Abstract
OBJECTIVES
Although no longer included in the American Academy of Pediatrics guideline, ribavirin was shown to be beneficial in a subset of adult patients with severe respiratory syncytial virus (RSV)–associated bronchiolitis. This study aimed to investigate risk factors for progression to severe acute respiratory tract infections in hospitalized pediatric patients with RSV-associated bronchiolitis to identify which patients may benefit from inhaled ribavirin therapy, despite its substantial cost, diffcult administration, and potential complications.
METHODS
Patients were identified by ICD-9 codes for RSV bronchiolitis and were only included if they had a confirmed positive result for RSV via polymerase chain reaction for detection and typing of respiratory viruses. Patient characteristics, including underlying conditions and comorbidities, were analyzed for the risk of severe acute respiratory tract infection.
RESULTS
A total of 299 patients were included in the study population. Ninety-six patients (32%) were admitted to the pediatric intensive care unit, and almost half of those patients (46%) required mechanical ventilation. Weight and presence of atrial septal defect were the only factors significantly associated with the need for mechanical ventilation, as identified by univariate analysis. Two patients required extracorporeal membrane oxygenation (ECMO), and a total of 5 patients, including one who received ECMO, died with RSV infection as the primary cause. Of these patients, all were less than 1 year of age. Two had a history of prematurity; however, no variables were associated with mortality.
CONCLUSIONS
Given the side effect profile and expense of ribavirin therapy, it is prudent to limit use to patients at risk for significant morbidity and mortality from RSV disease. Because we were unable to identify patients who would most likely benefit from ribavirin antiviral therapy, we cannot recommend the routine use of ribavirin to prevent mechanical ventilation, ECMO, or death from RSV bronchiolitis in our institution.
Keywords: antiviral, neonatal, respiratory syncytial virus, ribavirin, RSV, stewardship
Introduction
Bronchiolitis is one of the leading causes of hospitalizations for infants and children in the United States.1 Respiratory syncytial virus (RSV) is the most common viral pathogen causing bronchiolitis in this age group and accounts for 50% to 80% of cases.1–4 A study conducted by Leader and Kohlase5 found that during the period ranging from 1997 to 2000, RSV bronchiolitis led to 77,700 hospital admissions annually. It also is estimated that about 5% of hospitalized patients will require mechanical ventilation.4 The majority of children who require intubation caused by RSV-associated bronchiolitis have serious underlying conditions and comorbidities, such as cardiac and central nervous system anomalies, chromosomal abnormalities, low birth weight, and immunodeficiencies.6,7
Inhaled ribavirin, a nucleoside inhibitor of ribonucleic acid (RNA) synthesis, is the only drug approved by the Food and Drug Administration (FDA) for the treatment of RSV. Recent studies8,9 in adult patients who underwent allogeneic hematopoietic stem cell transplant showed that inhaled ribavirin significantly reduced the risk of RSV-associated mortality, all-cause mortality, and viral load. However, inhaled ribavirin is no longer included in the American Academy of Pediatrics guideline for the management of RSV bronchiolitis in children.10 While this antiviral agent may improve subjective outcomes of RSV bronchiolitis, such as respiratory scores or clinical assessments, pediatric studies supporting its efficacy are limited and only include patients with severe disease (i.e., those who require mechanical ventilation) or those at risk for severe disease, such as those patients who are immunocompromised and/or those with hemodynamically significant cardiopulmonary disease. Given the positive mortality benefit in a subset of adult patients and some evidence to suggest potential benefit in pediatric patients with severe disease, there has been renewed interest in the use of inhaled ribavirin within our institution. Given its significant side effect profile, administration difficulties, and cost, it is prudent to determine which patients are most likely to benefit from this therapy before considering its use, even in patients at high risk for severe RSV disease. Responsible use of ribavirin aligns with the new medication management standards of The Joint Commission addressing antimicrobial stewardship.11 While many antimicrobial stewardship programs focus largely on the inappropriate use of antibiotics and subsequent antibiotic resistance within the hospital setting, the standards are designed to promote responsible use of all antimicrobials, including antivirals. Antiviral strategies that improve patient outcomes while minimizing toxicities must be a key component of any antimicrobial stewardship program.
