Abstract
Dietary habits of healthy offspring with a positive family history of premature coronary artery disease (P-CAD) have not been studied so far. The aim of this study was twofold: (1) to identify dietary patterns in a sample of young healthy adults with (cases) and without (controls) family history of P-CAD, and (2) to study the association between dietary patterns and family history of P-CAD. The data came from the MAGNETIC case-control study. The participants were healthy adults aged 18–35 years old, with (n = 351) and without a family history of P-CAD (n = 338). Dietary data were collected with food frequency questionnaire FFQ-6. Dietary patterns (DP) were derived using principal component analysis (PCA). The associations between the adherence to DPs and family history of P-CAD were investigated using logistic regression. Two models were created: crude and adjusted for age, sex, smoking status, place of residence, financial situation, education, and physical activity at leisure time. Three DPs were identified: ‘prudent’, ‘westernized traditional’ and ‘dairy, breakfast cereals, and treats’. In both crude and adjusted models, subjects with family history of P-CAD showed higher adherence by 31% and 25% to ‘westernized traditional’ DP (odds ratio (OR) 1.31, 95% confidence interval (95% CI): 1.12–1.53; p < 0.005; per 1 unit of standard deviation (SD) of DP score and adjOR 1.25, 95% CI: 1.06–1.48; p = 0.007; per 1 unit of SD of DP score, respectively). Young healthy adults with family history of P-CAD present unfavorable dietary patterns and are potentially a target group for CAD primary prevention programs.
Keywords: dietary patterns, family history, FFQ-6, PCA, premature coronary artery disease, P-CAD
1. Introduction
Premature coronary artery disease (P-CAD) has a multifactorial etiology and is most likely a mixture of genetic and environmental factors. The relationship between family history of P-CAD in first-degree relatives and increased risk of atherosclerosis-based diseases is well described [1,2,3,4]. A particularly robust factor affecting the offspring is myocardial infarction (MI) occurrence before the age of 55 for men and 65 for women [2]. It has been estimated that the age-adjusted odds ratio of cardiovascular events is around 2.5 greater among individuals with family history of P-CAD [1], with a further 10-fold increase if the first-degree relative was affected under the age of 45 [3]. Among patients with family history of P-CAD, a more frequent occurrence of hypertension, hypercholesterolemia, abdominal obesity, and smoking has been reported [4]. A better understanding of the genetic and environmental background of the disease could result in a substantial progress in successful screening [5]. However, this also resulted in the focus of care provision being diverted from primary care to early diagnosis and pharmacological treatment. As highlighted by Jeemon et al. [6], a shift from a ‘reactive’ to a ‘proactive’ approach in the preventative management of patients with family history of P-CAD should receive more support.
Prevention mostly relies on lifestyle modifications. Poor dietary habits are one of the critical environmental factors for CAD occurrence in general population [7]. Conversely, healthy dietary patterns—characterized by high intake of low-fat dairy products, whole grains, vegetables, and fruits—are associated with a lower risk of death due to CAD and a lower risk of non-fatal MI incidence within nearly 28 years of observation [8]. Inadequate nutrition and smoking are highly prevalent among cardiovascular patients [9] and may cluster with other unfavorable behaviors such as excessive drinking and low activity level [10], increasing the cumulative effect of multiple risks. A study among young adults with P-CAD revealed that their smoking habit was correlated with parental smoking [11]. The study, however, did not provide information regarding characteristics of the diet in affected families. We hypothesized that, in a similar manner, unhealthy dietary habits may be passed on to the offspring in families with a history of P-CAD.
To our knowledge, dietary habits of the offspring of P-CAD patients have not been yet studied. Particularly, in the context of dietary patterns (DPs) derived with the use of multidimensional statistical techniques. Since no foods nor nutrients are consumed in isolation, the proposed DPs approach appears to better reflect the complexity of ‘real life’ diet, by analyzing the associations between combinations of different foods, specific to the studied population, and health outcomes [12]. Filling this research gap can provide an essential insight into the associations between diet and familial history in this specific group of patients.
The aim of this study was twofold: (1) to identify dietary patterns in a sample of young healthy adults with (cases) and without (controls) family history of P-CAD, and (2) to study the association between dietary patterns and family history of P-CAD.
2. Materials and Methods
2.1. Study Design and Sample
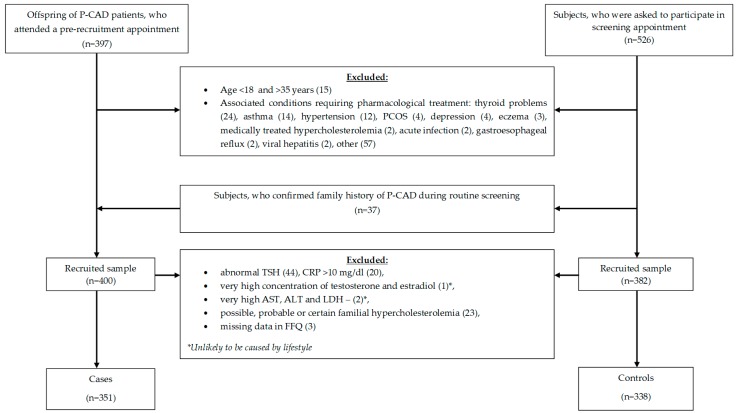
The following analysis is a part of the Metabolic and Genetic Profiling of Young Adults with and without a Family History of Premature Coronary Heart Disease (MAGNETIC) project, which is a case-control study that aims at analyzing classical and genetic risk factors of CAD in healthy young adults with and without a family history of P-CAD. Study design and methodology of the MAGNETIC project have been described previously [13]. In brief, the study sample was recruited between July 2015 and October 2017. The frequency matching method was used; gender as a matching variable. The inclusion criteria were: age ≥18 and ≤35 years old, P-CAD (myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting before the age of 55 in men, and 65 in women) in first-degree relatives (cases) or no P-CAD in first-degree relatives (control group). The exclusion criteria for both groups were: age <18 or >35 years, failure to provide informed consent, pregnancy, lactation, and acute or chronic diseases requiring pharmacotherapy. Subjects with a positive family history of P-CAD were recruited in two ways: (1) from healthy subjects aged 18–35 years, who were asked to participate in screening appointment at the Silesian Center for Heart Diseases, who provided documented proof of P-CAD history in their first-degree relatives, and (2) from the healthy offspring of the center patients, hospitalized in 2010–2017 due to P-CAD, who gave permission to be contacted once the treatment was completed. The control group was recruited from healthy subjects aged 18–35 years, who were asked to participate in screening appointment at the center, who confirmed no family history of P-CAD. In total, 689 subjects met the inclusion criteria and were included in the study. Of these, 351 (50.9%) had a parent with documented P-CAD (cases), and 338 (49.1%) subjects reported no family history of P-CAD (controls). The recruitment flow chart is presented in Figure 1.
