Abstract
While lasers have been used for many years for the treatment of glaucoma, proper indications and use of the procedures need to be considered before their application. This review summarizes the important laser procedures in Glaucoma.
Keywords: Cyclophotocoagulation, laser iridotomy, laser iridoplasty, laser trabeculoplasty
Glaucoma is currently the leading cause of irreversible blindness the world over.[1] Lasers have made the task of treating glaucoma simpler and these procedures are being used routinely by most ophthalmologists even at a very basic level. The most common procedure for angle closure is neodymium: yttrium-aluminum-garnet (Nd:YAG) laser peripheral iridotomy (LPI). The other procedures being used currently include laser trabeculoplasty (LTP), gonioplasty/iridoplasty, diode laser cyclophotocoagulation and endocyclophotocoagulation, laser suturolysis, bleb remodeling, iridolenticular synechiolysis and Nd:YAG laser hyaloidotomy. In this review, we highlight the current status of these procedures along with an update of the technique.
Nd:YAG Laser Peripheral Iridotomy (LPI)
The Indian and East Asian population has a high incidence and prevalence of primary angle closure glaucoma (PACG) which also has a greater blinding potential when compared to open angle glaucoma.[2,3]
The most common procedure for angle closure is YAG LPI, but of late controversy surrounds the ability to diagnose cases which actually require iridotomy. The lack of precise tests which prognosticate the need for an iridotomy has resulted in clinical examination being used to decide whether an eye has to undergo an iridotomy. Since not all primary angle closure suspects (PACS) will progress to primary angle closure (PAC) or to PACG, one must be judicious in advising an iridotomy. Among PACS, only those with high risk should be considered for LPI as given below.[4]
Indications
We may choose to do prophylactic PI in the following situations:
PACS: Among this group, those who cannot come for regular follow-up, require frequent pupillary dilatation, are found positive on provocative test, are one eyed with PACS, or have a family history of PAC/PACG should be considered for prophylactic iridotomy as should be those of fellow eyes of established PAC or PACG
PAC: Iridotomy is of greater benefit in this group of patients who are more likely to develop PACG[5,6,7]
In PACG cases where an iridotomy may reduce the incidence of further subacute attacks.
Pupillary block is a significant mechanism causing angle-closure glaucoma for which Nd:YAG LPI is effective in widening the drainage angle.[8,9,10] Previous studies have shown that LPI is effective for intraocular pressure (IOP) control in Caucasians.[10,11] However, reports from Asian populations[8,12] showed that Nd:YAG LPI might be inadequate to maintain IOP control in the long term. An iridotomy significantly reduces the pupillary block component of IOP response to provocative testing in PAC eyes. An iridotomy does not, however, significantly change mean IOP or diurnal phasing of IOP in PAC eyes. Eyes with a very narrow angle or a thick lens may continue to have angle closure due to other pathomechanisms for angle closure.[13]
Iridotomy is done for therapeutic purposes to relieve an acute angle closure attack and to relieve the raised IOP in eyes with iris bombe due to secondary angle closure.
Contraindications
Neovascular glaucoma (NVG): As iridotomy is likely to bleed if it is tried in presence of neovascularization of the iris.
Eyes with angle closure due to non-pupillary block mechanism such as lens-induced narrowing of the angle and drug-induced secondary angle closure (such as antipschychotic drug therapy), where the cause for angle narrowing is the choroidal swelling or choroidal effusion and not pupillary block.[14]
Procedure
The patient and relatives must be explained the need for the procedure, the procedure itself, and the possible complications along with the fact that the procedure may require multiple sittings. One must enquire whether the patient is on anticoagulants which may cause excessive bleeding during/after an iridotomy. These may need to be stopped in consultation with the cardiologist. The IOP should be well controlled on medications, and if it is a case of acute attack of angle closure then one may have to use systemic acetazolamide or intravenous mannitol so that the procedure is performed on an eye with as well controlled an IOP as possible. One drop of pilocarpine 2% is instilled into the eye 15 min before treatment. If there is not enough miosis or the iris does not look stretched, then one can put additional pilocarpine eye drops.
Most ophthalmologists perform a Nd:YAG iridotomy alone, while some prefer to do a sequential iridotomy with argon followed by YAG in eyes with thick irides. The patient is comfortably seated on the machine and the eye is anesthetized. An Abraham's contact lens or the Blumenthal lens is applied with a coupling fluid or gel. The Abraham's lens has a 10-mm diameter, 66D magnifying button to give a clear view of the iridotomy site. It provides a 1.5 × image magnification besides stabilizing the eye, neutralizing the corneal surface, and reaching the peripheral areas of the iris with greater concentration of laser power.
The PI is placed in the superior part in the area adequately covered by the lid, in the peripheral third of the iris, wherever the iris appears thinnest or a crypt is seen. In case the crypts or thin iris is present only in the inferior areas, one can choose that as well. While the area of the iris covered by the lid is the preferred site,[15] recent studies do not support this notion.[16,17] The site to be lasered is identified and depending on the thickness of the iris and the presence of a crypt, the energy is set at 3–9 mJ or a different setting depending on the personal choice of the ophthalmologist. The aim is to complete the iridotomy in two to four shots. If we increase energy levels substantially, then there are chances that the lens may get injured by the expanding plasma or rarely even get subluxated. If we keep the energy too low, then we would only be chipping at the superficial iris resulting in a lot of pigment release which will prevent adequate visualization of the iris and will block further laser application. The sure sign of full iris perforation is a sudden gush of aqueous with pigment from the posterior pigment epithelium into the anterior chamber (AC).
If a sequential argon–Nd:YAG LPI is performed, argon is set at 500 mW, increasing up to 1000 mW according to the tissue response, with a spot size of 50 μm and for a time of 0.1–0.3 s. When the iris appears to be adequately thin, the Nd:YAG laser beam is then focused within the iris stromal surface where pretreatment has been done with the help of argon laser and started at an initial setting of low energy of 2–4 mJ and then increased up to 6–10 mJ and shots given till full thickness hole is achieved. Argon pretreatment can be done in cases of thick iris where a circle of coagulative laser like argon/double-frequency YAG is used with a spot size of 300–500 μm, duration of 0.2–0.3 s, and a power of 0.3–0.5 W so that a drumhead is created and then a central Nd:YAG spot can easily perforate the stretched out iris.[18]
The size of an iridotomy to prevent an eye from developing an acute attack has been estimated to be around 150–200 μm. An acute attack developing in a number of cases even with a patent LPI which was of inadequate size is known.[19] The cause of the angle closure attack occurring after dilation even in the presence of a patent LPI may be due to angle crowding by the peripheral rolls of iris after dilation. It could also be due to early post-treatment edema or late pigment epithelium proliferation at the iris.[19]
Complications of iridotomy
Disturbing visual symptoms such as diplopia, transient blurring, glare, shadows, lines, and ghost images are the most common adverse effect especially in PACS/PAC eyes where a prophylactic PI is performed.[20,21]
There is controversy regarding the safety of this procedure to the corneal endothelium.[22,23,24] In a meta-analysis, Wang et al.[25] reported that though the LPI has been demonstrated to be a relatively safe procedure, there is still a potential long-term risk of corneal decompensation for which a corneal transplantation may be indicated eventually. The longest interval between laser iridotomy and corneal decompensation reported was 8 years. The mechanisms proposed for endothelial damage include direct focal injury, thermal damage, mechanical shock waves, iris pigment dispersion, transient rise in IOP, inflammation, turbulent aqueous flow, time-dependent shear stress on endothelium, chronic breakdown of blood–aqueous barrier, and damage from bubbles that settled onto the endothelium. There are variations in the reported changes in the endothelial damage depending on the race under study. In normal Caucasian eyes, the mean exponential cell loss over a 10-year period was reported to be 0.6%–0.5% per year.[26] In normal Indian and Chinese eyes, it was found to be around 0.3% per year.[26,27] The significance of the risk factors and their direct association with the development of corneal decompensation remain to be determined. Understanding these risk factors will allow the ophthalmologists to counsel their patients better. This is especially so in PACS where there is no existing damage or no clear benefit to the patient.[25]
There is a possibility of IOP elevation after an iridotomy and one should be careful in following up all eyes that have had iridotomies especially for those with a compromised disc which could further deteriorate following spikes in IOP. The peak IOP after an iridotomy usually occurs at 4–5 hours post laser.[28]
Cataract is also a known complication of iridotomy. Lim et al.[9] were the first to prospectively evaluate the changes in the lens opacity after LPI in the fellow eyes of subjects with acute PAC, using the Lens Opacities Classification System (LOCS) III and reported significant progression in 23.3% of eyes (95% confidence interval 16.9%–29.7%). In contrast, in a study from Mongolia, Yip et al.[29] reported no significant difference in cataract progression using the LOCS III classification system, between the LPI group and the rest of the study population, 6 years after the LPI for PAC. In the Chennai Eye Disease Incidence Study, 6 years after their baseline evaluation there was significant cortical cataract progression following LPI for PACS. Cataract progression was seen in 38.9% of eyes among those who had undergone LPI at baseline compared with 23.1% of eyes that had no intervention (P < 0.0001).[30]
The possibility of lens subluxation and lens dislocation following LPI is also known. Melamed et al.[31] first reported a case of inferior lens dislocation following a supratemporal LPI treatment that was initially caused by trauma. Kwon et al.[32] reported bilateral complete lens dislocation occurring (8 months in the right eye and 2 years in the left eye) after LPI in a patient with retinitis pigmentosa and phacodonesis. Seong et al.[33] reported complete lens dislocation 10 months after LPI in a high myopic eye. In these cases, the predisposed conditions may have already weakened the zonular fibers; the shock-wave effect from LPI was considered to cause further zonular damage and result in lens dislocation. Nevertheless, a few cases of spontaneous lens dislocation without any relevant predisposed condition besides a history of LPI treatment were also reported (one eye with posterior dislocation and four eyes with complete dislocation; intervals ranging from 1 month to 1 year).[34,35] Except for the traumatic lens dislocation reported by Melamed et al.,[31] clear evidence of causality between the LPI and lens dislocation is inadequate in these cases. There could also be an occult subluxation which may be diagnosed during a lens extraction only.[36]
Mild iris bleed is an invariable part of an iridotomy. Though the bleed appears significant when seen through the magnification of the lens, it usually is insignificant in most cases. Applying a little pressure with the iridotomy lens itself can control the bleed which usually stops by itself. If the bleed causes difficulty in visualization of the iris and further iris penetration, one can complete the iridotomy in another sitting or choose a different site for performing the LPI.
Long-term outcomes
He et al.[37] found that the mean IOP decreased by 0.2 mmHg for every 10° difference in angle width after iridotomy in PACS eyes. Ramani et al.[38] and Talajic et al.[39] found no significant change in IOP in PACS eyes after laser iridotomy. Sawada and Yamamoto[40] did a 10-year retrospective review and grouped PAC eyes depending on their baseline extent of peripheral anterior synechiae (PAS). They found that PAC eyes with less than two quadrants of PAS had an 89.8% control of IOP, and if baseline PAS was more than two quadrants, at 10 years 62.7% were on medication. The mean IOP in PAC eyes reflects the trabecular meshwork (TM) function/dysfunction, which an iridotomy cannot alter.