In keeping with the goals of antimicrobial stewardship, this study aimed to investigate risk factors for progression to severe acute respiratory tract infections in hospitalized pediatric patients with RSV-associated bronchiolitis and to identify which patients may benefit from inhaled ribavirin therapy to prevent widespread use of this drug in patients without the risk of severe disease. Acute respiratory tract infections were considered severe if patients required mechanical ventilation or extracorporeal membrane oxygenation (ECMO) or if the RSV infection resulted in death.
Methods
Patient Population. This was a retrospective cohort study design conducted at Children's Memorial Hermann Hospital, an academic medical center in Houston, Texas. Patients younger than the age of 18 years were included if they were hospitalized for RSV-associated bronchiolitis from April 1, 2012, to April 30, 2015. Patients were identified by ICD-9 codes for RSV bronchiolitis and were only included if they had a confirmed positive result for RSV via polymerase chain reaction (PCR) for detection and typing of respiratory viruses. Patients were excluded if they were pregnant or received palivizumab for the purpose of RSV treatment. The project was approved by the institutional review boards of The University of Texas McGovern Medical School and Memorial Hermann–Texas Medical Center, Houston, Texas.
Data Collection. Patient characteristics including age, sex, weight, height, admission diagnosis, and underlying conditions or comorbidities, including prematurity, cyanotic and acyanotic congenital heart diseases, chronic lung disease, cystic fibrosis, immunodeficiencies, neurologic disorders, Down syndrome, cerebral palsy, and inborn errors of metabolism, were recorded from the medical record. Laboratory results including RSV PCR and/or nasopharyngeal viral culture obtained at admission also were collected. Outcomes data including days of hospitalization, days in the pediatric intensive care unit (PICU), requirement for supplemental oxygen via invasive and non-invasive ventilatory support, requirement for ECMO, and mortality were recorded.
Statistical Analysis. Descriptive analyses were performed by using frequency distributions or rates. Medians with a range or interquartile range (25th–75th percentiles) were used to summarize patients' baseline characteristics. Univariate analysis was performed, with the presence of intubation, ECMO, or mortality as dependent variables and the following as independent variables: sex; weight; history of prematurity; and diagnosis of chronic lung disease, immunosuppressive disorders, congenital heart defects, Down syndrome, cerebral palsy, neurologic disorders, and inborn errors of metabolism. Predictor variables with a p value < 0.05 were considered significant. Multivariate analysis for the composite outcome of interest (mechanical ventilation, ECMO, or mortality) was to be performed by fitting logistic regression models with statistically significant variables identified by univariate analysis. All statistical analyses were performed using IBM SPSS Statistics Version 22 (IBM, Armonk, NY).