Figure 1.
Recruitment flow-chart.
2.2. Ethical Approval
The study was conducted following the Declaration of Helsinki and good clinical practice guidelines. The study protocol has been approved by the Ethics Committee at Institute of Occupational Medicine and Environmental Health, Sosnowiec (resolution No. 03/2013). Informed, written consent was obtained from all subjects enrolled to the study.
2.3. Dietary Data Collection
Dietary data were collected with a validated 61-item food frequency questionnaire (FFQ-6) for adolescents and adults [14]. The questionnaire covered a whole diet, including a wide variety of foods consumed in Poland. A validation procedure of the questionnaire was carried out in healthy females aged 13–21 years (data not published, paper in preparation). In brief, the internal compatibility of the questionnaire was tested by 97 respondents. The questionnaire was completed by respondents twice (test and retest after two weeks). Fleiss’ Kappa for food items ranged from 0.32 to 0.72 (on average, 0.52), and there were 94% of food items with Fleiss’ Kappa above 0.4 (cut-off for acceptable compatibility for dietary data) [15]. Compatible classification of subjects (into the same category in test and retest) ranged from 51% to 89% (on average, 68%). Therefore, the FFQ-6 proved ‘acceptable’ to ‘very good’ internal compatibility and was considered as an appropriate tool to evaluate dietary habits. The wide scope of FFQ-6 application was confirmed previously by its use in a randomized controlled trial among pediatric coeliac disease patients on a gluten-free diet [16], in the study regarding genetic-specific nutritional intervention in adult patients with non-alcoholic fatty liver disease [17], and in lung and breast cancer patients [18].
In this study, the self-administered version of FFQ-6 was used. Trained researchers were handing the questionnaires to the enrolled participants providing guidance and assistance as required, on the one-to-one basis. Questionnaires were completed and returned along with signed informed consents, prior to further data collection. Food frequency consumption covering the past 12 months was collected. Respondents could select one of six categories of frequency consumption, described in a range from ‘never or very rarely’ to ‘few times a day’. For further analysis, the frequency of consumption was recalculated and expressed as times/day as follows: ‘never or very rarely’ = 0; ‘once a month or less’ = 0.025; ‘several times a month’ = 0.1; ‘several times a week’ = 0.571; ‘daily’ = 1; ‘few times a day’ = 2 [14]. Some of the food items were combined by summing their daily frequency consumption (times/day) into 26 food groups (Table 1).
Table 1.
Food groups used in dietary patterns analysis
| Food Group | Questionnaire Item(s) | Remarks |
|---|---|---|
| Sugar | Sugar | Sugar added to beverages, such as tea, coffee, etc. |
| Honey | Honey | Honey added to dishes and added to beverages |
| Sweets and snacks | Bakers’ confectionery | Biscuits, cream cakes, sponge cakes, cheesecakes, doughnuts, poppy-seed cakes, croissants etc. |
| Ice creams and custard | Ice creams and custard | |
| Chocolates | Chocolate, chocolate sweets and chocolate bars | |
| Sugar confectionery | Boiled sweets, hard caramels, jellied sweets, fudge, etc. | |
| Savoury snacks | Crisps, crackers, pretzels | |
| Milk, fermented milk drinks, and curd cheese | Milk and milk beverages—natural | Milk and natural milk beverages (yoghurt, kefir, buttermilk), porridge, etc. |
| Cheese curds | Cheese curd, natural cottage cheese, soft cheese, mozzarella, cottage cheese with herbs, etc. | |
| Sweetened milk products | Milk beverages—sweetened | Fruit yoghurts, yoghurts with chocolate flakes, flavoured buttermilk, hot chocolate, etc. |
| Flavoured cheese curds | Flavored curds (with fruit, chocolate, vanilla), etc. | |
| Cheeses | Cheese | Hard cheese, blue cheese, processed cheese, cheese spreads, etc. |
| Eggs and egg dishes | Eggs and egg dishes | Scrambled eggs, omelette, egg salad, cooked eggs |
| Breakfast cereals | Breakfast cereals | Muesli, cornflakes, other cereals—sweetened or unsweetened, etc. |
| Wholegrain products | Wholemeal cereals | Wholemeal wheat or rye bread, seeded loafs, pumpernickel, wholemeal cracker bread, etc. |
| Coarse groats | Buckwheat groats, barley, brown rice, wholemeal pasta, etc. | |
| Refined grain products | Refined cereals | White bread, rye, wheat-rye bread, toast bread, white bread rolls, brioche, bagels, etc. |
| Fine groats | Semolina, milled barley, pasta, white rice, rice flakes, etc. | |
| Animal fats | Butter | Butter |
| Cream | Single, double, sour, used as an ingredient or added to beverages | |
| Other animal fats | Lard, pork fat, etc. | |
| Red meats | Red meat | Pork, beef, veal, etc. |
| Venison | Venison | Wild boar, venison, quail, mallard, hare, etc. |
| Processed meats | Sausages, bacon, reconstituted meat | Sausages, meat loaf, hot-dogs, smoked sausages, bacon, etc. |
| High quality cured meats | Ham, poultry and pork-beef good quality cold meats, etc. | |
| Offal products | Liver, blood sausage, sweetbread, liver pate, etc. | |
| Vegetables | All kind of vegetables (cruciferous, root, yellow-orange, leafy green, tomatoes, gourds, and squashes) | Cabbages, Brussel sprouts, cauliflower, broccoli, kale, carrots, peppers, spinach, chicory, lettuce, rocket, leek, celery, parsley, tomatoes, fresh cucumber, marrow, courgettes, pumpkins, aubergines, Parsnip, beetroots, onion, garlic, celeriac, radishes, turnip, salads, mixed vegetables |
| Potatoes | Potatoes | Boiled, baked, French fries, potato rosti, gnocchi, etc. |
| Vegetable oils | Vegetable based oil | |
| Other edible fats | Margarine | Margarine for baking, frying, spreading |
| Mayonnaise | Mayonnaise and salad dressings | |
| White meat | Poultry and rabbit | |
| Fish | Lean fish | Pollock, cod, perch, hake, carp to 1 kg, tuna, panga, trout etc. |
| Oily fish | Salmon, sardines, herring, mackerel, eel, large carp etc. | |
| Fruit | All kind of fruits (stone fruits, kiwi and citrus fruits, tropical fruits, berries, bananas, apples, and pears) | Apricots, cherries, nectarines, peaches, plums, grapes, kiwi, oranges, mandarins, grapefruit, lemons, pomelos, pineapples, watermelon, melons, fresh dates and figs, strawberries, raspberries, blackberries, blueberries, redcurrants, blackcurrants, bananas, apples, pears |
