Abstract
Plant hormones are master regulators of plant growth and development. Better knowledge of their spatial signaling and homeostasis (transport and metabolism) on the lowest structural levels (cellular and subcellular) is therefore crucial to a better understanding of developmental processes in plants. Recent progress in phytohormone analysis at the cellular and subcellular levels has greatly improved the effectiveness of isolation protocols and the sensitivity of analytical methods. This review is mainly focused on homeostasis of two plant hormone groups, auxins and cytokinins. It will summarize and discuss their tissue- and cell-type specific distributions at the cellular and subcellular levels.
Keywords: auxin, cytokinin, phytohormone metabolism, phytohormone transport, cellular level, subcellular level
1. Introduction
The most well-documented groups of plant hormones are auxins and cytokinins (CKs) (Figure A1) with reasonably well-described signaling, transport, and metabolism (biosynthesis, conjugation, and degradation). Moreover, mutual auxin–cytokinin regulation and/or crosstalk appear to control many developmental processes in plants [1]. Since the 1950s, both CKs and auxins have been known for their ability to effectively determine the type of organs regenerated in vitro from undifferentiated callus cultures [2]. High auxin-to-CK ratios stimulate root formation, whereas low ratios promote shoot formation. Müller and Sheen [3] showed that antagonism between CK and auxin is primarily realized at the molecular level and is important for specifying root stem cells during early embryogenesis. Moreover, recent transcriptomic data have shown that meristems reform in positions determined by antagonistic auxin and CK signaling domains during tissue repair [4]. On the other hand, synergistic effects of auxins and CKs have also been reported, an example being shoot apical meristem formation [5,6].
The importance of phytohormone homeostasis at the cellular level has become more prominent with the increasing sensitivity of analytical tools [7]. It is generally accepted that compartmentation is a key feature of eukaryotic cells. Plant cells contain admirably complex, albeit well-organized membrane systems dividing them into organelles or compartments. This partition provides possibilities to create appropriate microenvironments and conditions for specialized metabolic pathways. Thus, unique sets of enzymes, transporters, and other proteins are found separated into organelles.
Current advances in indirect or direct visualization methods and other sensitive analytical techniques enable us to visualize phytohormone distributions in vivo at the cellular and subcellular levels. In this review, the authors have connected homeostasis (transport and metabolism) of auxins and CKs with their tissue- and cell-type specific distributions at the cellular and subcellular levels. They are convinced that this topic will open completely new horizons in understanding how the balance of plant hormones is created and controlled.
2. Organelle-Specific Phytohormone Profiling
Analytical methods for quantitation of auxins and CKs have become increasingly sensitive and capable of discriminating not only the free hormones, but also many of their precursors, metabolites, and catabolites [7,8]. Nevertheless, at the subcellular level, organelle-specific phytohormone profiling is challenging and many factors need to be optimized, such as (i) leakage during isolation; (ii) purity of isolated compartments; and (iii) dynamic metabolic changes during isolation.
2.1. Subcellular Fractionation
The original idea of separating intracellular compartments to study the partition of enzyme processes was developed by De Duve and co-workers in the 1950s [9]. Methods of organelle isolation are mainly based on differential centrifugation or density gradient ultracentrifugation [10,11,12,13,14]. Even the simplest differential centrifugation can provide enriched fractions of crude organelles [15,16]. Higher purity organelle fractions can be achieved by density gradient ultracentrifugation yielding fractions enriched in endoplasmic reticulum (ER) [14], Golgi apparatus [17], vacuoles [11], mitochondria [16], and chloroplasts [12,18].
Alternative methods for compartment separation have been also described, for example, two-phase partitioning [19] and non-aqueous or aqueous fractionation [20,21]. Techniques such as flow cytometry can be used for more rapid sorting of organelles labelled by fluorescent probes, for example, nuclei [22], chloroplasts [23], and mitochondria [24]. Affinity capture or pull down by magnetic microparticles has been also used for isolating nuclei [25] or mitochondria [26].
2.2. Phytohormone Profiling in Organelles
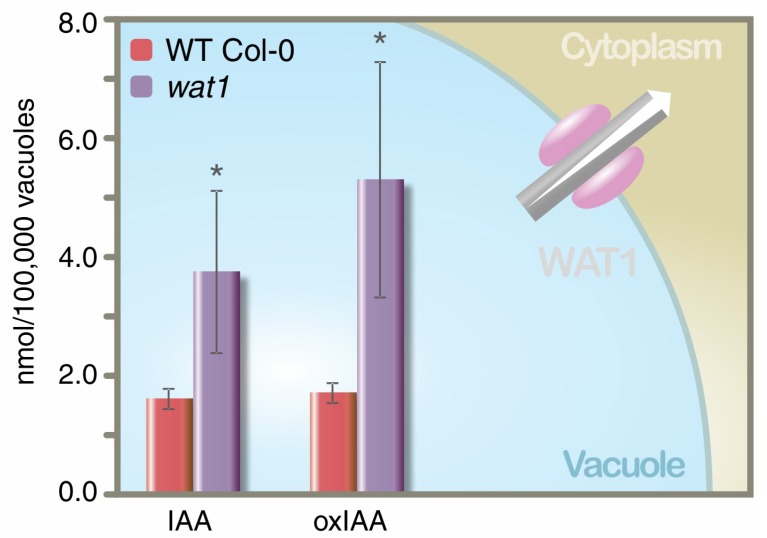
Currently, there are only few reports dealing with the determination of phytohormones in live-cell systems [7]. In addition, little is known about extra- and intracellular phytohormone distribution, or the phytohormone levels in individual cell compartments. Indole-3-acetic acid (IAA) has been determined in chloroplasts and mitochondria [27], whereas CKs, IAA, and abscisic acid concentrations have been determined in chloroplasts [28,29] (Table 1). The full profile of IAA and its metabolites has been described in wild-type Arabidopsis thaliana vacuoles [30] with determinations providing, for example, clear functional evidence of the vacuolar auxin transport protein WALLS ARE THIN 1 (WAT1) (Figure 1).
Table 1.
Auxin and cytokinin (CK) profiles at the subcellular level. Compounds are ordered according to their abundance in particular organelles. Abbreviations of auxins and CKs are listed in Figure A1.
| Organelles (Species 1) | Auxins | Cytokinins | Reference |
|---|---|---|---|
| Chloroplasts (Nicotiana tabacum) |
Precursors (n.a. 2) Active compounds (IAA) Metabolites (n.a.) |
Sum of CK bases (B) Sum of CK ribosides (R) Sum of CK N-glucosides (NG) Sum of CK O-glucosides (OG) Sum of CK phosphates (P) |
[29] |
| Chloroplasts (Nicotiana tabacum, Triticum aestivum) | n.a. |
B (iP, DHZ) R (ZR, iPR, DHZR) NG (Z9G, DHZ9G, iPNG, Z7G, DHZ7G) OG (n.a.) P (iPRMP, ZRMP, DHZRMP) |
[28] |
| Vacuoles (Arabidopsis) |
Precursors (Trp, IAN, ANT, TRA, IAM) Active compounds (IAA) Metabolites (IAA-Glc, oxIAA) |
n.a. | [30] |
| Vacuoles (Arabidopsis, Hordeum vulgare) | n.a. |
B (tZ, iP) R (cZR, iPR, tZR) NG (iP7G, tZ7G, DHZ7G, tZ9G, iP9G, cZ9G, DHZ9G) OG (cZROG, cZOG, tZOG, DHZOG, tZROG) P (iPRMP, tZRMP) |
[31] |
1 Phytohormone profiles are shown for species in bold. 2 “n.a.” indicates that the phytohormones were not profiled in the study.
Figure 1.
Indole-3-acetic acid (IAA) and 2-oxindole-3-acetic acid (oxIAA) contents were measured in vacuolar fractions isolated by density gradient ultracentrifugation from the wild-type (Arabidopsis Col-0) and the vacuolar auxin transporter mutant line (wat1-1). Plant tissues were grown, and vacuole isolation was performed as previously described [31]. Samples were purified by in-tip solid-phase microextraction [32] using a minor modification of the protocol described by Pěnčík et al. [33]. Quantification of IAA and oxIAA was performed by LC-MS/MS [34]. The bars represent averages (±SD) of four independent biological replicates; the asterisk indicates p-values of the genotype comparisons in an ANOVA analysis (* p < 0.05). White arrow indicates flux direction.
Profiling of CK metabolites at the subcellular level has been performed in both Arabidopsis and barley (Hordeum vulgare) [31]. Concentrations of 25 CK metabolites were determined from isolated apoplast, cytosol, and vacuoles (Table 1). Surprisingly, the highest proportion of CKs was located outside the cell (up to 90%, with a majority as O- and N-glucosides), and only about 10% was present in cytosol and vacuoles. In transgenic barley expressing the cytokinin oxidase gene AtCKX1, severe decreases in extracellular trans-zeatin (tZ) and tZ-7-glucoside (tZ7G) were accompanied by compensatory increases of isopentenyladenine (iP) and vacuolar isopentenyladenosine (iPR).
All these practical examples indicate that a far richer picture can be drawn of phytohormone homeostasis and fluxes with higher resolution data, but hormone profiling and quantitation remain challenging at the resolution required for reliable data about subcellular compartmentation.
3. Auxins
It is well described that cellular IAA concentrations are strictly regulated by its transport, biosynthesis, and catabolism [35]. Changes in auxin concentrations and morphogenic gradients are created in plant tissues and organs as a response to both exogenous and endogenous stimuli, resulting in various developmental events, but how homeostasis is managed in these systems is far from clear. While TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX proteins (TIR1/AFBs) are considered as proven auxin receptors, the clear contribution of AUXIN BINDING PROTEIN 1 (ABP1) and S-PHASE KINASE-ASSOCIATED PROTEIN 2A (SKP2A)-dependent perception to auxin signaling still remains controversial [36,37] (Figure 2).
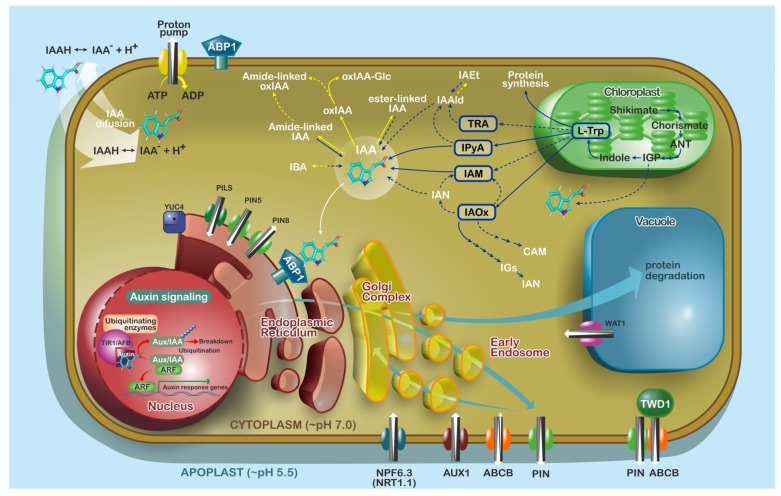
Figure 2.
