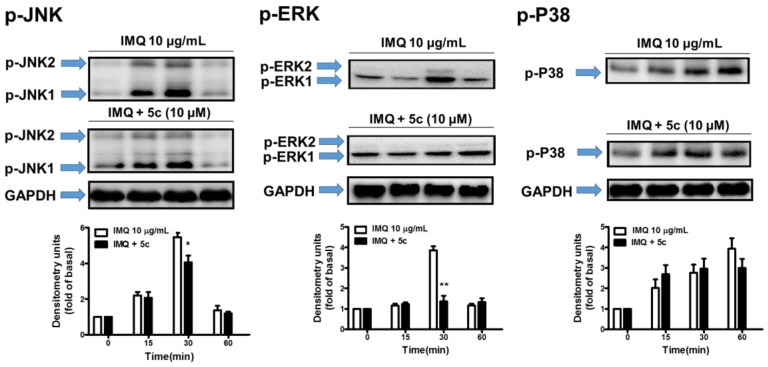
Mitogen-activated protein kinases (MAPKs), regulated key proinflammatory pathways following stimulation with environmental stimuli. MAPKs signaling pathways, including ERK, p38, and JNK, can regulate cellular responses to proliferation, apoptosis, differentiation, and inflammation in humans. Previous studies also found that MAPKs and NF-κB pathways play an important role in IMQ-induced psoriasiform dermatitis [38,39]. To confirm the actual pharmacological mechanism of compound 5c on psoriasis, p-JNK1/2, p-ERK1/2 and p-P38 were assessed by western blot using HaCaT cells treated with IMQ and compound 5c, with cells treated by IMQ alone used as control. The results are shown in Figure 5. Compared to the control, the test group incubated with compound 5c revealed significant decrease in p-JNK1/2 and p-ERK1/2 assay over 30 to 60 min; however, there was no inhibitory effect on p-P38 assay. The results provide strong evidence that the decrease of IL-1β, IL-6, IL-8 and IL-24 affected by compound 5c might act on MAPKs pathways in psoriasis via the disruption of JNK1/2 and ERK1/2, but not P38 (Figure 6).
Figure 5.
Effect of 5c on inhibition of p-JNK1/2, p-ERK1/2 and p-38 in differentiated HaCaT cells was done by Western Blot. The cells were incubated with IMQ alone, IMQ with different concentrations of 5c in media containing 2% FBS for 24 h at 37 °C. Data are presented as mean ± SD (n = 3).
Figure 6.
The supposition of mitogen-activated protein kinases (MAPKs) regulated proinflammatory pathways in psoriasis.
2.2.2. Molecular Docking Study on TNF-α, IL-6, JNK1, ERK2
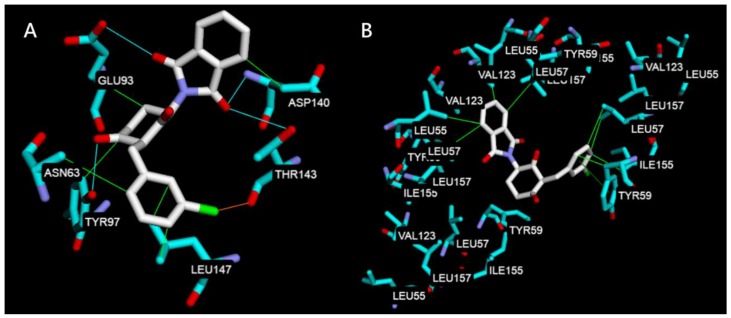
To rationalize the obtained biological results, a molecular docking study was used to evaluate the potential interaction mechanism between compound 5c and TNF-α, IL-6, JNK1, ERK2. Compound 5c was first docked at the IL-6 (PDB code 1ALU) [40] and TNF-α (PDB code 2AZ5) [41] subunit interface. The docking poses of compound 5c were examined and the pose that had the lowest binding energy is shown in Figure 7. In Figure 7A, compound 5c performs three kinds of interaction with IL-6 including hydrophobic interaction with Leu147, Asp140, Glu93, Asn63 and Tyr97, hydrogen bonds with Glu93, Asp140, Thr143 and Tyr97, and halogen bond with Thr143. However, the lowest binding energy score of IL-6 performed only −7.40 kcal/mol. The results indicate that the decrease of IL-1β, IL-6, IL-8, and IL-24 in biological analyses may involve in other mechanism but not act directly by compound 5c. In Figure 7B, compound 5c only interacts through hydrophobic interaction by the aromatic rings and the lowest binding energy score of TNF-α was −9.80 kcal/mol. The results could be explained in that compound 5c was able to dock into the middle of TNF-α dimer to form a stable complex and indicate that it may be the one of mechanisms of anti-inflammatory activity. The molecular docking study shown in Figure 7 shared the same results of the pharmacological test where inflammation of psoriasis may be inhibited by compound 5c through the MAPKinase pathway via the inhibitory effect(s) of ERK1/2 and JNK1/2.
Figure 7.
Docking poses of compound 5c at the (A) IL-6 (PDB code 1ALU) and (B) TNF-α (PDB code 2AZ5). The interaction illustrated by green lines represents hydrophobic interaction, blue lines represent hydrogen bonding, and orange line represents halogen bonding.
Figure 8A illustrates the docking results of compound 5c and ERK2 (PDB code 2Y9Q) [41] where hydrophobic interactions were formed between the aromatic rings and Tyr113, Trp 192 and hydrogen bonds were built to Ser153 and Lys151. Figure 8B shows the docking results of compounds 5c and JNK1 (PDB code 3PZE) [42] where hydrophobic interactions were also formed through the aromatic rings to Lys203, Glu204, His143, Val312 and pi-cation interaction was stacked to Lys203. The lowest binding energy score of ERK2 (−9.10 kcal/mol) was lower than JNK1 (−7.80 kcal/mol), indicating the same results of the western blot assays of p-ERK1/2 and p-JNK1/2.