Nolan et al.[8] found that an LPI lead to an increase in angle width by two Shaffer grades. They reported that PAC eyes with occludable angles that were normal in all other respects and underwent iridotomy did not develop glaucomatous optic neuropathy or symptomatic angle closure over 5 years. In a South Indian population with PACS or PAC/PACG, LPI was found to result in significant AC angle widening seen on both anterior segment optical coherence tomography (ASOCT) and gonioscopy, although some degree of persistent iridotrabecular contact was present in approximately half of PACS eyes and approximately two-thirds of PAC/PACG eyes on gonioscopy. The greatest widening on ASOCT was observed in eyes with features most consistent with greater baseline pupillary block.[41]
Laser Trabeculoplasty
LTP provides a viable option as an adjunct or replacement therapy to lower IOP in the management of primary open-angle glaucoma (POAG), ocular hypertension (OHT), and even PACG. It induces a mechanical effect by causing increased trabecular meshwork (TM) tension circumferentially, pulling the outer layers of the TM, and hence increasing the outflow facility.[42]
The original procedure was described using argon laser (major peaks at 488 and 514 nm) by Wise and Witter in 1979.[42] It can be performed with an argon laser, which emits blue-green radiation,[43] krypton red (wavelength 647.1 or 568.2 nm),[44] diode infrared-emitting laser (810 nm),[45] Nd:YAG (1064 nm) laser,[46] and frequency-doubled Q-switched Nd:YAG laser (532 nm).[47]
The Glaucoma Laser Trial (GLT) Research group indicated that the eyes initially treated with argon laser trabeculoplasty (ALT) tend to have lower IOP and better visual field and optic disc status than the fellow eyes receiving medical treatment. In the GLT follow-up study, after 7 years, patients with ALT had lower IOP than patients on medical treatment.[48,49] Trabeculoplasty has been shown to lower the IOP and slow visual field progression in several multicenter randomized trials, notably the Early Manifest Glaucoma Trial[50] and the Advanced Glaucoma Intervention Study.[51]
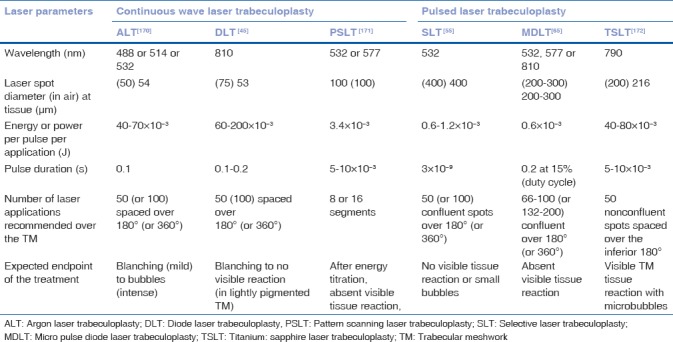
Table 1 summarizes the laser parameters used for different LTP techniques. We describe here the more currently performed selective laser and micropulse diode LTP.
Table 1.
Summary of the various laser treatment parameters used for laser trabeculoplasty
Indications
The most common indications are POAG and OHT – either as an adjunct to medical therapy or as a first-line treatment. Among this group of patients are those who are noncompliant or have difficulty to administer antiglaucoma medications, pregnant women, and patients who are not able to undergo glaucoma filtering surgery. Other indications are secondary OAG such as pseudophakic glaucoma, pseudoexfoliation, pigmentary glaucoma and PAC disease after an iridotomy has opened the angles.
Contraindications
Absolute contraindications are inadequate visualization of angle structures and glaucoma associated with uveitis, trauma, or angle dysgenesis. Some relative contraindications (because of potentially higher failure rate) are eyes with normal-tension glaucoma (NTG), aphakia, and PACG with PAS.
Selective Laser Trabeculoplasty (SLT)
In 1995, Latina and Park[47] introduced a procedure to selectively target pigmented TM cells while sparing adjacent non-pigmented cells and collagen TM beams from collateral thermal damage which can preserve the structural integrity of the TM. Selective targeting of pigmented TM cells can be obtained with pulse duration of 3 ns or less. Therefore, the technique has been named SLT, which delivers approximately 1% of total energy used by ALT. Clinically, the parameters used in SLT are too short for melanin (chromophore) to convert the electromagnetic energy to thermal energy, and hence no heat is generated.
Mechanism of action
SLT is based on the concept of selective photothermolysis, which ensures confinement of thermally mediated radiation damage to a selected pigmented cell population within a tissue with no collateral damage.[52] The mechanism by which SLT reduces IOP is unknown; however, the three main theories proposed by Van Buskirk et al.,[53] which apply to both SLT and ALT, include a mechanical, biochemical, and cellular effect on TM.
Procedure
The commercially available SLT device uses a Q-switched, frequency-doubled, 532-nm Nd:YAG laser, 400 μm diameter spot size and delivers energy in 3 ns. With the patient seated at the slit-lamp system, a Latina SLT single-mirror goniolens (Ocular Instruments, Bellevue, WA, USA) with a methylcellulose coupling medium is placed on the eye. The laser is focused on the TM using the helium–neon (He-Ne) aiming beams. An initial energy level of 0.7–0.8 mJ is typically used (with typical settings of 0.4–1.2 mJ) for lightly pigmented TM. The energy level used is titrated to the degree of trabecular pigmentation, that is, with the greater pigmentation, less energy is required. In more heavily pigmented TM, around 0.6 mJ initial energy may be used. If cavitation bubbles (”champagne bubbles”) appear, the laser energy is reduced by 0.1 mJ increments until no bubble formation is observed and the treatment is continued at this energy level. If no cavitation bubbles are observed at the TM after laser application, the pulse energy is increased by increments of 0.1 mJ until bubble formation is seen and then decreased as described above. An energy level just below that of bubble formation is then maintained. Confluent spots are applied for best results. A total of 360° of the TM is lasered (100 spots; 25 spots per quadrant), except in an eye with a heavily pigmented TM, when 180° is done first, and the remaining 180° later, if needed.
SLT outcomes
In a prospective study, Nagar et al.[54] randomized and compared patients to 90°, 180°, and 360° SLT treatment and compared with latanoprost 0.005% once daily at night in OHT and OAG with a mean follow-up of 10.3 months (range 1–12 months). Differences in success rates (20% or more and a 30% or more IOP reduction from baseline measurements with no additional antiglaucoma interventions) between latanoprost and 360° SLT did not reach statistical significance (P < 0.5). It was concluded that the success rates were higher with latanoprost 0.005% at night than with 90° and 180° SLT treatments. 90° SLT is generally not effective and 360° SLT appears to be an effective treatment with approximately 60% of eyes achieving an IOP reduction of 30% or more. Nagar et al.[55] compared the effect of reduction in IOP fluctuation between SLT and latanoprost 0.005% once daily in 40 patients, with a mean follow-up of 4–6 months. No significant differences could be found between the medication and the SLT groups. Wong et al.[56] conducted a meta-analysis (four randomized control trials comparing the efficacy of SLT and ALT which were conducted between 2011 and 2013) involving all studies on SLT effect in patients with OAG or OHT. All studies concluded that the IOP-lowering effect of SLT was comparable to ALT. Long-term effect of ALT diminishes over time and the 5-year success rate is reported to be about 50%.[57] Long-term effectiveness of SLT seems to show similar results. A prospective, nonrandomized study compared the efficacy of single-session 360° SLT for reduction in IOP in patients with pseudoexfoliation glaucoma (PXFG) and POAG.[58] A significantly higher percentage reduction in IOP in the first 6 months following SLT in PXFG eyes when compared with POAG (29% vs. 19%, P = 0.02) was noted. The efficacy of SLT in both types of glaucoma had decreased at the 1-year follow-up, with no significant difference in IOP reduction between the two types (16% vs. 16%, P = 0.9). Lee et al.[59,60] conducted two prospective studies demonstrating favorable clinical outcomes after a single session of SLT in Chinese patients with NTG at 1- and 2-year follow-up.[59] At 2-year follow-up, there was an 11.5% reduction in IOP and medication use was reduced by 41.1% (P < 0.0001) compared with pre-study levels.[60] Gupta et al.[61] in their study on SLT in eyes of young juvenile glaucoma patients, found good efficacy of SLT in 43% eyes which were already on medical therapy. Hence, SLT can be given a trial even among young patients with OAG.
Complications
Redness, discomfort, and AC reaction in the first week after SLT are very common. The transient IOP spike after LTP is supposed to be dependent on the energy used per pulse and the total energy administered. A recent review summarized some of the reported complications of SLT.[62] These include transient IOP spike (of up to 5 mmHg in 28% and up to 10 mmHg in 5.5%), iritis, hyphema, and macular edema., In addition, some uncommon complications of transient corneal thinning, changes in endothelial cell count, foveal burn, and corneal haze have been reported.
Micropulse Diode Laser Trabeculoplasty (MDLT)
Ingvoldstad et al.[63] first described MDLT in 2005, for LTP in patients with OAG. It is a low irradiance treatment that uses a large-spot size, 810-nm diode laser, which emits repetitive, short near-infrared 100 laser pulses in 200-ms long bursts and the micropulses have 1.7-ms interval in between. The interpulse separation of the MDLT by a long relaxation time is enough to allow the temperature to return to baseline prior to the arrival of the next pulse resulting in improved heat confinement around the pigmented tissue and does not cause observable coagulative damage to the TM.[64] This differentiates it from the conventional continuous wave DLT.
Theoretically, MDLT cannot produce micro-explosions, pigment dispersion, and IOP spikes in eyes with heavily pigmented TM.[63] Earlier studies used the 810-nm wavelength laser, but later studies have switched to using the 532- or 577-nm laser wavelength.
Procedure
With the patient seated at the slit lamp and after topical anesthesia, a laser antireflective coated Goldmann one-mirror lens or the Latina trabeculoplasty lens is inserted into the eye to be treated. MDLT is performed with the commercially available laser device, IRIS Medical Ocu Light SL × 810 diode laser system (IRIDEX Corporation, Mountain View, CA, USA). The micropulse laser settings are as follows: 200–300 μm spot size diameter, 2 W power (6.4 kW/cm2 irradiance), and 200 ms duration with 15% duty cycle (percentage of time that the laser will be active during treatment duration). The laser is focused on the anterior TM and approximately 70 confluent spots are administered over the inferior 180° of TM and 60 mJ of energy is delivered to each spot. As there is no visible laser-induced tissue change in TM, endpoint is relied on the operator's skill and judgment.
MDLT outcomes
In a short-term prospective study, 21 eyes were randomized to receive either MDLT or ALT and both the groups achieved around a 20% reduction in IOP at 3 months with no significant difference between the groups.[63]
In a phase II, prospective, interventional case series of 20 patients with OAG, it was found that MDLT was successful in 15 patients (75%) with a mean IOP reduction of around 20% at 12 months and 5 patients (25%) failed to achieve reduction in IOP (4 in the first week and 1 at 6 months).[65]
The preliminary data compared MDLT with SLT in 12 eyes which had MDLT and 14 eyes which underwent SLT. MDLT group achieved a mean IOP reduction of 3.9 mm Hg, while the reduction in SLT was 2.6 mm Hg. The mean change in the number of medication was 0.6 versus 0.1 in the MDLT and SLT groups, respectively.[66]
A retrospective review of 40 eyes of 29 MDLT-treated patients over a minimum follow-up period of 6 months found that only 1 of 40 patients (2.5%) had ≥20% reduction in IOP and only 3 of 40 patients (7.5%) had ≥3 mm Hg decrease in IOP at 19 months of follow-up.[67] The average time for failure of treatment was around 3 months. The result of the study suggested that 180° MDLT is ineffective in managing patients with OAG.
A prospective study investigated the safety and efficacy of MDLT in the treatment of 48 patients with OAG after a single session of unilateral MDLT treatment, using a 577-nm diode laser to 360° of the TM.[68] At 6 months, the IOP was reduced by 19.5% and antiglaucoma medications were reduced by 21.4%, compared to the pre-treatment levels.
There are no reported late post-laser complications arising from MDLT in literature. It has advantages over SLT, especially in patients at higher risk of post-laser IOP spikes, such as those with highly pigmented TM. MDLT has shown encouraging results in these early studies in the treatment of OAG; however, further studies are needed to compare its efficacy with other LTP treatment modalities.