Model of cellular and subcellular auxin homeostasis and signaling in Arabidopsis. IAA biosynthesis (indicated by dark blue arrows) could be mediated by l-tryptophan (l-Trp)-dependent or independent biosynthetic pathways [47]. Tryptophan as a substrate for the synthesis of IAA is synthesized in stroma of chloroplast [40]. There are already four described biosynthetic pathways named according to their first intermediates (in dark blue rectangles [60]). In Arabidopsis, IAA biosynthesis is running predominantly via the indole-3-pyruvic acid (IPyA) pathway including: cytoplasmic TRYPTOPHAN AMIDOTRANSFERASE OF ARABIDOPSIS (TAA1), TAA-Related (TAR1) localized on plasma membrane (PM), and YUCCA4 attached to the ER membrane [47,52]. Free IAA levels can be modulated via conjugation and/or oxidation, rarely via methylation (metabolic pathways are represented by yellow arrows [59,61]. Four main families of active auxin transporters are described: PM localized AUXIN1/LIKE-AUX1 (AUX1/LAX) auxin influx facilitators, and perhaps also into the ER [62]; PINs efflux carriers [36,63]; ATP-binding cassette type B (ABCB) proteins [64] involved in the influx or efflux of auxin [65,66]; and finally PIN-like (PILS) together with short PIN-FORMED proteins (PINs) (PIN5, 6, and 8) with confirmed localization at ER [67]. WALLS ARE THIN 1 (WAT1) is a recently described tonoplast-localized auxin transporter [30]. Similarly, NPF6.3 (NRT1.1) can control auxin influx (transport is marked by white arrows [68,69]. Nuclear TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX proteins (TIR1/AFBs) are considered as proven auxin receptors. However, the strong proof that putative AUXIN BINDING PROTEIN 1 (ABP1) and S-PHASE KINASE-ASSOCIATED PROTEIN 2A (SKP2A) receptors directly mediate auxin signaling still remains contentious (signaling is highlighted by red arrows [37,70]). Light blue arrows indicate protein trafficking. Solid arrows indicate known and well-described pathways, dashed arrows indicate not well-defined pathways. Abbreviations and structures of all IAA metabolites (precursors, catabolites, and conjugates) are listed in Figure A1a.
Polar auxin transport (PAT) is a regulated cell-to-cell transport of auxin that provides essential directional and positional information for all vital plant developmental processes, such as vascular differentiation, apical dominance, patterning, organ polarity, embryogenesis, organogenesis, phyllotaxis, and tropisms [38]. Disruption of such directional auxin movement by genetic or pharmacological manipulations results in severe developmental defects [39]. Local auxin production, frequently together with auxin transport, influences lateral root development, embryogenesis, and leaf and fruit development, whereas a strong reduction in auxin levels leads to defects in gravitropism, vasculature development, and reduced apical dominance [40]. All these activities are described at the tissue level, but little is known about how homeostasis, and perturbations to homeostasis, are affected at the subcellular level.
3.1. Locations of Auxin Biosynthesis and Metabolism
The first organelle-specific activity connected with auxin homeostasis described indole-3-butyric acid (IBA) enzymatic conversion to IAA in a peroxisome-dependent reaction [41]. However, whereas IBA has been recorded from a number of plant species [35], other labs have had difficulties detecting IBA or report it at much lower concentrations than IAA [34,42]. Certainly, IBA is a poor ligand for the receptor TIR1 [43,44], but IBA and/or its conjugates might still contribute to IAA homeostasis [45].
The biosynthesis of IAA as a natural auxin could be mediated by two main directions: via an l-tryptophan (l-Trp)-dependent or an l-Trp-independent pathway [46,47] (Figure 2). De novo synthesis through the l-Trp-independent pathway is well described in microorganisms [48] but still discussed in higher plants [49,50]. In contrast, l-Trp-dependent pathways are a significant source of endogenous IAA for higher plants [40], as l-Trp is synthesized by the shikimate pathway localized in the chloroplast stroma. Downstream, IAA biosynthesis is predominantly via the indole-3-pyruvic acid (IPyA) pathway with three main family proteins (Figure 2): TRYPTOPHAN AMIDOTRANSFERASE OF ARABIDOPSIS (TAA1 localized in cytoplasm) and TAA-Related (TAR1 localized on plasma membrane (PM)) that are responsible for the synthesis of IPyA from tryptophan, and flavin monooxygenases from the YUCCA family that are responsible for the conversion of IPyA to IAA [51,52] (Figure 2). The YUCCA enzymes are likely to be cytoplasmic, although Arabidopsis YUCCA4 can be localized both to the cytosol and to the cytosolic face of the ER membrane [18]. At least three of the maize auxin biosynthetic proteins are also localized to ER membranes [53] (Figure 2).
The indole-3-acetaldoxime (IAOx) pathway is a unique biosynthetic pathway in Brassicaceae with cytochrome P450 enzymes CYP79B2 and CYP79B3 localized in chloroplasts, where their substrate Trp is synthesized [54], converting Trp to IAOx, and then to indole-3-acetamide (IAM) or indole-3-acetonitrile (IAN) downstream. However, the enzymatic steps between IAOx and IAN have yet to be identified. The synthesis of IAM from IAOx has been directly demonstrated in assays with cyp79b2 cyp79b3 mutants [55,56], and IAM hydrolases have been isolated from Arabidopsis and tobacco BY-2 cells (AtAMI1 and NtAMI1) and shown to convert IAM to IAA in vitro, but the subcellular localization of these enzymes remains unclear [57,58], despite some evidence of AtAMI1-green fluorescent protein (GFP) fusion protein in the cytoplasm [59].
Free IAA levels are probably managed by activities in the cytoplasm, the compartment of synthesis and of arrival by transport. In the cytoplasm, IAA can be modulated via conjugation and/or oxidation, and rarely via methylation [59]. IAA can be conjugated via ester linkages to glucose by UDP-glucosyl transferases UGT74D1 and UGT84B1 to create 1-O-indole-3-acetyl-β-d-glucose (IAA-Glc) [71], or to amino acids by the GRETCHEN HAGEN 3 (GH3) family of IAA-amido synthases [72,73]. Interestingly, Barbez and Kleine-Vehn [74] later hypothesized that the localization of the GH3 family is in the ER, but this would place them in the same compartment as ILR1-like amidohydrolases (ILR1, ILR2, and ILR3 [75]), which will hydrolyze the products of GH3s. Unfortunately, clear evidence for GH3 localization to the ER is still missing. If IAA conjugates are synthesized in the cytoplasm, the authors can hypothesize that such conjugates are rapidly transported out of the cytoplasm for storage or derivative pathways.
The GH3 enzymes are induced strongly by elevated concentrations of auxin and this provides one level of homeostatic control [76,77], but the role of the ILRs in subsequently releasing free IAA back from amido-conjugates is not known. The steady state concentration of IAA in the cytoplasm is considered to be 5 μM when calculated in system models [78]. Rises in concentration above the steady state in the cytoplasm and nucleus will induce transcription and translation of GH3s, among many other genes, and the analysis above suggests that these reside in the cytoplasm ready to react with the elevated free IAA. Consideration of the kinetic properties of these enzymes suggests that GH3s will become increasingly active in the micromolar range of IAA concentrations (Km for OsGH3-8 = 182 μM, [77]; Km for AtGH3-5 = 700 μM, [79]). While these Km values suggest poor activity at the concentrations of IAA likely to be encountered in the cytoplasm, the enzymes do have very high catalytic efficiencies (kcat/Km) and so free IAA will be rapidly conjugated by resting levels of enzyme, and this will be rapidly supplemented as new enzyme is generated by the auxin response. In the ER, ILRs will become active at somewhat lower concentrations of the conjugates (Km AtILR1 = 14 μM; [75]), but the proper location of the conjugates remains to be determined.
IAA oxidation to 2-oxindole-3-acetic acid (oxIAA) is the major IAA catabolic pathway in Arabidopsis [34,80,81]. It was later shown that another oxidative metabolite in Arabidopsis, oxIAA-glucose (oxIAA-Glc), was synthesized via glycosylation of oxIAA and not via oxidation of IAA-Glc [82,83]. Oxidation of some IAA amides in Arabidopsis was also detected [80,84]. The first characterized IAA oxidases, DIOXYGENASE FOR AUXIN OXIDATION (DAO) in dicots, were rice OsDAO homologs in Arabidopsis AtDAO1 and AtDAO2 [83,85,86]. These dioxygenases are cytoplasmic [83] and so, again, responses to elevations of IAA concentration are targeted to the cytoplasm and one may expect the cytoplasmic concentration at homeostasis to be micromolar or lower given that OsDAO1 actively oxidized IAA when 1 μM IAA was supplied [85].
AtDAO1 was shown to be a primary determinant of auxin homeostasis [83]. However, the work on oxidases [83,86] showed that the loss of IAA oxidation in atdao1 mutants did not lead to a significant change in IAA levels, suggesting redundancy in homeostatic mechanisms. Moreover, the mathematical model from Mellor et al. [87] suggests that, in atdao1 mutant, IAA-aspartate (IAA-Asp) and IAA-glutamate (IAA-Glu) accumulate, compensating for the loss of IAA oxidation.
There are several reports indicating that methylation of IAA is highly relevant for some plant developmental processes, such as leaf development [88] and differential growth in the hypocotyl [89].
Taken together, these results suggest that plants possess redundant and sensitive mechanisms to catabolize cytoplasmic IAA [90]. It will be useful in future to know into which compartment the oxidation and other catabolic products are moved. The presence of the amidohydrolases in the ER suggests that this compartment is important, but it remains possible that this is only involved in feedback control of cytoplasmic IAA concentrations.
3.2. Auxin Transport
There are four main families of active auxin-specific transporters and by their nature, each is localized to specific membranes (Figure 2). Therefore, one can surmise their roles in auxin homeostasis in some detail: (i) AUXIN1/LIKE-AUX1 (AUX1/LAX) auxin-H+ symporters, responsible for auxin transport from the apoplast into the cell, and perhaps also into the ER [62,91,92]; (ii) PIN-FORMED proteins (PINs) that are gradient-driven secondary transporters (efflux carriers) [63]; (iii) ATP-binding cassette type B proteins (ABCBs) [64] uniformly localized at the PM are involved in the ATP-driven influx or efflux of auxin [65,66]; and (iv) the PIN-like (PILS) protein family with confirmed localization at ER [67] (Figure 2). Additionally, it has been demonstrated that the nitrate transceptor NPF6.3 (NRT1.1, Figure 2), which belongs to the NPF (NRT1/PTR) family in Arabidopsis [68,69], is involved in the auxin influx in heterologous systems of Xenopus oocytes, yeast, and tobacco BY-2 cells [93,94,95]. Finally, the tonoplast-localized auxin transporter WAT1 and endomembrane ADP1, that are involved in maintaining the intracellular auxin homeostasis, were also identified [30,96]. Experiments showed that WAT1 confers auxin efflux to yeast cells and Xenopus oocytes [30]. However, it is still not known which auxin-related compound(s) are transported in planta.