Figure 8.
Docking poses of compound 5c at the (A) ERK2 (PDB code 2Y9Q) and (B) JNK-1 (PDB code 3PZE). The interaction illustrated by green lines represents hydrophobic interaction, blue lines represent hydrogen bonding and red line represents pi-cation.
3. Conclusions
A series of novel thalidomide derivatives were synthesized and evaluated for anti-inflammatory activity. Among them, 2-[1-(3-chlorobenzyl)-2,6-dioxopiperidin-3-yl] isoindoline-1,3-dione (5c) was found to inhibit TNF-α and IL-6 expression in HaCaT cells with a higher potency than thalidomide and no significant cell cytotoxicity was detected at 10 µM. We also determined some structural activity relationships between the thalidomide derivatives and inhibitory effects of IL-6 and TNF-α. Anti-psoriasis mechanism studies have indicated that compound 5c reduced the secretion of IL-1β, IL-6, IL-8 and IL-24 in an IMQ-stimulated model and could also attenuate the activation of IMQ-induced MAPKs, including JNK1/2 and ERK1/2. These results suggest that compound 5c successfully amplified both anti-inflammatory and immunomodulatory activities. Compound 5c could be used as a candidate immunomodulatory agent in inflammatory skin disease, especially in psoriasis; however, its use in the case of TNF-α-overexpressing skin disease should be further investigated.
4. Materials and Methods
4.1. Chemistry Section
General Information. Commercial reagents were used as received without additional purification. Melting points were determined with Electrothermal IA9100 micro-melting point apparatus and uncorrected. NMR spectra were recorded with Varian Unity-400MHz spectrometer using DMSO-d6 as solvent and tetramethylsilane as internal standard. Chemical shifts were expressed as δ (ppm). Splitting patterns have been described as follows: s = singlet; d = doublet; t = triplet; q = quartet; dd = double doublet; m = multiplet. Analytical TLC was performed on Art. 5554 Kieselgel 60 GF254 produced by E. Merck and the spots of compounds were detected with UV light indicator irradiated at 254 and 366 nm. Art. 7734 Kieselgel 60 GF254 (70–400 mesh) made by E. Merck was used for column chromatography. The purity of compounds was determined with HPLC and elemental analysis (EA). HPLC analysis was performed on HITACHI Chromaster 5110 HPLC, fitted with a UV detector and an auto sampler. The compounds were tested on a Mightysil RP-18 GP 250-4.6 (5 mm) with mobile phase 10 mM KH2PO4:acetonitrile (35:65) pH = 2.89. The flow rate was 1 mL/min and the sample injection was 100 µL (5.00 mg dissolved in 1 mL DMSO, then diluted with methanol to 5 mL). The wavelength was set at 295 nm. Elemental Analysis was recorded on Heraeus CHN-O Rapid apparatus and the results were within ±0.4% of the theoretical value.
4.1.1. General Procedure for the Synthesis of Compounds 2a–3c
The mixture of the thalidomide (1, 0.52 g, 2.00 mmol), potassium carbonate (1.38 g, 10.0 mmol) and acetone (20.0 mL) was stirred at room temperature for 10 min. Selected phenacyl bromide (2.40 mmol) was then added into the mixture and stirred at room temperature for 24 h (monitored by TLC). The mixtures were concentrated under reduced pressure first and extracted twice by dichloromethane/water (1/1) 80 mL. The organic layers were dried with magnesium sulfate and concentrated under reduced pressure. The samples were further purified with silica gel column chromatography, using dichloromethane as the mobile phase, to produce compounds with variable yields (35–63%).
2-[2,6-Dioxo-1-(2-oxo-2-phenylethyl)piperidin-3-yl]isoindoline-1,3-dione (2a). Compound 2a was obtained in 48% yield as a light-yellow solid. Melting Point: 148.5–150.6 °C. Purity 98.0%. 1H NMR (400 MHz, DMSO-d6): δ 8.06–8.05 (m, 2H, Ar–H), 7.96–7.89 (m, 4H, Ar–H), 7.72–7.69. (m, 1H, Ar–H), 7.59–7.55 (m, 2H, Ar–H), 5.36 (dd, 1H, J = 13.2, 5.2 Hz, 3’-H), 5.24, 5.17 (AB system, 2H, J = 28 Hz, NCH2), 3.25–2.22 (4m, 4H, 4’-H and 5’-H).13C NMR (100 MHz, DMSO-d6): δ 191.91 (C=O), 171.30, 169.25 , 167.06 (C1, C3, C2’, C6’), 134.91 (2C), 134.27, 133.98, 131.19 (2C), 128.92 (2C), 127.97 (2C), 123.44 (2C), 49.61 (NCH2), 46.41 (C3’), 30.98 (C5’), 21.11 (C4’). Anal. calcd. for C21H16N2O5: C, 67.02; H, 4.28; N, 7.44. Found: C, 67.42; H, 4.44; N, 7.30.
2-{1-[2-(4-Fluorophenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl} isoindoline-1,3-dione (2b). Compound 2b was obtained in 48% yield as a light-yellow solid. Melting Point: 139.7–141.2 °C. Purity 98.9%. 1H NMR (400 MHz, DMSO-d6) δ 8.17–8.12 (m, 2H, Ar–H), 7.96–7.88 (m, 4H, Ar–H), 7.43–7.38 (m, 2H, Ar–H), 5.38 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.24, 5.17 (AB system, 2H, J = 26.8 Hz, NCH2), 3.18–2.19 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 190.63 (C=O), 171.30, 169.25, 167.06 (C1, C3, C2’, C6’), 165.41 (1JCF = 251.7 Hz), 134.91 (2C), 131.18 (2C), 131.11 (2C, 3JCF = 9.9 Hz), 123.43 (2C), 116.00 (2C, 2JCF = 22.0 Hz), 49.60 (NCH2), 46.34 (C3’), 30.95 (C5’), 21.10 (C4’). Anal. calcd. for C21H15FN2O5: C, 63.96; H, 3.83; N, 7.10. Found: C, 63.74; H, 3.93; N, 7.01.