Role of laser trabeculoplasty in angle closure glaucoma
Traditionally, LTP is not considered in ACG, as the procedure requires TM visualization for the treatment. Recent studies have investigated the efficacy and safety of SLT in patients with PACG, where portions of the angle are open and amenable to treatment.[54,55,69,70,71,72]
A retrospective case–control study compared the efficacy of SLT in eyes with PAC or PACG and POAG (59 eyes in each group), with an average of 10 and 11 months of follow-up.[69] In patients with uncontrolled preoperative IOP, SLT applied to the areas where the angle was open for at least 180° resulted in 38% mean IOP reduction in the PAC/PACG group. In patients who had controlled IOP in the PAC/PACG group (under medication but were intolerant to the medications), SLT achieved a reduction of 1.6 medications. The success rate of SLT in IOP reduction by at least 20% or more from baseline was 84.7% in the PAC/PACG group and 79.6% in the POAG group (P = 0.47).
A randomized clinical trial evaluated the efficacy of SLT in comparison to topical prostaglandin analogue medical therapy in patients with PAC/PACG.[70] After 6 months of follow-up, the mean IOP was significantly reduced from baseline in both the groups, and the IOP reduction was comparable between the two groups. This study suggests that SLT may be effective in patients with PAC and PACG in which sufficient extent of angle (ideally >180°) is visible after PI.
In a multicentric, prospective, non-controlled clinical trial, 60 eyes of 60 patients with chronic angle closure, post-LPI, IOP >21 mm Hg, and a gonioscopically visible pigmented TM for at least 90°, SLT was applied to the open segments of the angle.[71] At 6 months, IOP reduction of 3 or 4 mm Hg was seen in 82% and 72% of eyes, respectively, and IOP reduction of 20% or 30% was observed in 54% and 24% of eyes, respectively.
A recent review reported the histological changes and favorable outcomes of SLT in patients with PACG, specifically in areas of non-occluded angle.[72] However, it needs to be further substantiated through large controlled clinical trials.
Role of laser trabeculoplasty in secondary glaucoma
Few retrospective studies have demonstrated the safety and efficacy of SLT in treating different types of secondary glaucoma.[73,74] In a retrospective case series of 15 eyes with well-controlled uveitis with fluocinolone acetonide intravitreal implant and implant-induced steroid glaucoma. Seven eyes (46.7%) of seven patients achieved an IOP of < 22 mmHg and/or a 20% or greater IOP reduction from the pre-SLT IOP at 1, 6, and 12 months of follow-up period.[73,74] Another retrospective study of 42 patients (42 eyes) with silicone-oil-induced glaucoma, 360° of TM, was treated with SLT.[75] At 12-month follow-up, the mean IOP reduction was 60.7% (17 phakic eyes) and 57.1% (8 aphakic eyes). The mean number of glaucoma medications was significantly reduced, from 2.17 ± 1.21 to 1.25 ± 0.89 (P < 0.05).
Laser Peripheral Iridoplasty
In laser peripheral iridoplasty, laser burns of low energy are applied to the peripheral iris to widen the AC angle and/or break PAS.
Krasnov[76] was the first to use laser burns encompassing 90° of the angle, near the iris root to separate iris and TM. The laser parameters used often caused penetrating burns, resulting in insufficient retraction of the iris from the TM. Kimbrough et al.[77] described a technique, through a gonioscope, for the direct treatment of 360° of the peripheral iris and termed the procedure gonioplasty.
Argon laser peripheral iridoplasty (ALPI) is an effective means of opening an appositionally closed angle in situations in which LPI either cannot be performed or there is residual appositional closure after LPI, due to mechanisms other than pupillary block. It includes application of contraction burns (long duration, low energy, and large spot size) to the iris periphery to contract and compact the iris stroma at the site of the burn, thus pulling open the angle.[78,79]
Histopathological examination suggests that the short-term effect is related to heat shrinkage of collagen and the long-term effect is secondary to contraction of a fibroblastic membrane in the region of laser application.[78]
Indications
The most important indication of iridoplasty is controlling an acute attack of angle closure, if it is unresponsive to antiglaucoma medications or if corneal edema, very shallow AC and marked inflammation precludes immediate LPI, also in cases of an unresponsive attack despite patent LPI.[80] Others include PACG to open appositionally closed angle, plateau iris syndrome (PIS)[81] and as an initial treatment to break an attack of acute phacomorphic angle closure.[82]
Other indications are to improve visual function following multifocal intraocular lens (IOL) implantation, where the iris is encroaching onto the IOL,[83] in cases of optic obstruction by iris in eyes with Boston keratoprosthesis[84] and as an adjunct to gonio synechiolysis.[85,86]
Contraindications
Nonvisibility of the iris due to corneal edema or opacity or a flat AC.
Procedure
After an informed consent, topical pilocarpine 2% or 4% is applied to stretch the iris maximally. The procedure is performed under topical anesthesia. The argon laser is set to produce contraction burns (500 μm spot size, 0.5–0.7 s duration, and initially power is set at 240 mW). In the direct technique, which uses an Abraham lens (Ocular Instruments), the laser energy is applied perpendicular to the peripheral iris. With the indirect technique, which uses a single-mirror gonioscope (Ocular Instruments), the beam is directed at a low angle of incidence toward the peripheral iris and angle. It is useful to allow a thin crescent of the aiming laser beam to overlap the sclera at the limbus and the patient is asked to look in the direction of the beam to achieve more peripheral spot placement. The energy is increased in 40 mW increments until adequate iris stromal contraction is noted. Lighter irides generally require more power than darker ones. If there is pigment release or gas bubble formation, charring of iris, or production of “pop” sound, the energy should be reduced. Approximately 24 spots are placed over 360° (six to eight spots placed in each quadrant), leaving approximately two spot diameters between each spot.
The spots can also be applied without any contact lens. Apart from argon laser, the use of diode laser[85,86,87] and double-frequency Nd:YAG laser[88] has also been described for laser peripheral iridoplasty.
Complications
A mild iritis may be noticed which resolves over a week. Diffuse corneal endothelial burns may occur if ALPI is performed on patients with very shallow peripheral AC.[89] They can be minimized by placing an initial contraction burn more centrally on iris, before placing the peripheral burn (criss-cross iridoplasty). In all the cases, the endothelial burns disappear within several days. A transient rise in IOP may occur and must be looked for. There may be iris atrophy which can be avoided using the lowest laser power and not allowing the laser marks to become confluent.[90] Rarely, a Urrets-Zavalia syndrome may occur with dilated and fixed pupils, not responding to light, accommodation, or pilocarpine, after uneventful ALPI.[91]
Efficacy and long-term success
The efficacy of laser iridoplasty in PAC remains controversial. A retrospective case series reported long-term effect of ALPI in the eyes with plateau iris syndrome (PIS).[81] The angle in 87% eyes of 14 patients remained open during the mean follow-up period of 78.9 ± 8.0 months, after only one treatment. It was suggested that though ALPI is successful in long-term in eyes with PIS, these patients need to be followed closely for recurrence of appositional closure, which may require retreatment. In a study, 22 patients with bilateral PAC/PACS with occludable angles following LPI were randomized to receive ALPI (n = 11) or no further treatment (n = 11).[92] The temporal change in angle anatomy following ALPI in eyes with occludable angles post LPI were compared with control eyes using swept-source ASOCT. ALPI widened all the angle sections in eyes that remained occludable post LPI and the changes were maintained for 3 months. ALPI decreased diurnal IOP fluctuation in the treated eyes by lowering the maximum IOP. However, in a randomized controlled trial to determine the effectiveness of ALPI in PAC and PACG, ALPI was found to be associated with higher failure rates and lower IOP reduction compared with medical therapy.[93]
Laser Cyclophotocoagulation
Cyclodestructive procedures have been used for the treatment of refractory glaucoma since 1930s. Refractory glaucoma is defined as glaucomas which remains uncontrolled despite previous filtration surgery and/or laser treatment and/or under maximum tolerated medical treatment.[94] However in recent years, the spectrum of cyclophotocoagulation has expanded from end-stage glaucoma to glaucoma in patients with good visual acuity. Cyclophotocoagulation procedures have gained acceptance even in pediatric glaucoma.
Laser cyclophotocoagulation is now the current method of cyclodestruction. Various lasers have been used for this purpose which includes ruby, Nd:YAG, argon, krypton, and diode laser. Compared to cyclophotocoagulation, cyclocryotherapy is limited by significant intraocular inflammation, severe postoperative pain, hypotony, and phthisis.[74,95,96] Laser cycloablation is considered to be more effective and better tolerated by the patient than cyclocryotherapy.[97,98] Beckman et al. were the first to report on trans-sclera cyclophotocoagulation (TSCPC) using ruby laser (693 nm).[99] Since then a number of wavelengths have been used.
The most frequently used lasers are 1064 nm Nd:YAG[100,101,102] and the 810-nm diode laser.[103,104,105] Presently, diode laser is more commonly used than Nd:YAG for cyclophotocoagulation and can be performed with a contact probe (most common)[105] or as a noncontact procedure on the slit lamp.[106]
Indications
They are generally intractable glaucomas like NVG,[107] post-penetrating keratoplasty (PK) glaucoma,[108] post-traumatic glaucoma,[109] post-retinal detachment surgery glaucoma,[110] silicon-oil-induced glaucoma,[111] inflammatory glaucoma,[112] aphakic/pseudophakic glaucoma,[113] refractory pediatric glaucoma,[113,114] after multiple failed surgeries,[115,116,117] in the presence of severe conjunctival scarring, for pain relief in a painful blind eye due to elevated IOP,[92,118] as an urgent means to lower IOP when access to surgery is limited,[119] and for patients medically unfit for surgery.[120]
Mechanism of action
Various theories have been described like destruction of the ciliary epithelium resulting in decreased aqueous production,[121] destruction of ciliary blood vessels and coagulative necrosis leading to ciliary body ischemia,[98] intraocular inflammation which is thought to cause short-term decrease in IOP,[122] creation of a trans-scleral flow similar to cyclodialysis, or an increased uveo-scleral outflow.[123]
Trans-Scleral Cyclophotocoagulation
Procedure
Informed consent should be taken explaining the risks to the patient before the procedure. It is done under retrobulbar or peribulbar anesthesia. For children, it is usually done under general anesthesia and this is augmented by a retrobulbar or peribulbar block to prevent postoperative pain. An 810-nm semi-conductor diode laser is used with a G probe. The probe is a 600-μm diameter quartz fiber with a spherical protruding tip oriented by the footplate of the handpiece. It is designed to center treatment 1.2 mm behind the limbus. The handpiece footplate part which comes into contact with the sclera is spherically curved to match the contour of the sclera. The anterior, curved edge is designed to match the limbus.
Trans-scleral transillumination is used to demonstrate the position of the ciliary body, because its location varies considerably which may be so especially in buphthalmic eyes.[114] The power is kept between 1500 and 2000 mW with a time duration of 2 seconds. A “pop” sound denotes tissue disruption. If there is no “pop” sound, the power is increased by increments of 100 mW till the “pop” is heard, following which the power is reduced by 100 mW.
About 18–20 laser spots for 360° and about 10–12 for 180° (five spots per quadrant) are applied. One must avoid sites of previous filtering surgery/tubes, areas of thin sclera, and the 3 and 9′ O clock positions (to avoid the long posterior ciliary nerves).[124]
Post-laser treatment
After the procedure, patching of the eye is done for approximately 6 hours till the effect of the local anesthesia wears off. Topical antibiotic steroids and topical cycloplegics are prescribed, which are tapered as the inflammation subsides. The pre-laser glaucoma medications are continued, depending on IOP response. Analgesics (nonsteroidal anti-inflammatory drugs) may need to be prescribed for the pain. The patient is followed up at day 1, 1 week, 1 month, according to the response to treatment. Additional laser therapy, if needed, should be considered after 1 month.
