Abstract
Purpose:
To compare the visual outcomes and higher order aberrations (HOAs) following wavefront optimized (WFO) laser in situ keratomileusis (LASIK) versus topography-guided customized ablation (TCAT) LASIK for myopia and myopic astigmatism.
Methods:
Patients who underwent femtosecond-assisted LASIK for myopic correction between August 2016 and October 2017 were included in this interventional prospective case series. The following parameters were evaluated preoperatively and at 3 months’ postoperative visit: uncorrected distance vision acuity (UDVA) and corrected distance vision acuity (CDVA), manifest refraction, and HOAs.
Results:
Two hundred eyes of 100 patients were included in the study. At 3 months’ postoperative visit, 92% and 90% eyes in the TCAT and WFO groups, respectively, demonstrated a UDVA of 20/20 or better (P = 0.90). A residual manifest spherical equivalent within 0.5 D was achieved in 100% and 95% of the eyes in the TCAT and WFO groups, respectively (P = 0.10). No significant difference was observed in the HOAs induced in both the groups, with slightly lower induction of trefoil and horizontal coma in the TCAT group.
Conclusion:
Both groups demonstrated similar refractive efficacy and predictability, with greater gain of CDVA following TCAT ablation. HOAs induced were not significantly different between the two groups. Further studies are needed to validate the superiority of one procedure over the other.
Keywords: higher order aberrations, laser in situ keratomileusis, topography-guided customized ablation, wavefront optimized ablation
Corneal refractive surgery has witnessed various advancements over the past few decades, since its inception in the late 1990s. With a greater understanding of the optical aberrations of the eye, various excimer laser ablation patterns have evolved. Wavefront-guided (WFG) laser in situ keratomileusis (LASIK) produces a customized ablation profile to treat both the preoperative lower and higher order aberrations (HOAs) of the eye.[1] The wavefront optimized (WFO) approach minimizes treatment-induced spherical aberrations, with virtually no loss of contrast, postoperative glare or haloes.[2] However, it does not address the preexisting optical aberrations.[3] Topography-guided customized ablation (TCAT) profile is the latest modality of excimer laser correction that attempts to maintain the corneal aspheric profile and neutralizes corneal irregularities.[4,5]
The present literature demonstrating outcomes of TCAT in normal corneas has been promising. However, data comparing the outcomes following WFO and TCAT profiles are limited. The relative paucity of studies comparing the visual outcomes and HOAs following TCAT and WFO treatments, in femtosecond LASIK prompted the present study.[6,7] This prospective comparative interventional case series was designed to compare the safety, efficacy, refractive predictability, and postoperative HOAs following TCAT and WFO ablation.
Methods
Two hundred eyes of 100 consecutive patients were enrolled in this prospective, contralateral interventional study at a tertiary eye care hospital from August 2016 to October 2017. The study received approval from the Ethics Committee of our Institute and was conducted in accordance with the tenets of the Declaration of Helsinki. A written informed consent was obtained from all patients prior to the surgical procedure.
Study population
The study was conducted on patients who presented to our refractive services between August 2016 and October 2017 seeking refractive correction. The inclusion criteria were age >18 years, with a documented refractive stability for a minimum period of 1 year (a change of 0.25 diopters [D] or less) and discontinuation of soft contact lenses for at least 2 weeks. Patients with a corrected distance visual acuity (CDVA) of 20/25 or better and a spherical equivalent (SEQ) refraction of up to −10.00 D were included in the study. Exclusion criteria included a thinnest pachymetry lower than 490 μm, a residual stromal bed lower than 290 μm, topographic evidence of corneal ectasia, previous ocular surgery, history of herpetic eye disease, corneal scarring, collagen vascular disease, pregnancy, and lactation.
Study design
Preoperative evaluation included UDVA and CDVA, manifest refraction, slit lamp bio-microscopy, and dilated fundus evaluation. Corneal topography data for TCAT was obtained from the Vario Topolyser placido-based topography (WaveLight, Erlangen, Germany) and Scheimpflug corneal tomography with Oculyzer II (WaveLight). Higher order abberations were measured using the Zernicke polynomials obtained from the Oculyzer II for a standardised 6 mm zone.