Results
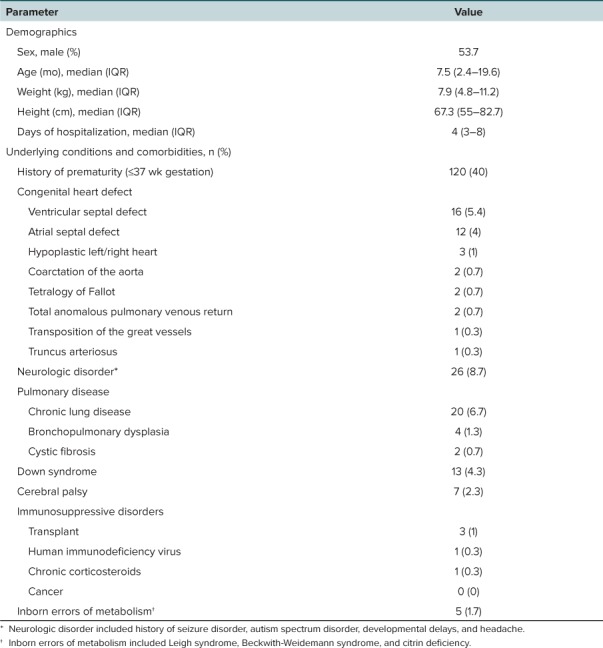
Baseline Characteristics. There were 324 patients screened for inclusion. Of those screened, 25 patients were excluded for either receiving a treatment dose of palivizumab or having a RSV diagnosis that was indeterminate. A total of 299 patients were included in the study population. Baseline characteristics of the study population are described in Table 1. All patients were under 2 years of age (median [IQR] = 7.5 months [2.4–19.6 months]), and the majority of patients were hospitalized for less than 1 week (median [IQR] = 4 days [3–8 days]). Forty percent of patients were born at =37 weeks' gestational age. Congenital heart disease was present in 13% of patients. Ventricular septal defect and atrial septal defect were the most common heart lesions (5.4% and 4%, respectively).
Table 1.
Characteristics of the Study Population (N = 299)
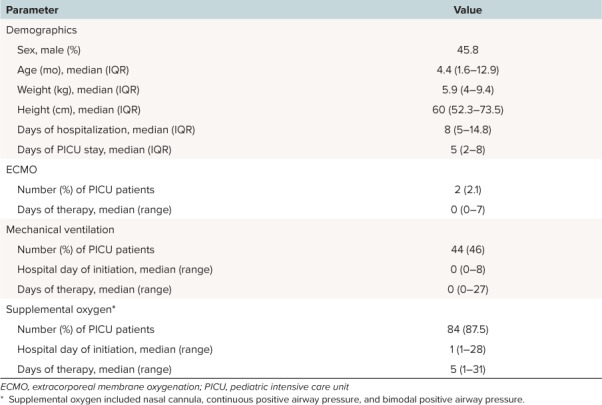
There were 96 patients (32%) admitted to the PICU (Table 2). The majority of these patients had a PICU stay of less than 1 week (median [IQR] = 5 days [2–8 days]). Almost half of the patients (46%) in the PICU required mechanical ventilation, but most patients remained intubated for less than 1 day (median [range] = 0 days [0–27 days]).
Table 2.
Characteristics of Patients Admitted to PICU (N = 96)
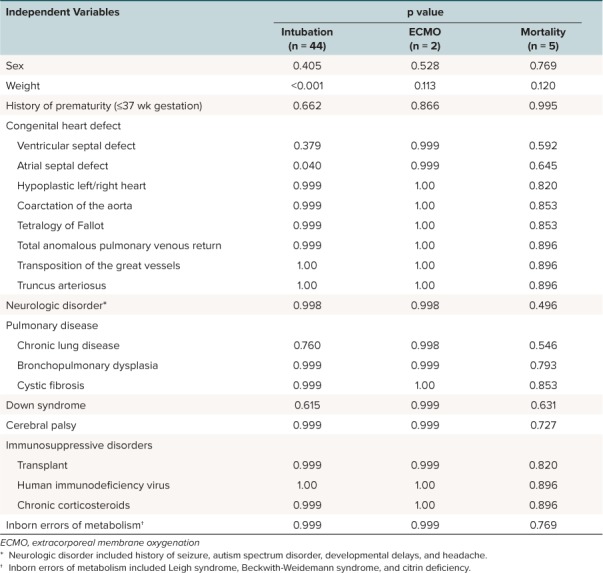
Risk Factors for Disease Progression. Forty-four patients in the study population required intubation. Weight and presence of atrial septal defect were the only factors significantly associated with the need for mechanical ventilation, as identified by univariate analysis (Table 3). Two patients required ECMO therapy during the study period. One patient who received ECMO was 17 days of age, and ECMO was initiated on day 6 of hospitalization. This patient expired on day 8 of hospitalization. No comorbid conditions were identified for this patient. The second patient who received ECMO was 159 days of age, was initiated on ECMO on hospital day 3, and was successfully withdrawn from ECMO on hospital day 7. This patient had a history of prematurity (32 weeks gestational age) and an atrial septal defect; however, these variables were not associated with the need for ECMO support (Table 3). A total of 5 patients, including one of the patients who received ECMO, died with RSV infection as the primary cause during the study period. All patients who expired were less than 1 year of age, and 2 had a history of prematurity, but these variables were not associated with mortality (Table 3). As a result of the small numbers of patients experiencing the composite outcome of interest (mechanical ventilation, ECMO, or mortality), multivariate analysis was not performed.