Complications
Pain can occur but is usually transient. Iridocyclitis (42%) is common after the procedure, due to a breakdown of the blood–aqueous barrier.[109,125,126,127] Transient rise of IOP can occur, along with corneal edema and corneal epithelial defects (9% of cases).[126] Cataract can develop in around 12% of cases.[126] The risk of hypotony varies among studies, but it is higher with cyclocryotherapy than with cyclophotocoagulation. The hypotony rate has been reported to be between 0% and 18%.[105,121,126,127,128,129,130]
The risk of phthisis increases with the number of procedures performed. Zonular damage[130] and staphyloma formation have been reported.[131,132] Pupillary distortion can occur as a result of a peripheral iris injury, caused by an anterior displacement of the laser spot. Even in normal, emmetropic eyes, the anterior margin of the ciliary body varies between 1.5 and 2 mm depending on the meridian.[133] Individual variations in the anatomical location of the pars plicata of the ciliary body also exist. The recommendations made by Bhola et al.[132] to avoid staphyloma and pupillary distortion are to avoid abnormally thinned or scarred sclera, to inspect and wipe the tip of the G probe between applications and to transilluminate the eye to identify the position of the ciliary body. In cases where transillumination is not possible, ultrasound biomicroscopy (UBM) or axial length of the eye can be used as a guide.
Macular edema is an important cause of decrease in visual acuity following cyclophotocoagulation.[134,135,136] Corneal endothelial decompensation may occur. When diode laser cyclophotocoagulation was performed in refractory glaucoma after PK, 16% of graft opacification was reported.[137]
A case of inadvertent sclerostomy with encysted bleb has also been reported.[138] Conjunctival surface burns can also occur, especially if debris is present at the end of the fiber optic.[136] Retinal detachment, serous, and hemorrhagic choroidal detachment have been reported, although patients who developed retinal detachment in these studies had aphakia.[113,114]
A case of panophthalmitis following contact diode laser cyclophotocoagulation using G-probe in a patient with failed trabeculectomy and trabeculotomy for congenital glaucoma has also been described.[139]
Diode laser cyclophotocoagulation in children with refractory glaucoma
Pediatric glaucomas, especially secondary pediatric glaucomas, are very difficult to manage. Drainage surgery may be complicated by hypotony when the eye is large and has a thin sclera and/or by failure to control IOP caused by an aggressive healing response.[113]
Kirwan et al.[113] in their study found that with repeat cyclodiode, 72% had a clinically useful reduction in IOP. In children with refractory glaucoma, cyclophotocoaguation has a role as a temporary measure, as an adjunct to surgery, or in managing selected patients in whom surgery is undesirable because of a high risk of surgical complications.
Endo Cyclophotocoagulation (ECP)
Norris and Cleasby[140] first described visualization of intraocular tissue through endoscopy. The ECP laser unit is a standalone unit which has four different components, the 810-nm diode laser, a 175-W xenon light source, a He-Ne laser aiming beam, and video monitor and recorder. The endoscopy probe contains all three fiber groupings and is available in 19, 20, or 23 gauge sizes with a field of view ranging from 70° to 140° and depth of focus spanning 1–30 mm. An advantage to the 23-gauge probe is its compatibility with all 23-gauge vitrectomy trocar systems. The probes can be sterilized and reused up to 25 times or more. Pantcheva et al.[123] compared the tissue effects of ECP to TSCPC in human autopsy eyes. The trans-scleral laser-treated areas showed destruction of pigmented and non-pigmented ciliary epithelium and capillaries in the ciliary processes, with pigment clumping, coagulative changes, and destruction of processes’ stroma. The TSCPC-treated tissue had extensive architectural disruption extending into the pars plana and iris stroma. In contrast, ECP-treated human tissue showed loss of lacy appearance of the stroma of the ciliary processes with destruction of non-pigmented epithelium and clumping of pigmented epithelium, with minimum to no coagulative changes in the tissue beyond the ciliary processes.
The indications for ECP are similar to the trans-scleral approach, but it is more commonly used for post vitreo-retinal surgery glaucoma, NVG, post-traumatic glaucoma, and post-PK glaucoma. Combined phacoemulsification–ECP can be done on patients with a visually significant cataract and coexisting glaucoma that is either uncontrolled with medications or medically controlled but with difficulty in affording or tolerating medications. ECP may be combined with other minimally invasive glaucoma surgery (MIGS) procedures such as Trabectome or the iStent to achieve IOP reduction.[141] Patients with PXFG are typically poor candidates due to buildup of fibrillar material on the ciliary processes that minimizes laser uptake.[142] Performing ECP in phakic patients is possible, but it can lead to cataract progression or zonular damage. Endo cycloplasty is a technique where shrinkage of ciliary processes with photocoagulation also helps in widening the AC angle in PIS and for treatment of irido ciliary cysts causing angle closure.[143,144] This is generally combined with a lens extraction.
Procedure
Limbal ECP can be done in phakic, aphakic, and pseudohphakic eyes. The limbal approach is generally recommended in patients undergoing ECP combined with cataract surgery and IOL implantation. After making two clear corneal incisions (superior or temporal), a highly retentive and cohesive viscoelastic (Healon GV) is injected under the iris, to widen the sulcus and enable visualization of the ciliary body. The laser settings are as follows: 0.2–0.5 W, continuous wave mode. The end point is whitening and shrinkage of the ciliary processes. If the probe is closer to the processes, a shorter duration and/or lower power will be needed. The entire visible area of each ciliary process should be treated including anterior and posterior edges as well as crypts in between processes. Treatment should be carried to the extent of visualization in one direction, and then the probe is rotated 180°, and treatment is continued as far as possible in the other direction. With a curved probe, a single incision allows treatment of approximately 270°. If more treatment is desired, a second incision may be placed 180° away from the initial wound to gain access to the subincisional processes and complete a 360° treatment for additional IOP lowering. Viscoelastic is aspirated at the end of the procedure.
Pars plana ECP is done in pseudophakic/aphakic eyes; the pars plana approach is advantageous since the ciliary processes are better visualized. After anterior vitrectomy, cyclophotocoagulation with the endolaser probe is performed. Pars plana ECP can be combined with vitreoretinal procedures, as in cases of NVG, silicone-oil-induced glaucoma, and post-traumatic glaucoma. In post-PK glaucoma, it has an advantage, as the procedure has no effect on corneal endothelium. ECP plus: It is ECP along with extension of the treatment 1–2 mm onto pars plana for a more aggressive IOP-lowering effect. The patient has to be pseudophakic or aphakic. It is done through a pars plana route with a pars plana vitrectomy.
Postoperative management
Postoperatively, the patient is started on cycloplegics and topical corticosteroids. If there is extensive AC inflammation, the steroid drops’ frequency can be increased. After ECP, topical antibiotics are necessary since it is an intraocular procedure. The pre-laser glaucoma medications are continued postoperatively and can be tapered based on IOP-lowering effect of the laser. Miotics should be stopped because they can enhance inflammatory response and cause posterior synechiae.
Advantages
Advantages of ECP are decreased energy, less inflammation, and less collateral tissue damage.
Complications
Complications include fibrin exudates (24%), hyphema (12%), cystoid macular edema (10%), and vision loss of two lines or more (6%).[145] Traumatic injury to the iris has been reported with ECP. This complication is due to the laser being improperly applied to the iris or through mechanical trauma. Therefore, the surgeon must be aware of the location of endolaser probe at all times and the laser should only be applied to the ciliary processes. The procedure also carries an increased risk of endophthalmitis, though not yet reported.
The disadvantages are the cost of the equipment, and the fact that the probes are semi-disposable.
Efficacy and long-term success
Chen et al.[146] in their study performed endoscopic cyclophotocoagulation on 68 eyes, 180°–360° of the ciliary body circumference, through a limbal incision (56 eyes, 12 of which underwent concurrent cataract extraction) or pars plana incision (12 eyes). In all, 61 eyes (90%) achieved an IOP ≤ 21 mm Hg. The mean number of glaucoma medications used by each patient was reduced from 3.0 ± 1.3 preoperatively to 2.0 ± 1.3 postoperatively (P < 0.0001). Best-corrected visual acuity was stable or improved in 64 eyes (94%), with 4 (6%) losing two or more lines of Snellen acuity. No case of hypotony or phthisis was observed. Uram[147] evaluated the potential efficacy of ophthalmic laser microendoscope photocoagulation of the ciliary processes in the management of intractable NVG. Ten patients underwent microendoscope ciliary process ablation through a pars plana incision. There was a decrease of 28.3 mmHg (65%) in the postoperative final IOP. Hypotony occurred in two eyes, although both these patients had chronic retinal detachment.
Compared to Ahmed's glaucoma valve in refractory glaucomas, ECP has been found to be equally efficacious.[148] Most of the long-term studies on combined phacoemulsification with ECP show that the procedure is efficacious in lowering IOP more than cataract surgery alone.[147,149,150,151,152,153,154,155] The most common complications noted have been fibrinous uveitis (11.1%), acute or chronic IOP elevation (3.2% and 7.9%, respectively), and CME (3.2%).[150]
Micropulse Diode Laser Trans-Scleral Cyclophotocoagulation (micropulse TSCPC)
Micropulse TSCPC uses a fractionated, continuous wave, diode laser, which allows a more focal delivery of heat energy to target the non-pigmented ciliary body epithelium. It delivers a series of short, repetitive pulses of laser with a duty cycle of 31.3% (0.5 ms of “on time” and 1.1 ms of “time”), which allows tissue to cool between the pulses, thereby minimizing collateral tissue damage.
Procedure
Procedure is performed under peribulbar anesthesia, with a contact probe (Cyclo G 6 laser system, with a P3 probe, Iridex) base at limbus, over the conjunctival surface and the notch is directed toward the cornea. Laser settings are as follows: energy of 2000 mW and time duration between 100 and 320 s. It is applied in a sweeping movement along the limbus, excluding 3 and 9 O′ clock positions, and then a second arc is performed in the untreated area to complete the session, depending on iris pigmentation and disease characteristics.
Long-term outcomes and efficacy
Tan et al.[156] evaluated the safety and efficacy of micropulse TSCPC in 40 eyes of 38 patients with refractory glaucoma and achieved a relative success of 80% at follow-up of 16.3 ± 4.5 months. None of the eyes had postoperative hypotony or visual loss.
In a study, 48 patients with refractory, end-stage glaucoma were randomized to either micropulse or continuous wave diode TSCPC treatment.[157] The mean IOP was reduced by 45% in both the groups (P = 0.7) from the baseline of 36.5 mmHg and 35 mmHg (P = 0.5) after a follow-up period of 17.5 ± 1.6 months. No significant difference in retreatment rates or number of antiglaucoma medications was noted between the two treatment groups. The ocular complication rate was higher in continuous wave TSCPC-treated eyes (P = 0.01).
Laser Suture Lysis (LSL) after Trabeculectomy
In 1984, Hoskins and Migliazzo[158] reported a technique for cutting the tight trabeculectomy scleral flap nylon sutures through the overlying conjunctiva, with the argon laser, to enhance filtration. It can be performed in the early post-operative period, a few days or few weeks after the surgery. If trabeculectomy is augmented with antimetabolite, LSL can be done 2–3 weeks after the surgery or later, as antimetabolites delay wound healing.[159] Pappa et al.[160] showed that the LSL can be effective even 21 weeks after trabeculectomy when intraoperative mitomycin C had been used.
Procedure
A lens (Hoskins or Blumenthal or Mandelkom or Zeiss four mirror) is used to enable clear visualization of the subconjunctival suture, and gentle pressure with the lens blanches the overlying conjunctival vessels. The parameters for the treatment depend on the bleb wall thickness and the type of laser (frequency-doubled Nd:YAG, argon, or diode) used. For argon LSL, a spot size of 50 μm, power of 250–600 mW, and duration of 0.1–0.2 s suffices. After LSL, digital pressure on the eye may be used, which forces the aqueous through sclerostomy and elevates the bleb.