In Arabidopsis, the PIN family consists of eight members and divides into two subfamilies according to the length of a hydrophilic loop located in the middle of their polypeptide chain. The “long” canonical PINs (PIN1-4, and 7) [97,98,99] act as auxin efflux carriers and are polarly localized at the PM where they direct auxin flow [100,101]. The “short” non-canonical PINs (PIN5-6 and PIN8) have the hydrophilic loop, either partially (PIN6) or significantly reduced (PIN5 and PIN8) [99]. “Short” PINs are predominantly localized to the ER where they presumably regulate auxin homeostasis by pumping auxin into (PIN5) or out (PIN8) of the ER lumen or hypothetically from the ER lumen into the nucleus (PIN6 and PIN8) [14,102,103,104,105]. However, Ganguly et al. [106,107] and Simon et al. [108] revealed dual localizations of PIN5, PIN6, and PIN8 at the PM and ER in Arabidopsis epidermal and root hair cells, as well as in tobacco BY-2 cells. PIN5::GFP was predominantly localized to the ER and PIN8::GFP, to the PM. However, in the epidermal and cortical cells of the root meristem region (the PIN2 domain), PIN5 showed a PM localization pattern [106,107]. Finally, Ganguly et al. [107] came up with the hypothesis that both PIN5 and PIN8, with their dual localization property, may act as linkers between the ER-based PILs and the PM-based canonical PINs. It is also clear that PINs do not stay static but undergo constitutive cycling through the clathrin-coated vesicle machinery between the PM and ER compartments [109,110].
ABCB, ABCD, and ABCG protein subfamilies are directly or indirectly involved in auxin transport. There is a clear and well-described functional interaction between members of the ABCB family (ABCB1 and ABCB19) and TWISTED DWARF1 (TWD1) which acts as a chaperone during PM trafficking [111]. Dudler and Hertig [112] were the first to determine the substrate specificity of ABCB1 and later Sidler et al. [113] pointed out the role of ABCB1 in the regulation of hypocotyl elongation and its localization to the PM. ABCB1 and ABCB19 show mainly apolar cellular localizations, although partial apical localization is found in different tissues [114,115,116]. Interestingly, PAT in abcb19 was highly reduced, in both inflorescence stems and hypocotyls [117], and by ~70% in the abcb1 abcb19 double mutant, whereas pin1 exhibited only a ~30% reduction [118,119]. Similar drastic reductions in PAT were found in twd1 [120]. This suggests that ABCBs primarily contribute to long-distance auxin transport and do not function in establishing the basal auxin flows that regulate organogenesis [111,118,119,121]. It has also been demonstrated that ABCB4 works as a unique auxin concentration-dependent switchable influx/efflux transporter [65,66], and this will clearly contribute to homeostatic control of cytoplasmic auxin concentrations.
The auxin carriers that are specifically localized to ER provide a clear link between auxin compartmentalization and auxin conjugation-based metabolism. Moreover, the role of auxin intracellular transport (PIN5, PIN8 and PILS) together with compartmentalization of auxin metabolism can be interfaced in maintaining and regulating intracellular auxin homeostasis [74]. Mravec et al. [102] were the first to realize that PIN5 increases cellular auxin retention in Arabidopsis protoplasts presumably via auxin transport from the cytosol into the ER lumen. Moreover, PIN5 activity decreases cellular levels of free IAA and increases levels of some auxin conjugates, namely, IAA-Asp, IAA-Glu, and IAA-Glc, suggesting a possible role for PIN5 in compartmentalized auxin metabolism. However, the picture for PIN8 is less clear [14,65,66,74,103]. As for PIN5, PILS2 and PILS5 in the ER increase cellular auxin accumulation, but reduce nuclear auxin signaling, and so one can speculate that they promote the sequestration of cytosolic auxin into the ER, where it is unavailable for nuclear auxin signaling [67].
It is clear that local directed transport activities contribute significantly to the regulation of cellular auxin metabolism. Indeed, Middleton et al. [122] have combined mathematical modelling with time course data from both auxin-mediated nuclear signaling and quantitative phenotyping at the single cell level, to show that an ER-to-nucleus auxin flux represents a major subcellular pathway to directly control nuclear auxin levels. Based on the preceding, the authors can propose that auxin-mediated responses are controlled by both maintenance of a homeostatic auxin pool in the ER together with regulated rapid auxin fluxes between ER and nucleus.
4. Cytokinins
CKs are divided according to the chemical character of their side chain on the prevalent isoprenoid group, such as tZ, cis-zeatin (cZ), dihydrozeatin (DHZ), and iP. The same classification applies to benzyladenine (BA) and topolins, which occur less in nature, which are CKs carrying an aromatic group instead of the isoprenoid (Figure A1b) [123,124,125,126,127]. Another group of naturally occurring CKs are derivatives modified at position C2 by the methylthio group [128]. CKs promote many responses at the cellular level (e.g., cell cycle and division [129], chloroplast development [130]) and are modulators of PIN formation and polarity [131]. These processes are dependent on CK perception mediated by three ARABIDOPSIS HISTIDINE KINASES (AHKs) which trigger a multistep phosphorelay cascade leading to gene transcription. The AHK receptors sit mainly in the ER, but the PM might be relevant in some circumstances as well (described in detail in [132,133]) (Figure 3).
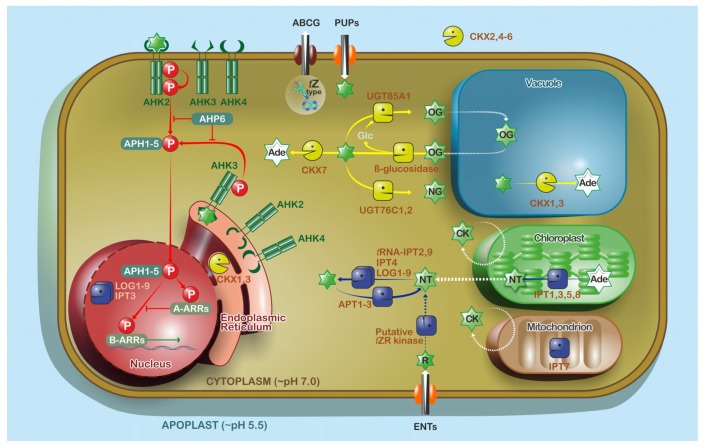
Figure 3.
Model of cellular and subcellular CK homeostasis and signaling in Arabidopsis. De novo synthesis of CKs is mediated by isopentenyl transferases (IPTs) mainly in chloroplasts; nevertheless, they are localized also in mitochondria, cytosol, and nuclei [137,138]. LONELY GUY enzymes (LOGs) present in cytosol and nuclei are other enzymes, which transform CK nucleotides to active form [140] (biosynthesis is highlighted by dark blue arrows). In contrast, APTs catalyze the opposite reaction [145,146,147]. Most of the active CKs can be modulated by uridine diphosphate glycosyltransferases (UGTs) [148] or β-glucosidase [144,165] (yellow arrows mark reversible/irreversible inactivation). The terminal degradation product of cytokinin dehydrogenases/oxidases (CKXs) is adenine (Ade, yellow arrows also mark CK degradation). CKXs are prevalent in the apoplast, although three homologs are intracellular [151,153,154]. Transport (represented by white arrows) of CK free bases and their ribosides to cytoplasm is facilitated by purine permeases (PUPs) [161,166,167] and equilibrative nucleoside transporters (ENTs) [158,168], respectively. Lomin et al. [169] proposed a model where ENTs are involved in tZR transport to cytosol and its subsequent conversion via a putative kinase and LOG into an active CK base, which enters to ER and triggers signaling. ABCG14 was described and proven as an exporter of tZ-types [163,164]. CK signaling pathways (marked by red arrows) are initiated by three ARABIDOPSIS HISTIDINE KINASES (AHKs) localized at PM [170] or ER [13,171]. Signal is transmitted via ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER1-5 (AHP1-5) [172,173] to nuclear type-A ARABIDOPSIS RESPONSE REGULATORS (A-ARRs) or B-ARRs (type-B). AHP6 is inhibitory [174,175]. Activation of B-ARRs leads to transcription [176,177] of CK inducible genes including A-ARRs which mediate a negative feedback loop [172,178,179,180]. Green stars indicate CK species; Glc—glucose; NT—cytokinin nucleotides; NG—cytokinin N-glucosides; OG—cytokinin O-glucosides; P—phosphate moiety; R—cytokinin riboside. Solid arrows indicate known and well-described pathways, dashed arrows indicate not well-defined pathways.
4.1. Locations of Cytokinin Biosynthesis and Metabolism
Key enzymes catalyzing the first step of CK biosynthesis are the isopentenyl transferases (IPTs). IPTs mediate the conjugation of an isopentenyl group to the N6-position of the adenine ribotide to form isopentenyladenosine-5′-di- or -triphosphate (iPRDP or iPRTP, respectively). Several IPTs were identified in Arabidopsis (AtIPT1-9) [134,135,136], where AtIPT2 and 9 can also catalyze isopentenylation of tRNA to provide a source for cZ-type CKs [136]. AtIPT1, 3, and 5 fused with GFP were localized to the chloroplasts of mesophyll cells [137], although AtIPT3 appears also in the nucleus. Specific localization depends on posttranslational modifications such as farnesylation, which may overcome the presence of chloroplast transit peptides [138] (Figure 3). GFP fusions of AtIPT2 and AtIPT4 point to cytosolic localization. This finding agrees with the idea that AtIPT4 may utilize isoprenoid precursors synthetized via the mevalonate pathway in the cytosol, but it is likely that the main pool of tZ arises from plastids. Additionally, AtIPT7::GFP was observed in mitochondria [137] (Figure 3).
Synthesis of iPRDP and iPRTP nucleotides via transmission of isoprenoid moieties to adenosine is followed by hydroxylation to produce tZ-type CKs, a reaction catalyzed by cytochrome P450 monooxygenases CYP735A1 and CYP735A2 [139]. CK nucleotides can get phosphoribohydrolased by “LONELY GUY” (LOG) enzymes into highly active free-base forms [140]. To date, nine AtLOG homologs targeted predominantly to the nucleus and cytosol have been identified [141].