| Nuts and seeds | Nuts and nut spreads | Peanuts, hazelnuts, walnuts, cashews, coconuts, chestnuts, etc. |
| Seeds and bran | Pumpkin seeds, sesame seeds, sunflower seeds, wheat germs, wheat bran, etc. | |
| Legumes | Fresh and tinned legumes | Corn, green peas, green beans, etc. |
| Dry and processed pulses | Beans (fava, butter kidney, broad, French, green), soya, peas, chickpea, and processed pulses (baked beans, hummus, other bread spreads) | |
| Juices | Fruit juices and nectars | Mixed fruit juice, orange, grapefruit, apple, pear, grape, blackcurrant, cherry juice |
| Vegetable and vegetable-fruit juices | Mixed vegetable juice, tomato, carrot and carrot-fruit juice | |
| Sweetened beverages and energy drinks | Sweetened beverages | |
| Energy drinks | ||
| Alcohol | Beer | Beer |
| Wine and cocktails | Wine and cocktails | |
| Spirits | Vodka and other spirits |
2.4. Confounding Factors
Based on the identified in the literature common risk factors of P-CAD, the following confounders were considered: age, sex, smoking status, place of residence, financial situation, level of education and physical activity at leisure time. Data on confounders was obtained with a validated questionnaire (KomPAN) using closed structured questions [19,20]. Physical activity at leisure time was categorized as low (sitting, screen time, reading, light housework, walking less than 2 h a week), moderate (walking, cycling, moderate exercise, working at home or other light physical activity performed 2–3 h/week), or high (cycling, running, working at home or other sports activities requiring physical effort over 3 h/week). Other variables were collected in three or four categories and then classified in dichotomous categories as follows: smoking (non-smoker or past smoker vs. current smoker), place of residence (city <20,000 inhabitants vs. city >20,000 inhabitants), financial situation (below average or average vs. above average, based on respondent’s declaration), and education (primary or secondary vs. higher).
2.5. Statistical Analysis
Smoking status and financial situation variables contained missing values for 2 (0.28%) and 1 (0.14%) subjects respectively. Food frequency questionnaire contained 51 missing values out of 42,029 element response matrix (0.1%). Missing values were imputed using the missForest data imputation algorithm [21], separately for clinical characteristics variables and food frequency questionnaire variables. All variables mentioned above, including the outcome variable, were entered into the multiple imputation algorithm; the missForest R–package was used [22]. The frequency of consumption of 26 food groups (times/day) was standardized so that that values had mean of 0 and standard deviation of 1. Principal component analysis (PCA) with varimax rotation, based on the correlation matrix of standardized variables, was used for dietary patterns identification and was described elsewhere in detail [23]. In brief, each component identifies a dietary pattern, that is a linear combination of questionnaire items. Components to retain were based on their interpretability and eigenvalues (>1) and a break-point identified in Scree test. The contribution of each questionnaire item to each dietary pattern is reflected by the item’s factor loading. Rotated factor loadings > |0.30| were considered to be of significant contribution to identified dietary patterns. Dietary patterns were labeled according to variables with highest loadings for each dietary pattern. For each subject, a dietary pattern score that reflects adherence to the dietary pattern was calculated (as a sum of the product of the food frequency consumption and factor loading for 26 food groups).
Unconditional logistic regression analysis was used to assess the association between adherence to identified dietary patterns and family history of P-CAD [24]. Logistic regression models were adjusted for potential confounders: age, sex, smoking status, place of residence, financial situation, education, physical activity at leisure time (as mentioned in the Section 2.4). Separate models were built for dietary pattern scores as a continuous term (per one unit of standard deviation (SD) of dietary pattern score) and for the tertile intervals calculated for each dietary pattern score. The bottom tertile of each dietary pattern score was treated as a reference. Two-sided Cochrane–Armitage test for proportions was used to assess a trend in the percentage of patients with family history of P-CAD across each dietary pattern score tertile [25,26]. p-values < 0.05 were considered to be statistically significant.
3. Results
3.1. Sample Characteristics
Compared with controls, patients with family history of P-CAD were older (average age 28.6 (standard deviation (SD) 4.6) vs. 27.3 (SD 4.2) years), more often current smokers (27.4% vs. 17.2%, respectively), and less often described their financial situation as above average (21.1% vs. 29.2%, respectively). No differences between the groups were observed regarding sex, place of residence, level of education, and physical activity at leisure time (Table 2).
Table 2.
Characteristics of the study sample.
| Variable | Total Sample | Family History of P-CAD | p | |
|---|---|---|---|---|
| With | Without | |||
| N (%) | 689 (100.0) | 351 (50.9) | 338 (49.1) | |
| Age (years) | 28.0 (SD 4.5) | 28.6 (SD 4.6) | 27.3 (SD 4.2) | 0.0001 |
| Female | 299 (43.4) | 142 (40.5) | 157 (46.4) | 0.11 |
| Current smoking (vs. past smoker or non-smoker) | 154 (22.4) | 96 (27.4) | 58(17.2) | 0.001 |
| Place of residence—city >20,000 inhabitants (vs. city <20,000) | 514 (74.6) | 252 (71.8) | 262 (77.5) | 0.08 |
| Financial situation above average (vs. average or below average) | 173 (25.1) | 74 (21.1) | 99 (29.2) | 0.01 |
| Higher education (vs. secondary or primary) | 389 (56.4) | 195 (55.6) | 194 (57.4) | 0.63 |
| Physical activity at leisure time | ||||
| Low | 171 (24.8) | 90 (25.6) | 81 (24.0) | 0.78 |
| Moderate | 328 (47.6) | 168 (47.9) | 160 (47.3) | |
| High | 190 (27.6) | 93 (26.5) | 97 (28.7) | |
Values are mean and standard deviation (SD) or N (%).
