Abstract
OBJECTIVES
Opioid pharmacotherapy is the cornerstone of postoperative analgesia. Despite its effectiveness, it has a variety of potential adverse effects. Therefore, a multimodal approach with non-opioid analgesics would be optimal. The aim of this study was to determine if intravenous (IV) acetaminophen would reduce opioid requirements and improve clinical outcomes in children after surgery.
METHODS
A single-center, randomized, double-blind study was conducted in 57 children (10–18 years old) undergoing posterior spine fusion surgery between July 2011 to May 2014. All subjects received either acetaminophen or placebo at the end of surgery, followed by repeated doses every 6 hours for a total of 8 doses.
RESULTS
In the first 24 postoperative hours, the average opioid consumption was lower for the active group compared with the placebo group (p = 0.02). The total unadjusted time to patient controlled analgesia (PCA) discontinuation was also longer in the placebo group than the active group (90 hours vs. 73 hours, p = 0.02); however, this was not statistically significant after normalizing for body weight. Additionally, time to first solid intake was longer without the use of acetaminophen (69 hours vs. 49 hours, p = 0.01).
CONCLUSIONS
Postoperative use of IV acetaminophen was associated with earlier time to diet advancement and discontinuation of IV analgesics and may result in lower opioid consumption.
Keywords: acetaminophen, analgesia, pediatric, postoperative pain
Introduction
Spinal fusion surgery for scoliosis can result in particularly severe postoperative pain, due to large incisions, muscle invasion, and bony manipulation. Adequate analgesia is essential for optimizing postoperative recovery, minimizing postoperative complications, promoting patient satisfaction, reducing hospital length of stay, and minimizing medical cost. Multimodal analgesic approaches with varying drug combinations have been studied to optimize postoperative pain control.1 Several studies have demonstrated improvement in analgesic efficacy with concomitant opioid and non-opioid analgesics.2,3 However, broad use of these adjuvants may be undermined by their lack of generalizable efficacy in certain patient populations and surgical procedures.
At present, opioid therapy remains the cornerstone of analgesic pharmacotherapy in the immediate postoperative period. Although effective, opioid therapy is associated with a variety of potential adverse effects, including pruritus, nausea, emesis, ileus, respiratory depression, sedation, and tolerance, with approximately 35% to 57% of pediatric intensive care unit patients experiencing opioid tolerance.4–6 Specifically in adolescents aged 12 to 17 years old, prescribing rates of opioids nearly doubled from 1994 to 2007 and over 200,000 adolescents were non-medical users of opioids, with approximately half of those having an addiction to prescription pain relievers.7,8 These adverse effects and tolerance potential may limit postoperative mobility, postpone return of bowel function, contribute to feeding intolerance, prolong hospitalization, decrease opioid efficacy for pain management, and delay recovery. Therefore, a multimodal approach using non-opioid analgesics would be optimal. Yet, a systematic review of analgesia after major spine surgery demonstrates paucity of evidence for overall benefit of non-opioids on postoperative pain control.9 Therefore, additional information is required to understand the extent that a specific non-opioid pain medication, such as acetaminophen, alleviates pain while also mitigating side effects in children undergoing different or specialized surgeries.
Acetaminophen has the potential to be an ideal opioid sparing drug in the pediatric postsurgical population due to its platelet sparing properties and excellent safety profile. It is widely used with a well-established safety profile and is recommended for first-line use in postoperative multimodal analgesic pharmacotherapy.10 Erratic absorption of oral and rectal formulations may occur in the immediate postoperative period during which time a parenteral formulation of drug is more appropriate.11–13 Intravenous (IV) acetaminophen provides rapid and predictable analgesic and antipyretic efficacy, producing earlier and higher peak serum concentrations along with a more rapid decay in serum concentrations.14 The consistent pharmacokinetics of IV acetaminophen avoids absorption lag times, erratic absorption due to postoperative nausea or inability to tolerate solids, and therefore is associated with consistent analgesic efficacy in children postoperatively. Many clinical studies have demonstrated both the analgesic efficacy and safety of IV acetaminophen, but little is known about its use in pediatric spinal fusion surgery.5,15–19 The aims of this study were to evaluate the analgesic efficacy and opioid sparing effects of IV acetaminophen in pediatric subjects following spine fusion surgery.