2-{1-[2-(4-Chlorophenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl}isoindoline-1,3-dione (2c). Compound 2c was obtained in 49% yield as a light-yellow solid. Melting Point: 147.2–149.6 °C. Purity 96.4%. 1H NMR (400 MHz, DMSO-d6) δ 8.08–8.05 (m, 2H, Ar–H), 7.95–7.89 (m, 4H, Ar–H), 7.66–7.63 (m, 2H, Ar–H), 5.36 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.23, 5.16 (AB system, 2H, J = 28.4 Hz, NCH2), 3.17–2.20 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 191.18 (C=O), 171.30, 169.25, 167.06 (C1, C3, C2’, C6’), 138.92, 134.92 (2C), 132.95, 131.18 (2C), 129.94 (2C), 129.06 (2C), 123.44 (2C), 49.61 (NCH2), 46.38 (C3’), 30.96 (C5’), 21.10 (C4’). Anal. calcd. for C21H15ClN2O5: C, 61.40; H, 3.68; N, 6.82. Found: C, 61.23; H, 3.80; N, 6.70.
2-{1-[2-(4-Bromophenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl}isoindoline-1,3-dione (2d). Compound 2d was obtained in 63% yield as a light-yellow solid. Melting Point: 115.1–116.9 °C. Purity 97.8%. 1H NMR (400 MHz, DMSO-d6) δ 8.00–7.97 (m, 2H, Ar–H), 7.96–7.89 (m, 4H, Ar–H), 7.80–7.77 (m, 2H, Ar–H), 5.37 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.22, 5.15 (AB system, 2H, J = 28.8 Hz, NCH2), 3.22–2.16 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 191.40 (C=O), 171.30, 169.25, 167.06 (C1, C3, C2’, C6’), 134.93 (2C), 133.27, 132.01 (2C), 131.19 (2C), 130.02 (2C), 128.15, 123.45 (2C), 49.59 (NCH2), 46.35 (C3’), 30.96 (C5’), 21.10 (C4’). Anal. calcd. for C21H15BrN2O5: 0.25 H2O: C, 54.85; H, 3.40; N, 6.09. Found: C, 54.63; H, 3.27; N, 5.86.
2-{1-[2-(4-Methoxyphenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl}isoindoline-1,3-dione (2e). Compound 2e was obtained in 49% yield as a yellow solid. Melting Point: 179.9–181.4 °C. Purity 96.2%. 1H NMR (400 MHz, DMSO-d6) δ 8.05–8.02 (m, 2H, Ar–H), 7.96–7.89 (m, 4H, Ar–H), 7.10–7.07 (m, 2H, Ar–H), 5.36 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.18, 5.06 (AB system, 2H, J = 27.8 Hz, NCH2), 3.86 (s, 3H, O–CH3), 3.18–2.17 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 190.05 (C=O), 171.30, 169.22, 167.07 (C1, C3, C2’, C6’), 163.64, 134.91 (2C), 131.19 (2C), 130.34 (2C), 127.21, 123.42 (2C), 114.12 (2C), 55.59 (OCH3), 49.64 (NCH2), 46.09 (C3’), 31.00 (C5’), 21.12 (C4’). Anal. calcd. for C22H18N2O6: C, 65.02; H, 4.46; N, 6.89. Found: C, 65.04; H, 4.49; N, 6.82.
2-{1-[2-(3-Fluorophenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl}isoindoline-1,3-dione (3b). Compound 3b was obtained in 25% yield as a yellow solid. Melting Point: 164.0–165.9 °C. Purity 95.9%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.89 (m, 4H, Ar–H), 7.88–7.85 (m, 2H, Ar–H), 7.65–7.58 (m, 2H, Ar–H), 5.39 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.25, 5.18 (AB system, 2H, J = 24 Hz, NCH2), 3.22–2.17 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 191.21 (C=O), 171.35, 169.29, 167.10 (C1, C3, C2’, C6’), 162.22 (1JCF = 244.1 Hz), 136.34 (3JCF = 6.8 Hz), 134.97 (2C), 131.28, 131.21 (2C), 124.31 (3JCF = 2.3 Hz), 123.48 (2C), 121.00 (2JCF = 21.2 Hz), 114.66 (2JCF = 22 Hz), 49.60 (NCH2), 46.60 (C3’), 31.00 (C5’), 21.12 (C4’). Anal. calcd. for C21H15FN2O5: 0.1 H2O: C, 63.66; H, 3.87; N, 7.07. Found: C, 63.69; H, 3.82; N, 6.69.
2-{1-[2-(3-Chlorophenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl}isoindoline-1,3-dione (3c). Compound 3c was obtained in 34% yield as a light-yellow solid. Melting Point: 123.7–125.0 °C. Purity 97.6%. 1H NMR (400 MHz, DMSO-d6) δ 8.08–8.01 (m, 2H, Ar–H), 7.96–7.89 (m, 4H, Ar–H), 7.79–7.77 (m, 1H, Ar–H), 7.61 (t, J = 8 Hz, 1H, Ar–H), 5.39 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.26, 5.19 (AB system, 2H, J = 28.4 Hz, NCH2), 3.22–2.17 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 191.29 (C=O), 171.32, 169.27, 167.08 (C1, C3, C2’, C6’), 136.05, 134.95 (2C), 133.91, 133.74, 131.19 (2C), 130.95, 127.70, 126.74, 123.46 (2C), 49.59 (NCH2), 46.50 (C3’), 30.96 (C5’), 21.11 (C4’). Anal. calcd. for C21H15ClN2O5 . 0.1H2O: C, 61.11; H, 3.72; N, 6.78. Found: C, 60.86; H, 3.98; N, 6.89.