Table 3.
Results of Univariate Analysis for Risk Factors
Discussion
Several studies previously analyzed potential risk factors associated with morbidity and mortality in children with RSV bronchiolitis. In a study of infants with RSV bronchiolitis performed by Holman and colleagues,7 the mortality rate was highest among infants weighing <1500 g at birth. Another study published by Howard et al12 concluded that mortality rates among infants and young children with RSV were highest for those with a cardiac comorbidity and/or chronic lung disease with a history of mechanical ventilation. In 2001, Buckingham and colleagues13 examined clinical characteristics of infants admitted to the PICU with RSV infection. The authors aimed to identify risk factors that predicted adverse outcomes among this population. The study found that mechanical ventilation and prolonged length of stay were associated with congenital heart disease, chronic lung disease, upper airway abnormalities, and non-cardiac congenital malformations.
Unlike these previous studies, no patient factors that were associated with severe RSV bronchiolitis were identified. However, this study defined severe RSV disease as illness resulting in mechanical ventilation, ECMO, or death rather than PICU admission14 or hospitalization.7,12,13 This study evaluated a group of children with significant illness requiring more specific, acute, and invasive support than those described in previous reports, as the authors' goal was to identify patients who would benefit from ribavirin therapy.
Ribavirin is currently the only medication approved by the FDA for the treatment of RSV. Unfortunately, ribavirin causes several adverse effects, including bronchospasm, dyspnea, chest pain, rash, and conjunctivitis.15,16 It also is a known teratogen and is contra-indicated in pregnancy.17 A technical consideration for administering ribavirin is the need for specially trained personnel to deliver the medication via a specialized aerosolization device. Additionally, ribavirin should only be administered in a well-ventilated room with at least 6 air exchanges per hour.15 Healthcare workers may still experience adverse effects despite the use of proper technique, equipment, and ventilation. In patients who are mechanically ventilated, ribavirin can potentially deposit into the ventilator delivery system. Ultimately this may lead to malfunction or obstruction of the expiratory valve and result in inadvertently high positive end-expiratory pressures. To help combat this problem, one-way valves in the inspiratory lines, breathing circuit filters in the expiratory lines, and frequent monitoring with filter changes are recommended.15 Lastly, ribavirin is very expensive, with an average cost of approximately $150,000 for a course of treatment.15
The 2006 American Academy of Pediatrics guideline18 for the management of bronchiolitis stated that ribavirin should not be used routinely in children with bronchiolitis, as the risks outweighed any potential benefit. But the authors of the guideline also stated that ribavirin may be considered in highly selected situations, such as in patients “with severe disease or in those who are at risk for severe disease (e.g., immunocompromised and/or hemodynamically significant cardiopulmonary disease).”18 A subsequent Cochrane review19 provided further evidence against a mortality benefit with the use of ribavirin therapy. This review comprised 12 randomized trials comparing ribavirin with placebo in infants and children with lower respiratory tract infection attributable to RSV. All of the trials enrolled infants below the age of 6 months. Four trials evaluated the effect of ribavirin therapy on mortality compared with placebo. In these 4 trials, mortality was seen in 5.8% of patients who received therapy with ribavirin and in 9.7% of patients who received a placebo. This difference in mortality was not statistically significant (OR 0.37; 95% CI, 0.12 to 1.18). In addition to mortality, several other acute outcomes were evaluated, including respiratory deterioration, length of hospitalization, ventilation and oxygen dependence, severity of illness in non-ventilated patients, improvement in oxygenation, pulmonary mechanics, and side effects. Overall