Materials and Methods
Intraoperative Procedures. This study was a single-center, double-blind, placebo-controlled, prospective randomized trial approved by the institutional review board at the Children's Hospital of Philadelphia (CHOP). Children (10–18 years of age) with idiopathic or neuromuscular scoliosis scheduled for posterior spine fusion surgery as inpatients at CHOP between July 2011 and May 2014 were included in this study. Exclusion criteria included any allergy to acetaminophen, hepatic dysfunction, severe neuromuscular scoliosis due to concern for postoperative requirements for mechanical ventilation, or mental impairment or developmental disability that precluded verbal assessment and responses to pain questionnaires. Consent was obtained by parents or guardians and assent obtained from minors when appropriate. This study was registered as a clinical trial (NCT01394718).
Subjects were randomized to receive 15 mg/kg IV acetaminophen or placebo (normal saline) with the first dose administered the time of skin closure intra-operatively with subsequent doses every 6 hours for 42 hours postoperatively (Figure 1). Placebo medication was indistinguishable from treatment drug in color, odor, packaging, and viscosity to ensure blinding. Subjects were block randomized in groups of 4 and assigned to either treatment or placebo, using a computer-based true random number generator system from randomization.org. Block randomization was performed and treatment group designation performed by the CHOP Investigational Pharmacy. After randomization and group assignment, subjects and group assignment were recorded in a master log and concealed from investigators, subjects, members of the research team, and clinical providers. Study treatment was drawn up in dose-specific volume in a syringe by an unblinded pharmacist, which was then dispensed to the clinical provider for administration, either anesthesiology (first dose) or nursing (subsequent doses). Aside from study treatment, clinical care teams were allowed to follow the established and successful pain management plan to decrease risk to patients.
Figure 1.
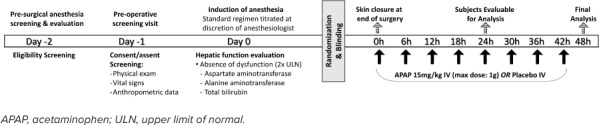
Study timeline.
Many subjects received 1 dose of IV methadone shortly after anesthesia induction. However, acetaminophen was not administered before or during surgery. During the surgery, subjects were managed with total IV anesthetic (no inhalational anesthetics) to allow for neurophysiologic monitoring per the institution's practice. At the conclusion of the surgery, all subjects received 1 dose of IV morphine at 0.05 to 0.1 mg/kg (not to exceed 0.1 mg/kg) or hydromorphone (0.01–0.02 mg/kg, not to exceed 0.02 mg/kg). A summary of intraoperative medications administered is provided in Table 1.
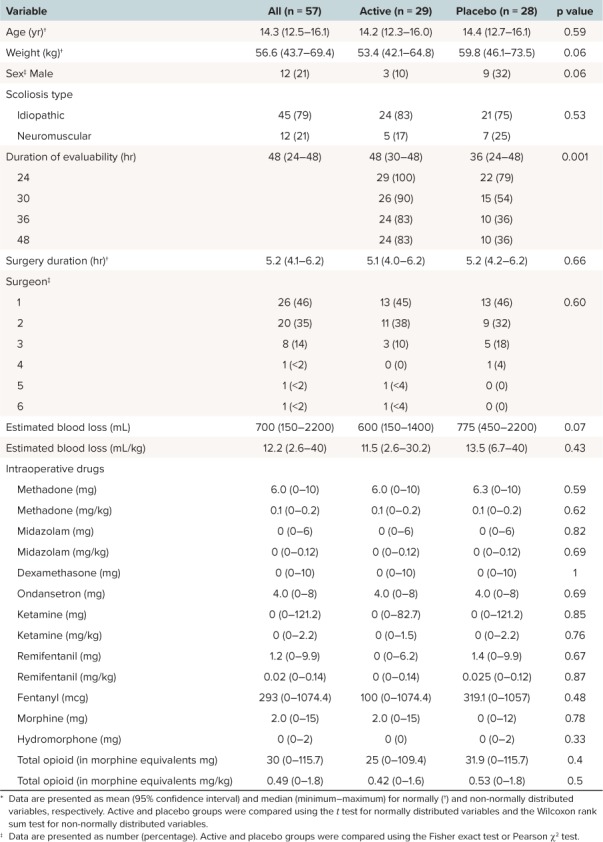
Table 1.