2-{1-[2-(3-Methoxyphenyl)-2-oxoethyl]-2,6-dioxopiperidin-3-yl}isoindoline-1,3-dione (3e). Compound 3e was obtained in 63% yield as a light-yellow solid. Melting Point: 139.7–140.3 °C. Purity 96.9%. 1H NMR (400 MHz, DMSO-d6) δ 7.96–7.89 (m, 4H, Ar–H), 7.67–7.65 (m, 1H, Ar–H), 7.53–7.47 (m, 2H, Ar–H), 7.29–7.26 (m, 1H, Ar–H), 5.37 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 5.23-5.17 (AB system, 2H, 24 Hz, NCH2), 3.83 (s, 3H, O-CH3), 3.22–2.17 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 191.74 (C=O), 171.32, 169.26, 167.09 (C1, C3, C2’, C6’), 159.52, 135.62, 134.92 (2C), 131.20 (2C), 130.14, 123.45 (2C), 120.44, 120.13, 112.42, 55.39 (OCH3), 49.64 (NCH2), 46.60 (C3’), 31.00 (C5’), 21.13 (C4’). Anal. calcd. for C22H18N2O6: C, 65.02; H, 4.46; N, 6.89. Found: C, 65.00; H, 4.43; N, 6.87.
4.1.2. General Procedure for the Synthesis of Compounds 4a–6e
The mixture of the thalidomide (1, 0.52 g, 2.00 mmol), potassium carbonate (1.38 g, 10.0 mmol) and acetone (20.0 mL) was stirred in room temperature for 10 min. Selected benzyl chloride (2.40 mmol) was then added into the mixture and stirred in room temperature for 4 days (monitored by TLC). The mixtures were concentrated under reduced pressure first and extracted twice by dichloromethane/water (1/1) 80 mL. The organic layers were dried with magnesium sulfate and concentrated under reduced pressure. The samples were further purified with silica gel column chromatography, using dichloromethane as the mobile phase, to produce compounds with variable yields (30–70%).
2-(1-Benzyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (4a). Compound 4a was obtained in 30% yield as a light-yellow solid. Melting Point: 172.2–173.5 °C. Purity 98.2%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.88 (m, 4H, Ar–H), 7.33–7.22 (m, 5H, Ar–H), 5.38 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.93, 4.80 (AB system, 2H, J = 49.8 Hz, NCH2), 3.09-2.12 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.55, 169.56, 167.11 (C1, C3, C2’, C6’), 136.96, 134.89 (2C), 131.19 (2C), 128.21 (2C), 127.05 (2C), 126.90, 123.45 (2C), 49.59 (NCH2), 42.74 (C3’), 31.09 (C5’), 21.15 (C4’). Anal. calcd. for C20H16N2O4: C, 68.96; H, 4.63; N, 8.04. Found: C, 68.74; H, 4.71; N, 7.86.
2-[1-(4-Fluorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (4b). Compound 4b was obtained in 48% yield as a light-yellow solid. Melting Point: 193.9–194.2 °C. Purity 98.8%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.88 (m, 4H, Ar–H), 7.33–7.28 (m, 2H, Ar–H), 7.17–7.11 (m, 2H, Ar–H), 5.37 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.89, 4.78 (AB system, 2H, J = 43.2 Hz, NCH2), 3.12–2.09 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.59, 169.58, 167.12 (C1, C3, C2’, C6’), 162.22 (1JCF = 241.1 Hz), 134.91 (2C), 133.18 (3JCF = 3.1 Hz), 131.19 (2C), 129.38 (2C, 2JCF = 7.6 Hz), 123.46 (2C), 114.97 (2C, 2JCF = 20.5 Hz), 49.56 (NCH2), 42.11 (C3’), 31.09 (C5’), 21.15 (C4’). Anal. calcd. for C20H15FN2O4: C, 65.57; H, 4.13; N, 7.65. Found: C, 65.56; H, 4.09; N, 7.58.
2-[1-(4-Chlorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (4c). Compound 4c was obtained in 32% yield as a white solid. Melting Point: 176.2–176.7 °C. Purity 98.0%. 1H NMR (400 MHz, DMSO-d6) δ 7.96–7.89 (m, 4H, Ar–H), 7.39–7.36 (m, 2H, Ar–H), 7.30–7.28 (m, 2H, Ar–H), 5.37 (dd, 1H, J = 5.2, 13 Hz, 3’-H), 4.89, 4.80 (AB system, 2H, J = 37.2 Hz, NCH2), 3.12-2.10 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.65, 169.63, 167.15 (C1, C3, C2’, C6’), 136.04, 134.97 (2C), 131.65, 131.22 (2C), 129.19 (2C), 128.23 (2C), 123.51 (2C), 49.59 (NCH2), 42.24 (C3’), 31.12 (C5’), 21.19 (C4’). Anal. calcd. for C20H15ClN2O4: C, 62.75; H, 3.95; N, 7.32. Found: C, 62.50; H, 3.87; N, 7.27.