It is expected that the metabolism of active free bases at least partially regulates CK homeostasis. CK bases can be reversibly conjugated with sugars (e.g., glucose or xylose) through their hydroxyl moiety on the N6-side chain of tZ, cZ, and DHZ via Arabidopsis uridine diphosphate glycosyltrasnferase (AtUGT) 85A1 which is located to the cytosol [142,143] (Figure 3). A pool of O-glucosides (OG) could serve as CK storage with the potential of rapid conversion back to active CKs via β-glucosidases [144]. Another reversible inactivation could be mediated by enzymes common with purine metabolism, such as adenine phosphoribosyltransferases (AtAPT1-3), which appear to be cytosolic and act antagonistically to other AtLOGs (Figure 3), switching bioactive CKs back to nucleotides [145,146,147]. Direct glycosylation at N7 or N9 might also be catalyzed by cytosolic UGT76C1 or UGT76C2, causing irreversible CK inactivation [148].
Cytokinin dehydrogenases/oxidases (CKXs) are recognized as the main enzymes mediating CK degradation [149,150] and they play a key role in the maintenance of endogenous CK levels. The seven CKX homologs in Arabidopsis have distinct subcellular localizations. It seems that the main site of CK inactivation is localized in the apoplast by CKX2 and CKX4-6 [151]. CKX1 and 3 were initially predicted as mitochondrial based on in silico experiments [152], although later GFP fusions showed that these two enzymes are predominantly targeted to vacuole, with some observed signal also in ER [151] where they are catalytically active [153]. In the case of CKX7, the lack of a signal peptide suggests that it is localized to the cytosol [154] (Figure 3).
As well as localizations, AtCKXs differ in their substrate specificity adding a level of complexity to cytokinin homeostasis above that for auxin. While CKXs prefer unsaturated isoprenoids, aromatic CKs can also be degraded but with lower turnover rates [155,156] and DHZ, OG, and almost all cZ-types are believed to be resistant to AtCKXs.
4.2. Cytokinin Transport
CKs are long-distance signals and the different CK forms appear to be moved differentially. For example, tZ riboside (tZR) is transported acropetaly in xylem sap, whereas iPR is mainly transferred basipetaly via phloem [157,158,159,160]. Despite the importance of CK transport, the facilitator proteins were not discovered until the beginning of the 21st century when three protein groups possessing CK translocation activity were described: purine permeases (PUPs) [161], equilibrative nucleoside transporters (ENTs) [162], and the G subfamily of ATP-binding cassette (ABCG) transporters [163,164] (Figure 3). CK transport seems to be shared with essential nucleobases, although the molecular basis of CK transport is still poorly understood compared with auxin transport.
The PUP family numbers 23 members [132,161] and some PUPs appear to mediate CK uptake at the PM (Figure 3). AtPUP1 was first examined in a yeast mutant deficient in adenine uptake. Results suggested that kinetin and tZ, but not tZR, were substrates by competitively inhibiting adenine uptake. A mildly acidic apoplast raised AtPUP1 activity, whereas proton pump inhibitors reduced it. These findings point to energy-dependent and potentially proton-coupled transport against the concentration gradient [161], later confirmed by the transport of radiolabeled tZ [166]. However, PUPs are promiscuous to other purines and even though PUP14, for example, was shown to be involved in the early stages of plant development [167], their role specifically as CK carriers remains to be elucidated.
AtENT1 was described as a putative nucleoside transporter based on shared similarity with human ENTs [181] and proton-dependent import was confirmed later [162,182]. AtENT1-8 are localized at the PM [162,181,183] (Figure 3), although AtENT1 was also identified in the tonoplast proteome [184]. Substrate specificity of some Arabidopsis ENTs has been examined using competition assays of adenosine uptake. As a result, AtENT6 and 8 may participate in CK riboside transport and AtENT6 preferred iPR to tZR [158,168].
ABCG14 was revealed as the first described CK exporter involved in root-to-shoot transport of CKs. It is highly expressed in Arabidopsis root vascular tissue and loss-of function abcg14 mutants resemble CK-deficient phenotypes [163,164], and measurements showed that mutant shoots contained decreased tZ-type CKs, despite abundant tZs in roots. Interestingly, iP-type and cZ-type CK contents were elevated in both shoots and roots, suggesting that the abcg14 plants are attempting to compensate for the loss of transport of root-synthetized tZ-type CKs for intrinsic CK homeostasis [163,164]. Undoubtedly, AtABCG14 represents an important element in the long-distance transport of CKs. Unfortunately, there is little information on local or subcellular compartmentation of cytokinins or cytokinin catabolites.
5. Future Perspectives
In spite of many recent studies on plant hormones, there are still gaps in our knowledge about the mechanisms of homeostasis. For instance, detailed information about intracellular CK transport is still missing. Cell- and organelle-specific distributions of auxin, CK, and their related compounds are also waiting for elucidation. Auxin and CK profiling at the subcellular level will definitely open new insights and provide a better understanding of the regulation of auxin and CK homeostasis, offering more precise inputs for mathematical modelling, the creation of biosensors, and other applications in plant biotechnologies.
Mass spectrometry imaging and living single-cell mass spectrometry analysis could soon provide powerful tools for studying hormone distribution, even though they are still limited for hormone profiling [8]. Cell-specific sorting has been employed to gain more accurate insight into auxin [33,185] and CK [186] distributions in Arabidopsis root tips, and flow cytometric techniques for the sorting of organelles may soon provide a better view on subcellular distributions. Another possible approach for visualizing auxin and CK distributions at the cellular or subcellular level is using novel synthetic analogues labelled with 7-nitro-2,1,3-benzoxadiazole (NBD), for example, that have been recently developed to mimic native phytohormones in vivo [187,188,189]. A combination of all these methodologies with the use of mathematical modelling [78,122,190] to parameterize auxin homeostasis at cellular and subcellular levels will undoubtedly lead to far more detailed insights into the secrets of plant developmental control.
Acknowledgments
The authors are thankful to Ioanna Antoniadi and Markéta Pernisová for their critical reading of the manuscript and to Ota Blahoušek for drawing the figures.
Appendix A
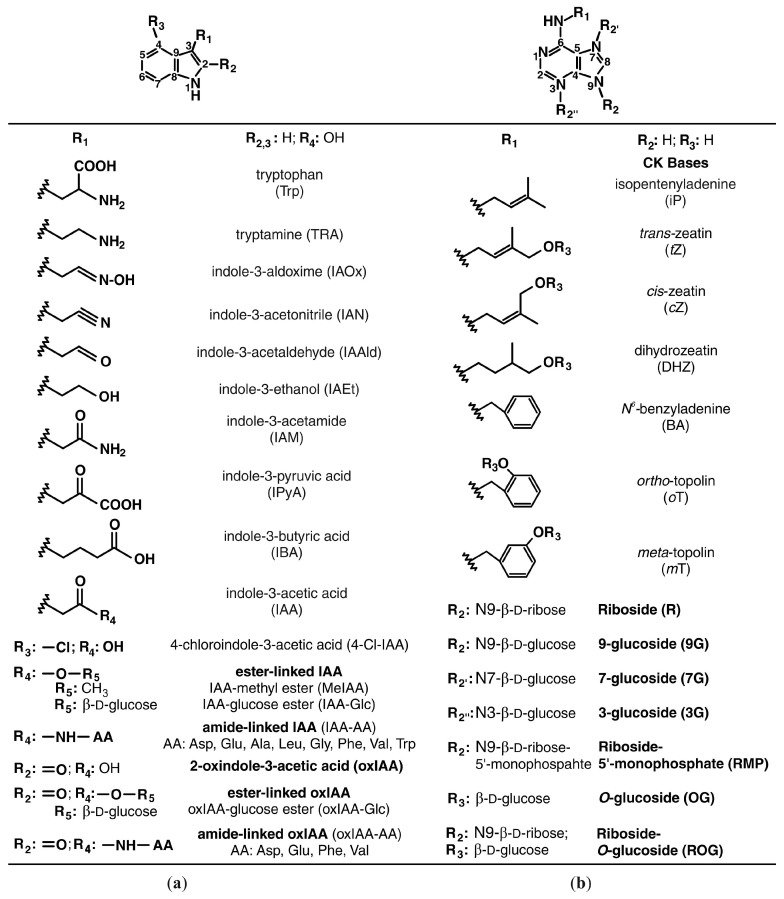
Figure A1.
Structures, names, and abbreviations of naturally occurring (a) auxins and (b) CKs. Wavy cuts indicate position of substituent attachment.
Author Contributions
All authors developed the idea and outline of the paper. V.S., M.K. and O.N. wrote the manuscript; R.N. and O.N. revised and edited the manuscript. All authors read the manuscript before submission.