Summary Demographics *
Postoperative Procedures. All subjects were placed on a standardized regimen of morphine or hydromorphone postoperatively to be managed by the anesthesia pain management service, who had discretion over adjuvant pain control regimens. Pain was quantified utilizing the numeric pain rating scale, which is a numeric version of the visual analog scale where patients select a whole number from 0 to 10 that represents their pain. This was the standard of care at our institution during the study period. The established regimen generally included patient controlled analgesia (PCA) with morphine doses of 0.02 mg/kg/dose and hydromorphone doses of 0.004 mg/kg/dose for up to 5 doses per hour. Basal infusions and/or scheduled morphine or hydromorphone were not routinely ordered for subjects, however could be added to the analgesia regimen at the discretion of the pain service. Scheduled bolus opioid on the second day postoperatively could be used as per the discretion of the anesthesia pain service, but were not routinely ordered immediately postoperatively. Rescue doses of morphine (typically 0.05 mg/kg/dose) or hydromorphone (typically 0.01 mg/kg/dose) for break-through pain were ordered and administered by nursing staff as indicated for optimized analgesic effect every 2 to 3 hours as needed. Doses of morphine or hydromorphone were increased as needed at the discretion of the pain service to achieve adequate pain control. Subjects enrolled in study were not administered any additional acetaminophen for the duration of study drug administration. Ondansetron as an antiemetic and nalbuphine as an antipruritic were administered as per pain service policy to address opioid-associated adverse effects. Non-steroidal analgesic therapy (ibuprofen, ketorolac) was routinely avoided for the initial time period of the study, but added to the postoperative regimen on an “as needed' basis later in the study period. This was recorded and accounted for in the final analysis. Total morphine equivalent requirements every 24 hours for the first 48 hours and up to 4 days postoperatively were recorded.
Subject Completion/Withdrawal. Subjects were deemed evaluable if a minimum of 24 hours of data was collected postoperatively with receipt of at least 4 doses of study drug prior to study withdrawal or unblinding with time zero being time of administration of first dose of either treatment drug or placebo. Subjects who developed a fever (temperature ≥ 38.5°C) during the study were immediately unblinded and removed from study to allow the clinical team to make decisions regarding fever treatment and to allow for administration of antipyretic therapy, as needed. Subjects needed to be unblinded because knowledge regarding treatment arm was essential to treating the fever. In the event that the child was already receiving acetaminophen, alternate medications for fever reduction were administered as guided by the clinical team. Acetaminophen could be administered as an antipyretic if the subject was receiving placebo.
Data Collection and Analysis. The primary analysis is based on an intention to treat approach and included all subjects randomized and determined evaluable. Based on prior experience at our institution, we hypothesized a 25% decrease in mean opioid requirement with acetaminophen. Therefore, a sample size of 29 subjects per group (total n = 58) would have 80% power to detect this 25% difference using a 2-sample-test with at 0.05 2-sided significance level at 24 hours. Statistical analyses were conducted using S-Plus (SolutionMetrics; Sydney, Australia).
The primary efficacy endpoint was reduction in total opioid consumption in the IV acetaminophen group when compared with the placebo group during the first 24 hours of study drug administration. Morphine and hydromorphone requirements were recorded for up to 4 days postoperatively. These measurements were totaled and recorded in mg/kg/day of morphine equivalents for purposes of reporting and data analysis. Total opioid exposure is expressed as morphine equivalent, with 1 mg of morphine equal to 1 morphine equivalent and 1 mg of hydromorphone equal to 5 morphine equivalents. Total opioid exposure was recorded for each evaluable subject after 24 hours, and again at the end of the study period (EOS) for at least 48 hours. The EOS varied across subjects based on when subjects were removed from study, but was always less than 48 hours. Opioid exposure for the first 24 hours was expressed as total morphine equivalents and as morphine equivalents/kg. Due to dropouts, opioid exposure for the study duration was expressed as total morphine equivalents/hr and as morphine equivalents/kg/hr to standardize for the variability in overall study duration.