Complications
Complications include conjunctival perforation, flat AC, hyphema, iris incarceration, bleb leak, prolonged hypotony due to overfiltration,[159] and malignant glaucoma.[161]
Iridolenticular Synechiolysis
It is done in cases of seclusio pupillae, where the iris does not allow for visualization of the posterior segment [Fig. 1]. We described a technique called iridolenticular synechiolysis where a phakic patient with seclusion pupillae can be dilated to bypass the pupil block of aqueous.[162] The pupil is dilated using topical mydriatics and though due to the bound down pupil the pupil will not dilate yet the area is brought under stretch. One applies a minimum YAG energy of 0.5 mJ and focuses the spot just anterior to the synechiae on the side of iris away from the lens such that the plasma generated helps sweep off the synechiae. If this does not work, one can go closer and directly cut the synechiae. One can increase the power to 2 mJ, but it is not prudent to increase it further as that is liable to cause lens damage. The procedure starts from the 6 O′ clock position and proceeds upward on either side of the pupil as it invariably bleeds and makes the media cloudy. It takes multiple sittings to achieve the objective of dilating this bound down pupil and one may not be able to do it completely if the synechiae are dense and the entire underside of the iris is plastered onto the lens. After each sitting, the pupil is to be dilated with phenylephrine cyclopentolate combination to move the area of the pupil where the synechiae have been successfully removed. A steroid eye drop along with dilating drops are given.[162]
Figure 1.
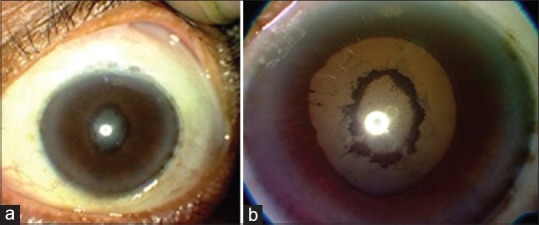
An eye before (a) and after (b) laser synechiolysis
Laser Management of Overhanging/Avascular Blebs
Overfiltration can lead to blebs which spread to the nasal and the temporal aspect with boggy conjunctiva, hypotony resulting in hypotonic maculopathy besides causing dysesthesia, irritation, and cosmetic blemish. Similarly, many chronic blebs may not be overfiltering but are elevated resulting in a dellen formation in the cornea adjacent to it along with lid movement problems and dysesthesia. We developed a technique in which we paint the conjunctiva in question with gentian violet.[163] A photocoagulative laser is used. The spot size is kept at 300–500 μm with 0.3–5 W power and 100 ms timing such that a superficial charring of the colored conjunctiva takes place without any perforation.[163]
If the bleb height is high and direct bleb painting with the laser is not working, one can puncture the bleb with a tiny spot of Nd:YAG laser to release the fluid and then perform the coagulation of the bleb which then becomes more effective.[164] One can thus avoid surgical procedures such as bleb sutures and conjunctival shortening by performing this outpatient procedure which can limit the bleb and overcome hypotony.
Laser Hyaloidotomy/Vitreolysis
In cases of malignant glaucoma in aphakic or pseudophakic eyes, Nd:YAG LPI with posterior capsulotomy/hyaloidotomy (2–3 mJ energy) can be performed to disrupt the intact posterior capsule and anterior hyaloid membrane, thus establishing a direct communication of aqueous between the AC and vitreous cavity [Fig. 2].[165] Several studies have reported success with this procedure in eyes refractory to medical therapy.[166,167] In a pseudophakic patient with a large IOL optic, the outcome can be improved by making the capsular opening through a dialing hole, if present.[168] If the procedure is effective, slight deepening of the AC should be seen over the next 24 h because of free aqueous flow between the posterior chamber and AC. However, the relapse rate may be high, in which case surgical options may be required.[169,172]
Figure 2.
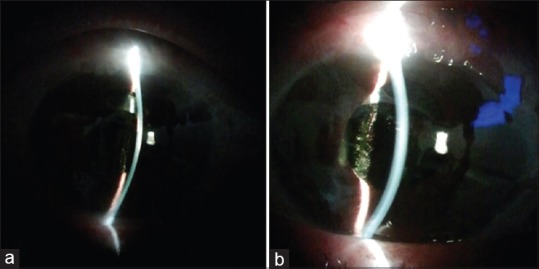
An eye with shallow anterior chamber and malignant glaucoma before (a) and after (b) laser hyaloidotomy
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO. Global Data on Visual Impairments 2010 (WHO/NMH/PBD/12.01) Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Day AC, Baio G, Gazzard G, Bunce C, Azuara-Blanco A, Munoz B, et al. The prevalence of primary angle closure glaucoma in European derived populations: A systematic review. Br J Ophthalmol. 2012;96:1162–7. doi: 10.1136/bjophthalmol-2011-301189. [DOI] [PubMed] [Google Scholar]
- 4.Ronnie G, Ve RS, Velumuri L, Asokan R, Vijaya L. Importance of population-based studies in clinical practice. Indian J Ophthalmol. 2011;59(Suppl):S11–8. doi: 10.4103/0301-4738.73681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol. 1996;114:1235–41. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- 6.Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Central corneal thickness and intraocular pressure in a Mongolian population. Ophthalmology. 1998;105:969–73. doi: 10.1016/S0161-6420(98)96021-3. [DOI] [PubMed] [Google Scholar]
- 7.Foster PJ, Devereux JG, Alsbirk PH, Lee PS, Uranchimeg D, Machin D, et al. Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: Modified grading scheme. Br J Ophthalmol. 2000;84:186–92. doi: 10.1136/bjo.84.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson GJ, Baasanhu J. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol. 2000;84:1255–9. doi: 10.1136/bjo.84.11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim LS, Husain R, Gazzard G, Seah SK, Aung T. Cataract progression after prophylactic laser peripheral iridotomy: Potential implications for the prevention of glaucoma blindness. Ophthalmology. 2005;112:1355–9. doi: 10.1016/j.ophtha.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Quigley HA. Long-term follow-up of laser iridotomy. Ophthalmology. 1981;88:218–24. doi: 10.1016/s0161-6420(81)35038-6. [DOI] [PubMed] [Google Scholar]
- 11.Robin AL, Pollack IP. Argon laser peripheral iridotomies in the treatment of primary angle closure glaucoma.Long-term follow-up. Arch Ophthalmol. 1982;100:919–23. doi: 10.1001/archopht.1982.01030030927004. [DOI] [PubMed] [Google Scholar]
- 12.Alsagoff Z, Aung T, Ang LP, Chew PT. Long-term clinical course of primary angle-closure glaucoma in an Asian population. Ophthalmology. 2000;107:2300–4. doi: 10.1016/s0161-6420(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 13.Sihota R, Rishi K, Srinivasan G, Gupta V, Dada T, Singh K. Functional evaluation of an iridotomy in primary angle closure eyes. Graefes Arch Clin Exp Ophthalmol. 2016;254:1141–9. doi: 10.1007/s00417-016-3298-x. [DOI] [PubMed] [Google Scholar]
- 14.Rajjoub LZ, Chadha N, Belyea DA. Intermittent acute angle closure glaucoma and chronic angle closure following topiramate use with plateau iris configuration. Clin Ophthalmol. 2014;8:1351–4. doi: 10.2147/OPTH.S65748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaeth GL, Idowu O, Seligsohn A, Henderer J, Fonatanarosa J, Modi A, et al. The effects of iridotomy size and position on symptoms following laser peripheral iridotomy. J Glaucoma. 2005;14:364–7. doi: 10.1097/01.ijg.0000177213.31620.02. [DOI] [PubMed] [Google Scholar]
- 16.Vera V, Naqi A, Belovay GW, Varma DK, Ahmed, II Dysphotopsia after temporal versus superior laser peripheral iridotomy: A prospective randomized paired eye trial. Am J Ophthalmol. 2014;157:929–35. doi: 10.1016/j.ajo.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Congdon N, Yan X, Friedman DS, Foster PJ, van den Berg TJ, Peng M, et al. Visual symptoms and retinal straylight after laser peripheral iridotomy: The Zhongshan Angle-Closure Prevention Trial. Ophthalmology. 2012;119:1375–82. doi: 10.1016/j.ophtha.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Kumar H, Sood NN, Kalra VK. Evaluation of Argon pre-treatment for mode locked Nd- YAG laser peripheral iridotomy in angle closure glaucoma. Glaucoma. 1990;12:126. [Google Scholar]
- 19.Fleck BW. How large must an iridotomy be? Br J Ophthalmol. 1990;74:583–8. doi: 10.1136/bjo.74.10.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung RS, Guan AE. Unusual visual disturbance following laser peripheral iridotomy for intermittent angle closure glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244:532–3. doi: 10.1007/s00417-005-0129-x. [DOI] [PubMed] [Google Scholar]
- 21.Murphy PH, Trope GE. Monocular blurring. A complication of YAG laser iridotomy. Ophthalmology. 1991;98:1539–42. doi: 10.1016/s0161-6420(91)32091-8. [DOI] [PubMed] [Google Scholar]
- 22.Jamali H, Jahanian S, Gharebaghi R. Effects of laser peripheral iridotomy on corneal endothelial cell density and cell morphology in primary angle closure suspect subjects. J Ophthalmic Vis Res. 2016;11:258–62. doi: 10.4103/2008-322X.188395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canning CR, Capon MR, Sherrard ES, Kerr Muir MG, Pearson R, Cooling RJ. Neodymium: YAG laser iridotomies – Short-term comparison with capsulotomies and long-term follow-up. Graefes Arch Clin Exp Ophthalmol. 1988;226:49–54. doi: 10.1007/BF02172718. [DOI] [PubMed] [Google Scholar]
- 24.Marraffa M, Marchini G, Pagliarusco A, Perfetti S, Toscano A, Brunelli C, et al. Ultrasound biomicroscopy and corneal endothelium in Nd:YAG-laser iridotomy. Ophthalmic Surg Lasers. 1995;26:519–23. [PubMed] [Google Scholar]
- 25.Wang PX, Koh VT, Loon SC. Laser iridotomy and the corneal endothelium: A systemic review. Acta Ophthalmol. 2014;92:604–16. doi: 10.1111/aos.12367. [DOI] [PubMed] [Google Scholar]
- 26.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779–82. [PubMed] [Google Scholar]
- 27.Rao SK, Ranjan Sen P, Fogla R, Gangadharan S, Padmanabhan P, Badrinath SS. Corneal endothelial cell density and morphology in normal Indian eyes. Cornea. 2000;19:820–3. doi: 10.1097/00003226-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H Sood NN, Kalra VK. Pressure dynamics after mode-locked Nd:YAG laser iridotomy in angle-closure glaucoma. Glaucoma. 1990;12:39–46. [Google Scholar]
- 29.Yip JL, Nolan WP, Gilbert CE, Uranchimeg D, Baassanhuu J, Lee PS, et al. Prophylactic laser peripheral iridotomy and cataract progression. Eye (Lond) 2010;24:1127–34. doi: 10.1038/eye.2010.59. quiz 1135. [DOI] [PubMed] [Google Scholar]
- 30.Vijaya L, Asokan R, Panday M, George R. Is prophylactic laser peripheral iridotomy for primary angle closure suspects a risk factor for cataract progression? The Chennai Eye Disease Incidence Study. Br J Ophthalmol. 2017;101:665–70. doi: 10.1136/bjophthalmol-2016-308733. [DOI] [PubMed] [Google Scholar]
- 31.Melamed S, Barraquer E, Epstein DL. Neodymium: YAG laser iridotomy as a possible contribution to lens dislocation. Ann Ophthalmol. 1986;18:281–2. [PubMed] [Google Scholar]