2-[1-(4-Bromobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (4d). Compound 4d was obtained in 46% yield as a light-yellow solid. Melting Point: 154.2–156.0 °C. Purity 97.5%. 1H NMR (400 MHz, DMSO-d6) δ 7.96–7.89 (m, 4H, Ar–H), 7.52–7.49 (m, 2H, Ar–H), 7.24–7.22 (m, 2H, Ar–H), 5.38 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.87, 4.78 (AB system, 2H, J = 37.2 Hz, NCH2), 3.12-2.10 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.64, 169.61, 167.13 (C1, C3, C2’, C6’), 136.46, 134.95 (2C), 131.21 (2C), 131.13 (2C), 129.54 (2C), 123.50 (2C), 120.10, 49.54 (NCH2), 42.25 (C3’), 31.10 (C5’), 21.16 (C4’). Anal. calcd. for C20H15BrN2O4 . 0.1H2O: C, 55.98; H, 3.58; N, 6.53. Found: C, 55.62; H, 3.58; N, 6.50.
2-[1-(4-Methoxybenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (4e). Compound 4e was obtained in 40% yield as a white solid. Melting Point: 150.6–151.5 °C. Purity 98.6%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.89 (m, 4H, Ar–H), 7.21–7.18 (m, 2H, Ar–H), 6.90–6.81 (m, 2H, Ar–H), 5.36 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.85, 4.72 (AB system, 2H, J = 52.4 Hz, NCH2), 3.73 (s, 3H, O-CH3), 3.11-2.08 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.54, 169.53, 167.15 (C1, C3, C2’, C6’), 158.32, 134.92 (2C), 131.22 (2C), 129.02, 128.87(2C), 123.47 (2C), 113.63 (2C), 55.04 (OCH3), 49.64 (NCH2), 42.24 (C3’), 31.15 (C5’), 21.16 (C4’). Anal. calcd. for C21H18N2O5: C, 66.66; H, 4.79; N, 7.40. Found: C, 66.99; H, 4.85; N, 7.33.
2-[1-(3-Fluorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (5b). Compound 17 was obtained in 55% yield as a white solid. Melting Point: 172.1–172.5 °C. Purity 96.0%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.89 (m, 4H, Ar–H), 7.40–7.34 (m, 1H, Ar–H), 7.12–7.05 (m, 3H, Ar–H), 5.41 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.94, 4.83 (AB system, 2H, J = 41.2 Hz, NCH2), 3.15-2.12 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.65, 169.68, 167.16 (C1, C3, C2’, C6’), 162.21 (1JCF = 241.9 Hz), 139.86 (3JCF = 7.6 Hz), 134.96 (2C), 131.22 (2C), 130.21 (2JCF = 8.3 Hz), 123.51 (2C), 123.08 (4JCF = 2.2 Hz), 113.83 (3JCF = 2.2 Hz), 113.62 (2JCF = 3.8 Hz), 49.56 (NCH2), 42.35 (C3’), 31.09 (C5’), 21.21 (C4’). Anal. calcd. for C20H15FN2O4: C, 65.57; H, 4.13; N, 7.65. Found: C, 65.62; H, 4.04; N, 7.60.
2-[1-(3-Chlorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (5c). Compound 5c was obtained in 51% yield as a white solid. Melting Point: 194.9–195.3 °C. Purity 96.1%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.88 (m, 4H, Ar–H), 7.38–7.30 (m, 3H, Ar–H), 7.24–7.22 (m, 1H, Ar–H), 5.41 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.92, 4.81 (AB system, 2H, J = 41.8 Hz, NCH2), 3.14-2.11 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.63, 169.65, 167.10 (C1, C3, C2’, C6’), 139.46, 134.91 (2C), 132.97, 131.19 (2C), 130.10, 127.00, 126.97, 125.79, 123.46 (2C), 49.51 (NCH2), 42.31 (C3’), 31.06 (C5’), 21.16 (C4’). Anal. calcd. for C20H15ClN2O4: C, 62.75; H, 3.95; N, 7.32. Found: C, 62.99; H, 4.09; N, 7.04.
2-[1-(3-methoxybenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (5e). Compound 5e was obtained in 70% yield as a white solid. Melting Point: 161.9–163.5 °C. Purity 96.4%. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.88 (m, 4H, Ar–H), 7.25–7.20 (m, 1H, Ar–H), 6.84–6.80 (m, 3H, Ar–H), 5.38 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.91, 4.77 (AB system, 2H, J = 55.4 Hz, NCH2), 3.75 (s, 3H, OCH3), 3.14–2.11 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.54, 169.59, 167.13 (C1, C3, C2’, C6’), 159.26, 138.53, 134.91 (2C), 131.20 (2C), 129.28, 123.45 (2C), 119.13, 112.55, 112.37, 54.92 (OCH3), 49.61 (NCH2), 42.70 (C3’), 31.09 (C5’), 21.17 (C4’). Anal. calcd. for C21H18N2O5: C, 66.66; H, 4.79; N, 7.40. Found: C, 66.68; H, 4.83; N, 7.07.
2-[1-(2-Fluorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (6b). Compound 6b was obtained in 58% yield as a white solid. Melting Point: 160.2–161.2 °C. Purity 97.9%. 1H NMR (400 MHz, DMSO-d6) δ 7.96–7.87 (m, 4H, Ar–H), 7.33–7.27 (m, 1H, Ar–H), 7.23–7.14 (m, 3H, Ar–H), 5.39 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.90 (s, 2H, NCH2), 3.15-2.12 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.58, 169.62, 167.15 (C1, C3, C2’, C6’), 159.76 (1JCF = 242.8 Hz), 134.97 (2C), 131.21 (2C), 128.91 (3JCF = 8.1 Hz), 127.98 (3JCF = 4.2 Hz), 124.35 (4JCF = 3.1 Hz), 123.69 (2JCF = 14.1 Hz), 123.52 (2C), 115.00 (2JCF = 21.0 Hz), 49.61 (NCH2), 36.66 (C3’), 31.13 (C5’), 21.19 (C4’). Anal. calcd. for C20H15FN2O4: C, 65.57; H, 4.13; N, 7.65. Found: C, 65.55; H, 4.06; N, 7.62.