Funding
This work was financially supported by the Ministry of Education, Youth and Sports, Czech Republic (Grant LO1204 from the National Programme of Sustainability I), by the Internal Grant Agency of Palacký University in Olomouc (IGA_PrF_2018_023), and by the Czech Science Foundation (17-21581Y). Vladimír Skalický was supported (in part) by the Endowment fund of Palacký University in Olomouc and Martin Kubeš was supported by the EU MSCA-IF project CrysPINs (792329).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schaller G.E., Bishopp A., Kieber J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell. 2015;27:44–63. doi: 10.1105/tpc.114.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skoog F., Miller C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- 3.Müller B., Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–1097. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efroni I., Mello A., Nawy T., Ip P.-L., Rahni R., DelRose N., Powers A., Satija R., Birnbaum K.D. Root regeneration triggers an embryo-like sequence guided by hormonal interactions. Cell. 2016;165:1721–1733. doi: 10.1016/j.cell.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leibfried A., To J.P.C., Busch W., Stehling S., Kehle A., Demar M., Kieber J.J., Lohmann J.U. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z., Andersen S.U., Ljung K., Doležal K., Miotk A., Schultheiss S.J., Lohmann J.U. Hormonal control of the shoot stem-cell niche. Nature. 2010;465:1089–1092. doi: 10.1038/nature09126. [DOI] [PubMed] [Google Scholar]
- 7.Novák O., Napier R., Ljung K. Zooming In on Plant Hormone Analysis: Tissue- and Cell-Specific Approaches. Annu. Rev. Plant Biol. 2017;68:323–348. doi: 10.1146/annurev-arplant-042916-040812. [DOI] [PubMed] [Google Scholar]
- 8.Pařízková B., Pernisová M., Novák O. What Has Been Seen Cannot Be Unseen—Detecting Auxin In Vivo. Int. J. Mol. Sci. 2017;18:2736. doi: 10.3390/ijms18122736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Duve C., Pressman B.C., Gianetto R., Wattiaux R., Appelmans F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber L.A., Pfaller K., Vietor I. Organelle Proteomics: Implications for Subcellular Fractionation in Proteomics. Circ. Res. 2003;92:962–968. doi: 10.1161/01.RES.0000071748.48338.25. [DOI] [PubMed] [Google Scholar]
- 11.Robert S., Zouhar J., Carter C.J., Raikhel N. Isolation of intact vacuoles from Arabidopsis rosette leaf–derived protoplasts. Nat. Protoc. 2007;2:259–262. doi: 10.1038/nprot.2007.26. [DOI] [PubMed] [Google Scholar]
- 12.Seigneurin-Berny D., Salvi D., Dorne A.-J., Joyard J., Rolland N. Percoll-purified and photosynthetically active chloroplasts from Arabidopsis thaliana leaves. Plant Physiol. Biochem. 2008;46:951–955. doi: 10.1016/j.plaphy.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Wulfetange K., Lomin S.N., Romanov G.A., Stolz A., Heyl A., Schmülling T. The cytokinin receptors of Arabidopsis are located mainly to the endoplasmic reticulum. Plant Physiol. 2011;156:1808–1818. doi: 10.1104/pp.111.180539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Z., Wang B., Moreno I., Dupláková N., Simon S., Carraro N., Reemmer J., Pěnčík A., Chen X., Tejos R., et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 2012;3:941. doi: 10.1038/ncomms1941. [DOI] [PubMed] [Google Scholar]
- 15.Somerville C.R., Somerville S.C., Ogren W.L. Isolation of photosynthetically active protoplasts and chloroplastids from Arabidopsis thaliana. Plant Sci. Lett. 1981;21:89–96. doi: 10.1016/0304-4211(81)90073-0. [DOI] [Google Scholar]
- 16.Keech O., Dizengremel P., Gardeström P. Preparation of leaf mitochondria from Arabidopsis thaliana. Physiol. Plant. 2005;124:403–409. doi: 10.1111/j.1399-3054.2005.00521.x. [DOI] [Google Scholar]
- 17.Parsons H.T., Christiansen K., Knierim B., Carroll A., Ito J., Batth T.S., Smith-Moritz A.M., Morrison S., McInerney P., Hadi M.Z., et al. Isolation and Proteomic Characterization of the Arabidopsis Golgi Defines Functional and Novel Components Involved in Plant Cell Wall Biosynthesis. Plant Physiol. 2012;159:12–26. doi: 10.1104/pp.111.193151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriechbaumer V., Wang P., Hawes C., Abell B.M. Alternative splicing of the auxin biosynthesis gene YUCCA4 determines its subcellular compartmentation. Plant J. 2012;70:292–302. doi: 10.1111/j.1365-313X.2011.04866.x. [DOI] [PubMed] [Google Scholar]
- 19.Minami A., Takahashi D., Kawamura Y., Uemura M. Methods in Molecular Biology. Volume 1511. Human Press; Clifton, NJ, USA: 2017. Isolation of plasma membrane and plasma membrane microdomains; pp. 199–212. [DOI] [PubMed] [Google Scholar]
- 20.Fürtauer L., Weckwerth W., Nägele T. A Benchtop Fractionation Procedure for Subcellular Analysis of the Plant Metabolome. Front. Plant Sci. 2016;7:1912. doi: 10.3389/fpls.2016.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietz K.-J. Subcellular metabolomics: The choice of method depends on the aim of the study. J. Exp. Bot. 2017;68:5695–5698. doi: 10.1093/jxb/erx406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovská B., Jeřábková H., Chamrád I., Vrána J., Lenobel R., Uřinovská J., Šebela M., Doležel J. Proteomic analysis of barley cell nuclei purified by flow sorting. Cytogenet. Genome Res. 2014;143:78–86. doi: 10.1159/000365311. [DOI] [PubMed] [Google Scholar]
- 23.Wolf P.G., Karol K.G., Mandoli D.F., Kuehl J., Arumuganathan K., Ellis M.W., Mishler B.D., Kelch D.G., Olmstead R.G., Boore J.L. The first complete chloroplast genome sequence of a lycophyte, Huperzia lucidula (Lycopodiaceae) Gene. 2005;350:117–128. doi: 10.1016/j.gene.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Cossarizza A., Ceccarelli D., Masini A. Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp. Cell Res. 1996;222:84–94. doi: 10.1006/excr.1996.0011. [DOI] [PubMed] [Google Scholar]
- 25.Deal R.B., Henikoff S. The INTACT method for cell type–specific gene expression and chromatin profiling in Arabidopsis thaliana. Nat. Protoc. 2011;6:56–68. doi: 10.1038/nprot.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W.W., Freinkman E., Wang T., Birsoy K., Sabatini D.M. Absolute Quantification of Matrix Metabolites reveals the dynamics of mitochondrial metabolism. Cell. 2016;166:1324–1337. doi: 10.1016/j.cell.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandberg G., Gardeström P., Sitbon F., Olsson O. Presence of indole-3-acetic acid in chloroplasts of Nicotiana tabacum and Pinus sylvestris. Planta. 1990;180:562–568. doi: 10.1007/BF02411455. [DOI] [PubMed] [Google Scholar]
- 28.Benková E., Witters E., Van Dongen W., Kolář J., Motyka V., Brzobohatý B., Van Onckelen H.A., Macháčková I. Cytokinins in tobacco and wheat chloroplasts. Occurrence and changes due to light/dark treatment. Plant Physiol. 1999;121:245–252. doi: 10.1104/pp.121.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polanská L., Vičánková A., Nováková M., Malbeck J., Dobrev P.I., Brzobohatý B., Vaňková R., Macháčková I. Altered cytokinin metabolism affects cytokinin, auxin, and abscisic acid contents in leaves and chloroplasts, and chloroplast ultrastructure in transgenic tobacco. J. Exp. Bot. 2007;58:637–649. doi: 10.1093/jxb/erl235. [DOI] [PubMed] [Google Scholar]
- 30.Ranocha P., Dima O., Nagy R., Felten J., Corratgé-Faillie C., Novák O., Morreel K., Lacombe B., Martinez Y., Pfrunder S., et al. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 2013;4:2625. doi: 10.1038/ncomms3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiskrová E., Novák O., Pospíšilová H., Holubová K., Karády M., Galuszka P., Robert S., Frébort I. Extra- and intracellular distribution of cytokinins in the leaves of monocots and dicots. New Biotechnol. 2016;33:735–742. doi: 10.1016/j.nbt.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Svačinová J., Novák O., Plačková L., Lenobel R., Holík J., Strnad M., Doležal K. A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: Pipette tip solid-phase extraction. Plant Methods. 2012;8:17. doi: 10.1186/1746-4811-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pěnčík A., Simonovik B., Petersson S.V., Henyková E., Simon S., Greenham K., Zhang Y., Kowalczyk M., Estelle M., Zažímalová E., et al. Regulation of auxin homeostasis and gradients in Arabidopsis roots through the formation of the indole-3-acetic acid catabolite 2-oxindole-3-acetic acid. Plant Cell. 2013;25:3858–3870. doi: 10.1105/tpc.113.114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novák O., Hényková E., Sairanen I., Kowalczyk M., Pospíšil T., Ljung K. Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. Plant J. 2012;72:523–536. doi: 10.1111/j.1365-313X.2012.05085.x. [DOI] [PubMed] [Google Scholar]
- 35.Korasick D.A., Enders T.A., Strader L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013;64:2541–2555. doi: 10.1093/jxb/ert080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grones P., Friml J. Auxin transporters and binding proteins at a glance. J. Cell Sci. 2015;128:1–7. doi: 10.1242/jcs.159418. [DOI] [PubMed] [Google Scholar]
- 37.Strader L.C., Zhao Y. Auxin perception and downstream events. Curr. Opin. Plant Biol. 2016;33:8–14. doi: 10.1016/j.pbi.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganguly A., Sasayama D., Cho H.-T. Regulation of the polarity of protein trafficking by phosphorylation. Mol. Cells. 2012;33:423–430. doi: 10.1007/s10059-012-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friml J. Auxin transport—Shaping the plant. Curr. Opin. Plant Biol. 2003;6:7–12. doi: 10.1016/S1369526602000031. [DOI] [PubMed] [Google Scholar]
- 40.Ljung K. Auxin metabolism and homeostasis during plant development. Development. 2013;140:943–950. doi: 10.1242/dev.086363. [DOI] [PubMed] [Google Scholar]