Linear regression models were performed to examine the treatment effects (acetaminophen versus placebo) on the total amount of morphine equivalents and morphine equivalents/kg for the first 24 hours, and total morphine equivalents/hr and morphine equivalents/kg/hr for the study duration with and without controlling for the administration of postoperative ketorolac and additional opioids such as fentanyl and methadone. Logarithmic transformation was applied to the morphine equivalents during the study period based on the exploratory data analyses before regression models were applied. Non-significant interaction effects of treatment groups with receiving any supplemental postoperative analgesics were not included in the models to achieve a more parsimonious model. Results from these models were expressed as regression coefficients with 95% confidence intervals and p values. Median and relative changes (exponentiated parameter estimates) were reported for log-transformed outcomes.
Secondary endpoints included opiate requirements for study duration, the average pain, nausea, and pruritus scores (0–12 hr, 0–24 hr, and 0–EOS), time to first oral liquid and solids (hours), time to first out of bed to chair, time to first mobilization with physical therapy, time to first rescue opioid, time to PCA discontinuation, and hospital length of stay. Indications for PCA discontinuation include patients tolerating oral liquids or solid foods with no nausea or vomiting and not associated with the administration and toleration of oral opioids. These secondary endpoints were compared between the IV acetaminophen and placebo groups using 2-sample t-test or Mann-Whitney U test. In addition, total antiemetic (ondansetron) and antipruritic (nalbuphine) consumption was recorded for at least 48 hours (the duration of treatment drug or placebo administration/effect). The reduction of opioid-associated adverse effects, as determined by total antiemetic and total antipruritic doses as measured in mg/kg/day were compared between IV acetaminophen and placebo groups using Mann-Whitney U tests. Adverse events ascribed to drug administration were monitored and addressed during the course of study.
Results
Overall, there was a total of 57 evaluable subjects for this study (Figure 2). The study was terminated at 57 subjects due to implementation of changes in the standardized approach to the perioperative management of these children, preventing continuation of the study protocol. Study demographics are demonstrated in Table 1. Overall, there was no statistical difference in demographics between active drug and placebo groups except for duration of evaluability. At 24 hours, all patients in the active group remained in the study compared with the placebo group, which experienced a 20% (n = 6) dropout. At the end of the study (48 hours), an additional 12 patients dropped out of the placebo group and 5 from the active group. Overall, patients in the active group remained in the study longer than those in the placebo group (p = 0.001).
Figure 2.
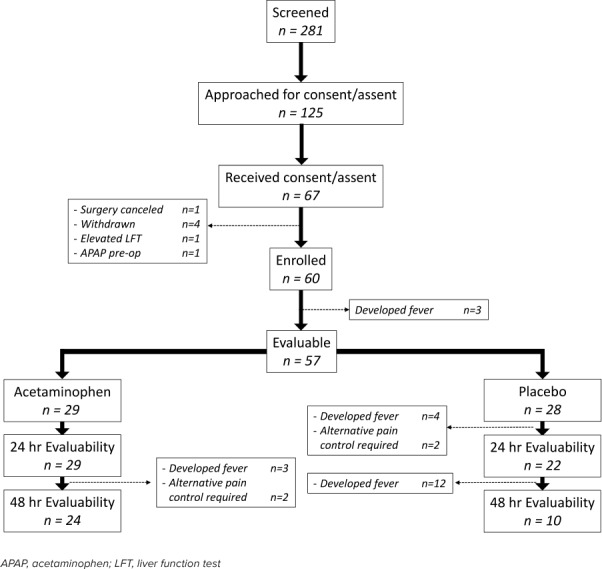
Trial randomization, enrollment, and inclusion/exclusion criteria.