2-[1-(2-Chlorobenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (6c). Compound 6c was obtained in 52% yield as a white solid. Melting Point: 166.1–167.0 °C. Purity 97.8%. 1H NMR (400 MHz, DMSO-d6) δ 7.96–7.88 (m, 4H, Ar–H), 7.47–7.45 (m, 1H, Ar–H), 7.35–7.28(m, 2H, Ar–H), 7.20–7.18 (m, 2H, Ar–H), 5.46 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.93, 4.88 (AB system, 2H, J = 19.2 Hz, NCH2), 3.19–2.16 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.60, 169.63, 167.10 (C1, C3, C2’, C6’), 134.94 (2C), 133.63, 131.44, 131.19 (2C), 129.14, 128.53, 127.22, 126.58, 123.48 (2C), 49.54 (NCH2), 40.70 (C3’), 31.09 (C5’), 21.19 (C4’). Anal. calcd. for C20H15ClN2O4: C, 62.75; H, 3.95; N, 7.32. Found: C, 62.30; H, 3.85; N, 7.23.
2-[1-(2-Methoxybenzyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione (6e). Compound 6e was obtained in 27% yield as a white solid. Melting Point: 202.2–204.0 °C. Purity 98.1%. 1H NMR (400 MHz, DMSO-d6) δ 7.96–7.89 (m, 4H, Ar–H), 7.25–7.21 (m, 1H, Ar–H), 7.00–6.97 (m, 2H, Ar–H), 6.92–6.88 (m, 1H, Ar–H), 5.43 (dd, 1H, J = 5.2, 13.2 Hz, 3’-H), 4.85, 4.77 (AB system, 2H, J = 30.4 Hz, N–CH2–Ar), 3.81 (s, 3H, OCH3), 3.18–2.15 (4m, 4H, 4’-H and 5’-H). 13C NMR (100 MHz, DMSO-d6) δ 171.57, 169.54, 167.13 (C1, C3, C2’, C6’), 156.22, 134.91 (2C), 131.21 (2C), 127.78, 125.21, 124.07, 123.47 (2C), 120.10, 110.33, 55.33 (OCH3), 49.61 (NCH2), 38.25 (C3’), 31.14 (C5’), 21.22 (C4’). Anal. calcd. for C21H18N2O5 . 0.05H2O: C, 66.49; H, 4.82; N, 7.38. Found: C, 66.79; H, 4.88; N, 6.98.
4.2. Biological Activity
4.2.1. Cell Culture
HaCaT cells (human keratinocytes cell) were a gift from Dr. Nan-Lin Wu at HsinChu Mackay Memorial Hospital. They were cultivated in DMEM complemented with 10% (v/v) FBS (fetal bovine serum), and penicillin/streptomycin mixture, in a water-jacketed CO2 incubator at 37 °C. Every 2 days, cells were passed using trypsin 0.05% (w/v).
4.2.2. Cell Viability Assays
The cytotoxicity assays of the compounds toward HaCaT cells were conducted using MTT assay. Briefly, 5 × 104 cells per well were seeded in a 96-well plate then incubated with different concentrations of the compounds at 37 °C for 24 h. Then, the cells were incubated with MTT solution (0.5 mg/mL) for 4 h at 37 °C. At the end of the incubation, the culture medium in each well was replaced by 150 μL of DMSO to dissolve the formazan-containing crystals. The plate was shaken for 5 min at room temperature, prior to measuring the absorbance of each well at 570 nm in a microplate reader. All the experiments were performed in triplicate.
4.2.3. ELISA
Cells (1 × 105/well) were seeded at least in triplicate into 12-well plates in 1ml medium with 10% FBS. After reaching confluence, the cells were washed and incubated with 0.5 ml serum-free medium containing the indicated concentration of imiquimod for 24 h. The culture supernatants were analyzed for IL-1β, IL-6 and IL-8 by ELISA (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions.
4.2.4. Western Blotting Analysis
Equal proteins were separated on SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane. Membranes were incubated with primary antibodies at 4 °C overnight, and then incubated with corresponding HRP-conjugated secondary antibodies for 1 h at room temperature. Protein bands were visualized using enhanced chemiluminescence reagent (NEN, Boston, MA, USA).
4.2.5. Neutral Red Staining
Briefly, HaCaT cells (1 × 105 cells/mL) were seeded into 6-well plates for overnight incubation at 37 °C with 5% CO2. Cells were treated with 5c (0, 1, 10, 50, 100, 500 μM), then further incubated for 24 h at 37 °C, washed twice with 1× PBS post-incubation, fixed with 3.7% paraformaldehyde, and stained with neutral red dye for 60 min. The cells were again washed thrice with 1× PBS and visualized under the phase-contrast microscope.
4.3. Molecular Docking Study
The crystal structures of IL-6 (PDB ID: 1ALU), TNF-α (PDB ID: 2AZ5), ERK2 (PDB ID: 2Y9Q) and JNK1 (PDB ID: 3PZE) were downloaded from the RCSB Protein Date Bank. The 3D conformation of hit compound 5c was generated by ChemBio 3D Ultra 14.0. The molecular docking was performed by Achilles Blind Docking Server (http://bio-hpc.ucam.edu/achilles/). The “blind docking” approach was used for the docking of the small molecule to the targets, which was done without a priori knowledge of the location of the binding site by the system [43]. Visual representation of molecules was created with 3Dmol by Nicholas Rego and David Koes [44].
Acknowledgments
Financial support of this work by the Minister of Science and Technology of the Republic of China (Most 106-2320-B-037-015, MOST 103-2320-B-037-011-MY3) and Kaohsiung Medical University (KMU-TP105E16, KMU-TP105H07, KMU-TP105H08, 105KMUOR02) is gratefully acknowledged. We also thank the Center for Research Resources and Development at Kaohsiung Medical University for the instrumentation and equipment support.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-0067/19/10/3061/s1.