there were no statistically significant differences in the outcomes of interest between ribavirin and placebo, including hospital length of stay, severity of illness, and improvement in oxygenation. This review also examined long-term outcomes, including oxygenation status, pulmonary function testing, and postviral recurrent wheezing. The single study evaluating long-term oxygenation status found comparable mean values for oxygen saturation in the ribavirin and placebo groups (98% and 99%, respectively). However, this study failed to report if the oxygen saturations were achieved on supplemental oxygen or room air. Rodriguez and colleagues20 studied pulmonary function in patients 5 years postdischarge. Twelve participants completed follow-up, and no statistically significant difference was seen in the ribavirin group compared to the placebo group. Additionally, the study examined postviral recurrent wheezing in the 3 years after initial illness, and no statistically significant difference was found in the incidence of recurrent wheezing with the use of ribavirin therapy. Overall the studies included had small sample sizes ranging from 26 to 53 patients, and the quality of the studies was highly variable. Of note, the updated 2014 American Academy of Pediatrics clinical practice guideline for the diagnosis and management of bronchiolitis does not include a discussion of ribavirin therapy.10 Although ribavirin is not mentioned in the current American Academy of Pediatrics guidelines for the diagnosis and management of bronchiolitis, there is still controversy over ribavirin use. An article8 published in 2013 in the Journal of Antimicrobial Chemotherapy showed positive outcomes (decreased RSV lower respiratory tract infections and all-cause mortality) with the use of aerosolized ribavirin in patients who underwent allogeneic hematopoietic stem cell transplant. An additional article published by Boeckh and colleagues9 outlined a study in which randomized hematopoietic cell transplant recipients received aerosolized ribavirin or supportive care for 10 days. The article concluded that preemptive aerosolized ribavirin treatment decreased viral load and was a safe therapeutic option. Given that subsets of patients (adult and pediatric) may benefit from this therapy, our institution was considering the use of this agent despite current American Academy of Pediatrics guideline recommendations to treat critically ill pediatric patients who are at a high risk of death.
While our study did not find any patient factors associated with severe acute lower respiratory tract infections in hospitalized pediatric patients with RSV-associated bronchiolitis who may be eligible for ribavirin therapy, several limitations to our study must be acknowledged. The first is that this was a retrospective chart review that relied solely on information from the electronic medical records. Inconsistent documentation could result in underreporting of comorbid conditions and concomitant therapies. This also was a single-centered study and therefore only reflects the population of a single institution rather than the general community. Lastly, we were fortunate in that in our sample population we only had 44 patients who required intubation and 6 patients who experienced ECMO and/or death. Since so few patients experienced our outcomes of interest, our ability to identify clinical characteristics associated with severe RSV bronchiolitis, and consequently those patients who may have benefitted from ribavirin therapy, was limited.
Conclusions
Given the side effect profile, difficulty of administration, and expense of ribavirin, it is prudent to limit use of this therapy to only those patients at risk for significant morbidity and mortality from RSV disease. Unfortunately, patient factors associated with progression to severe RSV bronchiolitis were not identified in our study population. One of the goals of an antimicrobial stewardship program is to promote the judicious use of antivirals. Because we were unable to identify patients who would most likely benefit from ribavirin antiviral therapy, we cannot recommend the routine use of ribavirin to prevent mechanical ventilation, ECMO, or death from RSV bronchiolitis in our institution.