Surgical procedure
All surgeries were performed by experienced refractive surgeons under topical anesthesia and aseptic conditions. Table of random numbers was used to determine the eye to be treated with WFO LASIK, whereas the fellow eye underwent TCAT LASIK. LASIK flaps were fashioned using the VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany) with a pulse energy of 140 nJ and a track spot distance of 3.0 μ and 1.5 μ for the flap bed and side cut, respectively. A flap diameter of 8.6 mm with an intended depth of 100 μ and a 4 mm superior hinge was constructed. The eye was positioned under the curved contact interface and the patient was asked to fixate at the “green blinking light.” This allowed auto-centration at the visual axis, following which suction was initiated. The flap cut was fashioned first from periphery to centre, followed by the side cut. Following blunt dissection and flap lift, the stromal bed was ablated with excimer laser (EX500 WaveLight) using an optic zone of 6.0 mm with a 1.25 mm transition zone. The corneal pachymetry and topographic data were imported from the Oculyzer II and Vario topolyzer.
Postoperatively, both groups received treatment with topical steroids (loteprednol etabonate 0.5% ophthalmic suspension) in tapering dose and lubricating drops (carboxymethylcellulose 0.5% ophthalmic drops). Follow-up visits included postoperative day 1, 6 weeks, and 3 months.
Statistical analysis
Data analysis was done with the help of a computer using SPSS software (version 17.0; SPSS, Inc., Chicago, IL) for Windows. Using this software, range, frequencies, percentages, means, standard deviations, and P values were calculated. The Kolmogorov–Smirnov test was used to analyze the normality of parameters. The independent sample t-test was performed for normally distributed data, whereas the nonparametric test was used for non-normal distributions. The correlation analyses were performed using the Pearson method based on the normality of parameters. A P value of < 0.05 denoted a significant relationship.
Results
Patient demographics
This study included 63 females and 37 males, with a mean age of 25.27 ± 4.03 years. The mean refractive spherical equivalent (MRSE) was −3.46 ± 2.14 D and −3.51 ± 1.94 D in the WFO and TCAT groups, respectively (P = 0.85), with a spherical error of −3.07 ± 2.10 D and −3.05 ± 2.02 D (P = 0.94) and a cylinder of −0.89 ± 0.99 D and −0.92 ± 1.08 D, respectively (P = 0.83). The preoperative patient demographics demonstrated age and spherical equivalent matched groups. There was no significant difference in the mesopic pupil diameter (2.84 ± 0.46 mm in WFO versus 2.91 ± 0.35 mm in TCAT, P > 0.05).
UDVA outcomes and stability
At the 3 months’ postoperative visit, 92 and 57% of the eyes demonstrated a UDVA of 20/20 and 20/16 or better, respectively, in the TCAT group [Fig. 1a]. Whereas in the WFO group, 90 and 58% of the eyes presented with a UDVA of 20/20 and 20/16 or better, respectively (P = 0.90) [Fig. 1b].
Figure 1.
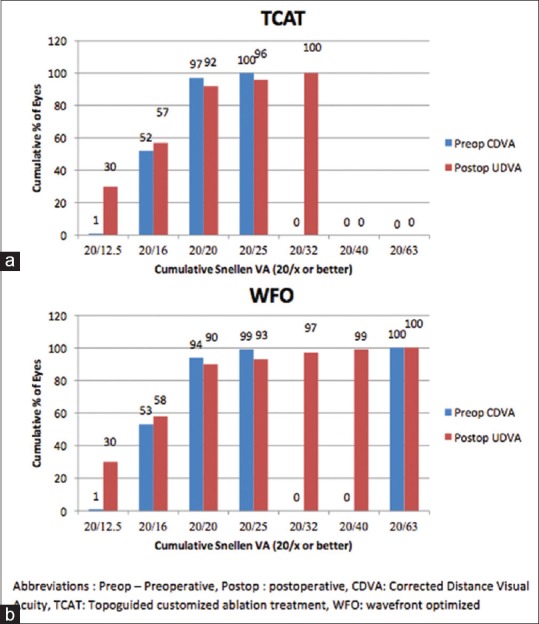
Percentage of eyes that achieved uncorrected visual acuity of 20/20 and better in the (a) TCAT and (b) WFO groups
Safety
The gain or loss of CDVA at 3 months’ follow-up indicated that 34% of the eyes in the TCAT group versus 32% in the WFO group gained one line (P = 0.080). A gain of two lines was noted in 8 and 7% of the eyes in the TCAT and WFO groups, respectively (P = 0.10). There was no loss of lines in either group [Fig. 2].