Author Contributions
K.-W.T. participated in synthesis, purification and characterization of the chemical compounds; Z.-C.L. participated in the biological activity, the interpretation of the results and in manuscript writing; Y.-L.C. and C.-C.T. participated in synthesis, the interpretation of the results and in manuscript writing; J.-Y.F. and C.-H.T. suggested the research idea, participated in the interpretation of the results and in manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shear N.H. Fulfilling an unmet need in psoriasis. Drug Saf. 2006;29:49–66. doi: 10.2165/00002018-200629010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lowes M.A., Bowcock A.M., Krueger J.G. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 3.Koo J., Marangell L.B., Nakamur M., Armstrong A., Jeon C., Bhutani T., Wu J.J. Depression and suicidality in psoriasis: Review of the literature including the cytokine theory of depression. J. Eur. Acad. Dermatol. Venereol. 2017;31:1999–2009. doi: 10.1111/jdv.14460. [DOI] [PubMed] [Google Scholar]
- 4.Strober B.E., Siu K., Menon K. Conventional systemic agents for psoriasis: A systematic review. J. Rheumatol. 2006;33:1442–1446. [PubMed] [Google Scholar]
- 5.Griffiths C.E., Clark C.M., Chalmers R.J., Li Wan Po A., Williams H.C. A systematic review of treatments for severe psoriasis. Health Technol. Assess. 2000;4:1–125. doi: 10.3310/hta4400. [DOI] [PubMed] [Google Scholar]
- 6.Woolacott N., Hawkins N., Mason A. Etanercept and efalizumab for the treatment of psoriasis: A systematic review. Health Technol. Assess. 2006;10:1–233. doi: 10.3310/hta10460. [DOI] [PubMed] [Google Scholar]
- 7.Bansback N., Sizto S., Sun H. Efficacy of systemic treatments for moderate to severe plaque psoriasis: Systematic review and meta-analysis. Dermatology. 2009;219:209–218. doi: 10.1159/000233234. [DOI] [PubMed] [Google Scholar]
- 8.Lin V.W., Ringold S., Devine E.B. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: A Bayesian network meta-analysis. Arch. Dermatol. 2012;148:1403–1410. doi: 10.1001/2013.jamadermatol.238. [DOI] [PubMed] [Google Scholar]
- 9.Rustin M.H. Long-term safety of biologics in the treatment of moderate-to-severe plaque psoriasis: review of current data. Br. J. Dermatol. 2012;167(Suppl. 3):3–11. doi: 10.1111/j.1365-2133.2012.11208.x. [DOI] [PubMed] [Google Scholar]
- 10.Randall T. Thalidomide has 37-year history. JAMA. 1990;263:1474. doi: 10.1001/jama.1990.03440110028006. [DOI] [PubMed] [Google Scholar]
- 11.Koren G., Pastuszak A., Ito S. Drugs in pregnancy. N. Engl. J. Med. 1998;338:1128–1137. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- 12.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin. Pharmacol. Ther. 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 13.Sampaio E.P., Kaplan G., Miranda A., Nery J.A., Miguel C.P., Viana S.M., Sarno E.N. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J. Infect. Dis. 1993;168:408–414. doi: 10.1093/infdis/168.2.408. [DOI] [PubMed] [Google Scholar]
- 14.D’Amato R.J., Loughnan M.S., Flynn E., Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira A.L., Sampaio E.P., Zmuidzinas A., Frindt P., Smith K.A., Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J. Exp. Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan G., Sampaio E.P. Method of Treating Abnormal Concentrations of TNFR. 5,385,901. U.S. Patent. 1992 (to Rockefeller University)
- 17.Richardson P., Hideshima T., Anderson K. Thalidomide: Emerging role in cancer medicine. Annu. Rev. Med. 2002;53:629–657. doi: 10.1146/annurev.med.53.082901.104043. [DOI] [PubMed] [Google Scholar]
- 18.Mei S.C., Wu R.T. The G-rich promoter and G-rich coding sequence of basic fibroblast growth factor are the targets of thalidomide in glioma. Mol. Cancer. Ther. 2008;7:2405–2414. doi: 10.1158/1535-7163.MCT-07-2398. [DOI] [PubMed] [Google Scholar]
- 19.Keifer J.A., Guttridge D.C., Ashburner B.P., Baldwin A.S. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J. Biol. Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 20.Wu J.J., Huang D.B., Pang K.R., Hsu S., Tyring S.K. Thalidomide: Dermatological indications, mechanisms of action and side-effects. Br. J. Dermatol. 2005;153:254–273. doi: 10.1111/j.1365-2133.2005.06747.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu J.H., Wu H.H., Zhao Y.K., Wang F., Gao Q., Luo D.Q. Thalidomide improves psoriasis-like lesions and inhibits cutaneous VEGF expression without alteration of microvessel density in imiquimod-induced psoriatic mouse model. Curr. Vasc. Pharmacol. 2017 doi: 10.2174/1570161115666171004123428. [DOI] [PubMed] [Google Scholar]
- 22.Furue M., Tsuji G., Chiba T., Kadono T. Cardiovascular and metabolic diseases comorbid with psoriasis: Beyond the skin. Intern. Med. 2017;56:1613–1619. doi: 10.2169/internalmedicine.56.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eleutherakis-Papaiakovou V., Bamias A., Dimopoulos M.A. Thalidomide in cancer medicine. Ann. Oncol. 2004;15:1151–1160. doi: 10.1093/annonc/mdh300. [DOI] [PubMed] [Google Scholar]