3.2. Dietary Patterns
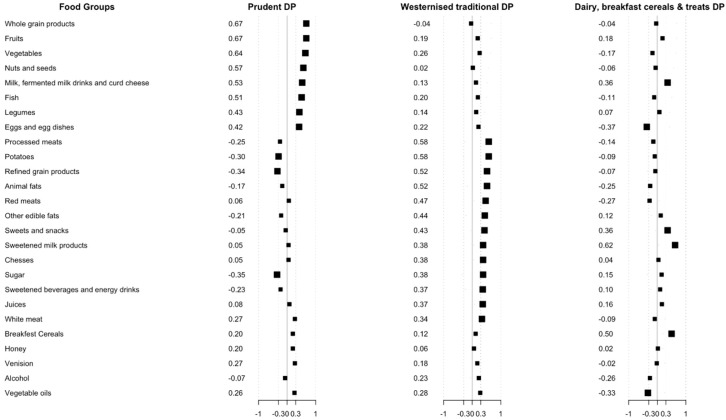
The principal component analysis led to the identification of three dietary patterns explaining 13, 12, and 6% of the variance of consumption in 26 food groups. Total explained variation of these three dietary patterns was 31%. The first dietary pattern—‘prudent’—was characterized by frequent consumption whole grain products (0.67); fruit (0.67); vegetables (0.64); nuts and seeds (0.57); milk, fermented milk drinks, and curd cheese (0.53); fish (0.0.51); legumes (0.43); eggs and egg dishes (0.42); and infrequent consumption of sugar (−0.35) and refined grain products (−0.34). The second dietary pattern, labelled ‘westernized traditional’ was characterized by frequent consumption of processed meats (0.58); potatoes (0.58); refined grain products (0.52); red meats (0.47); other edible fats (0.44); sweets and snacks (0.43); sweetened milk products (0.38); cheese (0.38); sugar (0.38); sweetened beverages and energy drinks (0.37); juices (0.37) and white meat (0.34). The third dietary pattern was based on frequent consumption of sweetened milk products (0.62); breakfast cereals (0.50); milk; fermented milk drinks, and curd cheese (0.36); sweets and snacks (0.36); and infrequent consumption of egg and egg dishes (−0.37) and vegetable oils (−0.33) and was labelled ‘dairy, breakfast cereals, and treats’ (Figure 2).
Figure 2.
Factors loadings for the three dietary patterns (DPs) identified by principal component analysis. The larger squares represent items with factor loading ≥ |0.3|.
3.3. Dietary Patterns and a Family History of P-CAD
The percentage of patients with family history of P-CAD decreased across tertiles of ‘prudent’ DP scores (test for trend p = 0.02), while an increase across tertiles of ‘westernized traditional’ DP scores (p = 0.0007) and ‘dairy, breakfast cereals, and treats’ DP scores (p = 0.02) was observed. Table 3.
Table 3.
Sample percentage (%), crude and adjusted odds ratios (ORs (95% CI)) of a family history of P-CAD, according to dietary patterns (n = 689).
| Dietary Patterns | Family History of P-CAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | |||||||
| % | p for Trend | OR | ORcrude | 95% CI | p | ORadj * | 95% CI | p | |
| ‘Prudent’ | |||||||||
| Bottom tertile (ref.) | 56.1 | 0.02 | 1.0 (ref.) | 1.0 (ref.) | ----------- | ---- | 1.0 (ref.) | -------- | ------- |
| Middle tertile | 51.3 | 1.0 | 0.82 | 0.57–1.19 | 0.30 | 0.94 | 0.64–1.39 | 0.75 | |
| Upper tertile | 45.4 | 1.0 | 0.65 | 0.45–0.94 | 0.02 | 0.72 | 0.48–1.09 | 0.11 | |
| Per 1 unit of SD of dietary pattern score | ----- | ------ | 1.0 | 0.82 | 0.70–0.95 | 0.01 | 0.85 | 0.72–1.01 | 0.07 |
| ‘Westernized traditional’ | |||||||||
| Bottom tertile (ref.) | 43.5 | 0.0007 | 1.0 | 1.0 (ref.) | ----------- | ------- | 1.0 (ref.) | ------------ | ------- |
| Middle tertile | 50.0 | 1.0 | 1.30 | 0.90–1.88 | 0.16 | 1.24 | 0.85–1.82 | 0.26 | |
| Upper tertile | 59.4 | 1.0 | 1.90 | 1.31–2.76 | 0.0007 | 1.72 | 1.16–2.57 | 0.007 | |
| Per 1 unit of SD of dietary pattern score | ----- | ------ | 1.0 | 1.31 | 1.12–1.53 | 0.0007 | 1.25 | 1.06–1.48 | 0.007 |
| ‘Dairy, breakfast cereals, and treats’ | |||||||||
| Bottom tertile (ref.) | 44.3 | 0.02 | 1.0 | 1.0 (ref.) | ------------ | -------- | 1.0 (ref.) | ----------- | ----- |
| Middle tertile | 53.5 | 1.0 | 1.44 | 1.00–2.09 | 0.05 | 1.76 | 1.20–2.61 | 0.004 | |
| Upper tertile | 55.0 | 1.0 | 1.54 | 1.06–2.21 | 0.02 | 1.75 | 1.19–2.58 | 0.005 | |
| Per 1 unit of SD of dietary pattern score | ------ | ------ | 1.0 | 1.11 | 0.95–1.29 | 0.19 | 1.16 | 0.99–1.36 | 0.07 |
* ORadj—odds ratio adjusted for age (years), sex, smoking status (never-smoker or former smoker, current smoker), place of residence (city <20,000 inhabitants, city >20,000 inhabitants), financial situation (below average or average, above average), education (primary or secondary, higher), physical activity at leisure time (low, moderate, high).