- 32.Kwon YA, Bae SH, Sohn YH. Bilateral spontaneous anterior lens dislocation in a retinitis pigmentosa patient. Korean J Ophthalmol. 2007;21:124–6. doi: 10.3341/kjo.2007.21.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seong M, Kim MJ, Tchah H. Argon laser iridotomy as a possible cause of anterior dislocation of a crystalline lens. J Cataract Refract Surg. 2009;35:190–2. doi: 10.1016/j.jcrs.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima M, Kawakita T, Shimazaki J. Complete spontaneous crystalline lens dislocation into the anterior chamber with severe corneal endothelial cell loss. Cornea. 2007;26:487–9. doi: 10.1097/ICO.0b013e3180303ae7. [DOI] [PubMed] [Google Scholar]
- 35.Mutoh T, Barrette KF, Matsumoto Y, Chikuda M. Lens dislocation has a possible relationship with laser iridotomy. Clin Ophthalmol. 2012;6:2019–22. doi: 10.2147/OPTH.S37972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu R, Wang X, Wang Y, Sun Y. Occult lens subluxation related to laser peripheral iridotomy: A case report and literature review. Medicine (Baltimore) 2017;96:e6255. doi: 10.1097/MD.0000000000006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He M, Friedman DS, Ge J, Huang W, Jin C, Cai X, et al. Laser peripheral iridotomy in eyes with narrow drainage angles: Ultrasound biomicroscopy outcomes. The Liwan Eye Study. Ophthalmology. 2007;114:1513–9. doi: 10.1016/j.ophtha.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 38.Ramani KK, Mani B, George RJ, Lingam V. Follow-up of primary angle closure suspects after laser peripheral iridotomy using ultrasound biomicroscopy and A-scan biometry for a period of 2 years. J Glaucoma. 2009;18:521–7. doi: 10.1097/IJG.0b013e318193c12d. [DOI] [PubMed] [Google Scholar]
- 39.Talajic JC, Lesk MR, Nantel-Battista M, Harasymowycz PJ. Anterior segment changes after pilocarpine and laser iridotomy for primary angle-closure suspects with Scheimpflug photography. J Glaucoma. 2013;22:776–9. doi: 10.1097/IJG.0b013e318259505a. [DOI] [PubMed] [Google Scholar]
- 40.Sawada A, Yamamoto T. Correlation between extent of preexisting organic angle closure and long-term outcome after laser peripheral iridotomy in eyes with primary angle closure. J Glaucoma. 2012;21:174–9. doi: 10.1097/IJG.0b013e3182070c98. [DOI] [PubMed] [Google Scholar]
- 41.Zebardast N, Kavitha S, Krishnamurthy P, Friedman DS, Nongpiur ME, Aung T, et al. Changes in anterior segment morphology and predictors of angle widening after laser iridotomy in South Indian eyes. Ophthalmology. 2016;123:2519–26. doi: 10.1016/j.ophtha.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Wise JB, Witter SL. Argon laser therapy for open-angle glaucoma. A pilot study. Arch Ophthalmol. 1979;97:319–22. doi: 10.1001/archopht.1979.01020010165017. [DOI] [PubMed] [Google Scholar]
- 43.Smith J. Argon laser trabeculoplasty: Comparison of bichromatic and monochromatic wavelengths. Ophthalmology. 1984;91:355–60. [PubMed] [Google Scholar]
- 44.Spurny RC, Lederer CM., Jr Krypton laser trabeculoplasty. A clinical report. Arch Ophthalmol. 1984;102:1626–8. doi: 10.1001/archopht.1984.01040031316015. [DOI] [PubMed] [Google Scholar]
- 45.McHugh D, Marshall J, Ffytche TJ, Hamilton PA, Raven A. Diode laser trabeculoplasty (DLT) for primary open-angle glaucoma and ocular hypertension. Br J Ophthalmol. 1990;74:743–7. doi: 10.1136/bjo.74.12.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Priore LV, Robin AL, Pollack IP. Long-term follow-up of neodymium: YAG laser angle surgery for open-angle glaucoma. Ophthalmology. 1988;95:277–81. doi: 10.1016/s0161-6420(88)33195-7. [DOI] [PubMed] [Google Scholar]
- 47.Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): A multicenter, pilot, clinical study. Ophthalmology. 1998;105:2082–8. doi: 10.1016/S0161-6420(98)91129-0. discussion 2089-90. [DOI] [PubMed] [Google Scholar]
- 48.The Glaucoma Laser Trial (GLT). 2. Results of argon laser trabeculoplasty versus topical medicines. The Glaucoma Laser Trial Research Group. Ophthalmology. 1990;97:1403–13. [PubMed] [Google Scholar]
- 49.The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Glaucoma Laser Trial Research Group. Am J Ophthalmol. 1995;120:718–31. doi: 10.1016/s0002-9394(14)72725-4. [DOI] [PubMed] [Google Scholar]
- 50.Heijl A, Peters D, Leske MC, Bengtsson B. Effects of argon laser trabeculoplasty in the Early Manifest Glaucoma Trial. Am J Ophthalmol. 2011;152:842–8. doi: 10.1016/j.ajo.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 51.Ederer F, Gaasterland DA, Dally LG, Kim J, Van Veldhuisen PC, Blackwell B, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology. 2004;111:651–64. doi: 10.1016/j.ophtha.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 52.Latina MA, Park C. Selective targeting of trabecular meshwork cells:In vitro studies of pulsed and CW laser interactions. Exp Eye Res. 1995;60:359–71. doi: 10.1016/s0014-4835(05)80093-4. [DOI] [PubMed] [Google Scholar]
- 53.Van Buskirk EM, Pond V, Rosenquist RC, Acott TS. Argon laser trabeculoplasty. Studies of mechanism of action. Ophthalmology. 1984;91:1005–10. doi: 10.1016/s0161-6420(84)34197-5. [DOI] [PubMed] [Google Scholar]
- 54.Nagar M, Ogunyomade A, O’Brart DP, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89:1413–7. doi: 10.1136/bjo.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: The effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol. 2009;93:497–501. doi: 10.1136/bjo.2008.148510. [DOI] [PubMed] [Google Scholar]
- 56.Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol. 2015;60:36–50. doi: 10.1016/j.survophthal.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Bovell AM, Damji KF, Hodge WG, Rock WJ, Buhrmann RR, Pan YI. Long term effects on the lowering of intraocular pressure: Selective laser or argon laser trabeculoplasty? Can J Ophthalmol. 2011;46:408–13. doi: 10.1016/j.jcjo.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Miraftabi A, Nilforushan N, Nassiri N, Nouri-Mahdavi K. Selective laser trabeculoplasty in patients with pseudoexfoliative glaucoma vs primary open angle glaucoma: A one-year comparative study. Int J Ophthalmol. 2016;9:406–10. doi: 10.18240/ijo.2016.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JW, Ho WL, Chan JC, Lai JS. Efficacy of selective laser trabeculoplasty for normal tension glaucoma: 1 year results. BMC Ophthalmol. 2015;15:1. doi: 10.1186/1471-2415-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JW, Shum JJ, Chan JC, Lai JS. Two-year clinical results after selective laser trabeculoplasty for normal tension glaucoma. Medicine (Baltimore) 2015;94:e984. doi: 10.1097/MD.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta V, Ghosh S, Sujeeth M, Chaudhary S, Gupta S, Chaurasia AK, et al. Selective laser trabeculoplasty for primary open-angle glaucoma patients younger than 40 years. Can J Ophthalmol. 2018;53:81–5. doi: 10.1016/j.jcjo.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Song J. Complications of selective laser trabeculoplasty: A review. Clin Ophthalmol. 2016;10:137–43. doi: 10.2147/OPTH.S84996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ingvoldstad DD, Krishna R, Willoughby L. Micropulse diode laser trabeculoplasty versus argon laser trabeculoplasty in the treatment of open-angle glaucoma.[Abstract] Invest Ophthalmol Vis Sci. 2005;46:123. [Google Scholar]
- 64.Fudemberg SJ MJ, Katz LJ. Trabecular meshwork tissue examination with scanning electron microscopy: A comparison of MicroPulse Diode Laser (MLT), Selective Laser (SLT), and Argon Laser (ALT) Trabeculoplasty in Human Cadaver Tissue [Abstract] Invest Ophthalmol Vis Sci. 2008;49:1236. [Google Scholar]
- 65.Fea AM, Bosone A, Rolle T, Brogliatti B, Grignolo FM. Micropulse diode laser trabeculoplasty (MDLT): A phase II clinical study with 12 months follow-up. Clin Ophthalmol. 2008;2:247–52. doi: 10.2147/opth.s2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coombs P, Radcliffe NM. Outcomes of micropulse laser trabeculoplasty vs. selective laser trabeculoplasty. ARVO. 2014 [Google Scholar]
- 67.Rantala E, Valimaki J. Micropulse diode laser trabeculoplasty – 180-degree treatment. Acta Ophthalmol. 2012;90:441–4. doi: 10.1111/j.1755-3768.2010.02026.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee JW, Yau GS, Yick DW, Yuen CY. MicroPulse laser trabeculoplasty for the treatment of open-angle glaucoma. Medicine (Baltimore) 2015;94:e2075. doi: 10.1097/MD.0000000000002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ali Aljasim L, Owaidhah O, Edward DP. Selective laser trabeculoplasty in primary angle-closure glaucoma after laser peripheral iridotomy: A case-control study. J Glaucoma. 2016;25:e253–8. doi: 10.1097/IJG.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 70.Narayanaswamy A, Leung CK, Istiantoro DV, Perera SA, Ho CL, Nongpiur ME, et al. Efficacy of selective laser trabeculoplasty in primary angle-closure glaucoma: A randomized clinical trial. JAMA Ophthalmol. 2015;133:206–12. doi: 10.1001/jamaophthalmol.2014.4893. [DOI] [PubMed] [Google Scholar]
- 71.Ho CL, Lai JS, Aquino MV, Rojanapongpun P, Wong HT, Aquino MC, et al. Selective laser trabeculoplasty for primary angle closure with persistently elevated intraocular pressure after iridotomy. J Glaucoma. 2009;18:563–6. doi: 10.1097/IJG.0b013e318193c2d1. [DOI] [PubMed] [Google Scholar]
- 72.Matos AG, Asrani SG, Paula JS. Feasibility of laser trabeculoplasty in angle closure glaucoma: A review of favourable histopathological findings in narrow angles. Clin Exp Ophthalmol. 2017;45:632–9. doi: 10.1111/ceo.12938. [DOI] [PubMed] [Google Scholar]
- 73.Maleki A, Swan RT, Lasave AF, Ma L, Foster CS. Selective laser trabeculoplasty in controlled uveitis with steroid-induced glaucoma. Ophthalmology. 2016;123:2630–2. doi: 10.1016/j.ophtha.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 74.Wagle NS, Freedman SF, Buckley EG, Davis JS, Biglan AW. Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. Ophthalmology. 1998;105:1921. doi: 10.1016/S0161-6420(98)91042-9. [DOI] [PubMed] [Google Scholar]
- 75.Zhang M, Li B, Wang J, Liu W, Sun Y, Wu X. Clinical results of selective laser trabeculoplasty in silicone oil-induced secondary glaucoma. Graefes Arch Clin Exp Ophthalmol. 2014;252:983–7. doi: 10.1007/s00417-014-2593-7. [DOI] [PubMed] [Google Scholar]
- 76.Krasnov MM. Q-switched laser iridectomy and Q-switched laser goniopuncture. Adv Ophthalmol. 1977;34:192–6. [PubMed] [Google Scholar]
- 77.Kimbrough RL, Trempe CS, Brockhurst RJ, Simmons RJ. Angle-closure glaucoma in nanophthalmos. Am J Ophthalmol. 1979;88(3 Pt 2):572–9. doi: 10.1016/0002-9394(79)90517-8. [DOI] [PubMed] [Google Scholar]