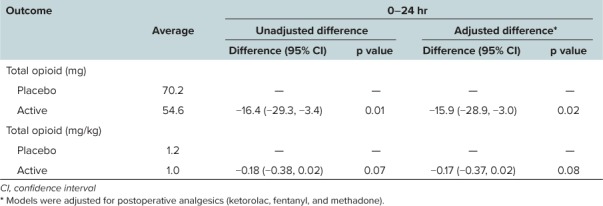
Table 2 demonstrates the differences in opioid equivalents (all routes of administration) between groups without adjusting for analgesic adjuncts, including rescue ketorolac, fentanyl, and methadone. Data for the primary outcomes until the end of study were normalized for hours due to the differences in dropout rates between the 2 arms. Table 2 also delineates the supplemental analgesia (besides morphine and hydromorphone) administered during the postoperative period. During both the initial 0- to 24-hour period and for duration of study evaluability, there were no significant differences in additional analgesics between the two groups. Table 3 presents the total opioid requirements after adjusting for all supplemental postoperative analgesics including ketorolac and fentanyl for the first 24 hours of the study. There was an approximate reduction in total opioid requirements (represented in terms of morphine equivalents) by 16 morphine equivalents (p = 0.01) and 0.17 morphine equivalents/kg (p = 0.07) in the first 24 hours.
Table 2.
Summary Statistics for the Morphine Equivalents and Postoperative Analgesics *
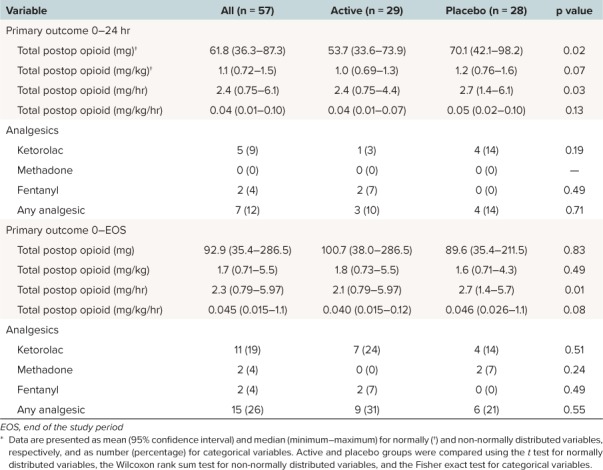
Table 3.
Comparisons of Morphine Equivalents Between the Treatment Groups
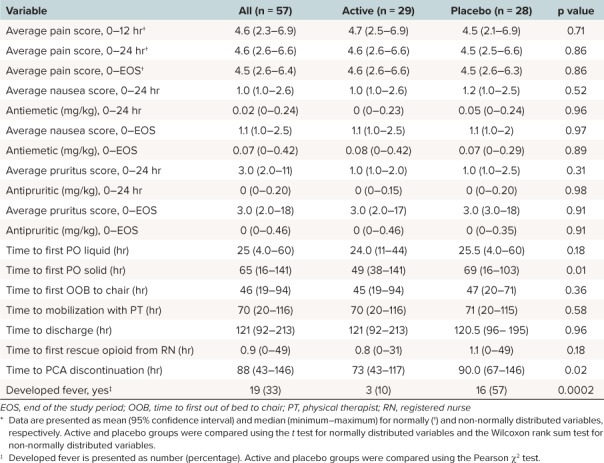
Secondary clinical course characteristics between treatment and placebo groups were comparable (Table 4). As anticipated, pain scores were similar between treatment and placebo groups as PCA allowed for self-titration of medications to achieve desired comfort. Although there was no difference between groups in time to mobilization or hospital discharge, time to first oral solids in hours was statistically significantly longer in the placebo group than the active treatment group (p = 0.012). Time to PCA discontinuation in hours was approximately 17 hours longer in the placebo group than the active treatment group (p = 0.018), resulting in earlier transition to oral analgesics in the treatment group. Nausea and pruritus scores were comparable between the 2 groups. In addition, 13 more patients developed a postoperative fever in the placebo group than in the active treatment group (p = 0.0002). No adverse effects or events were reported.
Table 4.