- 41.Zolman B.K., Martinez N., Millius A., Adham A.R., Bartel B. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics. 2008;180:237–251. doi: 10.1534/genetics.108.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X., Hegeman A.D., Gardner G., Cohen J.D. Protocol: High-throughput and quantitative assays of auxin and auxin precursors from minute tissue samples. Plant Methods. 2012;8:31. doi: 10.1186/1746-4811-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S., Sundaram S., Armitage L., Evans J.P., Hawkes T., Kepinski S., Ferro N., Napier R. Defining binding efficiency and specificity of auxins for SCF(TIR1/AFB)-Aux/IAA co-receptor complex formation. ACS Chem. Biol. 2014;9:673–682. doi: 10.1021/cb400618m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzunova V.V., Quareshy M., Del Genio C.I., Napier R. Tomographic docking suggests the mechanism of auxin receptor TIR1 selectivity. Open Biol. 2016;6:160139. doi: 10.1098/rsob.160139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frick E.M., Strader L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018;69:169–177. doi: 10.1093/jxb/erx298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodward A.W., Bartel B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mashiguchi K., Tanaka K., Sakai T., Sugawara S., Kawaide H., Natsume M., Hanada A., Yaeno T., Shirasu K., Yao H., et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 49.Nonhebel H.M. Tryptophan-independent indole-3-acetic acid synthesis: Critical evaluation of the evidence. Plant Physiol. 2015;169:1001–1005. doi: 10.1104/pp.15.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B., Chu J., Yu T., Xu Q., Sun X., Yuan J., Xiong G., Wang G., Wang Y., Li J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2015;112:4821–4826. doi: 10.1073/pnas.1503998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y., Christensen S.K., Fankhauser C., Cashman J.R., Cohen J.D., Weigel D., Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 52.Stepanova A.N., Yun J., Robles L.M., Novák O., He W., Guo H., Ljung K., Alonso J.M. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell. 2011;23:3961–3973. doi: 10.1105/tpc.111.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kriechbaumer V., Seo H., Park W.J., Hawes C. Endoplasmic reticulum localization and activity of maize auxin biosynthetic enzymes. J. Exp. Bot. 2015;66:6009–6020. doi: 10.1093/jxb/erv314. [DOI] [PubMed] [Google Scholar]
- 54.Hull A.K., Vij R., Celenza J.L. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA. 2000;97:2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y., Hull A.K., Gupta N.R., Goss K.A., Alonso J.M., Ecker J.R., Normanly J., Chory J., Celenza J.L. Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugawara S., Hishiyama S., Jikumaru Y., Hanada A., Nishimura T., Koshiba T., Zhao Y., Kamiya Y., Kasahara H. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:5430–5435. doi: 10.1073/pnas.0811226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollmann S., Neu D., Lehmann T., Berkowitz O., Schäfer T., Weiler E.W. Subcellular localization and tissue specific expression of amidase 1 from Arabidopsis thaliana. Planta. 2006;224:1241–1253. doi: 10.1007/s00425-006-0304-2. [DOI] [PubMed] [Google Scholar]
- 58.Nemoto K., Hara M., Suzuki M., Seki H., Muranaka T., Mano Y. The NtAMI1 gene functions in cell division of tobacco BY-2 cells in the presence of indole-3-acetamide. FEBS Lett. 2009;583:487–492. doi: 10.1016/j.febslet.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Ludwig-Müller J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011;62:1757–1773. doi: 10.1093/jxb/erq412. [DOI] [PubMed] [Google Scholar]
- 60.Mano Y., Nemoto K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012;63:2853–2872. doi: 10.1093/jxb/ers091. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J., Peer W.A. Auxin homeostasis: The DAO of catabolism. J. Exp. Bot. 2017;68:3145–3154. doi: 10.1093/jxb/erx221. [DOI] [PubMed] [Google Scholar]
- 62.Péret B., Swarup K., Ferguson A., Seth M., Yang Y., Dhondt S., James N., Casimiro I., Perry P., Syed A., et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell. 2012;24:2874–2885. doi: 10.1105/tpc.112.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrášek J., Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- 64.Verrier P.J., Bird D., Burla B., Dassa E., Forestier C., Geisler M., Klein M., Kolukisaoglu H.U., Lee Y., Martinoia E., et al. Plant ABC proteins—A unified nomenclature and updated inventory. Trends Plant Sci. 2008;13:151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Yang H., Murphy A.S. Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2009;59:179–191. doi: 10.1111/j.1365-313X.2009.03856.x. [DOI] [PubMed] [Google Scholar]
- 66.Kubeš M., Yang H., Richter G.L., Cheng Y., Młodzińska E., Wang X., Blakeslee J.J., Carraro N., Petrášek J., Zažímalová E., et al. The Arabidopsis concentration-dependent influx/efflux transporter ABCB4 regulates cellular auxin levels in the root epidermis. Plant J. 2012;69:640–654. doi: 10.1111/j.1365-313X.2011.04818.x. [DOI] [PubMed] [Google Scholar]
- 67.Barbez E., Kubeš M., Rolčík J., Béziat C., Pěnčík A., Wang B., Rosquete M.R., Zhu J., Dobrev P.I., Lee Y., et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature. 2012;485:119–122. doi: 10.1038/nature11001. [DOI] [PubMed] [Google Scholar]
- 68.Gojon A., Krouk G., Perrine-Walker F., Laugier E. Nitrate transceptor(s) in plants. J. Exp. Bot. 2011;62:2299–2308. doi: 10.1093/jxb/erq419. [DOI] [PubMed] [Google Scholar]
- 69.Corratgé-Faillie C., Lacombe B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 2017;68:3107–3113. doi: 10.1093/jxb/erw499. [DOI] [PubMed] [Google Scholar]
- 70.Powers S.K., Strader L.C. Up in the air: Untethered Factors of Auxin Response. F1000Research. 2016;5 doi: 10.12688/f1000research.7492.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin S.-H., Ma X.-M., Han P., Wang B., Sun Y.-G., Zhang G.-Z., Li Y.-J., Hou B.-K. UGT74D1 is a novel auxin glycosyltransferase from Arabidopsis thaliana. PLoS ONE. 2013;8:e61705. doi: 10.1371/annotation/457d7567-fc12-421c-9d79-880950ab10e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staswick P.E., Serban B., Rowe M., Tiryaki I., Maldonado M.T., Maldonado M.C., Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cano A., Sánchez-García A.B., Albacete A., González-Bayón R., Justamante M.S., Ibáñez S., Acosta M., Pérez-Pérez J.M. Enhanced conjugation of auxin by GH3 enzymes leads to poor adventitious rooting in carnation stem cuttings. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbez E., Kleine-Vehn J. Divide Et Impera—cellular auxin compartmentalization. Curr. Opin. Plant Biol. 2013;16:78–84. doi: 10.1016/j.pbi.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 75.LeClere S., Tellez R., Rampey R.A., Matsuda S.P.T., Bartel B. Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J. Biol. Chem. 2002;277:20446–20452. doi: 10.1074/jbc.M111955200. [DOI] [PubMed] [Google Scholar]
- 76.Okrent R.A., Brooks M.D., Wildermuth M.C. Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J. Biol. Chem. 2009;284:9742–9754. doi: 10.1074/jbc.M806662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Q., Westfall C.S., Hicks L.M., Wang S., Jez J.M. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. J. Biol. Chem. 2010;285:29780–29786. doi: 10.1074/jbc.M110.146431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kramer E.M., Ackelsberg E.M. Auxin metabolism rates and implications for plant development. Front. Plant Sci. 2015;6:150. doi: 10.3389/fpls.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Westfall C.S., Sherp A.M., Zubieta C., Alvarez S., Schraft E., Marcellin R., Ramirez L., Jez J.M. Arabidopsis thaliana GH3.5 acyl acid amido synthetase mediates metabolic crosstalk in auxin and salicylic acid homeostasis. Proc. Natl. Acad. Sci. USA. 2016;113:13917–13922. doi: 10.1073/pnas.1612635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ostin A., Kowalczyk M., Bhalerao R., Sandberg G. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 1998;118:285–296. doi: 10.1104/pp.118.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kowalczyk M., Sandberg G. Quantitative analysis of indole-3-acetic acid metabolites in Arabidopsis. Plant Physiol. 2001;127:1845–1853. doi: 10.1104/pp.010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanaka K., Hayashi K., Natsume M., Kamiya Y., Sakakibara H., Kawaide H., Kasahara H. UGT74D1 catalyzes the glucosylation of 2-oxindole-3-acetic acid in the auxin metabolic pathway in Arabidopsis. Plant Cell Physiol. 2014;55:218–228. doi: 10.1093/pcp/pct173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Porco S., Pěnčík A., Rashed A., Voß U., Casanova-Sáez R., Bishopp A., Golebiowska A., Bhosale R., Swarup R., Swarup K., et al. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2016;113:11016–11021. doi: 10.1073/pnas.1604375113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kai K., Horita J., Wakasa K., Miyagawa H. Three oxidative metabolites of indole-3-acetic acid from Arabidopsis thaliana. Phytochemistry. 2007;68:1651–1663. doi: 10.1016/j.phytochem.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Z., Zhang Y., Liu X., Zhang X., Liu S., Yu X., Ren Y., Zheng X., Zhou K., Jiang L., et al. A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev. Cell. 2013;27:113–122. doi: 10.1016/j.devcel.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., Lin J.E., Harris C., Campos Mastrotti Pereira F., Wu F., Blakeslee J.J., Peer W.A. DAO1 catalyzes temporal and tissue-specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2016;113:11010–11015. doi: 10.1073/pnas.1604769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mellor N., Band L.R., Pěnčík A., Novák O., Rashed A., Holman T., Wilson M.H., Voß U., Bishopp A., King J.R., et al. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc. Natl. Acad. Sci. USA. 2016;113:11022–11027. doi: 10.1073/pnas.1604458113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin G., Gu H., Zhao Y., Ma Z., Shi G., Yang Y., Pichersky E., Chen H., Liu M., Chen Z., et al. An Indole-3-Acetic Acid Carboxyl Methyltransferase Regulates Arabidopsis Leaf Development. Plant Cell. 2005;17:2693–2704. doi: 10.1105/tpc.105.034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abbas M., Hernández-García J., Pollmann S., Samodelov S.L., Kolb M., Friml J., Hammes U.Z., Zurbriggen M.D., Blázquez M.A., Alabadí D. Auxin methylation is required for differential growth in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2018;115:6864–6869. doi: 10.1073/pnas.1806565115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stepanova A.N., Alonso J.M. Auxin catabolism unplugged: Role of IAA oxidation in auxin homeostasis. Proc. Natl. Acad. Sci. USA. 2016;113:10742–10744. doi: 10.1073/pnas.1613506113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swarup R., Friml J., Marchant A., Ljung K., Sandberg G., Palme K., Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swarup K., Benková E., Swarup R., Casimiro I., Péret B., Yang Y., Parry G., Nielsen E., De Smet I., Vanneste S., et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- 93.Krouk G., Lacombe B., Bielach A., Perrine-Walker F., Malinska K., Mounier E., Hoyerová K., Tillard P., Leon S., Ljung K., et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Bouguyon E., Brun F., Meynard D., Kubeš M., Pervent M., Leran S., Lacombe B., Krouk G., Guiderdoni E., Zažímalová E., et al. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. plants. 2015;1:15015. doi: 10.1038/nplants.2015.15. [DOI] [PubMed] [Google Scholar]
- 95.Krouk G. Hormones and nitrate: A two-way connection. Plant Mol. Biol. 2016;91:599–606. doi: 10.1007/s11103-016-0463-x. [DOI] [PubMed] [Google Scholar]