In adjusted models, subjects with family history of P-CAD showed higher adherence by 72% to the ‘westernized traditional’ DP score in the upper tertile (OR 1.72, 95% CI (1.16–2.57), p = 0.007) when compared to the bottom tertile, and higher adherence by 25% per 1 unit of SD of dietary pattern score to this DP (OR 1.25, 95% CI (1.06–1.48), p = 0.007). Subjects with family history of P-CAD showed higher adherence by 76% or 75% in the middle or the upper tertile of ‘dairy, breakfast cereals, and treats’ DP (OR 1.76, 95% CI (1.20–2.61), p = 0.004 or 1.75, 95% CI (1.19–2.58), p = 0.005, respectively) when compared to the bottom tertile. Table 3.
In the crude models, subjects with family history of P-CAD showed lower adherence by 18% per 1 unit of SD of dietary pattern score to the ‘prudent’ DP (OR 0.82, 95% CI (0.70–0.95), p = 0.01) and higher adherence by 31% per 1 unit of SD of dietary pattern score to the ‘westernized traditional’ DP (OR 1.31, 95% CI (1.12–1.53), p = 0.0007). No significant associations per 1 unit of SD of dietary pattern score were observed in terms of ’dairy, breakfast cereals, and treats’. In the adjusted model, family history of P-CAD remained to be associated only with ’westernized traditional’ DP (ORadj 1.25, 95% CI 1.06–1.48; p = 0.007; per 1 unit of SD of dietary pattern score). A borderline association per one unit of SD of dietary pattern score were observed for ‘prudent’ and ‘dairy, breakfast cereals, and treats’ DPs and family history of P-CAD (p = 0.07 for both patterns). Table 3.
4. Discussion
A family history of P-CAD is often perceived as a hereditary burden as well as a non-modifiable risk factor. The results of our study revealed for the first time that family history of P-CAD was associated with unhealthy dietary patterns, a modifiable risk factor. Young healthy adults with a family history of P-CAD were more likely to adhere to the ‘westernized traditional’ dietary pattern, in comparison to subjects without the positive family history.
The ‘westernized traditional’ and ‘prudent’ DPs are consistent with previously reported two major patterns reported in cardiovascular-related reports. The ‘westernized traditional’ pattern reflects the diet of many Poles who combine traditional staple foods (e.g., meat, potatoes, cheese) with western influences (e.g., refined grains, sweets and snacks, sweetened milk products, sugar, sweetened beverages, and energy drinks) [27,28]. The results of the nationwide project (WOBASZ), indicated that the diet of only 15% of Polish people complies with the Healthy Diet Index (HDI), and 60% presents unfavorable dietary habits (i.e., excess consumption of foods containing saturated fatty acids, and insufficient intake of foods high in folates, calcium, potassium, and magnesium) [28]. The components of the ‘westernized traditional’ pattern coincide with the characteristics reported in previous studies, in which the ‘western’ pattern was associated with an increased risk of CAD [29,30,31] and increased concentrations of CRP, insulin, C-peptide, leptin, and homocysteine [32]. Also, an increasing trend of concentration of E-selectin, sICAM-1 and sVCAM-1 across the quintiles of the ‘western’ pattern was reported, suggesting that the pathogenic mechanisms may origin in endothelial dysfunction [33]. With the global westernization, this pattern is increasingly more commonly observed in children and has been linked to increased concentrations of LDL cholesterol, triglycerides, systolic blood pressure, and fasting glucose level as early as primary and secondary school age [27,34]. The profile of this diet can be therefore interpreted as pro-atherogenic and in the long-term perspective promoting the development of coronary artery disease. Therefore, the higher adherence to this dietary pattern among young adults with family history of P-CAD is a novel but also concerning finding, that warrants further investigation.
One of the possible explanations why the offspring of P-CAD patients were more likely to adhere to the ‘westernized traditional’ pattern is parental modelling, and the continuity of acquired habits to adult life. Parents’ behavior influences child’s dietary choices and was shown to have a lasting effect, even after the transition into independent living [35,36]. As shown by Dickens and Ogden [35] parental intake of unhealthy snacks and emotional eating predicted similar habit/behavior in young adults once they left home. Moreover, dietary patterns established in childhood and adolescence proved some consistencies over time, increasing the risk of diet-related diseases in later life [36,37]. Our results indicate that adult children of P-CAD patients may have inherited not only potential genetic susceptibility to the disease but also lifelong dietary habits, which combined with the influences of western lifestyle, may have a detrimental effect to their health, potentially accelerating disease onset.
In our study, the crude models revealed that subjects with family history of P-CAD were less likely to adhere to the ‘prudent’ DP (by 18% per 1 unit of the dietary pattern score), in comparison to those subjects without a family history. Previous studies discovered, that adherence to dietary patterns, often labelled as ‘prudent’, ‘mediterranean’, or ‘healthy’, characterized by frequent intake of fruit, vegetables, whole grains, dairy, fish and healthy fats, have a well-documented protective effect on both, healthy individuals, and those at increased risk [28,31,32,33,34,38,39]. Interestingly, some studies reported that this effect is more pronounced in women than in men [40]. Perhaps, sex-influenced traits [40] or environmental factors, more common in men (e.g., smoking, alcohol drinking) [10], attenuated the strength of this relationship. This effect was partly reflected in our study. The observed association lost its significance after the adjustment for the confounders. Although P-CAD has a strong familial genetic component, the onset of the disease is thought to be multifactorial. Especially at the early stage—when atherosclerotic changes begin to occur—the pathogenic process can be modulated by environmental factors, including nutrition [41]. Therefore, establishing healthy dietary habits (as early in life as possible) appears to be a useful preventative measure to modulate the onset or severity of the disease. The effectiveness of the family-based approach that includes screening, lifestyle interventions, and professional support in CAD affected families is currently being investigated (PROLIFIC Study) [6].