Figure 2.
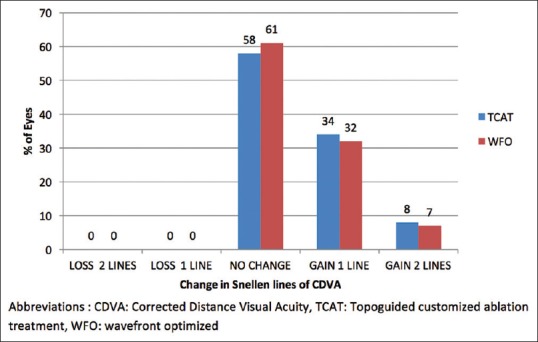
The number of lines lost and gained in the TCAT and WFO groups
Attempted versus achieved spherical equivalent correction
Fig. 3a and b demonstrates the scatter plot analysis comparing attempted and achieved spherical equivalent correction in the TCAT and WFO groups, respectively. The coefficient of determination between the attempted and achieved MRSE was similar between the TCAT (R2 = 0.99) and WFO (R2 = 0.99) groups. This was not statistically significant (P = 0.90).
Figure 3.
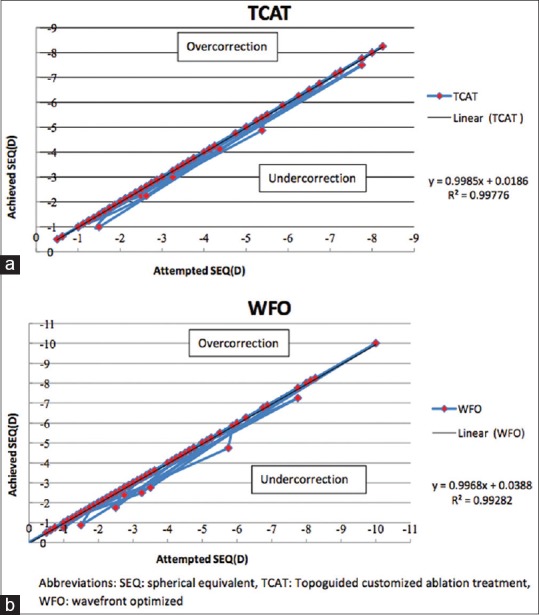
Attempted versus achieved SEQ in the form of a scatter plot in the (a) TCAT and (b) WFO ablation groups
Refractive predictability and accuracy
The residual manifest SEQ within ± 0.5D was achieved by 100% of eyes in the TCAT group compared to 95% in the WFO group (P = 0.10, Fig. 4).
Figure 4.
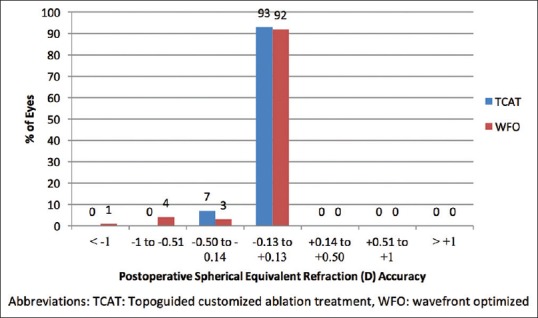
SEQ refractive accuracy in terms of number of eyes that were within ±0.50 diopters (D) of the attempted correction in the TCAT and WFO groups
Higher order aberrations
The higher order aberrations evaluated included trefoil, horizontal, and vertical coma and spherical aberrations. The preoperative and postoperative comparison of both the groups is shown in Table 1a. At the end of 3 months’ follow-up, the TCAT group demonstrated a decrease in trefoil (P = 0.55) as compared to an increase in the WFO group (P = 0.50). However, the difference was not statistically significant in either group. The induction of spherical aberration and coma, however, was significant in both the groups.