- 24.Varma S.R., Sivaprakasam T.O., Mishra A., Prabhu S., Rafiq M., Rangesh P. Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin. Eur. J. Pharmacol. 2017;813:33–41. doi: 10.1016/j.ejphar.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 25.Dastidar S.G., Rajagopal D., Ray A. Therapeutic benefit of PDE4 inhibitors in inflammatory diseases. Curr. Opin. Investig. Drugs. 2017;8:364. [PubMed] [Google Scholar]
- 26.Assis S.P., Araújo T.G., Sena V.L., Catanho M.T.J., Ramos M.N., Srivastava R.M., Lima V.L. Synthesis, hypolipidemic, and anti-inflammatory activities of arylphthalimides. Med. Chem. Res. 2014;23:708–716. doi: 10.1007/s00044-013-0673-6. [DOI] [Google Scholar]
- 27.Fumian M.M., da Motta N.A., Maia R., Fraga C.A., Barreiro E.J., Ferreira de Brito F.C. LASSBio-1425, an analog of thalidomide, decreases triglyceride and increases HDL cholesterol levels by inhibition of TNF-alpha production. Int. J. Cardiol. 2016;202:497–499. doi: 10.1016/j.ijcard.2015.09.071. [DOI] [PubMed] [Google Scholar]
- 28.Tseng C.H., Lin C.S., Shih P.K., Tsao L.T., Wang J.P., Cheng C.M., Tzeng C.C., Chen Y.L. Furo[3, 2 :3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg. Med. Chem. 2009;17:6773–6779. doi: 10.1016/j.bmc.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 29.Tseng C.H., Tzeng C.C., Shih P.K., Yang C.N., Chuang Y.C., Peng S.I., Lin C.S., Wang J.P., Cheng C.M., Chen Y.L. Identification of furo[3′,2′:3,4]naphtho[1,2-d] imidazole derivatives as orally active and selective inhibitors of microsomal prostaglandin E(2) synthase-1 (mPGES-1) Mol. Divers. 2012;16:215–229. doi: 10.1007/s11030-011-9347-9. [DOI] [PubMed] [Google Scholar]
- 30.Tseng C.H., Cheng C.M., Tzeng C.C., Peng S.I., Yang C.L., Chen Y.L. Synthesis and anti-inflammatory evaluations of -lapachone derivatives. Bioorg. Med. Chem. 2013;21:523–531. doi: 10.1016/j.bmc.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.R., Tseng C.H., Chen Y.L., Hwang T.L., Tzeng C.C. Discovery of benzo[f]indole-4,9-dione derivatives as new type of anti-inflammatory agents. Int. J. Mol. Sci. 2015;16:6532–6544. doi: 10.3390/ijms16036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng C.H., Tung C.W., Wu C.H., Tzeng C.C., Chen Y.H., Hwang T.L., Chen Y.L. Discovery of indeno[1,2-c]quinoline derivatives as potent dual antituberculosis and anti-Inflammatory agents. Molecules. 2017;22:1001. doi: 10.3390/molecules22061001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao D.R., Kankan R.N., Surve M., Birari D. Processes for the Preparation of Thalidomide. WO 2009083724 A1. 2009
- 34.Luzzio F.A., Mayorov A.V., Ng S.S., Kruger E.A., Figg W.D. Thalidomide metabolites and analogues. 3. Synthesis and antiangiogenic activity of the teratogenic and TNF-α modulatory thalidomide analogue 2-(2,6-dioxopiperidine-3-yl) phthalimidine. J. Med. Chem. 2003;46:3793–3799. doi: 10.1021/jm020079d. [DOI] [PubMed] [Google Scholar]
- 35.Baliwag J., Barnes D.H., Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342–350. doi: 10.1016/j.cyto.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkes J.E., Gudjonsson J.E., Ward N.L. The snowballing literature on imiquimod-induced skin inflammation in mice: A critical appraisal. J. Investig. Dermatol. 2017;137:546–549. doi: 10.1016/j.jid.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schön M.P., Schön M., Klotz K.N. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J. Investig. Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 38.Johansen C., Kragballe K., Westergaard M., Henningsen J., Kristiansen K., Iversen L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br. J. Dermatol. 2005;152:37–42. doi: 10.1111/j.1365-2133.2004.06304.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W., Xiao S., Li H., Zheng T., Huang J., Hu R., Zhang B., Liu X., Huang G. MAPK phosphatase-1 deficiency exacerbates the severity of imiquimod-induced psoriasiform skin disease. Front. Immunol. 2018;9:569. doi: 10.3389/fimmu.2018.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayakar C., Kumar B.S., Sneha G., Sagarika G., Meghana K., Ramakrishna S., Prakasham R.S., Raju B.C. Synthesis, pharmacological activities and molecular docking studies of pyrazolyltriazoles as anti-bacterial and anti-inflammatory agents. Bioorg. Med. Chem. 2017;25:5678–5691. doi: 10.1016/j.bmc.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 41.Singh P., Kaur S., Sharma A., Kaur G., Bhatti R. TNF-α and IL-6 inhibitors: Conjugates of N-substituted indole and aminophenylmorpholin-3-one as anti-inflammatory agents. Eur. J. Med. Chem. 2017;140:92–103. doi: 10.1016/j.ejmech.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Hisanaga A., Mukai R., Sakao K., Terao J., Hou D.X. Anti-inflammatory effects and molecular mechanisms of 8-prenyl quercetin. Mol. Nutr. Food Res. 2016;60:1020–1032. doi: 10.1002/mnfr.201500871. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Linares I., Pérez-Sánchez H., Cecilia J.M., García J.M. High-Throughput parallel blind Virtual Screening using BINDSURF. BMC Bioinform. 2012;13(Suppl. 14):S13. doi: 10.1186/1471-2105-13-S14-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rego N., Koes D. 3Dmol.js: Molecular visualization with WebGL. Bioinformatics. 2015;31:1322–1324. doi: 10.1093/bioinformatics/btu829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.