- 78.Ritch R, Tham CC, Lam DS. Argon laser peripheral iridoplasty (ALPI): An update. Surv Ophthalmol. 2007;52:279–88. doi: 10.1016/j.survophthal.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Ritch R. Argon laser peripheral iridoplasty. J Glaucoma. 1992;1:206–13. doi: 10.1097/00061198-199201040-00006. [DOI] [PubMed] [Google Scholar]
- 80.Ritch R. Argon laser treatment for medically unresponsive attacks of angle-closure glaucoma. Am J Ophthalmol. 1982;94:197–204. doi: 10.1016/0002-9394(82)90075-7. [DOI] [PubMed] [Google Scholar]
- 81.Ritch R, Tham CC, Lam DS. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmology. 2004;111:104–8. doi: 10.1016/j.ophtha.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Yip PP, Leung WY, Hon CY, Ho CK. Argon laser peripheral iridoplasty in the management of phacomorphic glaucoma. Ophthalmic Surg Lasers Imaging. 2005;36:286–91. [PubMed] [Google Scholar]
- 83.Solomon R, Barsam A, Voldman A, Holladay J, Bhogal M, Perry HD, et al. Argon laser iridoplasty to improve visual function following multifocal intraocular lens implantation. J Refract Surg. 2012;28:281–3. doi: 10.3928/1081597X-20120209-01. [DOI] [PubMed] [Google Scholar]
- 84.Kang JJ, Allemann N, Cortina MS, de la Cruz J, Aref AA. Argon laser iridoplasty for optic obstruction of Boston keratoprosthesis. Arch Ophthalmol. 2012;130:1051–4. doi: 10.1001/archophthalmol.2012.858. [DOI] [PubMed] [Google Scholar]
- 85.Lai JS, Tham CC, Chua JK, Lam DS. Efficacy and safety of inferior 180 degrees goniosynechialysis followed by diode laser peripheral iridoplasty in the treatment of chronic angle-closure glaucoma. J Glaucoma. 2000;9:388–91. doi: 10.1097/00061198-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Lai JS, Tham CC, Chua JK, Lam DS. Immediate diode laser peripheral iridoplasty as treatment of acute attack of primary angle closure glaucoma: A preliminary study. J Glaucoma. 2001;10:89–94. doi: 10.1097/00061198-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 87.Lai JS, Tham CC, Lam DS. The efficacy and safety of combined phacoemulsification, intraocular lens implantation, and limited goniosynechialysis, followed by diode laser peripheral iridoplasty, in the treatment of cataract and chronic angle-closure glaucoma. J Glaucoma. 2001;10:309–15. doi: 10.1097/00061198-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 88.Zhang HC, Yao K. [Peripheral iridoplasty with doubled-frequency Nd:YAG laser as treatment for angle-closure glaucoma] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2002;31:388–90. doi: 10.3785/j.issn.1008-9292.2002.05.020. [DOI] [PubMed] [Google Scholar]
- 89.Lam DS, Lai JS, Tham CC, Chua JK, Poon AS. Argon laser peripheral iridoplasty versus conventional systemic medical therapy in treatment of acute primary angle-closure glaucoma: A prospective, randomized, controlled trial. Ophthalmology. 2002;109:1591–16. doi: 10.1016/s0161-6420(02)01158-2. [DOI] [PubMed] [Google Scholar]
- 90.Lai JS, Tham CC, Chua JK, Poon AS, Lam DS. Laser peripheral iridoplasty as initial treatment of acute attack of primary angle-closure: A long-term follow-up study. J Glaucoma. 2002;11:484–7. doi: 10.1097/00061198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Espana EM, Ioannidis A, Tello C, Liebmann JM, Foster P, Ritch R. Urrets-Zavalia syndrome as a complication of argon laser peripheral iridoplasty. Br J Ophthalmol. 2007;91:427–9. doi: 10.1136/bjo.2006.105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bourne RRA, Zhekov I, Pardhan S. Temporal ocular coherence tomography-measured changes in anterior chamber angle and diurnal intraocular pressure after laser iridoplasty: IMPACT study. Br J Ophthalmol. 2017;101:886–91. doi: 10.1136/bjophthalmol-2016-308720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Narayanaswamy A, Baskaran M, Perera SA, Nongpiur ME, Htoon HM, Tun TA, et al. Argon laser peripheral iridoplasty for primary angle-closure glaucoma: A randomized controlled trial. Ophthalmology. 2016;123:514–21. doi: 10.1016/j.ophtha.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Abe RY, Tavares CM, Schimiti RB, Vasconcellos JP, Costa VP. Ahmed glaucoma valve implantation for refractory glaucoma in a tertiary hospital in Brazil. J Ophthalmol. 2015;2015:850785. doi: 10.1155/2015/850785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krupin T, Mitchell KB, Becker B. Cyclocryotherapy in neovascular glaucoma. Am J Ophthalmol. 1978;86:24–6. doi: 10.1016/0002-9394(78)90008-9. [DOI] [PubMed] [Google Scholar]
- 96.Bellows AR, Grant WM. Cyclocryotherapy of chronic open-angle glaucoma in aphakic eyes. Am J Ophthalmol. 1978;85(5 Pt 1):615–21. doi: 10.1016/s0002-9394(14)77093-x. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki Y, Araie M, Yumita A, Yamamoto T. Transscleral Nd:YAG laser cyclophotocoagulation versus cyclocryotherapy. Graefes Arch Clin Exp Ophthalmol. 1991;229:33–6. doi: 10.1007/BF00172258. [DOI] [PubMed] [Google Scholar]
- 98.van der Zypen E, England C, Fankhauser F, Kwasniewska S. The effect of transscleral laser cyclophotocoagulation on rabbit ciliary body vascularization. Graefes Arch Clin Exp Ophthalmol. 1989;227:172–9. doi: 10.1007/BF02169792. [DOI] [PubMed] [Google Scholar]
- 99.Beckman H, Kinoshita A, Rota AN, Sugar HS. Transscleral ruby laser irradiation of the ciliary body in the treatment of intractable glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:423–36. [PubMed] [Google Scholar]
- 100.Brancato R, Carassa R, Trabucchi G. Diode laser compared with argon laser for trabeculoplasty. Am J Ophthalmol. 1991;112:50–5. doi: 10.1016/s0002-9394(14)76212-9. [DOI] [PubMed] [Google Scholar]
- 101.Cantor LB, Nichols DA, Katz LJ, Moster MR, Poryzees E, Shields JA, et al. Neodymium-YAG transscleral cyclophotocoagulation. The role of pigmentation. Invest Ophthalmol Vis Sci. 1989;30:1834–7. [PubMed] [Google Scholar]
- 102.Assia EI, Hennis HL, Stewart WC, Legler UF, Carlson AN, Apple DJ. A comparison of neodymium:yttrium aluminum garnet and diode laser transscleral cyclophotocoagulation and cyclocryotherapy. Invest Ophthalmol Vis Sci. 1991;32:2774–8. [PubMed] [Google Scholar]
- 103.Youn J, Cox TA, Herndon LW, Allingham RR, Shields MB. A clinical comparison of transscleral cyclophotocoagulation with neodymium: YAG and semiconductor diode lasers. Am J Ophthalmol. 1998;126:640–7. doi: 10.1016/s0002-9394(98)00228-1. [DOI] [PubMed] [Google Scholar]
- 104.Pastor SA, Singh K, Lee DA, Juzych MS, Lin SC, Netland PA, et al. Cyclophotocoagulation: A report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:2130–8. doi: 10.1016/s0161-6420(01)00889-2. [DOI] [PubMed] [Google Scholar]
- 105.Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, et al. “Cyclodiode.” Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104:1508. doi: 10.1016/s0161-6420(97)30109-2. [DOI] [PubMed] [Google Scholar]
- 106.Agarwal HC, Gupta V, Sihota R. Evaluation of contact versus non-contact diode laser cyclophotocoagulation for refractory glaucomas using similar energy settings. Clin Exp Ophthalmol. 2004;32:33–8. doi: 10.1046/j.1442-9071.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- 107.Oguri A, Takahashi E, Tomita G, Yamamoto T, Jikihara S, Kitazawa Y. Transscleral cyclophotocoagulation with the diode laser for neovascular glaucoma. Ophthalmic Surg Lasers. 1998;29:722–7. [PubMed] [Google Scholar]
- 108.Rodriguez-Garcia A, Gonzalez-Gonzalez LA, Carlos Alvarez-Guzman J. Trans-scleral diode laser cyclophotocoagulation for refractory glaucoma after high-risk penetrating keratoplasty. Int Ophthalmol. 2016;36:373–83. doi: 10.1007/s10792-015-0130-2. [DOI] [PubMed] [Google Scholar]
- 109.Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2007;91:1631–5. doi: 10.1136/bjo.2007.116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gedde SJ. Management of glaucoma after retinal detachment surgery. Curr Opin Ophthalmol. 2002;13:103–9. doi: 10.1097/00055735-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Honavar SG, Goyal M, Majji AB, Sen PK, Naduvilath T, Dandona L. Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology. 1999;106:169–76. doi: 10.1016/S0161-6420(99)90017-9. discussion 177. [DOI] [PubMed] [Google Scholar]
- 112.Schlote T, Derse M, Thiel HJ, Jean B. Pupillary distortion after contact transscleral diode laser cyclophotocoagulation. Br J Ophthalmol. 2000;84:337–8. doi: 10.1136/bjo.84.3.337a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: Role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109:316–23. doi: 10.1016/s0161-6420(01)00898-3. [DOI] [PubMed] [Google Scholar]
- 114.Autrata R, Rehurek J. Long-term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients. Ophthalmologica. 2003;217:393–400. doi: 10.1159/000073068. [DOI] [PubMed] [Google Scholar]
- 115.Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J AAPOS. 2009;13:379–83. doi: 10.1016/j.jaapos.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ness PJ, Khaimi MA, Feldman RM, Tabet R, Sarkisian SR, Jr, Skuta GL, et al. Intermediate term safety and efficacy of transscleral cyclophotocoagulation after tube shunt failure. J Glaucoma. 2012;21:83–8. doi: 10.1097/IJG.0b013e31820bd1ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bloom PA, Clement CI, King A, Noureddin B, Sharma K, Hitchings RA, et al. A comparison between tube surgery, Nd:YAG laser and diode laser cyclophotocoagulation in the management of refractory glaucoma. Biomed Res Int 2013. 2013:371951. doi: 10.1155/2013/371951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin KR, Broadway DC. Cyclodiode laser therapy for painful, blind glaucomatous eyes. Br J Ophthalmol. 2001;85:474–6. doi: 10.1136/bjo.85.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manna A, Foster P, Papadopoulos M, Nolan W. Cyclodiode laser in the treatment of acute angle closure. Eye (Lond) 2012;26:742–5. doi: 10.1038/eye.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shakir M, Bokhari SA, Zafar S, Zeeshan K, Rizvi SF. Transscleral diode laser cyclophotoco-aglation for refractory glaucoma. Pak J Ophthalmol. 2012;28:22–6. [Google Scholar]
- 121.Walland MJ. Diode laser cyclophotocoagulation: Longer term follow up of a standardized treatment protocol. Clin Exp Ophthalmol. 2000;28:263–7. doi: 10.1046/j.1442-9071.2000.00320.x. [DOI] [PubMed] [Google Scholar]
- 122.Schubert HD, Agarwala A, Arbizo V. Changes in aqueous outflow after in vitro neodymium:yttrium aluminum garnet laser cyclophotocoagulation. Invest Ophthalmol Vis Sci. 1990;31:1834–8. [PubMed] [Google Scholar]
- 123.Pantcheva MB, Kahook MY, Schuman JS, Noecker RJ. Comparison of acute structural and histopathological changes in human autopsy eyes after endoscopic cyclophotocoagulation and trans-scleral cyclophotocoagulation. Br J Ophthalmol. 2007;91:248–52. doi: 10.1136/bjo.2006.103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gupta V, Agarwal HC. Contact trans-scleral diode laser cyclophotocoagulation treatment for refractory glaucomas in the Indian population. Indian J Ophthalmol. 2000;48:295–300. [PubMed] [Google Scholar]