The profile of ‘dairy, breakfast cereals, and treats’ DP is somewhat unique, with only few studies reporting similar results. A pattern labelled “Sweet & Dairy” (added sugar, cakes, ice-cream, coffee, eggs, butter, milk and cheese) was previously described in reports from the Italian arm of the EPIC (European Prospective Investigation into Cancer and Nutrition) study [42,43]. Also, Nobbs et al. discovered a pattern characterized by frequent consumption of breads and cereals, sweet bakery goods, and milk products [44]. However, both studies were conducted in elderly cohorts and patterns have shown only partial similarity the one described in this paper, which makes the comparison challenging. In our study, individuals with family history of P-CAD presented higher adherence to both middle and upper tertile of ‘dairy, breakfast cereals, and treats’ pattern in both, crude and adjusted model. However, the odds ratios of adherence to this pattern calculated per 1 unit of the dietary pattern score did not differ between subjects with and without a family history of P-CAD. It can be explained by no linear association between this pattern and family history. Further research could evaluate the associations between the adherence to this pattern and biomarkers of CAD in general population of young adults, regardless of their family history status.
The main limitation of this study is that data regarding dietary habits of the parents diagnosed with P-CAD were not recorded. This information would allow confirming whether unhealthy dietary habits observed in the offspring were acquired at home and sustained over time or developed in young adulthood. However, when the study was being conducted, parents affected by P-CAD were already after a first cardiovascular event. Data collected at that time would not reflect their habitual diet prior to the onset of the disease. As research shows, a diagnosis is often a trigger for dietary and lifestyle changes [45]. Secondly, the cases had less favorable financial situation, were older and more often smokers than the controls. However, these factors were addressed in the adjusted model, where they served as confounding factors. Higher prevalence of smokers among cases can create a concern as to whether cases were less health-orientated than controls. Nonetheless, previous studies showed that patients with P-CAD are more often smokers and have less favorable dietary patterns, than patients without P-CAD [6,9,11,29,30,31]. Therefore, higher prevalence of smokers among cases may be caused by parental modelling and parenting behavior effects on offspring, rather than control group selection [35,36,37,46]. The strengths include a novel approach in investigating the associations between hereditary and environmental risks factors, and taking into account entire diet (food-based dietary patterns), rather than particular nutrients. This holistic approach allows us to form food-based recommendations fundamental to health education programs and prevention of diet-related diseases. Explained variation of dietary patterns (31%) was more than satisfactory, considering that the extend found in previous studies ranged between 13% to 30% [47]. Other assets of the study include a well-matched control group, homogenous offspring group, and verification of family history with angiographic documentation rather than self-reported data.
5. Conclusions
Young healthy adults with family history of P-CAD had less favorable dietary patterns than those with negative family history. The unhealthy habits might have been established while living in the family home and tracked into the adult life, increasing already-existing familial cardiovascular risks. Primary care interventions should focus on stressing the importance of healthy diet among adults with potential hereditary susceptibility, as well as educating CAD patients about the influence of their own diet on their offspring health status.
Author Contributions
T.O., N.P., K.O., J.K.S., L.P., and M.G. (Mariusz Gąsior), made substantial contributions to the design of the study. T.O., N.P., K.O., K.B., M.F., R.R., M.G. (Marcin Gawlita), M.G. (Marta Góral), and M.G. (Marek Gierlotka), were involved in the data acquisition. T.O. and L.W. analyzed and contributed to the interpretation of the data. T.O., L.W., M.L. (Marta Lonnie) and M.L. (Mateusz Lejawa). interpreted the data and wrote the manuscript. All authors were involved in critically revising the manuscript, and have given their approval to the manuscript submitted.
Funding
The study is financed by the National Science Center (2014/13/B/NZ5/03166, OPUS 7).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Lloyd-Jones D.M., Nam B.H., D’Agostino R.B., Levy D., Murabito J.M., Wang T.J., Wilson P.W.F., O’Donnell C.J. Parental Cardiovascular Disease as a Risk Factor for Cardiovascular Disease in Middle-aged Adults. JAMA. 2004;291:2204. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 2.Sivapalaratnam S., Boekholdt S.M., Trip M.D., Sandhu M.S., Luben R., Kastelein J.J., Wareham N.J., Khaw K.T. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96:1985–1989. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulders T.A., Sivapalaratnam S., Stroes E.S.G., Kastelein J.J., Guerci A.D., Pinto-Sietsma S.J. Asymptomatic Individuals With a Positive Family History for Premature Coronary Artery Disease and Elevated Coronary Calcium Scores Benefit From Statin Treatment: A Post Hoc Analysis From the St. Francis Heart Study. JACC Cardiovasc. Imaging. 2012;5:252–260. doi: 10.1016/j.jcmg.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Hurrell C., Wietlisbach V., Jotterand V., Volet M., Lenain V., Nicod P., Darioli R., Paccaud F., Waeber G., Mooser V. High prevalence of major cardiovascular risk factors in first-degree relatives of individuals with familial premature coronary artery disease—The GENECARD project. Atherosclerosis. 2007;194:253–264. doi: 10.1016/j.atherosclerosis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Superko H.R., Roberts R., Agatston A., Frohwein S., Reingold J.S., White T.J., Sninsky J.J., Margolis B., Momary K.M., Garrett B.C., et al. Genetic Testing for Early Detection of Individuals at Risk of Coronary Heart Disease and Monitoring Response to Therapy: Challenges and Promises. Curr. Atheroscler. Rep. 2011;13:396–404. doi: 10.1007/s11883-011-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeemon P., Harikrishnan S., Sanjay G., Sivasubramonian S., Lekha T.R., Padmanabhan S., Tandon N., Prabhakaran D. A PROgramme of Lifestyle Intervention in Families for Cardiovascular risk reduction (PROLIFIC Study): Design and rationale of a family based randomized controlled trial in individuals with family history of premature coronary heart disease. BMC Public Health. 2017;17:10. doi: 10.1186/s12889-016-3928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelfriet P., Hoekstra J., Hoogenveen R., Büchner F., van Rossum C., Verschuren M. Food and vessels: The importance of a healthy diet to prevent cardiovascular disease. Eur. J. Cardiovasc. Prev. Rehabil. 2010;17:50–55. doi: 10.1097/HJR.0b013e32832f3a76. [DOI] [PubMed] [Google Scholar]