- 96.Li R., Li J., Li S., Qin G., Novák O., Pěnčík A., Ljung K., Aoyama T., Liu J., Murphy A.S., et al. ADP1 affects plant architecture by regulating local auxin biosynthesis. PLoS Genet. 2014;10:e1003954. doi: 10.1371/journal.pgen.1003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanaka H., Dhonukshe P., Brewer P.B., Friml J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vieten A., Sauer M., Brewer P.B., Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Křeček P., Skůpa P., Libus J., Naramoto S., Tejos R., Friml J., Zažímalová E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009;10:249. doi: 10.1186/gb-2009-10-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrášek J., Mravec J., Bouchard R., Blakeslee J.J., Abas M., Seifertová D., Wisniewska J., Tadele Z., Kubeš M., Covanová M., et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 101.Wisniewska J., Xu J., Seifertová D., Brewer P.B., Růžička K., Blilou I., Rouquié D., Benková E., Scheres B., Friml J. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 102.Mravec J., Skůpa P., Bailly A., Hoyerová K., Křeček P., Bielach A., Petrášek J., Zhang J., Gaykova V., Stierhof Y.-D., et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- 103.Dal Bosco C., Dovzhenko A., Palme K. Intracellular auxin transport in pollen. Plant Signal. Behav. 2012;7:1504–1505. doi: 10.4161/psb.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bender R.L., Fekete M.L., Klinkenberg P.M., Hampton M., Bauer B., Malecha M., Lindgren K., Maki J., Perera M.A.D., Nikolau B.J., et al. PIN6 is required for nectary auxin response and short stamen development. Plant J. 2013;74:893–904. doi: 10.1111/tpj.12184. [DOI] [PubMed] [Google Scholar]
- 105.Sawchuk M.G., Edgar A., Scarpella E. Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet. 2013;9:e1003294. doi: 10.1371/journal.pgen.1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ganguly A., Lee S.H., Cho M., Lee O.R., Yoo H., Cho H.-T. Differential Auxin-Transporting Activities of PIN-FORMED Proteins in Arabidopsis Root Hair Cells. Plant Physiol. 2010;153:1046–1061. doi: 10.1104/pp.110.156505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ganguly A., Park M., Kesawat M.S., Cho H.-T. Functional Analysis of the Hydrophilic Loop in Intracellular Trafficking of Arabidopsis PIN-FORMED Proteins. Plant Cell. 2014;26:1570–1585. doi: 10.1105/tpc.113.118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simon S., Skůpa P., Viaene T., Zwiewka M., Tejos R., Klíma P., Čarná M., Rolčík J., De Rycke R., Moreno I., et al. PIN6 auxin transporter at endoplasmic reticulum and plasma membrane mediates auxin homeostasis and organogenesis in Arabidopsis. New Phytol. 2016;211:65–74. doi: 10.1111/nph.14019. [DOI] [PubMed] [Google Scholar]
- 109.Dhonukshe P., Aniento F., Hwang I., Robinson D.G., Mravec J., Stierhof Y.-D., Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 110.Kleine-Vehn J., Dhonukshe P., Sauer M., Brewer P.B., Wiśniewska J., Paciorek T., Benková E., Friml J. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr. Biol. 2008;18:526–531. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 111.Geisler M., Aryal B., di Donato M., Hao P. A Critical View on ABC Transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017;58:1601–1614. doi: 10.1093/pcp/pcx104. [DOI] [PubMed] [Google Scholar]
- 112.Dudler R., Hertig C. Structure of an mdr-like gene from Arabidopsis thaliana. Evolutionary implications. J. Biol. Chem. 1992;267:5882–5888. [PubMed] [Google Scholar]
- 113.Sidler M., Hassa P., Hasan S., Ringli C., Dudler R. Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell. 1998;10:1623–1636. doi: 10.1105/tpc.10.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geisler M., Blakeslee J.J., Bouchard R., Lee O.R., Vincenzetti V., Bandyopadhyay A., Titapiwatanakun B., Peer W.A., Bailly A., Richards E.L., et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- 115.Terasaka K., Blakeslee J.J., Titapiwatanakun B., Peer W.A., Bandyopadhyay A., Makam S.N., Lee O.R., Richards E.L., Murphy A.S., Sato F., et al. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;17:2922–2939. doi: 10.1105/tpc.105.035816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cho M., Lee S.H., Cho H.-T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2007;19:3930–3943. doi: 10.1105/tpc.107.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Noh B., Murphy A.S., Spalding E.P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.13.11.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blakeslee J.J., Bandyopadhyay A., Lee O.R., Mravec J., Titapiwatanakun B., Sauer M., Makam S.N., Cheng Y., Bouchard R., Adamec J., et al. Interactions among PIN-FORMED and P-Glycoprotein Auxin Transporters in Arabidopsis. Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bandyopadhyay A., Blakeslee J.J., Lee O.R., Mravec J., Sauer M., Titapiwatanakun B., Makam S.N., Bouchard R., Geisler M., Martinoia E., et al. Interactions of PIN and PGP auxin transport mechanisms. Biochem. Soc. Trans. 2007;35:137–141. doi: 10.1042/BST0350137. [DOI] [PubMed] [Google Scholar]
- 120.Geisler M., Kolukisaoglu H.U., Bouchard R., Billion K., Berger J., Saal B., Frangne N., Koncz-Kalman Z., Koncz C., Dudler R., et al. TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell. 2003;14:4238–4249. doi: 10.1091/mbc.e02-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bailly A., Sovero V., Vincenzetti V., Santelia D., Bartnik D., Koenig B.W., Mancuso S., Martinoia E., Geisler M. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J. Biol. Chem. 2008;283:21817–21826. doi: 10.1074/jbc.M709655200. [DOI] [PubMed] [Google Scholar]
- 122.Middleton A.M., Dal Bosco C., Chlap P., Bensch R., Harz H., Ren F., Bergmann S., Wend S., Weber W., Hayashi K.-I., et al. Data-driven modeling of intracellular auxin fluxes indicates a dominant role of the ER in controlling nuclear auxin uptake. Cell Rep. 2018;22:3044–3057. doi: 10.1016/j.celrep.2018.02.074. [DOI] [PubMed] [Google Scholar]
- 123.Miller C.O., Skoog F., Okumura F.S., Von Saltza M.H., Strong F.M. Structure and synthesis of kinetin. J. Am. Chem. Soc. 1955;77:2662–2663. doi: 10.1021/ja01614a108. [DOI] [Google Scholar]
- 124.Miller C.O., Skoog F., Von Saltza M.H., Strong F.M. Kinetin, a cell division factor from deoxyribonucleic acid. J. Am. Chem. Soc. 1955;77:1392. doi: 10.1021/ja01610a105. [DOI] [Google Scholar]
- 125.Horgan R., Hewett E.W., Purse J., Wareing P.F. A new cytokinin from Populus x robusta. Tetrahedron Lett. 1973:2827–2828. doi: 10.1016/S0040-4039(01)96062-9. [DOI] [Google Scholar]
- 126.Horgan R., Hewett E.W., Horgan J.M., Purse J., Wareing P.F. A new cytokinin from Populus x robusta. Phytochemistry. 1975;14:1005–1008. doi: 10.1016/0031-9422(75)85176-4. [DOI] [Google Scholar]
- 127.Strnad M. The aromatic cytokinins. Physiol. Plant. 1997;101:674–688. doi: 10.1111/j.1399-3054.1997.tb01052.x. [DOI] [Google Scholar]
- 128.Persson B.C., Esberg B., Olafsson O., Björk G.R. Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie. 1994;76:1152–1160. doi: 10.1016/0300-9084(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 129.Davies P.J. In: Plant Hormones: Biosynthesis, Signal Transduction, Action! 3rd ed. Davies P.J., editor. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2010. [Google Scholar]
- 130.Cortleven A., Schmülling T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015;66:4999–5013. doi: 10.1093/jxb/erv132. [DOI] [PubMed] [Google Scholar]
- 131.Armengot L., Marquès-Bueno M.M., Jaillais Y. Regulation of polar auxin transport by protein and lipid kinases. J. Exp. Bot. 2016;67:4015–4037. doi: 10.1093/jxb/erw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zürcher E., Müller B. Cytokinin Synthesis, Signaling, and Function-Advances and New Insights. Volume 324. Elsevier; Amsterdam, The Netherlands: 2016. [DOI] [PubMed] [Google Scholar]
- 133.Romanov G.A., Lomin S.N., Schmülling T. Cytokinin signaling: From the ER or from the PM? That is the question! New Phytol. 2018 doi: 10.1111/nph.14991. [DOI] [PubMed] [Google Scholar]
- 134.Kakimoto T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001;42:677–685. doi: 10.1093/pcp/pce112. [DOI] [PubMed] [Google Scholar]
- 135.Takei K., Sakakibara H., Sugiyama T. Identification of Genes Encoding Adenylate Isopentenyltransferase, a Cytokinin Biosynthesis Enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001;276:26405–26410. doi: 10.1074/jbc.M102130200. [DOI] [PubMed] [Google Scholar]
- 136.Miyawaki K., Tarkowski P., Matsumoto-Kitano M., Kato T., Sato S., Tarkowská D., Tabata S., Sandberg G., Kakimoto T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kasahara H., Takei K., Ueda N., Hishiyama S., Yamaya T., Kamiya Y., Yamaguchi S., Sakakibara H. Distinct Isoprenoid Origins of cis- and trans-Zeatin Biosyntheses in Arabidopsis. J. Biol. Chem. 2004;279:14049–14054. doi: 10.1074/jbc.M314195200. [DOI] [PubMed] [Google Scholar]
- 138.Galichet A., Hoyerová K., Kamínek M., Gruissem W. Farnesylation directs AtIPT3 subcellular localization and modulates cytokinin biosynthesis in Arabidopsis. Plant Physiol. 2008;146:1155–1164. doi: 10.1104/pp.107.107425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takei K., Yamaya T., Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyse the biosynthesis of trans-Zeatin. J. Biol. Chem. 2004;279:41866–41872. doi: 10.1074/jbc.M406337200. [DOI] [PubMed] [Google Scholar]
- 140.Kurakawa T., Ueda N., Maekawa M., Kobayashi K., Kojima M., Nagato Y., Sakakibara H., Kyozuka J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- 141.Kuroha T., Tokunaga H., Kojima M., Ueda N., Ishida T., Nagawa S., Fukuda H., Sugimoto K., Sakakibara H. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell. 2009;21:3152–3169. doi: 10.1105/tpc.109.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jin S.-H., Ma X.-M., Kojima M., Sakakibara H., Wang Y.W., Hou B.-K. Overexpression of glucosyltransferase UGT85A1 influences trans-zeatin homeostasis and trans-zeatin responses likely through O-glucosylation. Planta. 2013;237:991–999. doi: 10.1007/s00425-012-1818-4. [DOI] [PubMed] [Google Scholar]
- 143.Šmehilová M., Dobrůšková J., Novák O., Takáč T., Galuszka P. Cytokinin-Specific Glycosyltransferases Possess Different Roles in Cytokinin Homeostasis Maintenance. Front. Plant Sci. 2016;7:1264. doi: 10.3389/fpls.2016.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brzobohatý B., Moore I., Kristoffersen P., Bako L., Campos N., Schell J., Palme K. Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science. 1993;262:1051–1054. doi: 10.1126/science.8235622. [DOI] [PubMed] [Google Scholar]
- 145.Moffatt B., Pethe C., Laloue M. Metabolism of Benzyladenine is Impaired in a Mutant of Arabidopsis thaliana Lacking Adenine Phosphoribosyltransferase Activity1. Plant Physiol. 1991;95:900–908. doi: 10.1104/pp.95.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Allen M., Qin W., Moreau F., Moffatt B. Adenine phosphoribosyltransferase isoforms of Arabidopsis and their potential contributions to adenine and cytokinin metabolism. Physiol. Plant. 2002;115:56–68. doi: 10.1034/j.1399-3054.2002.1150106.x. [DOI] [PubMed] [Google Scholar]