Acknowledgments
We wish to acknowledge David Guervil, PharmD, Memorial Hermann Hospital, for his assistance with the study concept and patient identification.
ABBREVIATIONS
- ECMO
extracorporeal membrane oxygenation
- FDA
Food and Drug Administration
- PICU
pediatric intensive care unit
- PCR
polymerase chain reaction
- RSV
respiratory syncytial virus
Footnotes
Disclosure The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Jessica Hoover has access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Hall CB, Weinger GA, Blumkin AK et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):341–348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 2.Paes BA, Mitchell I, Baerji A et al. A decade of respiratory syncytial virus epidemiology and prophylaxis: translating evidence into everyday clinical practice. Can Respir J. 2001;18(2):e10–e19. doi: 10.1155/2011/493056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaurin KK, Leader S. Growing impact of RSV hospitalizations among infants in the US, 1997–2002. Pediatric Academic Societies Annual Meeting; Washington, DC. May 15–17, 2005. Abstract 936. [Google Scholar]
- 4.Tristram DA, Welliver RC. Principles and Practice of Infectious Diseases. 2nd ed. New York, NY: Churchill Livingston; 2003. Respiratory Syncytial Virus; pp. 213–218. [Google Scholar]
- 5.Leader S, Kohlase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(suppl 5):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 6.Panickar JR, Dodd SR, Smyth RL et al. Trends in deaths from respiratory illness in England and Wales from 1968–2000. Thorax. 2005;60(12):1035–1038. doi: 10.1136/thx.2005.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holman RC, Shay DK, Curns AT et al. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;22(6):483–489. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 8.Shah DP, Ghantoji SS, Shah JN et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68(8):1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeckh M, Englund J, Li Y et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007;44(2):245–249. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 10.Ralson SL, Lieberthal AS, Meissner NC et al. Clinical practice guideline: the diagnosis, management and prevention of bronchiolitis. Pediatrics. 2014;134(5):1474–1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 11.Joint Commission on Accreditation of Healthcare Organizations New Antimicrobial Stewardship Standard. Oakbrook Terrace, IL: Joint Commission Resources; 2016. [PubMed] [Google Scholar]
- 12.Howard RS, Hoffman LH, Stang PE et al. Respiratory syncytial virus pneumonia in the hospital setting: length of stay, charges, and mortality. J Pediatr. 2000;137(2):227–232. doi: 10.1067/mpd.2000.107525. [DOI] [PubMed] [Google Scholar]
- 13.Buckingham SC, Quasney MW, Bush AJ et al. Respiratory syncytial virus outbreak in a pediatric intensive care unit: clinical characteristics and risk factors for adverse outcome. Pediatr Crit Care Med. 2001;2(4):318–323. doi: 10.1097/00130478-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child. 2009;94(2):99–103. doi: 10.1136/adc.2008.139188. [DOI] [PubMed] [Google Scholar]
- 15.Lexicomp Online®, Pediatric & Neonatal Lexi-Drugs®. Hudson, Ohio: Lexi-Comp, Inc; Jan 29, 2018. [Google Scholar]
- 16.Department of Health and Human Services; Centers for Disease Control and Prevention; National Institute for Occupational Safety and Health NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2016. https://www.cdc.gov/niosh/docs/2016-161/pdfs/2016-161.pdf?id=10.26616/NIOSHPUB2016161 Updated September 2016. Accessed July 19, 2018.
- 17.Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 10th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2015. [Google Scholar]
- 18.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 19.Ventre K, Randolph AG. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2007;1:CD000181. doi: 10.1002/14651858.CD000181.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez WJ, Arrobio J, Fink R et al. Prospective follow-up and pulmonary functions from a placebo-controlled randomized trial of ribavirin therapy in respiratory syncytial virus bronchiolitis. Arch Pediatr Adolesc Med. 1999;153(5):469–474. doi: 10.1001/archpedi.153.5.469. [DOI] [PubMed] [Google Scholar]