- 8.Fung T.T., Pan A., Hou T., Rexrode K.M., Willett W.C., Hu F.B. Food quality score and the risk of coronary artery disease: A prospective analysis in 3 cohorts. Am. J. Clin. Nutr. 2016;104:65–72. doi: 10.3945/ajcn.116.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaram A.A., Shah S. Risk factors, clinical features, angiographic characteristics and treatment outcomes of young myocardial infarction patients. JICC. 2015;5:203–208. doi: 10.1016/j.jicc.2015.05.002. [DOI] [Google Scholar]
- 10.Noble N., Paul C., Turon H., Oldmeadow C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity (‘SNAP’) health risk factors. Prev. Med. 2015;81:16–41. doi: 10.1016/j.ypmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Mansur Ade P., Mattar A.P., Rolim A.L., Yoshi F.R., Marin J.F., César L.A., Ramires J.A. Distribution of risk factors in parents and siblings of patients with early coronary artery disease. Arq. Bras. Cardiol. 2003;80:582–584. doi: 10.1590/S0066-782X2003000600001. [DOI] [PubMed] [Google Scholar]
- 12.United States Department of Agriculture (USDA) A Series of Systematic Reviews on the Relationship between Dietary Patterns and Health Outcome. [(accessed on 4 July 2018)]; Available online: https://www.cnpp.usda.gov/
- 13.Osadnik T., Osadnik K., Pawlas N., Strzelczyk J.K., Kasperczyk J., Gąsior M. Metabolic and genetic profiling of young adults with and without family history of premature coronary heart disease (MAGNETIC). Study design and methodology. Arch. Med. Sci. 2018 doi: 10.5114/aoms.2018.75895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadolowska L., Niedzwiedzka E., Kowalkowska J. Kwestionariusz Częstotliwości Spożycia Zywności FFQ-6 [Food Frequency Questionnaire FFQ-6] [(accessed on 4 July 2018)]; Available online: http://www.uwm.edu.pl/edu/lidiawadolowska/
- 15.Masson L.F., McNeill G., Tomany J.O., Simpson J.A., Peace H.S., Wei L., Grubb D.A., Bolton-Smith C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003;6:313–321. doi: 10.1079/PHN2002429. [DOI] [PubMed] [Google Scholar]
- 16.Drabinska N., Jarocka-Cyrta E., Markiewicz L.H., Krupa-Kozak U. The Effect of Oligofructose-Enriched Inulin on Faecal Bacterial Counts and Microbiota-Associated Characteristics in Celiac Disease Children Following a Gluten-Free Diet: Results of a Randomized, Placebo-Controlled Trial. Nutrients. 2018;10:201. doi: 10.3390/nu10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachowska E., Ryterska K., Maciejewska D., Banaszczak M., Milkiewicz P., Milkiewicz M., Gutowska I., Ossowski P., Kaczorowska M., Jamioł-Milc D., et al. Nutritional Strategies for the Individualized Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) Based on the Nutrient-Induced Insulin Output Ratio (NIOR) Int. J. Mol. Sci. 2016;17:1192. doi: 10.3390/ijms17071192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krusinska B., Hawrysz I., Wadolowska L., Slowinska M.A., Biernacki M., Czerwinska A., Golota J.J. Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients. 2018;10:470. doi: 10.3390/nu10040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jezewska-Zychowicz M., Gawecki J., Wadolowska L., Czarnocinska J., Galinski G., Kollajtis-Dolowy A., Roszkowski W., Wawrzyniak A., Przybylowicz K., Krusinska B., et al. Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data. The Committee of Human Nutrition, Polish Academy of Sciences. [(accessed on 4 July 2018)]; Available online: http://www.knozc.pan.pl/
- 20.Kowalkowska J., Wadolowska L., Czarnocinska J., Czlapka-Matyasik M., Galinski G., Jezewska-Zychowicz M., Bronkowska M., Dlugosz A., Laboda D., Wyka J. Analiza Zgodności Wewnętrznej Kwestionariusza do Badania Poglądów I Zwyczajów Żywieniowych (KomPAN) [Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN)] [(accessed on 4 July 2018)]; doi: 10.3390/nu10121845. Available online: http://www.knozc.pan.pl/ [DOI] [PMC free article] [PubMed]
- 21.Stekhoven D.J., Buhlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 22.R Foundation for Statistical Computing. R Core Team; Vienna, Austria: 2013. [(accessed on 4 July 2018)]. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ [Google Scholar]
- 23.Melaku Y.A., Gill T.K., Taylor A.W., Adams R., Shi Z. A comparison of principal component analysis, partial least-squares and reduced-rank regressions in the identification of dietary patterns associated with bone mass in ageing Australians. Eur. J. Nutr. 2017;57:1969–1983. doi: 10.1007/s00394-017-1478-z. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C.L., Duan Y., Grady J. Unconditional or Conditional Logistic Regression Model for Age-Matched Case–Control Data? Front. Public Health. 2018;6:57. doi: 10.3389/fpubh.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasior M., Gierlotka M., Pyka L., Zdrojewski T., Wojtyniak B., Chlebus K., Rozentryt P., Niedziela J., Jankowski P., Nessler J., et al. Temporal Trends in Secondary Prevention in myocardial infarction patients discharged with left ventricular dysfunction in Poland. Eur. J. Prev. Cardiol. 2018;25:960–969. doi: 10.1177/2047487318770830. [DOI] [PubMed] [Google Scholar]
- 26.Gierlotka M., Gasior M., Tajstra M., Hawranek M., Osadnik T., Wilczek K., Kalarus Z., Lekston A., Zembala M., Poloński L. Outcomes of invasive treatment in very elderly Polish patients with non-ST-segment-elevation myocardial infarction from 2003–2009 (from the PL-ACS registry) Cardiol. J. 2013;20:34–43. doi: 10.5603/CJ.2013.0007. [DOI] [PubMed] [Google Scholar]