Table 1a.
Intragroup comparison of higher order aberrations following WFO and TCAT ablation
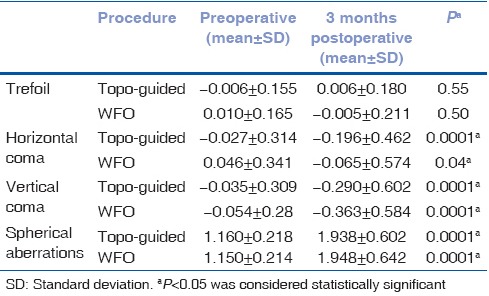
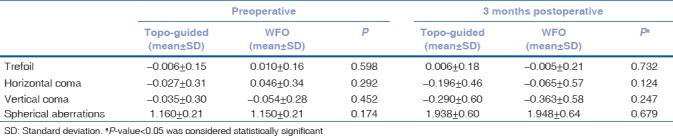
Intergroup comparison demonstrated no significant difference between the groups, although the induced trefoil and coma was slightly lower in the TCAT group [Table 1b]. No significant difference in postoperative dryness on Schirmers tear test strips was noted at 6 weeks and 3 months’ postoperative visit.
Table 1b.
Intergroup comparison of higher order aberrations following WFO and TCAT ablation
Low- and high-contrast sensitivity (ETDRS Contrast, Aurochart, Aurolab, India) for both mesopic and scotopic conditions demonstrated no statistically significant difference between the two groups (P = 0.26).
Discussion
Femtosecond LASIK entails the creation of a flap with subsequent excimer laser stromal ablation. Various changes ensue, including asymmetric anterior surface ablation with subsequent change in corneal asphericity, biomechanical changes, and wound healing-induced epithelial hyperplasia.[8] These changes are responsible for inducing spherical aberrations following keratoablative procedures. Additionally, HOAs including coma and trefoil further effect the visual quality with resultant phenomenon such as glare and halos.[9]
Traditional LASIK aims at treating the lower order aberrations, however, an increase in the HOAs particularly coma and spherical aberrations is induced.[10,11] Topographic-guided corneal regularization in highly aberrated keratoconic eyes offers promising outcomes.[12] TCAT LASIK for myopic refractive correction offers topo-guided regularization of HOAs, including coma and trefoil, along with the correction of refractive error utilizing the WFO approach. This intuitively would result in lower induction of HOAs, with subsequently better contrast and reduced glare and halos.
Comparison between laser platforms or ablation algorithms is often hampered by lack of standardization in patient selections, variations in wound healing, and corneal biomechanics. A contralateral protocol aids to limit intersubjective bias, including healing properties, environmental, psychological, and compliance issues. Published literatures comparing outcomes between WFO and topo-guided LASIK in contralateral eyes are limited.[6,7,13]
Earlier data demonstrated the induction of significantly lower spherical aberrations following TCAT LASIK as compared to WFO ablation.[6,7] Although our study demonstrates a lower induction of spherical aberrations following TCAT, the difference was not statistically significant. Additionally, a significantly lower coma following TCAT ablation was demonstrated in previous data,[6,13] while other cohorts demonstrated no significant difference between the two groups.[7] In our cohort, we noted reduced postoperative coma and trefoil following TCAT ablation; however, the difference was not significant between the two groups postoperatively.
Our study demonstrated equivalent outcomes following WFO and TCAT ablation for myopic LASIK, both in terms of refractive efficacy and predictability. 92% of the eyes in the TCAT group versus 90% in the WFO cohort achieved an UDVA of 20/20 or better (P > 0.05). The results were similar to the data published by Shetty and co-workers in a contralateral study including 30 patients.[7] The predictability of refractive outcomes between the two groups was similar wherein all eyes (TCAT group) versus 95% eyes (WFO group) achieved a postoperative MRSE within 0.50 D.