- 125.Schuman JS, Bellows AR, Shingleton BJ, Latina MA, Allingham RR, Belcher CD, et al. Contact transscleral Nd:YAG laser cyclophotocoagulation. Midterm results. Ophthalmology. 1992;99:1089–194. doi: 10.1016/s0161-6420(92)31846-9. discussion 1095. [DOI] [PubMed] [Google Scholar]
- 126.Jennings BJ, Mathews DE. Complications of neodymium: YAG cyclophotocoagulation in the treatment of open-angle glaucoma. Optom Vis Sci. 1999;76:686–91. doi: 10.1097/00006324-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 127.Liu W, Chen Y, Lv Y, Wang L, Xing X, Liu A, et al. Diode laser transscleral cyclophotocoagulation followed by phacotrabeculectomy on medically unresponsive acute primary angle closure eyes: The long-term result. BMC Ophthalmol. 2014;14:26. doi: 10.1186/1471-2415-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh K, Jain D, Veerwal V. Diode laser cyclophotocoagulation in Indian eyes: Efficacy and safety. Int Ophthalmol. 2017;37:79–84. doi: 10.1007/s10792-016-0228-1. [DOI] [PubMed] [Google Scholar]
- 129.Spencer AF, Vernon SA. “Cyclodiode”: Results of a standard protocol. Br J Ophthalmol. 1999;83:311–6. doi: 10.1136/bjo.83.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rao VJ, Dayan M. Lens subluxation following contact transscleral cyclodiode. Arch Ophthalmol. 2002;120:1393–4. [PubMed] [Google Scholar]
- 131.Lai JS, Tham CC, Lam DS. Pupillary distortion and staphyloma following transscleral contact diode laser cyclophotocoagulation: A clinicopathological study of three patients. Eye (Lond) 2002;16:674. doi: 10.1038/sj.eye.6700178. author reply 675. [DOI] [PubMed] [Google Scholar]
- 132.Bhola RM, Prasad S, McCormick AG, Rennie IG, Talbot JF, Parsons MA. Pupillary distortion and staphyloma following trans-scleral contact diode laser cyclophotocoagulation: A clinicopathological study of three patients. Eye (Lond) 2001;15(Pt 4):453–47. doi: 10.1038/eye.2001.154. [DOI] [PubMed] [Google Scholar]
- 133.Brown AJ, Tripathi RC, Tripathi BJ. London: Chapman and Hall Medical; 1997. Wolff's Anatomy of the Eye and the Orbit; p. 229. [Google Scholar]
- 134.Ishida K. Update on results and complications of cyclophotocoagulation. Curr Opin Ophthalmol. 2013;24:102–10. doi: 10.1097/ICU.0b013e32835d9335. [DOI] [PubMed] [Google Scholar]
- 135.Schlote T, Derse M, Zierhut M. Transscleral diode laser cyclophotocoagulation for the treatment of refractory glaucoma secondary to inflammatory eye diseases. Br J Ophthalmol. 2000;84:999–1003. doi: 10.1136/bjo.84.9.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001;10:294–301. doi: 10.1097/00061198-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 137.Shah P, Lee GA, Kirwan JK, Bunce C, Bloom PA, Ficker LA, et al. Cyclodiode photocoagulation for refractory glaucoma after penetrating keratoplasty. Ophthalmology. 2001;108:1986–91. doi: 10.1016/s0161-6420(01)00767-9. [DOI] [PubMed] [Google Scholar]
- 138.Gupta V, Sony P, Sihota R. Inadvertent sclerostomy with encysted bleb following trans-scleral contact diode laser cyclophotocoagulation. Clin Exp Ophthalmol. 2006;34:86–7. doi: 10.1111/j.1442-9071.2006.01125.x. [DOI] [PubMed] [Google Scholar]
- 139.Venkatesh P, Gogoi M, Sihota R, Agarwal H. Panophthalmitis following contact diode laser cyclophotocoagulation in a patient with failed trabeculectomy and trabeculotomy for congenital glaucoma. Br J Ophthalmol. 2003;87:508. doi: 10.1136/bjo.87.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Norris JL, Cleasby GW. An endoscope for ophthalmology. Am J Ophthalmol. 1978;85:420–2. doi: 10.1016/s0002-9394(14)77741-4. [DOI] [PubMed] [Google Scholar]
- 141.Seibold LK, SooHoo JR, Kahook MY. Endoscopic cyclophotocoagulation. Middle East Afr J Ophthalmol. 2015;22:18–24. doi: 10.4103/0974-9233.148344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Francis BA, Kwon J, Fellman R, Noecker R, Samuelson T, Uram M, et al. Endoscopic ophthalmic surgery of the anterior segment. Surv Ophthalmol. 2014;59:217–31. doi: 10.1016/j.survophthal.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 143.Francis BA, Pouw A, Jenkins D, Babic K, Vakili G, Tan J, et al. Endoscopic cycloplasty (ECPL) and lens extraction in the treatment of severe plateau iris syndrome. J Glaucoma. 2016;25:e128–33. doi: 10.1097/IJG.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 144.Pathak-Ray V, Ahmed, II Phaco-endocycloplasty: A novel technique for management of ring iridociliary cyst presenting as acute angle closure. Oman J Ophthalmol. 2016;9:63–5. doi: 10.4103/0974-620X.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin S. Endoscopic cyclophotocoagulation. Br J Ophthalmol. 2002;86:1434–8. doi: 10.1136/bjo.86.12.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol. 1997;124:787–96. doi: 10.1016/s0002-9394(14)71696-4. [DOI] [PubMed] [Google Scholar]
- 147.Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology. 1992;99:1823–8. doi: 10.1016/s0161-6420(92)31718-x. [DOI] [PubMed] [Google Scholar]
- 148.Lima FE, Magacho L, Carvalho DM, Susanna R, Jr, Avila MP. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;13:233–7. doi: 10.1097/00061198-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 149.Gayton JL, Van Der Karr M, Sanders V. Combined cataract and glaucoma surgery: Trabeculectomy versus endoscopic laser cycloablation. J Cataract Refract Surg. 1999;25:1214–9. doi: 10.1016/s0886-3350(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 150.Clement CI, Kampougeris G, Ahmed F, Cordeiro MF, Bloom PA. Combining phacoemulsification with endoscopic cyclophotocoagulation to manage cataract and glaucoma. Clin Exp Ophthalmol. 2013;41:546–51. doi: 10.1111/ceo.12051. [DOI] [PubMed] [Google Scholar]
- 151.Lindfield D, Ritchie RW, Griffiths MF. “Phaco-ECP”: Combined endoscopic cyclophotocoagulation and cataract surgery to augment medical control of glaucoma. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roberts SJ, Mulvahill M, SooHoo JR, Pantcheva MB, Kahook MY, Seibold LK. Efficacy of combined cataract extraction and endoscopic cyclophotocoagulation for the reduction of intraocular pressure and medication burden. Int J Ophthalmol. 2016;9:693–8. doi: 10.18240/ijo.2016.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40:1313–21. doi: 10.1016/j.jcrs.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 154.Lima FE, Carvalho DM, Avila MP. [Phacoemulsification and endoscopic cyclophotocoagulation as primary surgical procedure in coexisting cataract and glaucoma] Arq Bras Oftalmol. 2010;73:419–22. doi: 10.1590/s0004-27492010000500006. [DOI] [PubMed] [Google Scholar]
- 155.Marco S, Damji KF, Nazarali S, Rudnisky CJ. Cataract and glaucoma surgery: Endoscopic cyclophotocoagulation versus trabeculectomy. Middle East Afr J Ophthalmol. 2017;24:177–82. doi: 10.4103/meajo.MEAJO_232_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan AM, Chockalingam M, Aquino MC, Lim ZI, See JL, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38:266–72. doi: 10.1111/j.1442-9071.2010.02238.x. [DOI] [PubMed] [Google Scholar]
- 157.Aquino MC, Barton K, Tan AM, Sng C, Li X, Loon SC, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol. 2015;43:40–6. doi: 10.1111/ceo.12360. [DOI] [PubMed] [Google Scholar]
- 158.Hoskins HD, Jr, Migliazzo C. Management of failing filtering blebs with the Argon laser. Ophthalmic Surg. 1984;15:731–3. [PubMed] [Google Scholar]
- 159.Kapetansky FM. Laser suture lysis after trabeculectomy. J Glaucoma. 2003;12:316–20. doi: 10.1097/00061198-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Pappa KS, Derick RJ, Weber PA, Kapetansky FM, Baker ND, Lehmann DM. Late argon laser suture lysis after mitomycin C trabeculectomy. Ophthalmology. 1993;100:1268–71. doi: 10.1016/s0161-6420(93)31494-6. [DOI] [PubMed] [Google Scholar]
- 161.DiSclafani M, Liebmann JM, Ritch R. Malignant glaucoma following argon laser release of scleral flap sutures after trabeculectomy. Am J Ophthalmol. 1989;108:597–8. doi: 10.1016/0002-9394(89)90441-8. [DOI] [PubMed] [Google Scholar]
- 162.Kumar H, Ahuja S, Garg SP. Neodymium: YAG laser iridolenticular synechiolysis in uveitis. Ophthalmic Surg. 1994;25:288–91. [PubMed] [Google Scholar]
- 163.Sony P, Kumar H, Pushker N. Treatment of overhanging blebs with frequency-doubled Nd:YAG laser. Ophthalmic Surg Lasers Imaging. 2004;35:429–32. [PubMed] [Google Scholar]
- 164.Kumar H, Dangda S. Bleb reduction using combined photodisruptive and photocoagulative neodymium-doped yttrium-aluminum-garnet laser. Indian J Ophthalmol. 2016;64:934–6. doi: 10.4103/0301-4738.198846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Epstein DL, Steinert RF, Puliafito CA. Neodymium-YAG laser therapy to the anterior hyaloid in aphakic malignant (ciliovitreal block) glaucoma. Am J Ophthalmol. 1984;98:137–43. doi: 10.1016/0002-9394(87)90347-3. [DOI] [PubMed] [Google Scholar]
- 166.Brown RH, Lynch MG, Tearse JE, Nunn RD. Neodymium-YAG vitreous surgery for phakic and pseudophakic malignant glaucoma. Arch Ophthalmol. 1986;104:1464–6. doi: 10.1001/archopht.1986.01050220058026. [DOI] [PubMed] [Google Scholar]
- 167.Little BC, Hitchings RA. Pseudophakic malignant glaucoma: Nd:YAG capsulotomy as a primary treatment. Eye (Lond) 1993;7(Pt 1):102–4. doi: 10.1038/eye.1993.21. [DOI] [PubMed] [Google Scholar]
- 168.Melamed S, Ashkenazi I, Blumenthal M. Nd-YAG laser hyaloidotomy for malignant glaucoma following one-piece 7 mm intraocular lens implantation. Br J Ophthalmol. 1991;75:501–3. doi: 10.1136/bjo.75.8.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Debrouwere V, Stalmans P, Van Calster J, Spileers W, Zeyen T, Stalmans I. Outcomes of different management options for malignant glaucoma: A retrospective study. Graefes Arch Clin Exp Ophthalmol. 2012;250:131–41. doi: 10.1007/s00417-011-1763-0. [DOI] [PubMed] [Google Scholar]
- 170.Wise JB. Long-term control of adult open angle glaucoma by argon laser treatment. Ophthalmology. 1981;88:197–202. doi: 10.1016/s0161-6420(81)35049-0. [DOI] [PubMed] [Google Scholar]
- 171.Turati M, Gil-Carrasco F, Morales A, Quiroz-Mercado H, Andersen D, Marcellino G, et al. Patterned laser trabeculoplasty. Ophthalmic Surg Lasers Imaging. 2010;41:538–45. doi: 10.3928/15428877-20100910-02. [DOI] [PubMed] [Google Scholar]
- 172.Goldenfeld M, Melamed S, Simon G, Ben Simon GJ. Titanium: sapphire laser trabeculoplasty versus argon laser trabeculoplasty in patients with open-angle glaucoma. Ophthalmic Surg Lasers Imaging. 2009;40:264–9. doi: 10.3928/15428877-20090430-07. [DOI] [PubMed] [Google Scholar]