- 27.Dlugosz A. Dietary Patterns, Adverse Health Outcomes, Socioeconomic Situation and Lifestyle of Adolescents from Less Urbanised Regions of Poland [Dissertations and Monographs] Wydawnictwo UWM; Olsztyn, Poland: 2017. [Google Scholar]
- 28.Waskiewicz A., Szczesniewska D., Szostak-Wegierek D., Kwaśniewska M., Pająk A., Stepaniak U., Kozakiewicz K., Tykarski A., Zdrojewski T., Zujko M.E., et al. Are dietary habits of the Polish population consistent with the recommendations for prevention of cardiovascular disease?—WOBASZ II project. Kardiol. Pol. 2016;74:969–977. doi: 10.5603/KP.a2016.0003. [DOI] [PubMed] [Google Scholar]
- 29.Fung T.T., Willett W.C., Stampfer M.J., Manson J.E., Hu F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001;161:1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 30.Oikonomou E., Psaltopoulou T., Georgiopoulos G.G., Siasos G., Kokkou E., Antonopoulos A., Vogiatzi G., Tsalamandris S., Gennimata V., Papanikolaou A., et al. Western Dietary Pattern is Associated with Severe Coronary Artery Disease. Angiology. 2018;69:339–346. doi: 10.1177/0003319717721603. [DOI] [PubMed] [Google Scholar]
- 31.Heidemann C., Schulze M.B., Franco O.H., van Dam R.M., Mantzoros C.S., Hu F.B. Dietary Patterns and Risk of Mortality from Cardiovascular Disease, Cancer, and All-Causes in a Prospective Cohort of Women: Heidemann-Dietary Patterns and Mortality. Circulation. 2008;118:230–237. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung T.T., Rimm E.B., Spiegelman D., Rifai N., Tofler G.H., Willet W.C., Hu F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001;73:61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Garcia E., Schulze M.B., Fung T.T., Meigs J.B., Rifai N., Manson J.E., Hu F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 34.Shang X., Li Y., Liu A., Zhang Q., Hu X., Du S., Ma J., Xu G., Li Y., Guo H., et al. Dietary Pattern and Its Association with the Prevalence of Obesity and Related Cardiometabolic Risk Factors among Chinese Children. PLoS ONE. 2012;7:e43183. doi: 10.1371/journal.pone.0043183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickens E., Ogden J. The role of parental control and modelling in predicting a child’s diet and relationship with food after they leave home. A prospective study. Appetite. 2014;76:23–29. doi: 10.1016/j.appet.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Mikkilä V., Räsänen L., Raitakari O.T., Pietinen P., Viikari J. Consistent dietary patterns identified from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr. 2005;93:923–931. doi: 10.1079/BJN20051418. [DOI] [PubMed] [Google Scholar]
- 37.Wadolowska L., Ulewicz N., Sobas K., Wuenstel J.W., Slowinska M.A., Niedzwiedzka E., Czlapka-Matyasik M. Dairy-Related Dietary Patterns, Dietary Calcium, Body Weight and Composition: A Study of Obesity in Polish Mothers and Daughters, the MODAF Project. Nutrients. 2018;10:90. doi: 10.3390/nu10010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez-González M.A., Zazpe I., Razquin C., Sánchez-Tainta A., Corella D., Salas-Salvadó J., Toledo E., Ros E., Muñoz M.Á., Recondo J., Gómez-Gracia E., et al. Empirically-derived food patterns and the risk of total mortality and cardiovascular events in the PREDIMED study. Clin. Nutr. 2015;34:859–867. doi: 10.1016/j.clnu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira A., Rodríguez-Artalejo F., Gaio G., Santos A.C., Ramos E., Lopes C. Major Habitual Dietary Patterns Are Associated with Acute Myocardial Infarction and Cardiovascular Risk Markers in a Southern European Population. J. Am. Diet. Assoc. 2011;111:241–250. doi: 10.1016/j.jada.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 40.Yang C., Wang X., Ding H. Is coronary artery disease a multifactorial inherited disorder with a sex-influenced trait? Med. Hypotheses. 2008;71:449–452. doi: 10.1016/j.mehy.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Bao W., Srinivasan S.R., Valdez R., Greenlund K.J., Wattigney W.A., Berenson G.S. Longitudinal Changes in Cardiovascular Risk from Childhood to Young Adulthood in Offspring of Parents with Coronary Artery Disease the Bogalusa Heart Study. JAMA. 1997;278:1749–1754. doi: 10.1001/jama.1997.03550210047037. [DOI] [PubMed] [Google Scholar]
- 42.Pala V., Sieri S., Masala G., Palli D., Panico S., Vineis P., Sacerdote C., Mattiello A., Galasso R., Salvini S., et al. Associations between dietary pattern and lifestyle, anthropometry and other health indicators in the elderly participants of the EPIC-Italy cohort. Nutr. Metab. Cardiovasc. Dis. 2006;16:186–201. doi: 10.1016/j.numecd.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Masala G., Ceroti M., Pala V., Krogh V., Vineis P., Sacerdote C., Saieva C., Salvini S., Sieri S., Berrino F., et al. A dietary pattern rich in olive oil and raw vegetables is associated with lower mortality in Italian elderly subjects. Br. J. Nutr. 2007;98:406–415. doi: 10.1017/S0007114507704981. [DOI] [PubMed] [Google Scholar]
- 44.Nobbs H.M., Yaxley A., Thomas J., Delaney C., Koczwara B., Luszcz M., Miller M. Do dietary patterns in older age influence the development of cancer and cardiovascular disease: A longitudinal study of ageing. Clin. Nutr. 2016;35:528–535. doi: 10.1016/j.clnu.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Murphy B.M., Worcester M.U., Elliott P.C., Le Grande M.R., Higgins R.O., Goble A.J. Change in women’s dietary fat intake following an acute cardiac event: Extent, predictors and comparison with noncardiac Australian women and older adults. Eur. J. Cardiovasc. Nurs. 2006;5:206–213. doi: 10.1016/j.ejcnurse.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 46.White H.R., Johnson V., Buyske S. Parental modeling and parenting behavior effects on offspring alcohol and cigarette use. A growth curve analysis. J. Subst. Abuse. 2000;12:287–310. doi: 10.1016/S0899-3289(00)00056-0. [DOI] [PubMed] [Google Scholar]
- 47.McCann S.E., Marshall J.R., Brasure J.R., Graham S., Freudenheim J.L. Analysis of patterns of food intake in nutritional epidemiology (Food classification in principal components analysis and the subsequent impact on estimates for endometrial cancer) Public Health Nutr. 2001;4:989–997. doi: 10.1079/PHN2001168. [DOI] [PubMed] [Google Scholar]