Conclusion
Refractive outcomes in terms of achieved spherical equivalent, efficacy, and safety were similar in both the WFO and TCAT groups. Additionally, no significant reduction was noted in induced HOAs (spherical aberration, coma, and trefoil) following TCAT in normal corneas. This study demonstrates the nonsuperiority of TCAT LASIK over traditional WFO ablation profile for regular corneas, contrary to data published thus far. Further studies are needed to analyze corneal response to customized ablations and ascertain the superiority of one ablation profile over the other. Till such time, the advantages of TCAT ablation may be limited to highly aberrated eyes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Schallhorn SC, Farjo AA, Huang D, Wachler B, Trattler WB, Tanzer DJ, et al. Wavefront-guided LASIK for the correction of primary myopia and astigmatism a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1249–61. doi: 10.1016/j.ophtha.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 2.El Awady HE, Ghanem AA, Saleh SM. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: Comparative study of higher order aberrations. Ophthalmic Surg Lasers Imaging. 2011;42:314–20. doi: 10.3928/15428877-20110421-01. [DOI] [PubMed] [Google Scholar]
- 3.Tan J, Simon D, Mrochen M, Por YM. Clinical results of topography-based customized ablations for myopia and myopic astigmatism. J Refract Surg. 2012;28:S829–S836. doi: 10.3928/1081597X-20121005-04. [DOI] [PubMed] [Google Scholar]
- 4.Stulting D, Fant BS the T-CAT Study Group. Results of topography-guided laser in situ keratomileusis custom ablation treatment with refractive excimer laser. J Cataract Refract Surg. 2016;42:11–8. doi: 10.1016/j.jcrs.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Sekundo W, Gertnere J, Bertelmann T, Solomatin I. One-year refractive results, contrast sensitivity, high-order aberrations and complications after myopic small-incision lenticule extraction (ReLEx SMILE) Graefes Arch Clin Exp Ophthalmol. 2014;252:837–43. doi: 10.1007/s00417-014-2608-4. [DOI] [PubMed] [Google Scholar]
- 6.Farooqui MA, Al-Muammar AR. Topography-guided CATz versus conventional LASIK for myopia with the NIDEK EC-5000: a bilateral eye study. J Refract Surg. 2006;22:741–5. doi: 10.3928/1081-597X-20061001-03. [DOI] [PubMed] [Google Scholar]
- 7.Shetty R, Shroff R, Deshpande K, Gowda R, Lahane S, Jayadev C. A prospective study to compare visual outcomes between wavefront-optimized and topography-guided ablation profiles in contralateral eyes with myopia. J Refract Surg. 2017;33:6–10. doi: 10.3928/1081597X-20161006-01. [DOI] [PubMed] [Google Scholar]
- 8.Robert C. The cornea is not a piece of plastic. J Refract Surg. 2000;16:407–13. doi: 10.3928/1081-597X-20000701-03. [DOI] [PubMed] [Google Scholar]
- 9.Chalita MR, Chavala S, Xu M, Kreuger RR. Wavefront analysis is post-LASIK eyes and its correlation with visual symptoms, refraction and topography. Ophthalmology. 2004;111:447–53. doi: 10.1016/j.ophtha.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001;42:1396–403. [PubMed] [Google Scholar]
- 11.Mrochen M, Kaemmerer M, Mierdel P, Seiler T. Increased higher-order optical aberrations after laser refractive surgery: A problem of subclinical decentration. J Cataract Refract Surg. 2001;27:362–9. doi: 10.1016/s0886-3350(00)00806-3. [DOI] [PubMed] [Google Scholar]
- 12.Kanellopoulos AJ, Asemellis G. Keratoconus management: long term stability of topography-guided normalization combined with high-fluence CXL stabilization (the Athens Protocol) J Refract Surg. 2014;30:88–93. doi: 10.3928/1081597X-20140120-03. [DOI] [PubMed] [Google Scholar]
- 13.El Awady HE, Ghanem AA, Saleh SM. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: Comparative study of higher order aberrations. Ophthalmic Surg Lasers Imaging. 2011;42:314–20. doi: 10.3928/15428877-20110421-01. [DOI] [PubMed] [Google Scholar]