Summary Statistics of the Secondary Outcomes by Treatment Groups *
Discussion
The primary objective of this randomized, placebo controlled clinical trial demonstrated that multimodal treatment with IV acetaminophen for spine fusion surgery reduces the cumulative opioid dose requirement for pain management. Although the decrease in opioid requirements in morphine equivalents was not statistically significant, there was a reduction in total opioid requirements in the treatment group. This difference was large enough to show a difference in opioid related side effects, for instance the time to first solid intake. This demonstrates that a small reduction in opioid dosing can produce beneficial downstream effects. Additionally, while there is some controversy with the use of the visual analog scale in pediatric patients, it is the standard of care for assessing pain and is considered appropriate for the age of patients in our population.
Three of the secondary findings were statistically significant, including time to PCA discontinuation, time to first solid food, and the development of fever. These differences demonstrate the clinical impact of acetaminophen on patient recovery. The time to discontinuing PCA, along with the relative reduction in opioid requirements, establishes that acetaminophen adequately treats pain in the postoperative period thereby decreasing the reliance on opioids. Decreasing opioid requirements is important to the lasting impact on patient safety and opioid dependence. In addition, patients will be disconnected from IV pumps, possibly hastening their ability to ambulate freely. Furthermore, switching from IV to oral medications lowers complications from IV administration as well as lowering the cost of hospital stays, and occurs once the patient can tolerate solid foods. Finally, fevers are common during healing after surgeries. The first line of treatment for fevers is acetaminophen. Thus, administering acetaminophen allows clinicians to treat the patient for both pain control and fever prevention.
The lack of statistical significance for the remaining secondary outcomes are not unexpected. For both pain and nausea, medications were given to control these symptoms based on hospital protocols. Therefore, the lack of difference demonstrates that despite having lower opioid requirements, patients in the acetaminophen group still achieved target pain and nausea scores. In addition, mobilization was strictly regulated by established postoperative protocols that direct activity based on previous outcomes and would not differ between the 2 groups. However, the results of this study could potentially provide a basis to reassess mobilization protocols, especially if other non-opioid pain medications can be added to this regimen to further reduce the opioid requirement and reliance on PCA.
Our study findings are similar to those reported by other groups.19–27 These studies investigated the potential of IV, oral, and rectal acetaminophen as opioid-sparing pain management in both adults and children. The degree of clinically significant benefit with administration of acetaminophen, in regards to analgesic augmentation, mitigation of opioid-related side effects, and reduction in opioid consumption varies greatly across studies. This can, in part, be attributed to differences in subject population and surgical procedure, drug formulation, drug dosing, and duration of therapy. It is possible that acetaminophen may have varying analgesic efficacy in differing surgical populations. Although spine surgery patients with significant bone and muscle pain may receive greater benefit from non-steroidal anti-inflammatory drug (NSAID) therapy, other surgical populations may have greater benefit from acetaminophen therapy. The discrepancies found between these studies might be due to the central-acting mechanism of action or weaker inhibition of prostaglandins in acetaminophen versus NSAIDs. However, our study provides evidence that IV acetaminophen can be combined with opioids as part of a multimodal regimen for pain control, fever reduction, and reduced time to switch to oral intake. These findings are supported by the results of a study by Hansen et al,28 that found that IV acetaminophen is associated with lower costs, lower opioid consumption, and fewer resources used when compared with oral acetaminophen in the postoperative period. Rectal and oral routes of administration of acetaminophen were not included in this analysis due to the high doses required by erratic absorption via the rectal route and the inability to tolerate solids in the postoperative period. These routes could be investigated as part of the multimodal approach after the first 48 hours postoperatively.
Our study demonstrated some limitations. This was a single-center study with subjects confined to a single surgical population, potentially limiting the generalizability of these results. However, this study population constitutes one with high postoperative analgesic requirements. The study was limited to opioid usage in the first 48 hours following surgery, and did not evaluate analgesic requirements after discharge. Due to changes in clinical practice, introduction of routine NSAID therapy to the postoperative analgesic regimen after the first 24 postoperative hours occurred toward the end of study enrollment. Although there were only a few subjects who received ketorolac, this may have impacted study results. Within the first 24 hours, 4 subjects in the placebo group and 1 in the active group received ketorolac. This would bias the results toward the null by providing the placebo group additional long-acting analgesia, potentially minimizing their morphine requirements. Another limitation was due to the high dropout rate in the placebo group. By 48 hours, only 10 of the initial 28 subjects were still evaluable, making statistical comparisons at 48 hours unreliable. The clinical and research teams determined that subjects who were on study and developed fever should be unblinded so that they may receive acetaminophen as first-line antipyretic as administered as standard of care. The decision by the clinical team to use routine ketorolac postoperatively combined with the high dropout rate in the placebo group necessitated early termination of the study.