- 147.Zhang X., Chen Y., Lin X., Hong X., Zhu Y., Li W., He W., An F., Guo H. Adenine phosphoribosyl transferase 1 is a key enzyme catalyzing cytokinin conversion from nucleobases to nucleotides in Arabidopsis. Mol. Plant. 2013;6:1661–1672. doi: 10.1093/mp/sst071. [DOI] [PubMed] [Google Scholar]
- 148.Mok D.W., Mok M.C. Cytokinin metabolism and action. Annu. Rev. Plant Physiol. 2001;52:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- 149.Pačes V., Werstiuk E., Hall R.H. Conversion of N-(Delta-Isopentenyl)adenosine to adenosine by enzyme activity in tobacco tissue. Plant Physiol. 1971;48:775–778. doi: 10.1104/pp.48.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Werner T., Köllmer I., Bartrina y Manns I., Holst K., Schmülling T. New insights into the biology of cytokinin degradation. Plant Biol. 2006;8:371–381. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- 151.Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H.A., Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schmülling T., Werner T., Riefler M., Krupková E., Bartrina y Manns I. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J. Plant Res. 2003;116:241–252. doi: 10.1007/s10265-003-0096-4. [DOI] [PubMed] [Google Scholar]
- 153.Niemann M.C.E., Weber H., Hluska T., Leonte G., Anderson S.M., Novák O., Senes A., Werner T. The cytokinin oxidase/dehydrogenase CKX1 is a membrane-bound protein requiring homooligomerization in the endoplasmic reticulum for its cellular activity. Plant Physiol. 2018 doi: 10.1104/pp.17.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Köllmer I., Novák O., Strnad M., Schmülling T., Werner T. Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. Plant J. 2014;78:359–371. doi: 10.1111/tpj.12477. [DOI] [PubMed] [Google Scholar]
- 155.Galuszka P., Popelková H., Werner T., Frébortová J., Pospíšilová H., Mik V., Köllmer I., Schmülling T., Frébort I. Biochemical Characterization of Cytokinin Oxidases/Dehydrogenases from Arabidopsis thaliana Expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007;26:255–267. doi: 10.1007/s00344-007-9008-5. [DOI] [Google Scholar]
- 156.Kowalska M., Galuszka P., Frébortová J., Šebela M., Béreš T., Hluska T., Šmehilová M., Bilyeu K.D., Frébort I. Vacuolar and cytosolic cytokinin dehydrogenases of Arabidopsis thaliana: Heterologous expression, purification and properties. Phytochemistry. 2010;71:1970–1978. doi: 10.1016/j.phytochem.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 157.Corbesier L., Prinsen E., Jacqmard A., Lejeune P., Van Onckelen H.A., Périlleux C., Bernier G. Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during floral transition. J. Exp. Bot. 2003;54:2511–2517. doi: 10.1093/jxb/erg276. [DOI] [PubMed] [Google Scholar]
- 158.Hirose N., Takei K., Kuroha T., Kamada-Nobusada T., Hayashi H., Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 159.Kudo T., Kiba T., Sakakibara H. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 2010;52:53–60. doi: 10.1111/j.1744-7909.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- 160.Osugi A., Kojima M., Takebayashi Y., Ueda N., Kiba T., Sakakibara H. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat. Plants. 2017;3:17112. doi: 10.1038/nplants.2017.112. [DOI] [PubMed] [Google Scholar]
- 161.Gillissen B., Bürkle L., André B., Kühn C., Rentsch D., Brandl B., Frommer W.B. A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell. 2000;12:291–300. doi: 10.1105/tpc.12.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wormit A., Traub M., Flörchinger M., Neuhaus H.E., Möhlmann T. Characterization of three novel members of the Arabidopsis thaliana equilibrative nucleoside transporter (ENT) family. Biochem. J. 2004;383:19–26. doi: 10.1042/BJ20040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ko D., Kang J., Kiba T., Park J., Kojima M., Do J., Kim K.Y., Kwon M., Endler A., Song W.-Y., et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. USA. 2014;111:7150–7155. doi: 10.1073/pnas.1321519111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang K., Novák O., Wei Z., Gou M., Zhang X., Yu Y., Yang H., Cai Y., Strnad M., Liu C.-J. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 2014;5:3274. doi: 10.1038/ncomms4274. [DOI] [PubMed] [Google Scholar]
- 165.Kiran N.S., Polanská L., Fohlerová R., Mazura P., Válková M., Šmeral M., Zouhar J., Malbeck J., Dobrev P.I., Macháčková I., et al. Ectopic over-expression of the maize β-glucosidase Zm-p60.1 perturbs cytokinin homeostasis in transgenic tobacco. J. Exp. Bot. 2006;57:985–996. doi: 10.1093/jxb/erj084. [DOI] [PubMed] [Google Scholar]
- 166.Bürkle L., Cedzich A., Döpke C., Stransky H., Okumoto S., Gillissen B., Kühn C., Frommer W.B. Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J. 2003;34:13–26. doi: 10.1046/j.1365-313X.2003.01700.x. [DOI] [PubMed] [Google Scholar]
- 167.Zürcher E., Liu J., di Donato M., Geisler M., Müller B. Plant development regulated by cytokinin sinks. Science. 2016;353:1027–1030. doi: 10.1126/science.aaf7254. [DOI] [PubMed] [Google Scholar]
- 168.Sun J., Hirose N., Wang X., Wen P., Xue L., Sakakibara H., Zuo J. Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in Planta. J. Integr. Plant Biol. 2005;47:588–603. doi: 10.1111/j.1744-7909.2005.00104.x. [DOI] [Google Scholar]
- 169.Lomin S.N., Myakushina Y.A., Arkhipov D. V., Leonova O.G., Popenko V.I., Schmülling T., Romanov G.A. Studies of cytokinin receptor–phosphotransmitter interaction provide evidences for the initiation of cytokinin signalling in the endoplasmic reticulum. Funct. Plant Biol. 2018;45:192. doi: 10.1071/FP16292. [DOI] [PubMed] [Google Scholar]
- 170.Kim H.J., Ryu H., Hong S.H., Woo H.R., Lim P.O., Lee I.C., Sheen J., Nam H.-G., Hwang I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:814–819. doi: 10.1073/pnas.0505150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Caesar K., Thamm A.M.K., Witthöft J., Elgass K., Huppenberger P., Grefen C., Horak J., Harter K. Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. J. Exp. Bot. 2011;62:5571–5580. doi: 10.1093/jxb/err238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hwang I., Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. 2001;413:383–389. doi: 10.1038/35096500. [DOI] [PubMed] [Google Scholar]
- 173.Punwani J.A., Hutchison C.E., Schaller G.E., Kieber J.J. The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. Plant J. 2010;62:473–482. doi: 10.1111/j.1365-313X.2010.04165.x. [DOI] [PubMed] [Google Scholar]
- 174.Mähönen A.P., Bishopp A., Higuchi M., Nieminen K.M., Kinoshita K., Törmäkangas K., Ikeda Y., Oka A., Kakimoto T., Helariutta Y. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006;311:94–98. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- 175.Suzuki T., Sakurai K., Imamura A., Nakamura A., Ueguchi C., Mizuno T. Compilation and characterization of histidine-containing phosphotransmitters implicated in His-to-Asp phosphorelay in plants: AHP signal transducers of Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2000;64:2486–2489. doi: 10.1271/bbb.64.2486. [DOI] [PubMed] [Google Scholar]
- 176.Kiba T., Taniguchi M., Imamura A., Ueguchi C., Mizuno T., Sugiyama T. Differential expression of genes for response regulators in response to cytokinins and nitrate in Arabidopsis thaliana. Plant Cell Physiol. 1999;40:767–771. doi: 10.1093/oxfordjournals.pcp.a029604. [DOI] [PubMed] [Google Scholar]
- 177.Mason M.G., Li J., Mathews D.E., Kieber J.J., Schaller G.E. Type-B response regulators display overlapping expression patterns in Arabidopsis. Plant Physiol. 2004;135:927–937. doi: 10.1104/pp.103.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.D’Agostino Ingrid B., Deruère J., Kieber J.J. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000;124:1706–1717. doi: 10.1104/pp.124.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rashotte A.M., Carson S.D.B., To J.P.C., Kieber J.J. Expression profiling of cytokinin action in Arabidopsis. Plant Physiol. 2003;132:1998–2011. doi: 10.1104/pp.103.021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.To J.P.C., Haberer G., Ferreira F.J., Deruère J., Mason M.G., Schaller G.E., Alonso J.M., Ecker J.R., Kieber J.J. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li J., Wang D. Cloning and in vitro expression of the cDNA encoding a putative nucleoside transporter from Arabidopsis thaliana. Plant Sci. 2000;157:23–32. doi: 10.1016/S0168-9452(00)00261-2. [DOI] [PubMed] [Google Scholar]
- 182.Möhlmann T., Mezher Z., Schwerdtfeger G., Neuhaus H.E. Characterisation of a concentrative type of adenosine transporter from Arabidopsis thaliana (ENT1,At) FEBS Lett. 2001;509:370–374. doi: 10.1016/S0014-5793(01)03195-7. [DOI] [PubMed] [Google Scholar]
- 183.Li G., Liu K., Baldwin S.A., Wang D. Equilibrative nucleoside transporters of Arabidopsis thaliana. cDNA cloning, expression pattern, and analysis of transport activities. J. Biol. Chem. 2003;278:35732–35742. doi: 10.1074/jbc.M304768200. [DOI] [PubMed] [Google Scholar]
- 184.Jaquinod M., Villiers F., Kieffer-Jaquinod S., Hugouvieux V., Bruley C., Garin J., Bourguignon J. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteom. 2007;6:394–412. doi: 10.1074/mcp.M600250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Petersson S.V., Johansson A.I., Kowalczyk M., Makoveychuk A., Wang J.Y., Moritz T., Grebe M., Benfey P.N., Sandberg G., Ljung K. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell. 2009;21:1659–1668. doi: 10.1105/tpc.109.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Antoniadi I., Plačková L., Simonovik B., Doležal K., Turnbull C., Ljung K., Novák O. Cell-type-specific cytokinin distribution within the Arabidopsis primary root apex. Plant Cell. 2015;27:1955–1967. doi: 10.1105/tpc.15.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hayashi K., Nakamura S., Fukunaga S., Nishimura T., Jenness M.K., Murphy A.S., Motose H., Nozaki H., Furutani M., Aoyama T. Auxin transport sites are visualized in planta using fluorescent auxin analogs. Proc. Natl. Acad. Sci. USA. 2014;111:11557–11562. doi: 10.1073/pnas.1408960111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bieleszová K., Pařízková B., Kubeš M., Husičková A., Kubala M., Ma Q., Sedlářová M., Robert S., Doležal K., Strnad M., et al. New fluorescently labeled auxins exhibit promising anti-auxin activity. New Biotechnol. 2018 doi: 10.1016/j.nbt.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 189.Kubiasová K., Mik V., Nisler J., Hönig M., Husičková A., Spíchal L., Pěkná Z., Šamajová O., Doležal K., Plíhal O., et al. Design, synthesis and perception of fluorescently labeled isoprenoid cytokinins. Phytochemistry. 2018;150:1–11. doi: 10.1016/j.phytochem.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 190.Hošek P., Kubeš M., Laňková M., Dobrev P.I., Klíma P., Kohoutová M., Petrášek J., Hoyerová K., Jiřina M., Zažímalová E. Auxin transport at cellular level: New insights supported by mathematical modelling. J. Exp. Bot. 2012;63:3815–3827. doi: 10.1093/jxb/ers074. [DOI] [PMC free article] [PubMed] [Google Scholar]