In conclusion, scheduled, intermittent acetaminophen may reduce cumulative total opioid consumption in the immediate postoperative period in pediatric patients who have undergone posterior spine fusion. In addition, compared with opioid-only treatment, this multimodal regimen using acetaminophen demonstrated equal analgesic efficacy, with associated facilitation of additional elements of clinical recovery, including diet advancement and transition from IV to oral opioid administration. These data support previous findings for use of multimodal analgesia with acetaminophen for perioperative pain management in children. Follow-up studies are needed to determine other opioid-sparing agents that can further reduce the cumulative opioid dose and facilitate reassessment of postoperative recovery goals. Quantification of clinically significant benefits, including reduction in opioid-related adverse effects, time to clinical recovery, postdischarge functional outcome, and patient satisfaction is likely to require much larger or multicenter studies that are sufficiently powered to detect these differences, as well as more prolonged follow-up.
Acknowledgments
All phases of this study were supported in part by Cadence/Mallinckrodt. Dr. Rizkalla, Dr. Elci, and Dr. Zuppa conceptualized and designed the study, and reviewed and revised the manuscript. Dr. Zane carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. Zuppa, Dr. Rizkalla, Ms. DiLiberto, Ms. Prodell, and Dr. Maxwell coordinated and supervised data collection, collected data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ABBREVIATIONS
- CHOP
Children's Hospital of Philadelphia
- EOS
end of the study period
- IV
intravenous
- NSAID
non-steroidal anti-inflammatory drug
- PCA
patient controlled analgesia
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all data and take responsibility for the integrity and accuracy of the data analysis.
REFERENCES
- 1.Palmer GM, Pirakalathanan P, Skinner AV. A multi-centre multi-national survey of anaesthetists regarding the range of anaesthetic and surgical practices for paediatric scoliosis surgery. Anaesth Intensive Care. 2010;38(6):1077–1084. doi: 10.1177/0310057X1003800619. [DOI] [PubMed] [Google Scholar]
- 2.Lui F, Ng KF. Adjuvant analgesics in acute pain. Expert Opin Pharmacother. 2011;12(3):363–385. doi: 10.1517/14656566.2011.521743. [DOI] [PubMed] [Google Scholar]
- 3.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22(5):588–593. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 4.Walder B, Schafer M, Henzi I et al. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand. 2001;45(7):795–804. doi: 10.1034/j.1399-6576.2001.045007795.x. [DOI] [PubMed] [Google Scholar]
- 5.Sinatra RS, Jahr JS, Reynolds LW et al. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102(4):822–831. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Anand KJ, Willson DF, Berger J et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5):e1208–e1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2016. HHS Publication SMA 16-4984, NSDUH Series H-51. [Google Scholar]
- 8.Fortuna RJ, Robbins BW, Caiola E et al. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics. 2010;126(6):1108–1116. doi: 10.1542/peds.2010-0791. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Balireddy RK, Vorenkamp KE et al. Beyond opioid patient-controlled analgesia: a systematic review of analgesia after major spine surgery. Reg Anesth Pain Med. 2012;37(1):79–98. doi: 10.1097/AAP.0b013e3182340869. [DOI] [PubMed] [Google Scholar]
- 10.Bertolini A, Ferrari A, Ottani A et al. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12(3–4):250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108(4):1020–1024. doi: 10.1542/peds.108.4.1020. [DOI] [PubMed] [Google Scholar]
- 12.Cullen S, Kenny D, Ward OC et al. Paracetamol suppositories: a comparative study. Arch Dis Child. 1989;64(10):1504–1505. doi: 10.1136/adc.64.10.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn TW, Mogensen T, Lund C et al. High-dose rectal and oral acetaminophen in postoperative patients--serum and saliva concentrations. Acta Anaesthesiol Scand. 2000;44(3):302–306. doi: 10.1034/j.1399-6576.2000.440314.x. [DOI] [PubMed] [Google Scholar]
- 14.Chidambaran V, Sadhasivam S. Pediatric acute and surgical pain management: recent advances and future perspectives. Int Anesthesiol Clin. 2012;50(4):66–82. doi: 10.1097/AIA.0b013e31826f3284. [DOI] [PubMed] [Google Scholar]
- 15.Polomano RC, Dunwoody CJ, Krenzischek DA et al. Perspective on pain management in the 21st century. J Perianesth Nurs. 2008;23(1 suppl):S4–S14. doi: 10.1016/j.jopan.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Manyike PT, Kharasch ED, Kalhorn TF et al. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther. 2000;67(3):275–282. doi: 10.1067/mcp.2000.104736. [DOI] [PubMed] [Google Scholar]
- 17.Alhashemi JA, Alotaibi QA, Mashaat MS et al. Intravenous acetaminophen vs oral ibuprofen in combination with morphine PCIA after Cesarean delivery. Can J Anaesth. 2006;53(13):1200–1206. doi: 10.1007/BF03021581. [DOI] [PubMed] [Google Scholar]
- 18.Capici F, Ingelmo PM, Davidson A et al. Randomized controlled trial of duration of analgesia following intravenous or rectal acetaminophen after adenotonsillectomy in children. Br J Anaesth. 2008;100(2):251–255. doi: 10.1093/bja/aem377. [DOI] [PubMed] [Google Scholar]
- 19.Ceelie I, de Wildt SN, van Dijk M et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309(2):149–154. doi: 10.1001/jama.2012.148050. [DOI] [PubMed] [Google Scholar]
- 20.Korpela R, Korvenoja P, Meretoja OA. Morphine-sparing effect of acetaminophen in pediatric day-case surgery. Anesthesiology. 1999;91(2):442–447. doi: 10.1097/00000542-199908000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013;23(6):475–495. doi: 10.1111/pan.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong JY, Kim WO, Koo BN et al. Fentanyl-sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent-/nurse-controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology. 2010;113(3):672–677. doi: 10.1097/ALN.0b013e3181e2c34b. [DOI] [PubMed] [Google Scholar]
- 23.Bremerich DH, Neidhart G, Heimann K et al. Prophylactically-administered rectal acetaminophen does not reduce postoperative opioid requirements in infants and small children undergoing elective cleft palate repair. Anesth Analg. 2001;92(4):907–912. doi: 10.1097/00000539-200104000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Hiller A, Helenius I, Nurmi E et al. Acetaminophen improves analgesia but does not reduce opioid requirement after major spine surgery in children and adolescents. Spine (Phila Pa 1976) 2012;37(20):E1225–E1231. doi: 10.1097/BRS.0b013e318263165c. [DOI] [PubMed] [Google Scholar]
- 25.Hong JY, Won Han S, Kim WO et al. Fentanyl sparing effects of combined ketorolac and acetaminophen for outpatient inguinal hernia repair in children. J Urol. 2010;183(4):1551–1555. doi: 10.1016/j.juro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 26.Korpela R, Silvola J, Laakso E et al. Oral naproxen but not oral paracetamol reduces the need for rescue analgesic after adenoidectomy in children. Acta Anaesthesiol Scand. 2007;51(6):726–730. doi: 10.1111/j.1399-6576.2007.01319.x. [DOI] [PubMed] [Google Scholar]
- 27.van der Marel CD, Peters JW, Bouwmeester NJ et al. Rectal acetaminophen does not reduce morphine consumption after major surgery in young infants. Br J Anaesth. 2007;98(3):372–379. doi: 10.1093/bja/ael371. [DOI] [PubMed] [Google Scholar]
- 28.Hansen RN, Pham AT, Boing EA et al. Comparative analysis of length of stay, hospitalization costs, opioid use, and discharge status among spine surgery patients with postoperative pain management including intravenous versus oral acetaminophen. Curr Med Res Opin. 2017;33(5):943–948. doi: 10.1080/03007995.2017.1297702. [DOI] [PubMed] [Google Scholar]


